Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Animal Model
2.3. Intraperitoneal Glucose Tolerance Test (ipGTT)
2.4. Analysis of Cellular Lipid Content
2.5. RNA Expression Analysis
2.6. (Immuno) Histological Analyses
2.7. HPLC Analysis of XN in Serum Samples
2.8. Statistical Analysis
3. Results
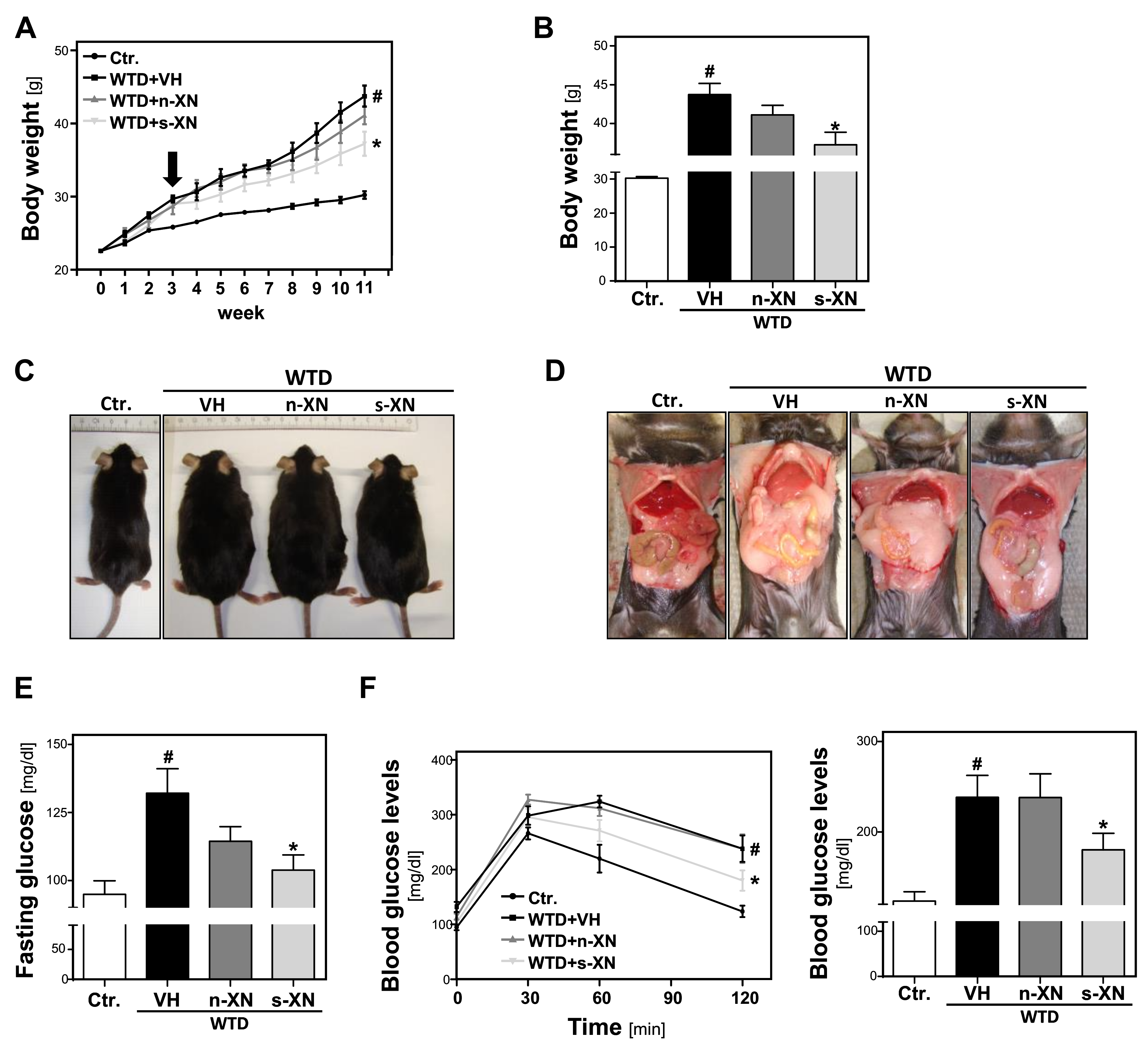
3.1. Effects of Solubilized Xanthohumol on Body Weight Gain in Mice Fed with a Western-Type Diet (WTD)
3.2. Serum Concentrations of Xanthohumol (XN) in Mice Fed with a Western-Type Diet
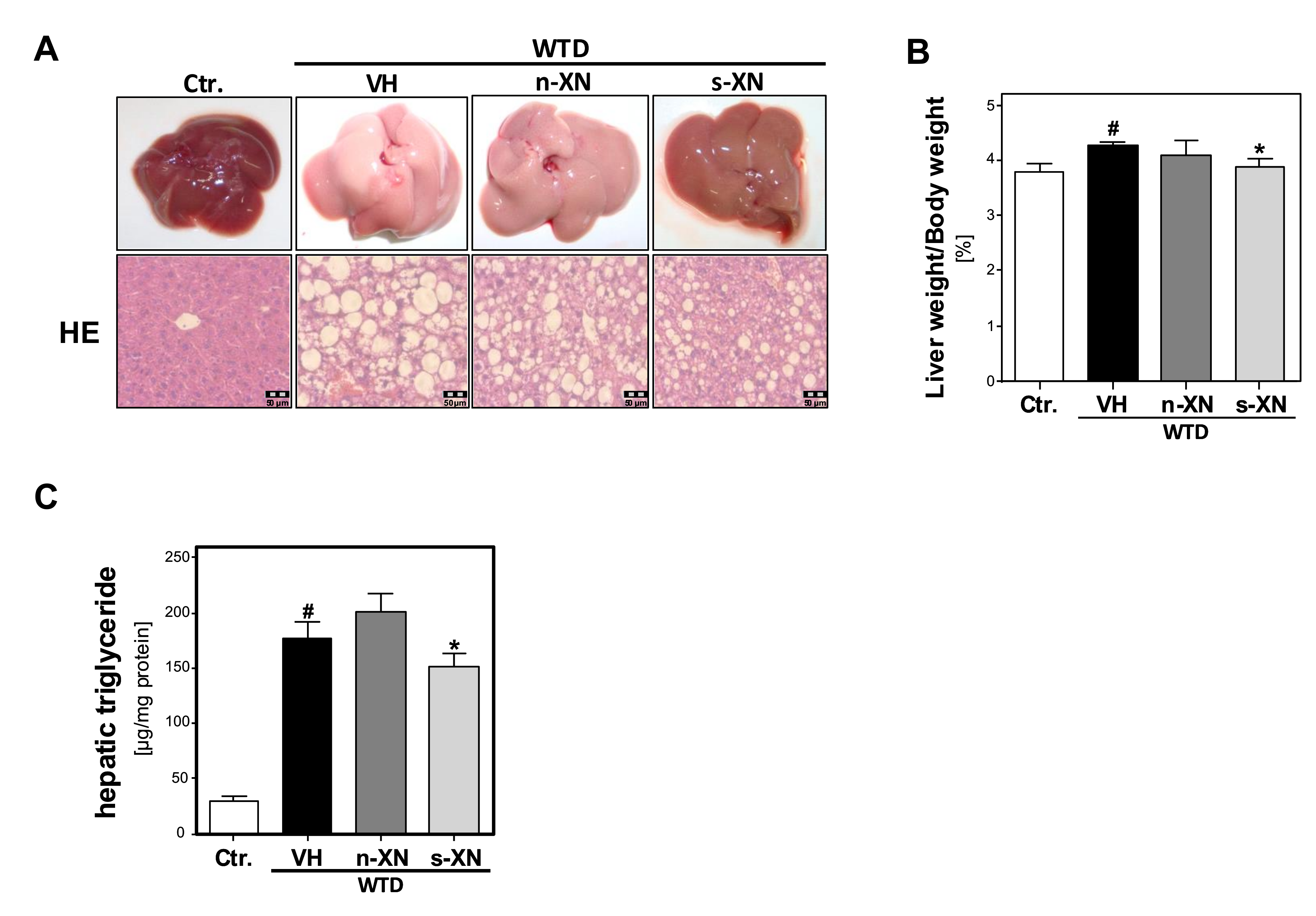
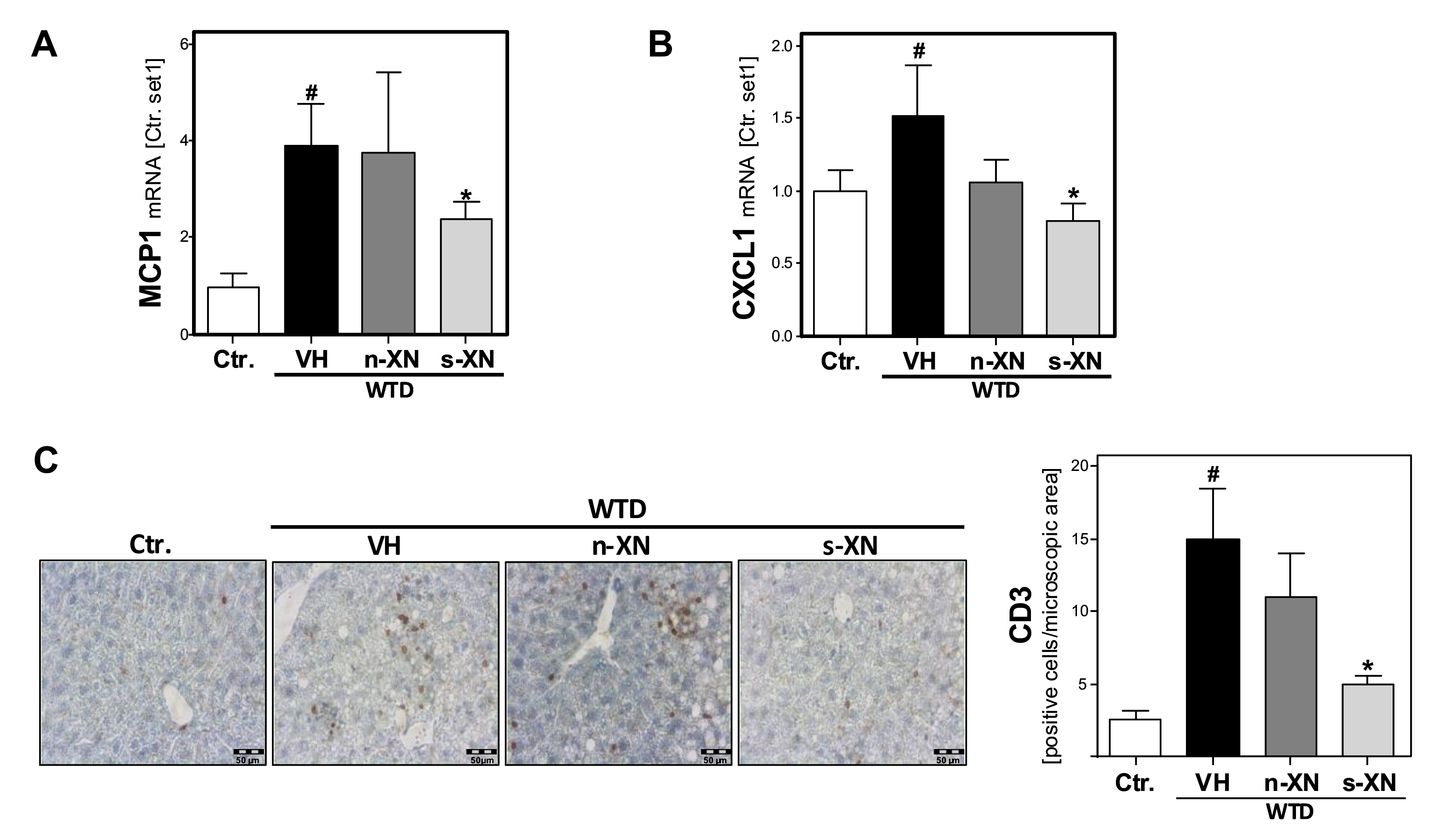
3.3. Effects of Solubilized Xanthohumol on Hepatic Steatosis, Inflammation and Fibrosis in Mice Fed with a Western-Type Diet
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Powell, E.E.; Cooksley, W.G.; Hanson, R.; Searle, J.; Halliday, J.W.; Powell, L.W. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology 1990, 11, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Anstee, Q.M.; Valenti, L. Genetic predisposition in NAFLD NASH: Impact on severity of liver disease response to treatment. Curr. Pharm. Des. 2013, 19, 5219–5238. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Yannakoulia, M.; Chan, J.L.; Mantzoros, C.S. Management of the metabolic syndrome type 2 diabetes through lifestyle modification. Annu. Rev. Nutr. 2009, 29, 223–256. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjostrom, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, K.J.; Allard, J.P. Clinical approaches to non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.B.; Cole, S.W.; Nuzum, D.S. Alternative dosing strategies for liraglutide in patients with type 2 diabetes mellitus. Am. J. Health Syst. Pharm. 2014, 71, 223–226. [Google Scholar] [CrossRef]
- Sus, N.; Schlienz, J.; Calvo-Castro, L.A.; Burkard, M.; Venturelli, S.; Busch, C.; Frank, J. Validation of a rapid and sensitive reversed-phase liquid chromatographic method for the quantification of prenylated chalcones and flavanones in plasma and urine. NFS J. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Mahli, A.; Weiskirchen, S.; Hellerbrand, C. The hop constituent xanthohumol exhibits hepatoprotective effects and inhibits the activation of hepatic stellate cells at different levels. Front. Physiol. 2015, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rodrigues, I.; Guardao, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhaes, J.; Ferreira-de-Almeida, M.; Negrao, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef]
- Legette, L.L.; Luna, A.Y.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Osada, K. Effect of dietary purified xanthohumol from Hop (Humulus lupulus L.) pomace on adipose tissue mass, fasting blood glucose level, and lipid metabolism in KK-Ay mice. J. Oleo Sci. 2017, 66, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Kraus, B.; Motyl, M.; Weiss, T.S.; Gehrig, M.; Scholmerich, J.; Heilmann, J.; Hellerbrand, C. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol. Nutr. Food Res. 2010, 54, S205–S213. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Heilmann, J.; Hellerbrand, C. Protective effect of xanthohumol on toxin-induced liver inflammation and fibrosis. Int. J. Clin. Exp. Pathol. 2012, 5, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Massinger, S.; Wuzik, A.; Heilmann, J.; Hellerbrand, C. Xanthohumol suppresses inflammatory response to warm ischemia-reperfusion induced liver injury. Exp. Mol. Pathol. 2013, 94, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, N.; Li, F.; Zhu, Q.; Liu, X.; Han, Q.; Wang, Y.; Chen, Y.; Zeng, X.; Lv, Y.; et al. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int. Immunopharmacol. 2013, 16, 466–474. [Google Scholar] [CrossRef]
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2014, 58, 248–255. [Google Scholar] [CrossRef]
- Dorn, C.; Engelmann, J.C.; Saugspier, M.; Koch, A.; Hartmann, A.; Muller, M.; Spang, R.; Bosserhoff, A.; Hellerbrand, C. Increased expression of c-Jun in nonalcoholic fatty liver disease. Lab. Investig. 2014, 94, 394–408. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The oral bioavailability of trans-resveratrol from a grapevine-shoot extract in healthy humans is significantly increased by micellar solubilization. Mol. Nutr. Food Res. 2018, 62, 1701057. [Google Scholar] [CrossRef]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial transport of curcumin in Caco-2 cells is significantly enhanced by micellar solubilisation. Plant Food Hum. Nutr. 2017, 72, 48–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kocher, A.; Bohnert, L.; Schiborr, C.; Frank, J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016, 60, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Koch, A.; Fresse, K.; Schiergens, T.; Thasler, W.E.; Schonberger, C.; Bergheim, I.; Bosserhoff, A.; Hellerbrand, C. Iso-alpha acids from hops (Humulus lupulus) inhibit hepatic steatosis, inflammation, and fibrosis. Lab. Investig. 2018, 98, 1614–1626. [Google Scholar] [CrossRef]
- Mahli, A.; Thasler, W.E.; Patsenker, E.; Muller, S.; Stickel, F.; Muller, M.; Seitz, H.K.; Cederbaum, A.I.; Hellerbrand, C. Identification of cytochrome CYP2E1 as critical mediator of synergistic effects of alcohol and cellular lipid accumulation in hepatocytes in vitro. Oncotarget 2015, 6, 41464–41478. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Mahli, A.; Freese, K.; Schiergens, T.S.; Kuecuekoktay, F.S.; Teufel, A.; Thasler, W.E.; Muller, M.; Bosserhoff, A.K.; Hellerbrand, C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget 2017, 8, 13059–13072. [Google Scholar] [CrossRef] [PubMed]
- Hellerbrand, C.; Muhlbauer, M.; Wallner, S.; Schuierer, M.; Behrmann, I.; Bataille, F.; Weiss, T.; Scholmerich, J.; Bosserhoff, A.K. Promoter-hypermethylation is causing functional relevant downregulation of methylthioadenosine phosphorylase (MTAP) expression in hepatocellular carcinoma. Carcinogenesis 2006, 27, 64–72. [Google Scholar] [CrossRef]
- Mahli, A.; Saugspier, M.; Koch, A.; Sommer, J.; Dietrich, P.; Lee, S.; Thasler, R.; Schulze-Luehrmann, J.; Luehrmann, A.; Thasler, W.E.; et al. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut 2018, 67, 746–756. [Google Scholar]
- Mahli, A.; Koch, A.; Czech, B.; Peterburs, P.; Lechner, A.; Haunschild, J.; Müller, M.; Hellerbrand, C. Hepatoprotective effect of oral application of a silymarin extract in carbon tetrachloride-induced hepatotoxicity in rats. Clin. Phytosci. 2015, 1, 5. [Google Scholar] [CrossRef]
- Arndt, S.; Wacker, E.; Dorn, C.; Koch, A.; Saugspier, M.; Thasler, W.E.; Hartmann, A.; Bosserhoff, A.K.; Hellerbrand, C. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut 2015, 64, 973–981. [Google Scholar] [CrossRef]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2012, 56, 466–474. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Gambino, R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies. Nat. Rev. Drug Discov. 2016, 15, 249–274. [Google Scholar] [CrossRef]
- Hellerbrand, C. Hepatic stellate cells—The pericytes in the liver. Pflüg. Arch. Eur. J. Physiol. 2013, 465, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; McKillop, I.H.; Schrum, L.W. Targeting collagen expression in alcoholic liver disease. World J. Gastroenterol. 2011, 17, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Riener, M.O.; Kirovski, G.; Saugspier, M.; Steib, K.; Weiss, T.S.; Gabele, E.; Kristiansen, G.; Hartmann, A.; Hellerbrand, C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2010, 3, 505–514. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Johnson, L.A.; de Montgolfier, O.; Elias, V.D.; Ullrich, L.S.; Hay, J.J.; Paraiso, I.L.; Choi, J.; Reed, R.L.; Revel, J.S.; et al. Non-estrogenic xanthohumol derivatives mitigate insulin resistance and cognitive impairment in high-fat diet-induced obese mice. Sci. Rep. 2018, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-A(y) mice. Biochem. Biophys. Res. Commun. 2005, 336, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Inoue, J.; Shimizu, M.; Sato, R. Xanthohumol improves diet-induced obesity and fatty liver by suppressing sterol regulatory element-binding protein (SREBP) activation. J. Biol. Chem. 2015, 290, 20565–20579. [Google Scholar] [CrossRef] [PubMed]
- Doddapattar, P.; Radovic, B.; Patankar, J.V.; Obrowsky, S.; Jandl, K.; Nusshold, C.; Kolb, D.; Vujic, N.; Doshi, L.; Chandak, P.G.; Goeritzer, M.; Ahammer, H.; et al. Xanthohumol ameliorates atherosclerotic plaque formation, hypercholesterolemia, and hepatic steatosis in ApoE-deficient mice. Mol. Nutr. Food Res. 2013, 57, 1718–1728. [Google Scholar] [PubMed]
- Yui, K.; Kiyofuji, A.; Osada, K. Effects of xanthohumol-rich extract from the hop on fatty acid metabolism in rats fed a high-fat diet. J. Oleo Sci. 2014, 63, 159–168. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahli, A.; Seitz, T.; Freese, K.; Frank, J.; Weiskirchen, R.; Abdel-Tawab, M.; Behnam, D.; Hellerbrand, C. Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease. Cells 2019, 8, 359. https://doi.org/10.3390/cells8040359
Mahli A, Seitz T, Freese K, Frank J, Weiskirchen R, Abdel-Tawab M, Behnam D, Hellerbrand C. Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease. Cells. 2019; 8(4):359. https://doi.org/10.3390/cells8040359
Chicago/Turabian StyleMahli, Abdo, Tatjana Seitz, Kim Freese, Jan Frank, Ralf Weiskirchen, Mona Abdel-Tawab, Dariush Behnam, and Claus Hellerbrand. 2019. "Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease" Cells 8, no. 4: 359. https://doi.org/10.3390/cells8040359
APA StyleMahli, A., Seitz, T., Freese, K., Frank, J., Weiskirchen, R., Abdel-Tawab, M., Behnam, D., & Hellerbrand, C. (2019). Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease. Cells, 8(4), 359. https://doi.org/10.3390/cells8040359





