1. Introduction
It is well established that gravity has had a great impact on the development of all organisms since the emergence of life [
1]. Indeed, mechanical stimuli mediated by gravity induce signal transduction pathways, affecting several cellular biological processes [
2]. More recently, attention has been focused on the unique medical problems of astronauts. During space flights, astronauts are exposed to several major stress stimuli, including microgravity and radiation. Astronauts exposed to microgravity for several weeks suffer from many health-related complications. Based on the observations of the three Skylab missions of 28, 56, and 84 days, each involving three astronauts, it was concluded that microgravity induced loss of bone density, muscle atrophy, and cardiovascular, hematic, metabolic, and endocrine complications, which are all associated with increased senescence [
1,
3]. More recently, it has become clear that both microgravity and radiation induce oxidative stress in various living organisms [
1,
3].
Notably, the mortality rate of Apollo astronauts was reported to be very high because of heart failure and myocardial infarction problems [
4,
5]. It is hoped that progress in aerospace medical research will minimize health risks for astronauts. In the long-term, the vision of future space settlement drives several scientific disciplines to investigate the influence of different gravity conditions on human life and health [
6]. However, there is a significant gap in developing strategies for fundamental pharmacological interventions for potential microgravity-induced health problems. One of the critical questions that remains to be evaluated is how therapeutics are effective under altered gravity. Fundamental medical pharmaceutical studies under space conditions are cost-intensive and challenging to plan, with very restricted spaceflight opportunities. However, microgravity in the range of 0.01
g can be achieved on parabolic flights, offering an alternative means to address pharmaceutical questions, at least under short-term altered gravity conditions (
Figure 1). In this scenario, a specially equipped aircraft repeats a parabolic flight path 31 times (completing 31 parabolas), in which each parabola consists of short periods (20–25-s) of 1.8
g (hypergravity), ~0.01
g (microgravity), and a second 1.8
g (hypergravity) period. After completion of the first parabola (P0), a 2-min 1
g-recovery period occurs, before starting on subsequent parabolas. In addition, between the 15th and 16th parabola, a 1
g-recovery phase of 8 min occurs.
Clearly, human-relevant pharmacological studies require human cellular models to monitor any potential effect of a compound on cellular function. However, there are experimental and ethical limitations to using ex vivo human cells. These limitations can be overcome by investigating functional somatic cell types, derived from human induced pluripotent stem cells (hiPSCs). It is well established that hiPSCs can be randomly differentiated into several somatic cells, including cardiomyocytes [
7]. Therefore, hiPSCs offer an unlimited cellular source of functional human cardiomyocytes. We performed fundamental pharmacological studies under different gravity conditions during the parabolic flight campaign by determining the beating rate (BR) of hiPSCs-derived cardiomyocytes (hCMs) in the presence and absence of different agonists, i.e., isoprenalin (ISO), which is a β-adrenoceptor agonist, and Bay-K8644 (Bay-K), which is an L-type Ca
2+ channel (LTCC) agonist. To measure the BR, we used two identical xCELLigence
® real-time cell analyzer (RTCA) Cardio Instruments (ACEA Biosciences, Inc., San Diego, CA, USA), one to monitor cardiomyocyte activity on the ground and another to monitor it during the parabolic flight. Cells were pre-incubated with the active compounds and loaded into both xCELLigence RTCA Cardio Instruments. Observations were carried out for the 3:30-h flight duration to evaluate the effect of repetitive cycles of acute hyper- and micro-gravity. Such fundamental and human-relevant experiments are urgently needed to determine the mechanisms of pharmacological agents targeting cardiovascular receptors and channels under altered gravity conditions, and to determine how microgravity affects cardiac functions. These studies contribute to the development of preventive strategies for astronauts.
2. Materials and Methods
2.1. Cardiomyocyte Cell Culture
Purified human iCell Cardiomyocytes® (Cellular Dynamics International, Madison, WI, USA), derived from hiPSCs, were used for all experiments. The cardiomyocytes were supplied as a cryopreserved single cell suspension with a ~98% pure population, comprising a mixture of electrically active, atrial-, nodal-, and ventricular-like myocytes. These cells exhibited the typical biochemical, electrophysiological, and mechanical characteristics of normal human heart cells, with expected responses on exposure to exogenous agents. For functional studies, cryopreserved hiPSC-CMs were thawed in iCell Cardiomyocyte Plating Medium (iCell-PM; Cellular Dynamics International, Madison, WI, USA) and directly plated on a fibronectin-coated (5 μg/cm2, 2 h at 37 °C) E-plate Cardio 96 (ACEA Biosciences, Inc.) at a cell density of approximately 25 × 103 cells per well, using iCell-PM. For other studies, thawed cells were plated on fibronectin-coated (5 μg/cm2, 2 h at 37 °C) 6-well or 96-well plates at a density of 25 × 103 cells per well, respectively. From Day 2 onwards, cells were maintained in iCell Cardiomyocyte Maintenance Medium (iCell-MM; Cellular Dynamics International), with a fresh medium change every 2 days. We observed beating activity 48 h after cell seeding. The hCMs were cultured in a standard cell culture incubator at 5% CO2 and 37 °C. Cardiomyocytes cultured for 48 h were treated with ISO (1 µM; Sigma-Aldrich, Corp., St. Louis, MO, USA), LTCC agonist Bay-K (5 μM; Sigma-Aldrich, Corp.), or LTCC blocker nifedipine (1 μM; Sigma-Aldrich, Corp.). These chemicals were added ~40 min prior to performing the parabolic flight experiments.
2.2. The xCELLigence RTCA Cardio Instrument
The xCELLigence RTCA Cardio Instrument is an impedance-based platform for monitoring the real-time beating function of the cardiomyocytes [
8]. The E-plate Cardio 96 (ACEA Biosciences, Inc.) xCELLigence plates were equilibrated using iCell-PM (50 μL per well) and inserted into the xCELLigence station to measure background impedance and to ensure that all wells and connections were working within acceptable limits. After equilibration, the cells were harvested and seeded at the required density. Impedance measurements were monitored at regular time intervals. The growth area on the E-plate Cardio 96 related to cell adhesion was defined as the Cell Index (CI). A high CI indicated more cell adhesion and higher cell viability. The raw data and statistical information, such as mean and standard error of the mean (SEM) for CI and BR values were acquired using RTCA Cardio software version 1.0 (ACEA Biosciences, Inc.).
2.3. Experimental Design
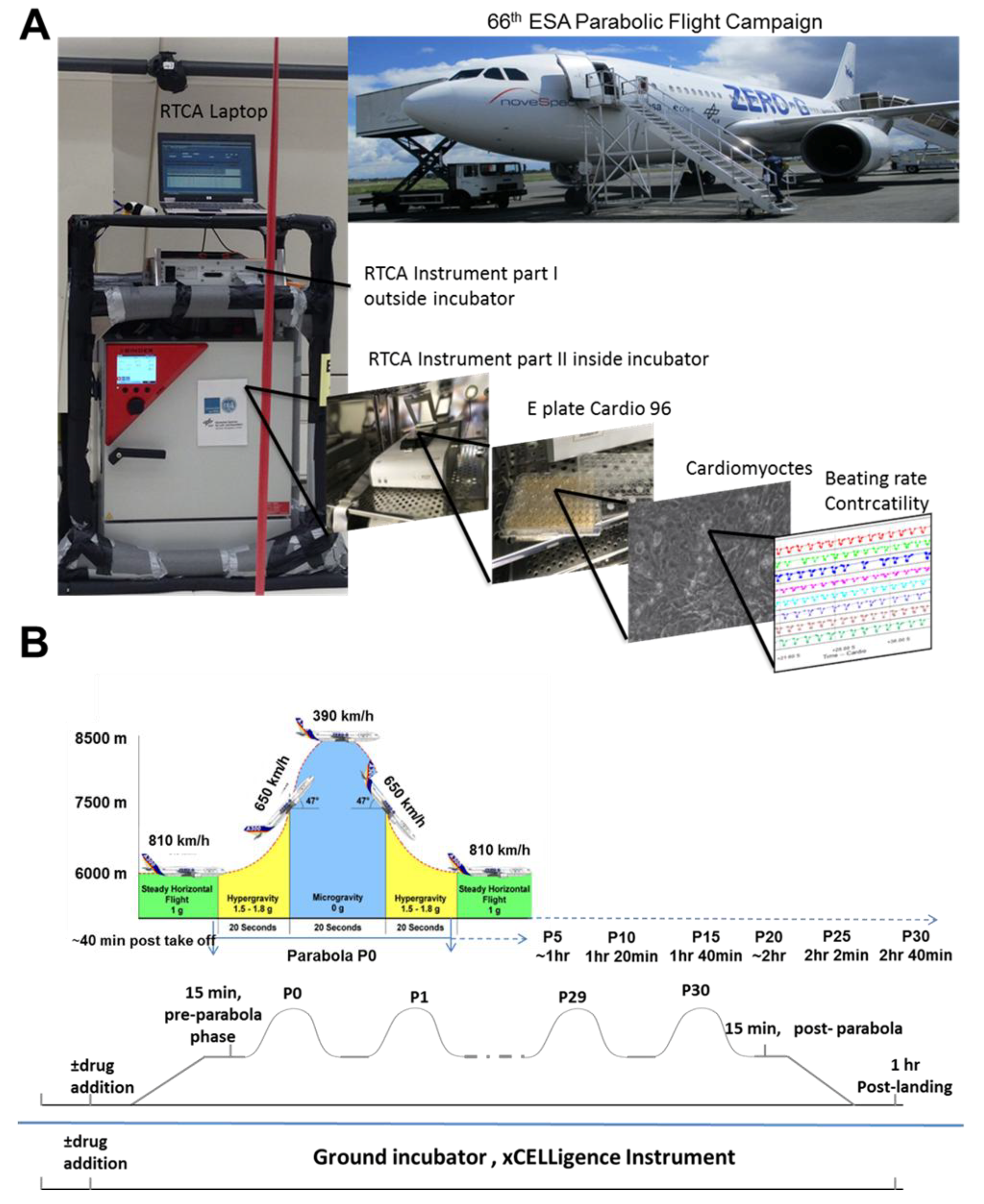
The parabolic flight experiments were performed using the Airbus A310 Zero-G (Novespace, Bordeaux Mérignac Airport, France) during the 66th Parabolic Flight Campaign of the European Space Agency. The experimental hardware consisted of a parabolic flight rack, including a normal cell culture incubator (CB 060; Binder, Tuttlingen, Germany) (
Figure 1A). The xCELLigence RTCA Cardio Instrument containing the hCMs was placed within the incubator. Changes in the BR of the hCMs were monitored with a laptop, using the RTCA Cardio software version 1.0 [
9]. A typical parabolic flight experiment comprised 31 parabolas (
Figure 1B), with alternating acceleration levels of regular gravity (1
g). Gravity conditions consisted of an approximately 22-s hypergravity phase (1.8
g), a 22-s microgravity phase (~0.01
g), followed by another 22-s hypergravity phase (1.8
g). The first maneuver of the Airbus A310 Zero-G to create a parabola always counts as the P0, while subsequent maneuvers are recorded as P1 to P30. One campaign consists of three consecutive flight days, with 31 parabolas carried out on each day. Ground experiments were carried out in parallel using a second xCELLigence RTCA Cardio Instrument.
2.4. Statistical Analysis
Our statistical analysis was based on Student’s t-test (p ≤ 0.05).
4. Discussion
Recently, embryonic stem cells have been used to study the effects of different small signaling molecules [
10] and microgravity [
11] on the embryonic development processes. To do this, we established in vitro methodologies allowing differentiation of mouse embryonic stem cells under simulated microgravity within a fast-rotating clinostat (clinorotation) [
12] and under parabolic flight conditions [
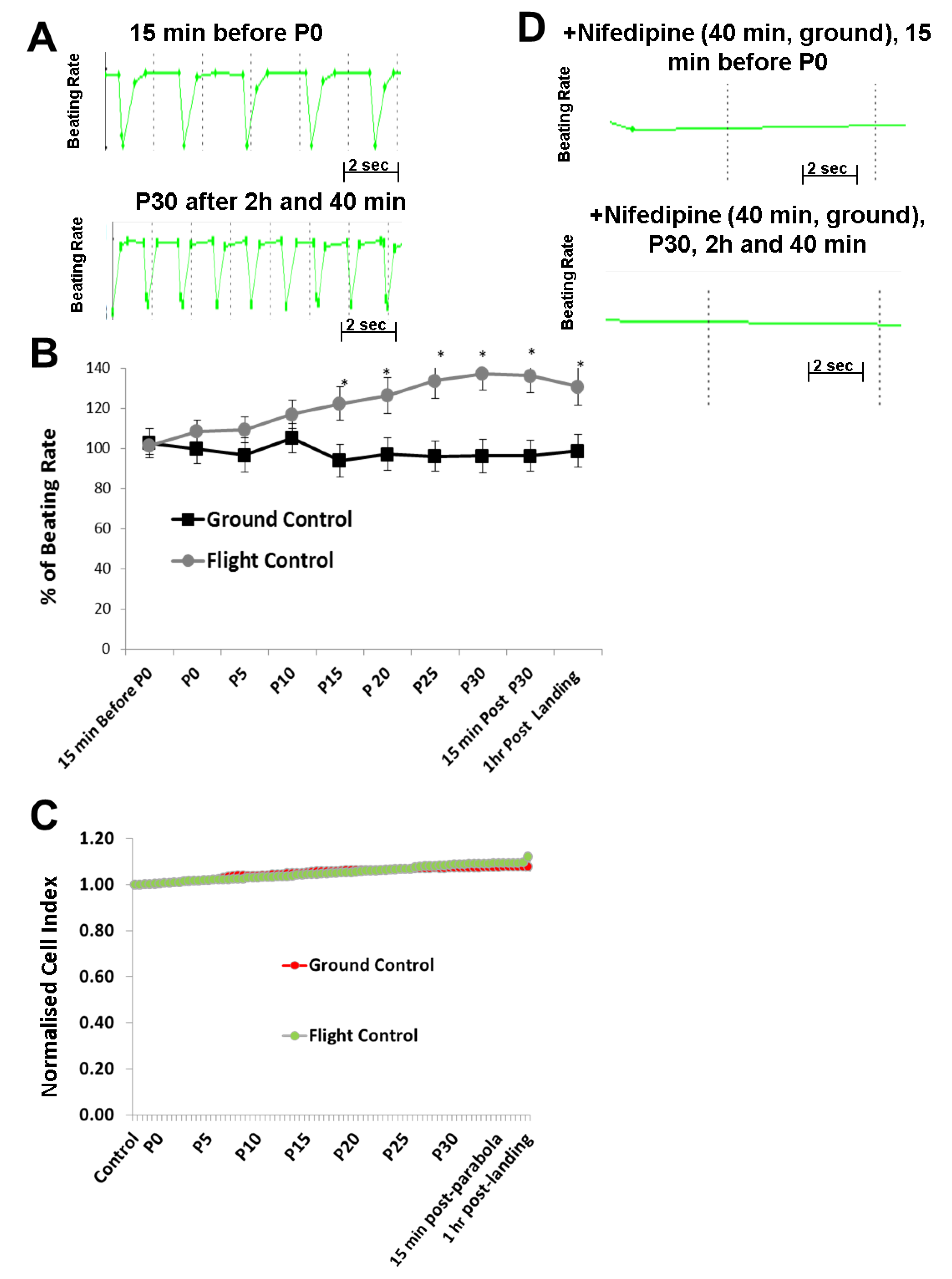
11]. Using these methods, we identified developmental processes affected by microgravity using microarray technologies. To determine the mechanisms of cardiovascular-acting drugs under different gravity conditions, hCMs were exposed to alternate short periods of hypergravity (22-s), microgravity (22-s), and hypergravity (22-s) during a single parabolic loop for 31 parabolas. During exposure of the hCMs to this increasing number of parabolas, the BR and CI, representing hCM functionality and viability, were monitored using the xCELLigence RTCA Cardio Instrument. In parallel, corresponding control experiments were carried out on the ground (1
g) using the same experimental set up. Interestingly, our results indicated that the BR of hCMs was significantly accelerated as the number of parabolic cycles increased. However, the Cl values were not affected, demonstrating that the mechanical stress did not modify hCMs viability. The BR was mediated by the LTCC, allowing an influx of extracellular Ca
2+ which normally binds to the ryanodine receptor of the sarcoplasmic reticulum, thereby inducing a massive Ca
2+ release from the sarcoplasmic reticulum, a process known as the Ca
2+-induced Ca
2+-release mechanism. In another set of the experiments, we preincubated the hCMs with nifedipine prior to the parabolic flight experiment. As expected, prior to lift off, nifedipine completely blocked the beating of the hCMs. Notably, no beating activity was observed during the parabolic flight in the presence of nifedipine, even after 31 parabolas. This finding clearly indicates that the elevation of the BR observed with an increasing number of parabolas was mediated by the LTCC. We surmise that the mechanical stress induced by the high number of parabolas increased the activity of the LTCC. This is supported by the observation that mechanical stress modulates the stability and activity of cardiomyocytes, e.g., acute mechanical stress induces opening of the LTCC [
13]. Mechanical stress also appears to induce elevation of cytosolic Ca
2+ in rat cardiomyocytes, mediated by the LTCC via the Ca
2+-induced Ca
2+-release mechanism [
14]. Therefore, we postulate that the mechanical stress induced by parabolic-flight-altered gravity conditions initiated an increase in BR via activation of the LTCC, although the detailed mechanism is yet to be determined.
Recently, it was demonstrated that gravity impacts on membrane fluidity, raising new ideas about how cells might experience gravity [
15]. Clearly, altered gravity (during parabolic flight conditions) alters membrane fluidity, voltage-gated K
+ channel activity, and signal transduction pathways mediated by some transmembrane proteins (e.g., receptors) [
16]. It is also well known that changes in membrane fluidity of the sarcoplasmic reticulum affect the activity of the ryanodine receptor, which releases Ca
2+ from the sarcoplasmic reticulum that regulates the BR of cardiomyocytes [
17]. Gravity-related changes in cell membranes are a very interesting aspect, but they do not provide an explanation for distinct changes in the activity for a specific channel, as observed herein for the LTCC in hCMs.
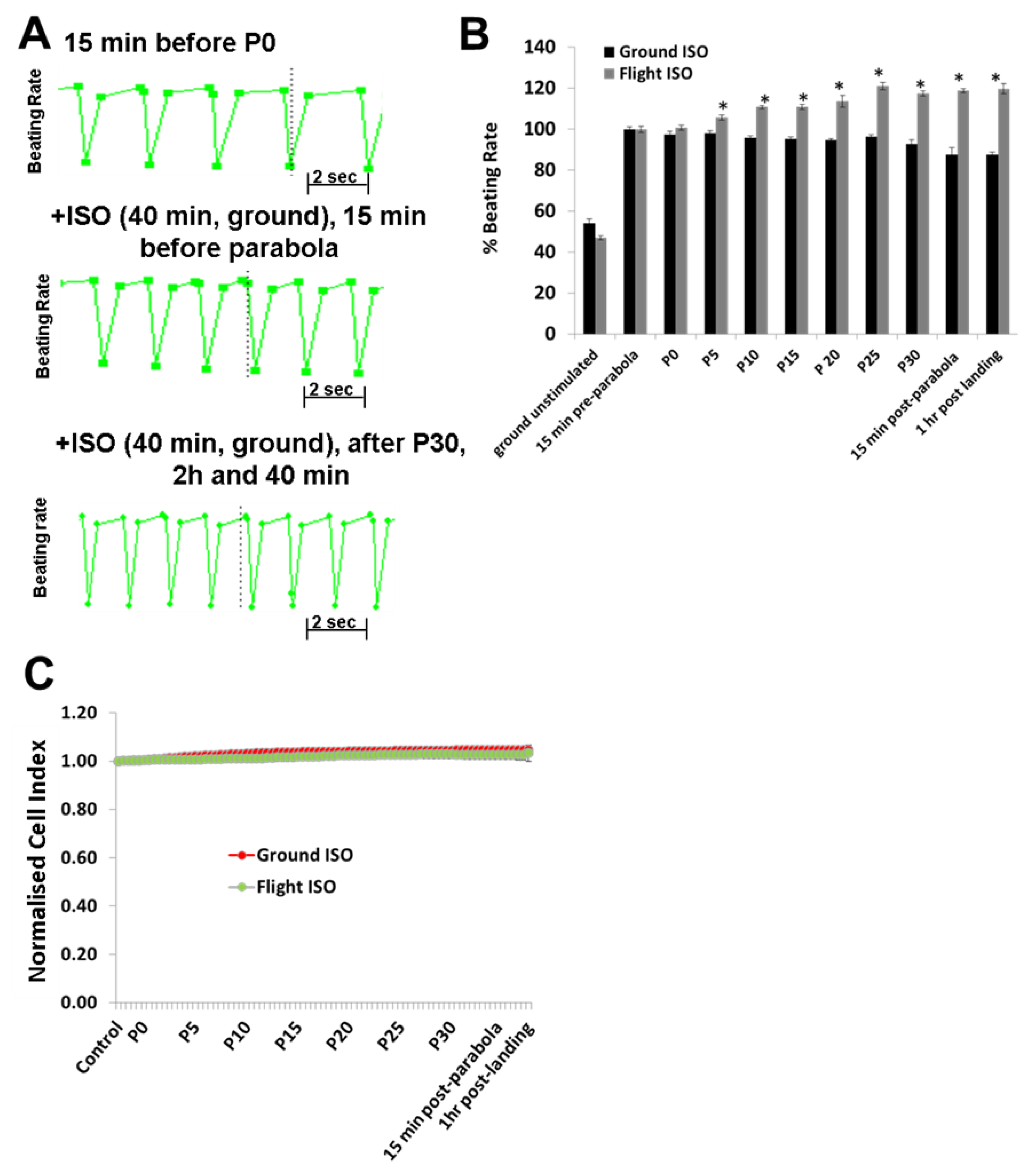
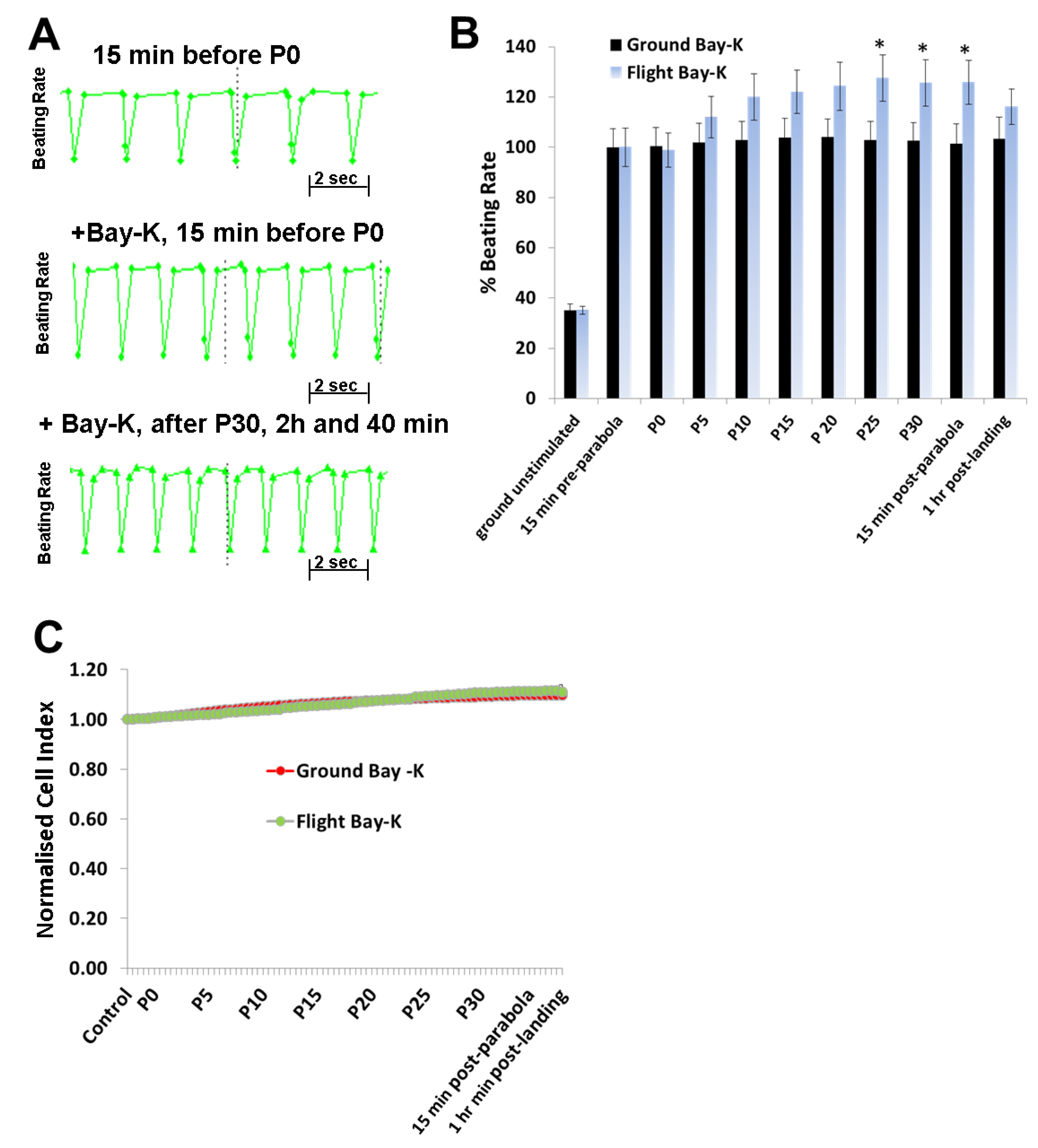
To investigate the effects of hyper- and micro-gravity on the interactions of ISO with the β-adrenoceptor of the cardiomyocytes and of Bay-K with the LTCC, we prestimulated the hCMs with agonists and exposed them to parabolic flight conditions. Both ISO and Bay-K increased the BR of the hCMs under 1
g ground control and pre-parabola conditions. However, we observed an additional increase of about 20% after 25 parabolas during the experiments. We assume that this additional effect is related to mechanical stress accumulated after 25 parabolas. We also investigated the effect of Carbachol (1 µM) on the BR under 1
g conditions. However, we did not observe any effects on the basal BR, although carbachol mitigated the additional effect of ISO (data not shown). Similar results with Carbachol were described recently [
18].
In conclusion, alternate hypergravity and microgravity phases increased the BR of hCMs, most probably mediated by the LTCC. Moreover, alternate hypergravity and microgravity phases had no effects on the interactions of agonists with the β-adrenoceptor or the LTCC, given that we only observed an additional stimulating effect of approximately 20%, which could be induced by mechanical stress during the parabola flight conditions.
Several experiments have been conducted with subjects exposed to parabolic flights. Standing subjects exposed to parabolic flight conditions indicated an increase of 12 to 16 heart beats per minute (bpm) within the first 1.8
g phase, a decrease of 27 to 15 bpm within the microgravity phase (0.01
g) and an increase of 20 to 24 bpm during the second 1.8
g phase [
19,
20,
21]. Interestingly, using the electrocardiography (ECG) method for determining the heart rate, an increase in the bpm was observed after completion of the 15 parabolas, with a maximum effect after completion of five parabolas [
19]. This study demonstrated that, although there were fluctuations in the bpm within the three phases of one parabolic cycle, the mean bpm was maximally increased after completion of five parabolas [
19]. These results can be explained by an activation of the sympathetic and competing parasympathetic neuronal systems during the parabolic flight, with a higher activity of the sympathetic system after at least 15 parabolas. Indeed, the in vivo neuronal mechanisms regulating the beating activity of the heart are completely absent in our in vitro system. However, although we did not observe differences in the BR over the three different phases of each parabola (
supplementary Figure S1), we found that the increase in the BR of hCMs was proportional to the number of the parabolas completed.
A clear understanding of the effects of microgravity on cardiomyocyte functions may only be obtained by culturing them under long-term (several weeks) microgravity conditions, which would need to be performed on the International Space Station. We believe that our pilot work under parabolic flight conditions provides worthwhile data for the design of experiments under a long-term microgravity environment. Such experiments would contribute to our ongoing understanding of the underlying molecular mechanisms of gravity-induced cardiomyocyte altered functioning and to the development of preventive pharmacological applications for microgravity-associated cardiovascular diseases.