Microrna-130a Downregulates HCV Replication through an atg5-Dependent Autophagy Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. MicroRNA Mimics, siRNAs, CRISPR/cas9 gRNAs, Plasmids and Transfection
2.3. RNA Isolation, Rt² Profiler™ Pcr Array and Gene Quantification
2.4. Western Blotting and Antibodies
2.5. Luciferase Reporter Assay
2.6. Statistical Analysis
3. Results
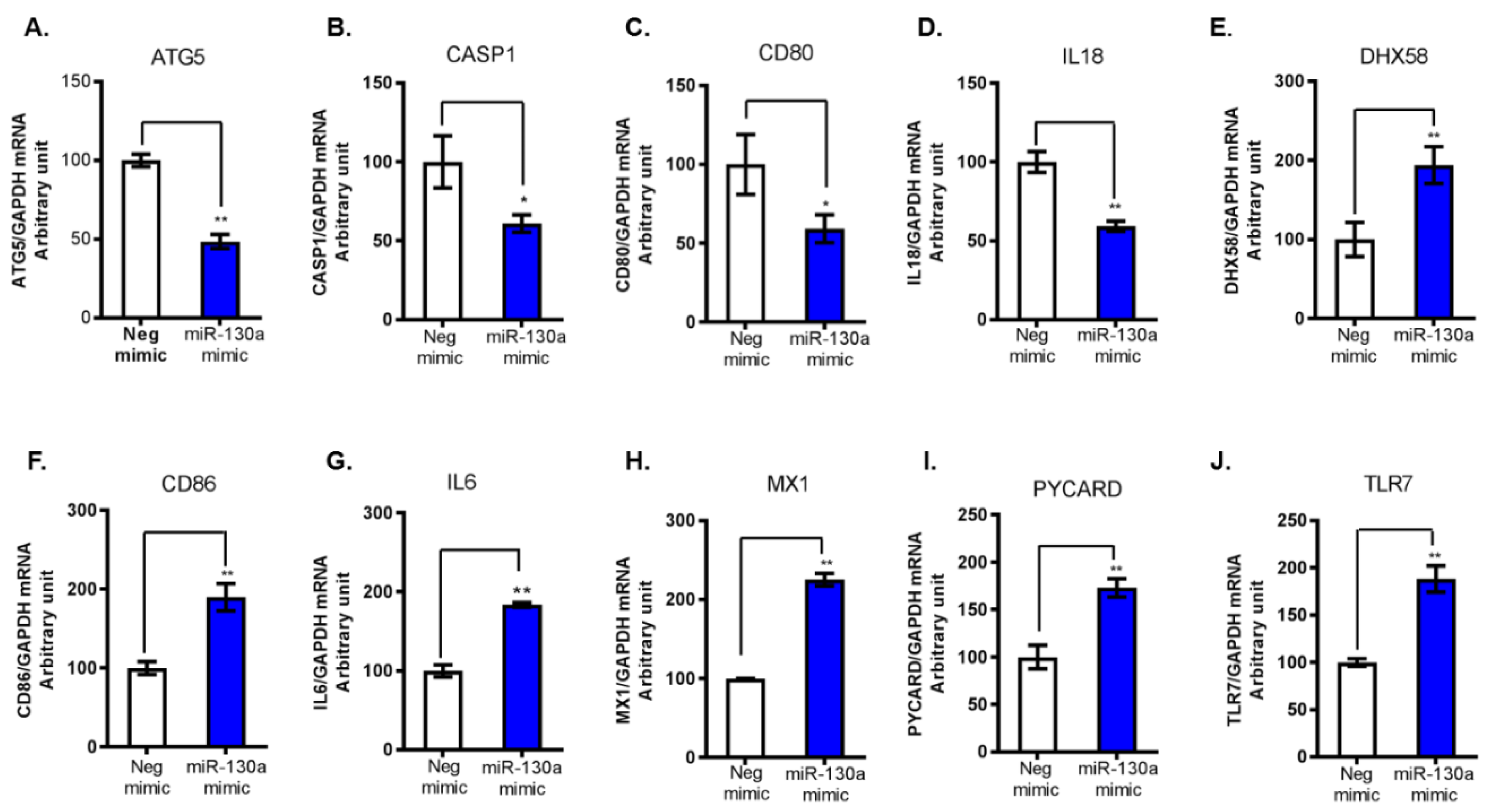
3.1. miR-130a Regulates Host Antiviral Response Genes
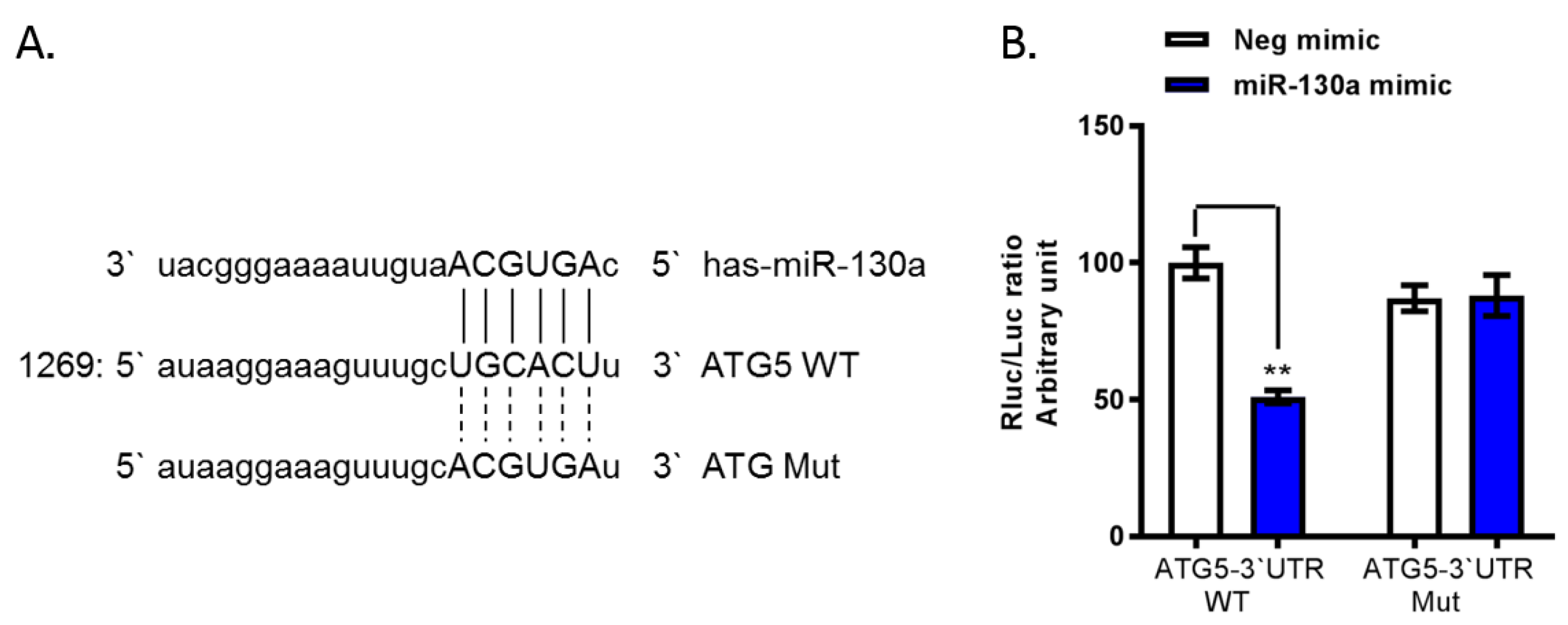
3.2. ATG5 Is a Target Gene for miR-130a
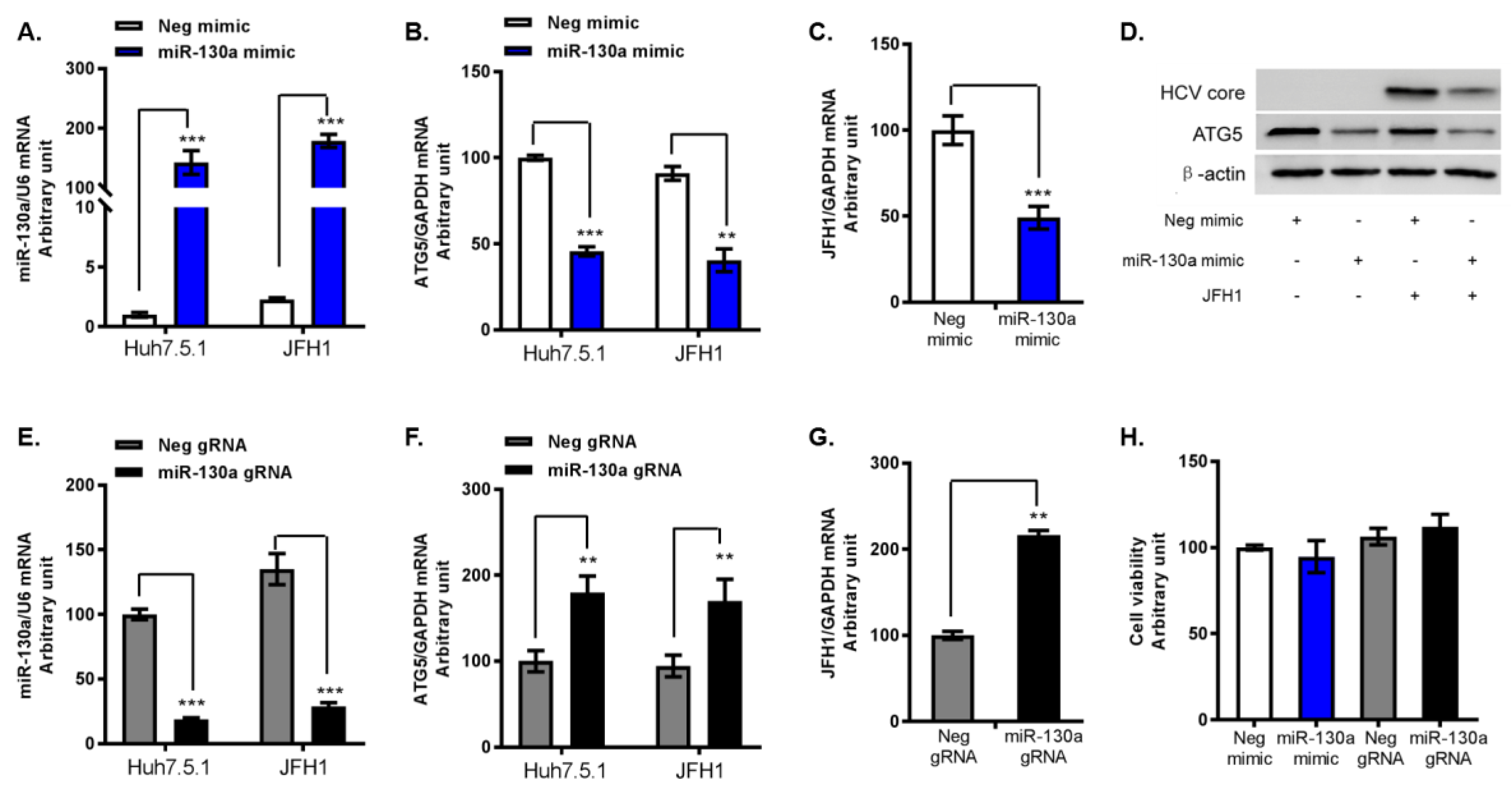
3.3. miR-130a Downregulates ATG5 Expression and HCV Replication
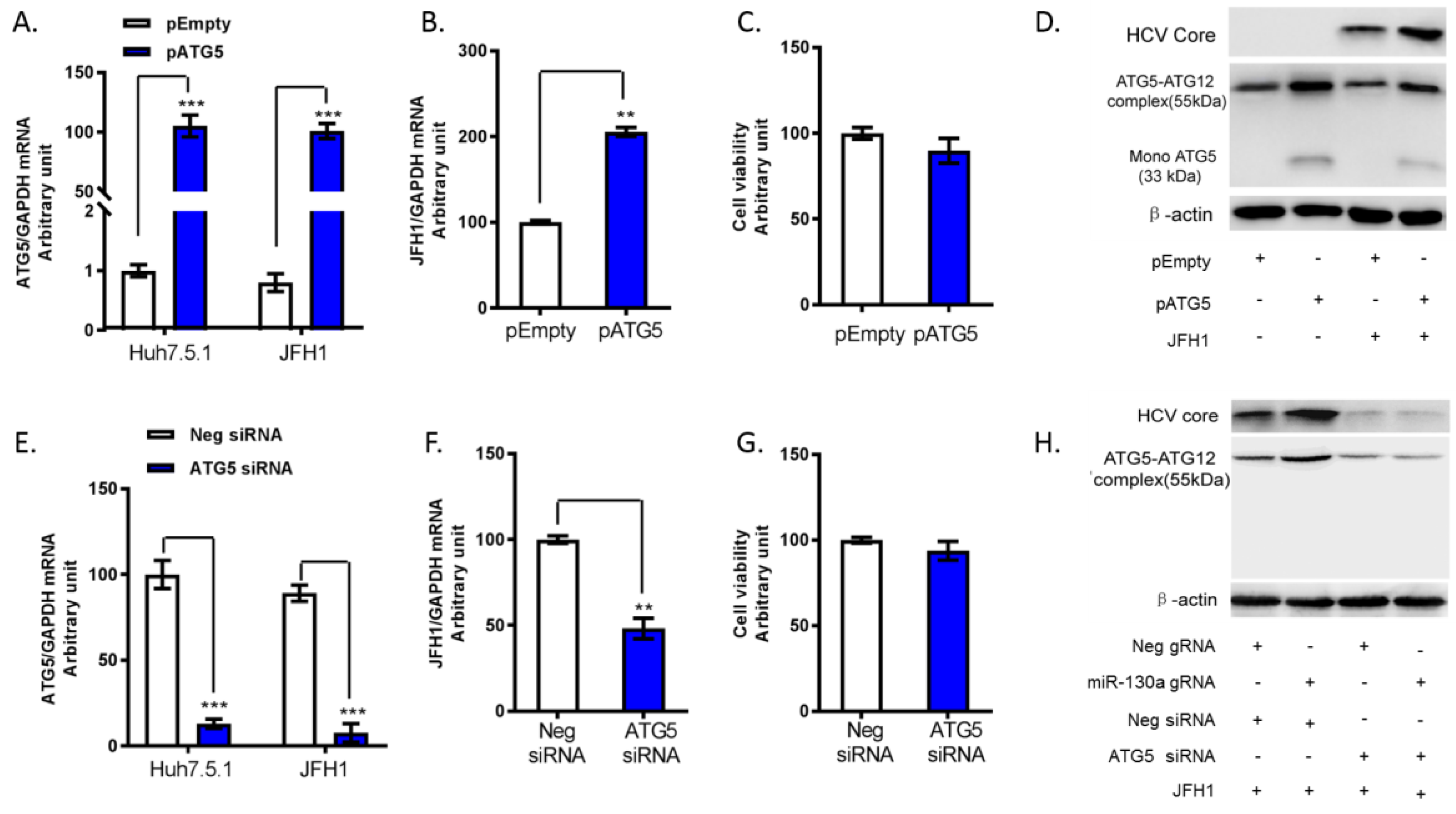
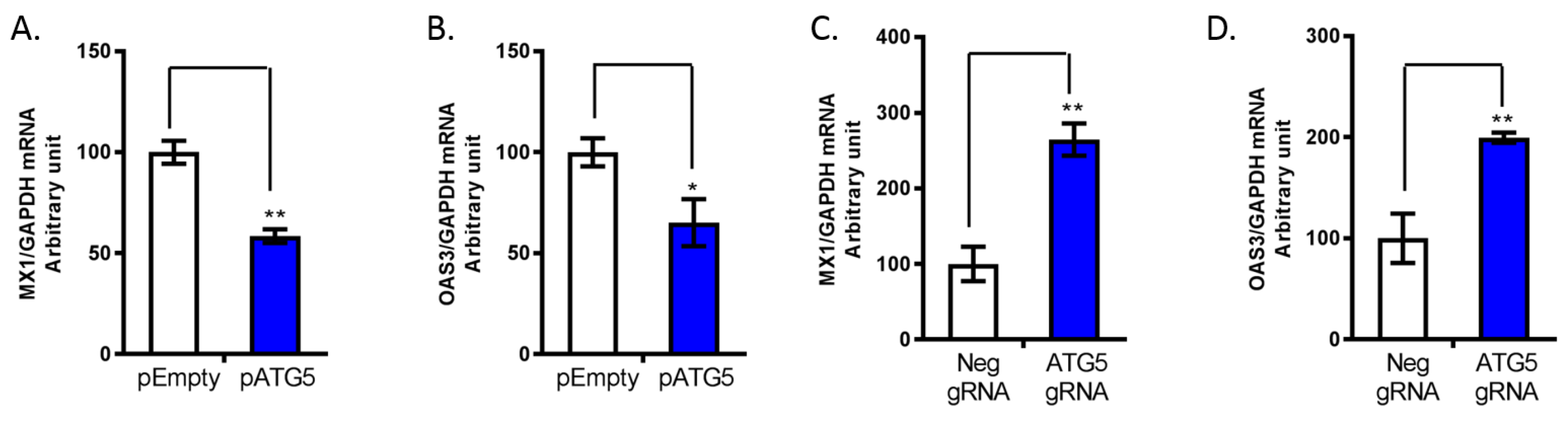
3.4. ATG5 Upregulates HCV Replication through Downregulation of ISG Expression
4. Discussion
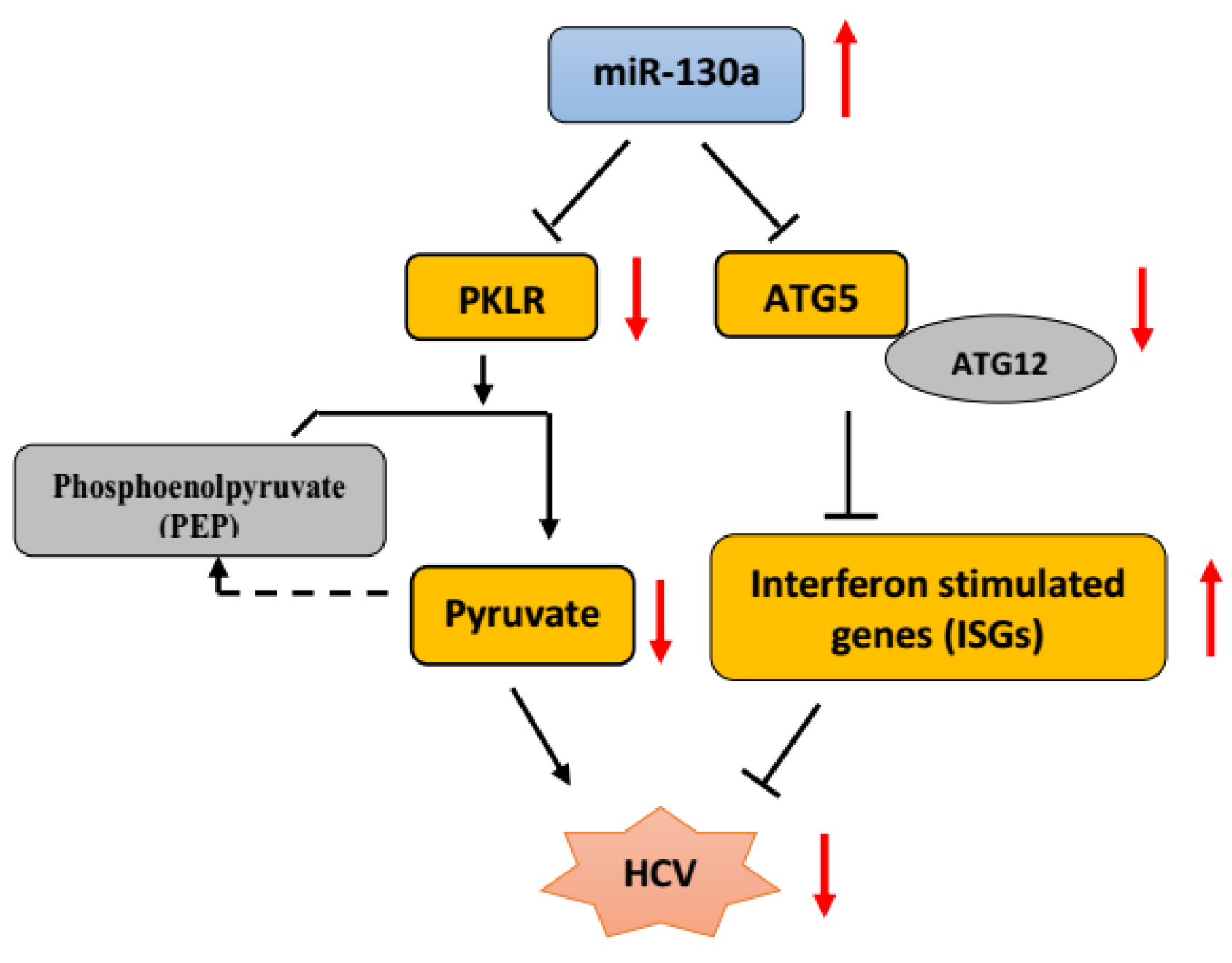
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Duan, X.; Holmes, J.A.; Li, W.; Lee, S.H.; Tu, Z.; Zhu, C.; Salloum, S.; Lidofsky, A.; Schaefer, E.A.; et al. A novel lncRNA regulates HCV infection through IFI6. Hepatology 2018. [Google Scholar] [CrossRef]
- Li, S.; Duan, X.; Li, Y.; Liu, B.; McGilvray, I.; Chen, L. MicroRNA-130a inhibits HCV replication by restoring the innate immune response. J. Viral Hepat. 2014, 21, 121–128. [Google Scholar] [CrossRef]
- Duan, X.; Li, S.; Holmes, J.A.; Tu, Z.; Li, Y.; Cai, D.; Liu, X.; Li, W.; Yang, C.; Jiao, B.; et al. MicroRNA-130a regulates both HCV and HBV replication through a central metabolic pathway. J. Virol. 2018. [Google Scholar] [CrossRef]
- Lai, X.; Wolkenhauer, O.; Vera, J. Understanding microRNA-mediated gene regulatory networks through mathematical modelling. Nucleic Acids Res. 2016, 44, 6019–6035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Akiyama, Y.; Yuasa, Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE 2013, 8, e62589. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Klionsky, D.J. Autophagy in health and disease: A double-edged sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Sir, D.; Kuo, C.F.; Tian, Y.; Liu, H.M.; Huang, E.J.; Jung, J.U.; Machida, K.; Ou, J.H. Replication of hepatitis C virus RNA on autophagosomal membranes. J. Biol. Chem. 2012, 287, 18036–18043. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Kuzmanovic, T.; Zhang, Y.; Peter, C.B.; Veleeparambil, M.; Chakravarti, R.; Sen, G.C.; Chattopadhyay, S. A new mechanism of interferon’s antiviral action: Induction of autophagy, essential for paramyxovirus replication, is inhibited by the interferon stimulated gene, TDRD7. PLoS Pathog. 2018, 14, e1006877. [Google Scholar] [CrossRef]
- Pratt, Z.L.; Sugden, B. How human tumor viruses make use of autophagy. Cells 2012, 1, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Choe, W.H.; Hiasa, Y.; Kamegaya, Y.; Blackard, J.T.; Schmidt, E.V.; Chung, R.T. Hepatitis C virus expression suppresses interferon signaling by degrading STAT1. Gastroenterology 2005, 128, 1034–1041. [Google Scholar] [PubMed]
- Lin, W.; Tsai, W.L.; Shao, R.X.; Wu, G.; Peng, L.F.; Barlow, L.L.; Chung, W.J.; Zhang, L.; Zhao, H.; Jang, J.Y.; et al. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology 2010, 138, 2509–2518, 2518 e2501. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kim, S.S.; Yeung, E.; Kamegaya, Y.; Blackard, J.T.; Kim, K.A.; Holtzman, M.J.; Chung, R.T. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J. Virol. 2006, 80, 9226–9235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xiao, F.; Hong, J.; Wang, K.; Liu, X.; Cai, D.; Fusco, D.N.; Zhao, L.; Jeong, S.W.; Brisac, C.; et al. EFTUD2 Is a Novel Innate Immune Regulator Restricting Hepatitis C Virus Infection through the RIG-I/MDA5 Pathway. J. Virol. 2015, 89, 6608–6618. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Zhu, C.; Hong, J.; Zhao, L.; Jilg, N.; Fusco, D.N.; Schaefer, E.A.; Brisac, C.; Liu, X.; Peng, L.F.; et al. The spliceosome factor SART1 exerts its anti-HCV action through mRNA splicing. J. Hepatol. 2015, 62, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Y.; Ou, J.H. HCV induces the expression of Rubicon and UVRAG to temporally regulate the maturation of autophagosomes and viral replication. PLoS Pathog. 2015, 11, e1004764. [Google Scholar] [CrossRef] [PubMed]
- Dreux, M.; Gastaminza, P.; Wieland, S.F.; Chisari, F.V. The autophagy machinery is required to initiate hepatitis C virus replication. Proc. Natl. Acad. Sci. USA 2009, 106, 14046–14051. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, P.; Blanchard, E.; Roingeard, P. Ultrastructural and biochemical analyses of hepatitis C virus-associated host cell membranes. J. Gen. Virol. 2010, 91, 2230–2237. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, A.M.; Labonte, P. The autophagy elongation complex (ATG5-12/16L1) positively regulates HCV replication and is required for wild-type membranous web formation. Sci. Rep. 2017, 7, 40351. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.; Devhare, P.; Sujijantarat, N.; Steele, R.; Kwon, Y.C.; Ray, R.; Ray, R.B. Knockdown of Autophagy Inhibits Infectious Hepatitis C Virus Release by the Exosomal Pathway. J. Virol. 2016, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanida, I.; Fukasawa, M.; Ueno, T.; Kominami, E.; Wakita, T.; Hanada, K. Knockdown of autophagy-related gene decreases the production of infectious hepatitis C virus particles. Autophagy 2009, 5, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Jounai, N.; Takeshita, F.; Kobiyama, K.; Sawano, A.; Miyawaki, A.; Xin, K.Q.; Ishii, K.J.; Kawai, T.; Akira, S.; Suzuki, K.; et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14050–14055. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Lund, J.M.; Ramanathan, B.; Mizushima, N.; Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 2007, 315, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Raychoudhuri, A.; Steele, R.; Ray, R.; Ray, R.B. Knockdown of autophagy enhances the innate immune response in hepatitis C virus-infected hepatocytes. Hepatology 2011, 53, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Meng, S.; Liu, B.; Li, M.Q.; Li, Y.; Fang, L.; Li, Y.G. MicroRNA-130a regulates autophagy of endothelial progenitor cells through Runx3. Clin. Exp. Pharmacol. Physiol. 2014, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, V.; Mora, R.; Park, Y.J.; Plass, C.; Chiramel, A.I.; Bartenschlager, R.; Dohner, H.; Stilgenbauer, S.; Pscherer, A.; Lichter, P.; et al. miRNA-130a targets ATG2B and DICER1 to inhibit autophagy and trigger killing of chronic lymphocytic leukemia cells. Cancer Res. 2012, 72, 1763–1772. [Google Scholar] [CrossRef]
- Matsushita, M.; Suzuki, N.N.; Obara, K.; Fujioka, Y.; Ohsumi, Y.; Inagaki, F. Structure of Atg5.Atg16, a complex essential for autophagy. J. Biol. Chem. 2007, 282, 6763–6772. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017, 23, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Flynt, A.S.; Lai, E.C. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat. Rev. Genet. 2008, 9, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidigal, J.A.; Ventura, A. The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol. 2015, 25, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Barathan, M.; Mohamed, R.; Yong, Y.K.; Kannan, M.; Vadivelu, J.; Saeidi, A.; Larsson, M.; Shankar, E.M. Viral Persistence and Chronicity in Hepatitis C Virus Infection: Role of T-Cell Apoptosis, Senescence and Exhaustion. Cells 2018, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Guevin, C.; Manna, D.; Belanger, C.; Konan, K.V.; Mak, P.; Labonte, P. Autophagy protein ATG5 interacts transiently with the hepatitis C virus RNA polymerase (NS5B) early during infection. Virology 2010, 405, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Perot, B.P.; Boussier, J.; Yatim, N.; Rossman, J.S.; Ingersoll, M.A.; Albert, M.L. Autophagy diminishes the early interferon-beta response to influenza A virus resulting in differential expression of interferon-stimulated genes. Cell Death Dis. 2018, 9, 539. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| ATG5 | TGTGCTTCGAGATGTGTGGTT | ACCAACGTCAAATAGCTGACTC |
| CASP1 | CAGCCCTGGTGTGGTGTG | AAAATCCTTCTCTATGTGGGCTTTC |
| CD80 | CACCTGGCTGAAGTGAC | GTCAGGCAGCATATCAC |
| IL18 | CTTCCAGATCGCTTCCTCTC | TCAAATAGAGGCCGATTTCC |
| DHX58 | GGGCCTCCAAACTCGATGG | TTCTGGGGTGACATGATGCAC |
| MX1 | GTTTCCGAAGTGGACATCGCA | GAAGGGCAACTCCTGACAGT |
| OAS3 | GAAGGAGTTCGTAGAGAAGGCG | CCCTTGACAGTTTTCAGCACC |
| PYCARD | TGGTCAGCTTCTACCTGGAG | CAGCCACTCAACGTTTGTGA |
| CD86 | GGGCCGCACAAGTTTTGA | GCCCTTGTCCTTGATCTGAA |
| IL6 | GACAACTTTGGCATTGTGG | ATGCAGGGATGATGTTCTG |
| TLR7 | TCCTTGGGGCTAGATGGTTTC | TCCACGATCACATGGTTCTTTG |
| JFH1 | TCTGCGGAACCGGTGAGTA | TCAGGCAGTACCACAAGGC |
| GAPDH | ACCTTCCCCATGGTGTCTGA | GCTCCTCCTGTTCGACAGTCA |
| Gene Name | Relative Expression * | Gene Name | Relative Expression |
|---|---|---|---|
| DHX58 | 4.27 | MYD88 | 1.65 |
| PYCARD | 3.44 | ISG15 | 1.63 |
| TLR7 | 2.99 | RELA | 1.63 |
| MX1 | 2.67 | DDX58 | 1.62 |
| CD86 | 2.38 | TRADD | 1.61 |
| IL6 | 2.3 | MAPK3 | 1.6 |
| PYDC1 | 1.95 | CASP10 | 1.58 |
| MAPK14 | 1.95 | CTSS | 1.58 |
| MAP3K1 | 1.95 | MAPK1 | 1.56 |
| IRAK1 | 1.95 | TBK1 | 1.55 |
| SPP1 | 1.9 | IRF5 | 1.54 |
| FADD | 1.85 | TLR9 | 1.53 |
| JUN | 1.83 | IL18 | 0.54 |
| NFKBIA | 1.77 | ATG5 | 0.55 |
| CXCL11 | 1.69 | CD80 | 0.65 |
| IKBKB | 1.66 | CASP1 | 0.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Liu, X.; Li, W.; Holmes, J.A.; Kruger, A.J.; Yang, C.; Li, Y.; Xu, M.; Ye, H.; Li, S.; et al. Microrna-130a Downregulates HCV Replication through an atg5-Dependent Autophagy Pathway. Cells 2019, 8, 338. https://doi.org/10.3390/cells8040338
Duan X, Liu X, Li W, Holmes JA, Kruger AJ, Yang C, Li Y, Xu M, Ye H, Li S, et al. Microrna-130a Downregulates HCV Replication through an atg5-Dependent Autophagy Pathway. Cells. 2019; 8(4):338. https://doi.org/10.3390/cells8040338
Chicago/Turabian StyleDuan, Xiaoqiong, Xiao Liu, Wenting Li, Jacinta A. Holmes, Annie J. Kruger, Chunhui Yang, Yujia Li, Min Xu, Haiyan Ye, Shuang Li, and et al. 2019. "Microrna-130a Downregulates HCV Replication through an atg5-Dependent Autophagy Pathway" Cells 8, no. 4: 338. https://doi.org/10.3390/cells8040338
APA StyleDuan, X., Liu, X., Li, W., Holmes, J. A., Kruger, A. J., Yang, C., Li, Y., Xu, M., Ye, H., Li, S., Liao, X., Sheng, Q., Chen, D., Shao, T., Cheng, Z., Kaj, B., Schaefer, E. A., Li, S., Chen, L., ... Chung, R. T. (2019). Microrna-130a Downregulates HCV Replication through an atg5-Dependent Autophagy Pathway. Cells, 8(4), 338. https://doi.org/10.3390/cells8040338






