Studying Heterotypic Cell–Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration
Abstract
:1. Introduction
2. The Physiological Role of Vascular Cells in the Central Nervous System
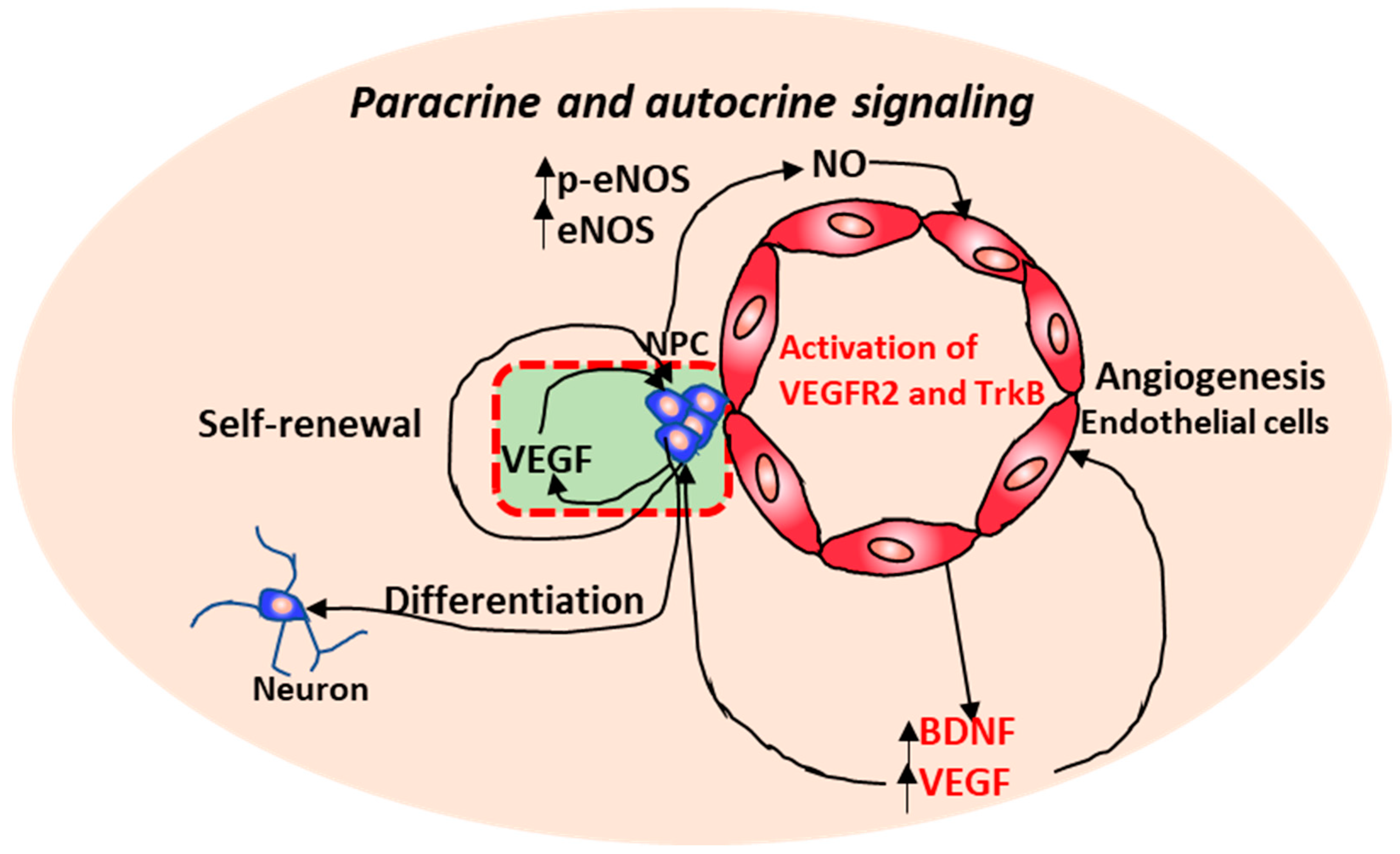
2.1. Neurovascular Interactions
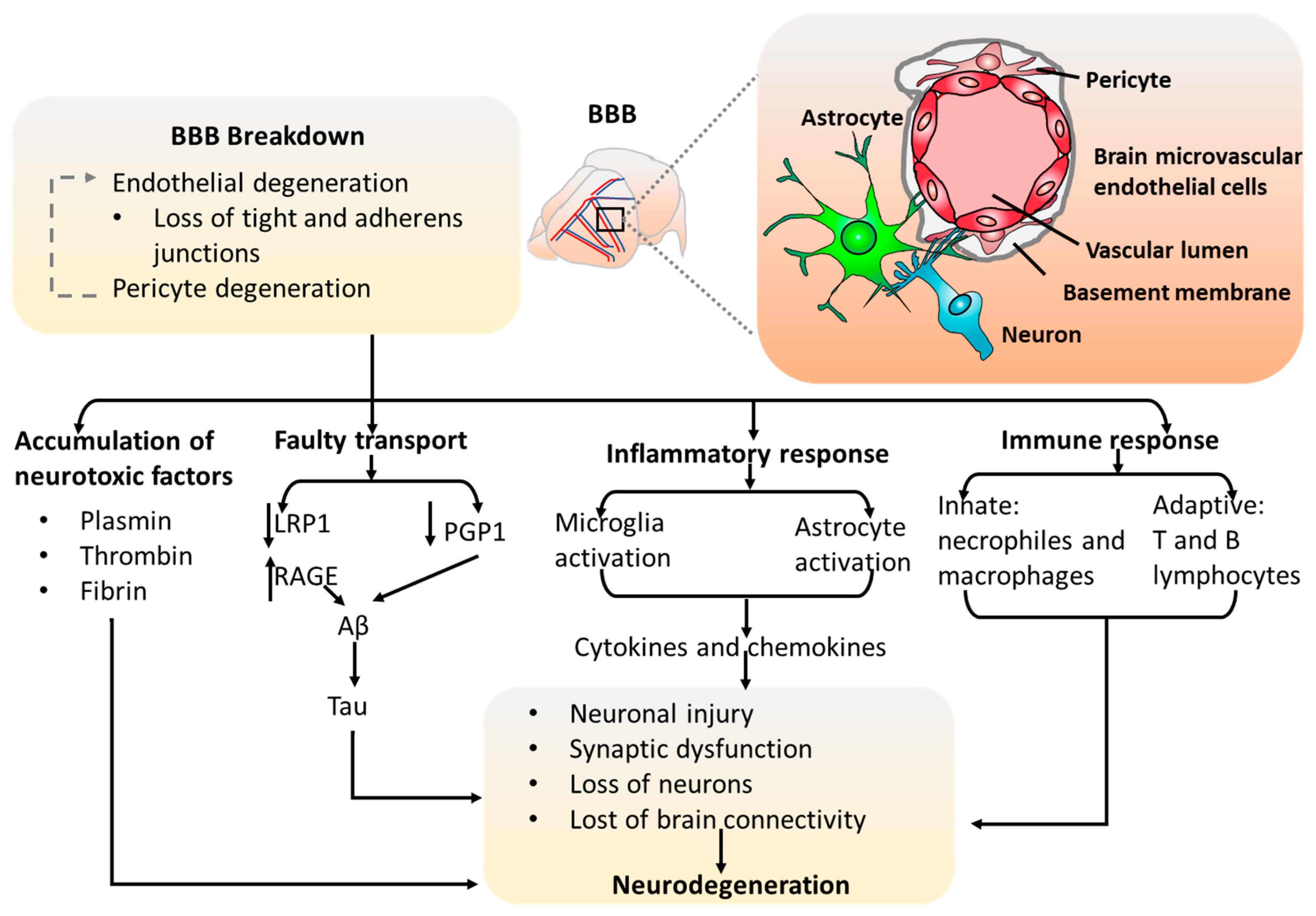
2.2. The Blood–Brain Barrier (BBB) Models
2.3. In Vitro Co-Culture Modeling of BBB Using Human-Induced Pluripotent Stem Cells (hiPSCs)
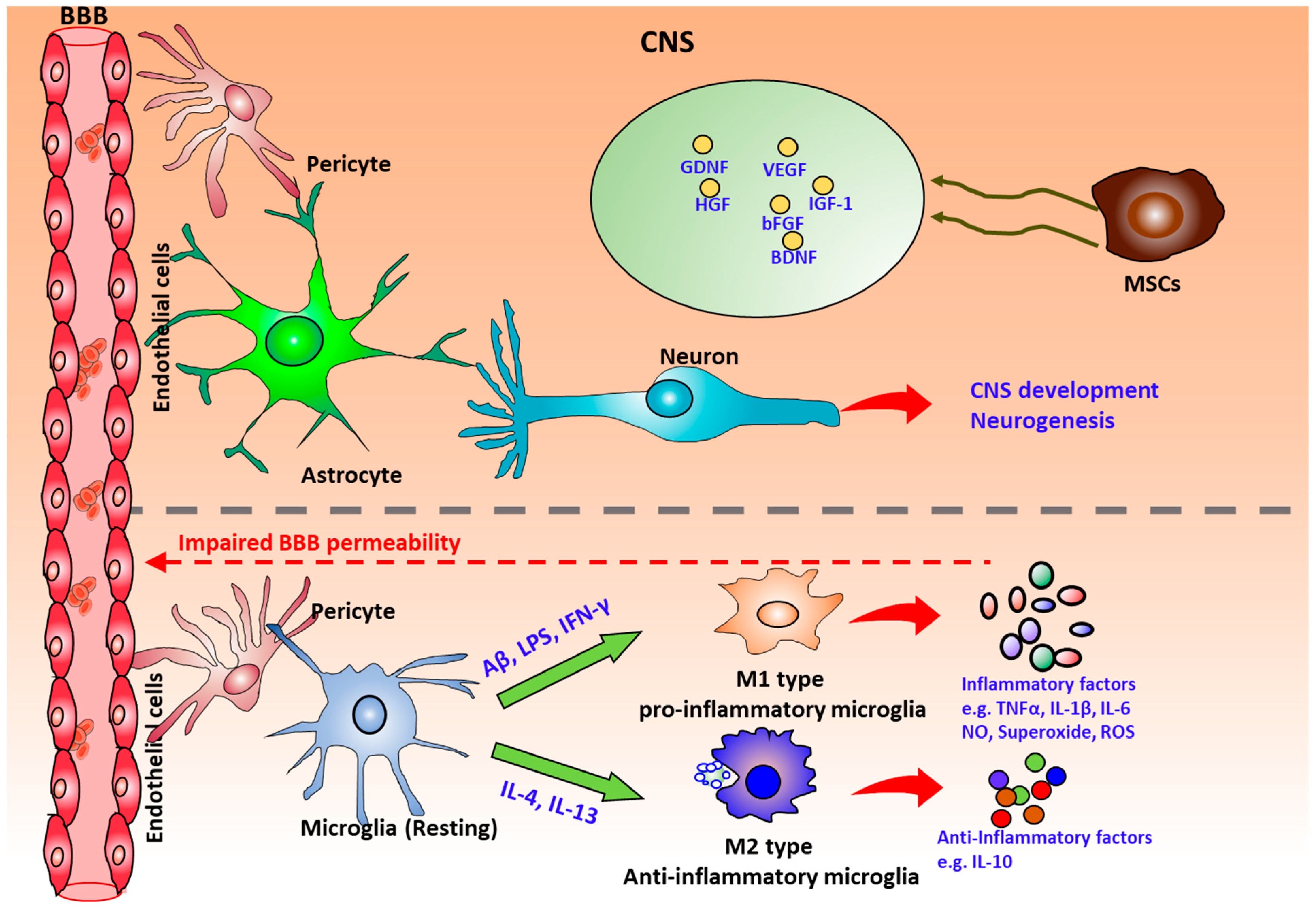
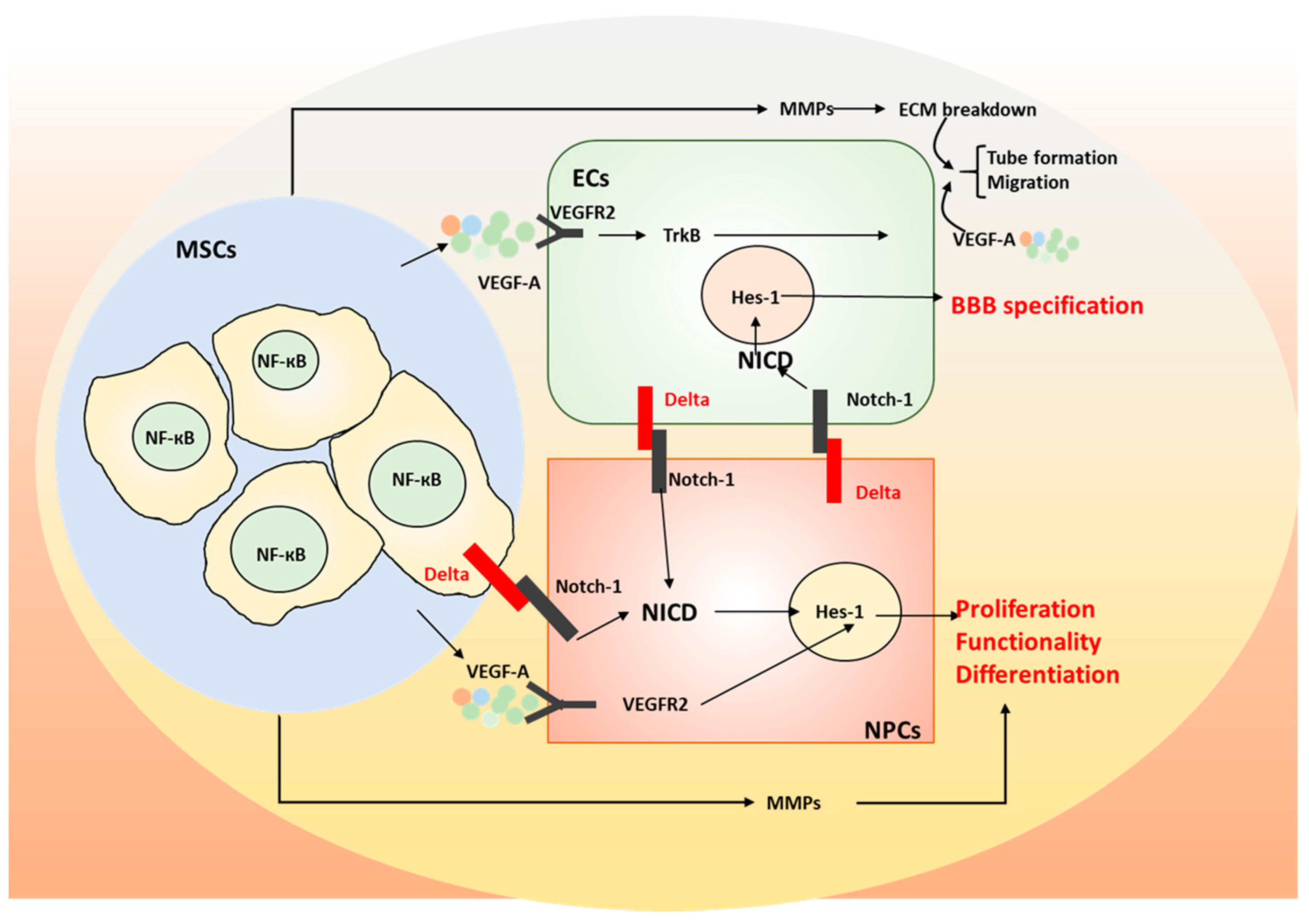
3. The Role of Mesenchymal Stem Cells (MSCs) on Neurodegeneration
4. The Physiological Role of Microglia in the Human Brain
4.1. Microglial Phenotype
4.2. The Role of Microglia in Alzheimer’s Disease
4.3. Generation of Microglia-Like Cells from Humna Pluripotent Stem Cells (hPSCs)
4.4. Neuronal-Microglial Crosstalk
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, A.A.; Goncalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.A.; Reumann, D.; Bian, S.; Levi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chen, J.; Kindberg, A.A.; Bendriem, R.M.; Spivak, C.E.; Williams, M.P.; Richie, C.T.; Handreck, A.; Mallon, B.S.; Lupica, C.R. CYP3A5 mediates effects of cocaine on human neocorticogenesis: Studies using an in vitro 3D self-organized hPSC model with a single cortex-like unit. Neuropsychopharmacology 2017, 42, 774. [Google Scholar] [CrossRef] [PubMed]
- Pasca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.J.; Elahi, L.S.; Pasca, A.M.; Marton, R.M.; Gordon, A.; Revah, O.; Miura, Y.; Walczak, E.M.; Holdgate, G.M.; Fan, H.C.; et al. Reliability of human cortical organoid generation. Nat. Methods 2019, 16, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.; Kanton, S.; Kyrousi, C.; Ayo-Martin, A.C.; Di Giaimo, R.; Riesenberg, S.; O’Neill, A.C.; Camp, J.G.; Tocco, C.; Santel, M.; et al. Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat. Med. 2019. [Google Scholar] [CrossRef]
- Stachowiak, E.K.; Benson, C.A.; Narla, S.T.; Dimitri, A.; Chuye, L.E.B.; Dhiman, S.; Harikrishnan, K.; Elahi, S.; Freedman, D.; Brennand, K.J.; et al. Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef]
- Ilieva, M.; Fex Svenningsen, A.; Thorsen, M.; Michel, T.M. Psychiatry in a Dish: Stem Cells and Brain Organoids Modeling Autism Spectrum Disorders. Biol. Psychiatry 2018, 83, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering stem cell organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.P. The rise of three-dimensional human brain cultures. Nature 2018, 553, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 2015, 16, 556–565. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449.e434. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Nzou, G.; Wicks, R.T.; Wicks, E.E.; Seale, S.A.; Sane, C.H.; Chen, A.; Murphy, S.V.; Jackson, J.D.; Atala, A.J. Human Cortex Spheroid with a Functional Blood Brain Barrier for High-Throughput Neurotoxicity Screening and Disease Modeling. Sci. Rep. 2018, 8, 7413. [Google Scholar] [CrossRef] [PubMed]
- Woodford, C.; Zandstra, P.W. Tissue engineering 2.0: Guiding self-organization during pluripotent stem cell differentiation. Curr. Opin. Biotechnol. 2012, 23, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Asthana, A.; Cheng, K.; Kisaalita, W.S. Neural cell 3D microtissue formation is marked by cytokines’ up-regulation. PLoS ONE 2011, 6, e26821. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev. 2015, 11, 288–297. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.-C.; Li, Y.; Ma, T. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev 2012, 21, 937–947. [Google Scholar] [CrossRef]
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159. [Google Scholar] [CrossRef]
- Xu, J.; Gong, T.; Heng, B.C.; Zhang, C.F. A systematic review: Differentiation of stem cells into functional pericytes. FASEB J. 2017, 31, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Faal, T.; Phan, D.T.T.; Davtyan, H.; Scarfone, V.M.; Varady, E.; Blurton-Jones, M.; Hughes, C.C.W.; Inlay, M.A. Induction of Mesoderm and Neural Crest-Derived Pericytes from Human Pluripotent Stem Cells to Study Blood-Brain Barrier Interactions. Stem Cell Rep. 2019, 12, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S.; Butler, J.M.; Ding, B.S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hammerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.A.; Argraves, W.S.; Gentile, C.; Neagu, A.; Forgacs, G.; Drake, C.J. Fusion of uniluminal vascular spheroids: A model for assembly of blood vessels. Dev. Dyn. 2010, 239, 398–406. [Google Scholar] [CrossRef]
- Moldovan, L.; Barnard, A.; Gil, C.H.; Lin, Y.; Grant, M.B.; Yoder, M.C.; Prasain, N.; Moldovan, N.I. iPSC-Derived Vascular Cell Spheroids as Building Blocks for Scaffold-Free Biofabrication. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from heart development: Biomimetic development of functional human cardiac organoids. Biomaterials 2017, 142, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Appelt-Menzel, A.; Cubukova, A.; Gunther, K.; Edenhofer, F.; Piontek, J.; Krause, G.; Stuber, T.; Walles, H.; Neuhaus, W.; Metzger, M. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Rep. 2017, 8, 894–906. [Google Scholar] [CrossRef]
- Canfield, S.G.; Stebbins, M.J.; Morales, B.S.; Asai, S.W.; Vatine, G.D.; Svendsen, C.N.; Palecek, S.P.; Shusta, E.V. An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J. Neurochem. 2017, 140, 874–888. [Google Scholar] [CrossRef]
- Lauschke, K.; Frederiksen, L.; Hall, V.J. Paving the Way Toward Complex Blood-Brain Barrier Models Using Pluripotent Stem Cells. Stem Cells Dev. 2017, 26, 857–874. [Google Scholar] [CrossRef] [Green Version]
- Ribecco-Lutkiewicz, M.; Sodja, C.; Haukenfrers, J.; Haqqani, A.S.; Ly, D.; Zachar, P.; Baumann, E.; Ball, M.; Huang, J.; Rukhlova, M.; et al. A novel human induced pluripotent stem cell blood-brain barrier model: Applicability to study antibody-triggered receptor-mediated transcytosis. Sci. Rep. 2018, 8, 1873. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Lloyd, A.F.; Davies, C.L.; Miron, V.E. Microglia: Origins, homeostasis, and roles in myelin repair. Curr. Opin. Neurobiol. 2017, 47, 113–120. [Google Scholar] [CrossRef]
- Claes, C.; Van den Daele, J.; Verfaillie, C.M. Generating tissue-resident macrophages from pluripotent stem cells: Lessons learned from microglia. Cell Immunol. 2018, 330, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler-Waldkirch, S.; d’Errico, P.; Sauer, J.F.; Erny, D.; Savanthrapadian, S.; Loreth, D.; Katzmarski, N.; Blank, T.; Bartos, M.; Prinz, M.; et al. Seed-induced Abeta deposition is modulated by microglia under environmental enrichment in a mouse model of Alzheimer’s disease. EMBO J. 2018, 37, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martinez-Cerdeno, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [Green Version]
- Pandya, H.; Shen, M.J.; Ichikawa, D.M.; Sedlock, A.B.; Choi, Y.; Johnson, K.R.; Kim, G.; Brown, M.A.; Elkahloun, A.G.; Maric, D. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 2017, 20, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Li, X.; Bao, X.; Wang, R. Microglia-targeted stem cell therapies for Alzheimer disease: A preclinical data review. J. Neurosci. Res. 2017, 95, 2420–2429. [Google Scholar] [CrossRef]
- Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. Modeling the blood-brain barrier: Beyond the endothelial cells. Curr. Opin. Biomed. Eng. 2018, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tsai, A.C.; Yuan, X.; Bejoy, J.; Sart, S.; Ma, T.; Li, Y. Neural differentiation of spheroids derived from human induced pluripotent stem cells-mesenchymal stem cells co-culture. Tissue Eng. Part A 2018, 24, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E. Grafted human-induced pluripotent stem-cell–derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallon, M.; Chang, J.; Zhang, H.; Kuo, C.J. Developmental and pathological angiogenesis in the central nervous system. Cell. Mol. Life Sci. 2014, 71, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-W.; Mo, J.-L.; Kou, Z.-W.; Liu, Q.; Lv, L.-L.; Lei, Y.; Sun, F.-Y. Neurovascular Interaction Promotes the Morphological and Functional Maturation of Cortical Neurons. Front. Cell. Neurosci. 2017, 11, 290. [Google Scholar] [CrossRef]
- Shen, Q.; Goderie, S.K.; Jin, L.; Karanth, N.; Sun, Y.; Abramova, N.; Vincent, P.; Pumiglia, K.; Temple, S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004, 304, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Sinden, J.D.; Couraud, P.-O.; Modo, M. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PLoS ONE 2014, 9, e106346. [Google Scholar] [CrossRef]
- Sosa, M.A.G.; De Gasperi, R.; Rocher, A.B.; Perez, G.M.; Simons, K.; Cruz, D.E.; Hof, P.R.; Elder, G.A. Interactions of primary neuroepithelial progenitor and brain endothelial cells: Distinct effect on neural progenitor maintenance and differentiation by soluble factors and direct contact. Cell Res. 2007, 17, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.E.; Kramer, L.L.; Livi, L.L.; Brown, T.; Moore, C.; Hoffman-Kim, D. A three-dimensional neural spheroid model for capillary-like network formation. J. Neurosci. Methods 2018, 299, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ford, M.C.; Lavik, E.B.; Madri, J.A. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J. Neurosci. Res. 2006, 84, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-W.; Duan, C.-L.; Chen, X.-H.; Wang, Y.-Q.; Sun, X.; Zhang, Q.-W.; Cui, H.-R.; Sun, F.-Y. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology 2016, 108, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Cui, H.-R.; Yang, S.-Z.; Sun, H.-P.; Qiu, M.-H.; Feng, X.-Y.; Sun, F.-Y. VEGF enhance cortical newborn neurons and their neurite development in adult rat brain after cerebral ischemia. Neurochem. Int. 2009, 55, 629–636. [Google Scholar] [CrossRef]
- Zhang, H.; Vutskits, L.; Pepper, M.S.; Kiss, J.Z. VEGF is a chemoattractant for FGF-2–stimulated neural progenitors. J. Cell Biol. 2003, 163, 1375–1384. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.-L.; Baker, K.; Han, J.; Calvo, C.; Nurmi, H.; Eichmann, A.C.; Alitalo, K. Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell. Mol. Life Sci. 2013, 70, 1779–1792. [Google Scholar] [CrossRef] [Green Version]
- Sandström, J.; Eggermann, E.; Charvet, I.; Roux, A.; Toni, N.; Greggio, C.; Broyer, A.; Monnet-Tschudi, F.; Stoppini, L. Development and characterization of a human embryonic stem cell-derived 3D neural tissue model for neurotoxicity testing. Toxicol. In Vitro 2017, 38, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wu, S.; Lin, H.; Kuss, M.A.; Lei, Y.; Krasnoslobodtsev, A.; Ahmed, S.; Zhang, C.; Kim, H.J.; Jiang, P.; et al. Establishment of a Human iPSC- and Nanofiber-based Microphysiological Blood-Brain Barrier System. ACS Appl. Mater. Interfaces 2018, 10, 21825–21835. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 7. [Google Scholar] [CrossRef]
- Katt, M.E.; Xu, Z.S.; Gerecht, S.; Searson, P.C. Human brain microvascular endothelial cells derived from the BC1 iPS cell line exhibit a blood-brain barrier phenotype. PLoS ONE 2016, 11, e0152105. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, M.J.; Lippmann, E.S.; Faubion, M.G.; Daneman, R.; Palecek, S.P.; Shusta, E.V. Activation of RARalpha, RARgamma, or RXRalpha Increases Barrier Tightness in Human Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells. Biotechnol. J. 2018, 13. [Google Scholar] [CrossRef]
- Clark, P.A.; Al-Ahmad, A.J.; Qian, T.; Zhang, R.R.; Wilson, H.K.; Weichert, J.P.; Palecek, S.P.; Kuo, J.S.; Shusta, E.V. Analysis of Cancer-Targeting Alkylphosphocholine Analogue Permeability Characteristics Using a Human Induced Pluripotent Stem Cell Blood-Brain Barrier Model. Mol. Pharm. 2016, 13, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, E.K.; Bailey, A.K.; Potharazu, A.V.; Neely, M.D.; Bowman, A.B.; Lippmann, E.S. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Maguire, S.E.; Canfield, S.G.; Bao, X.; Olson, W.R.; Shusta, E.V.; Palecek, S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017, 3, e1701679. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.G.; Quan, C.; Reyes-Ortiz, A.M.; Lutz, S.E.; Kedaigle, A.J.; Gipson, T.A.; Wu, J.; Vatine, G.D.; Stocksdale, J.; Casale, M.S.; et al. Huntington’s Disease iPSC-Derived Brain Microvascular Endothelial Cells Reveal WNT-Mediated Angiogenic and Blood-Brain Barrier Deficits. Cell Rep. 2017, 19, 1365–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Vatine, G.D.; Al-Ahmad, A.; Barriga, B.K.; Svendsen, S.; Salim, A.; Garcia, L.; Garcia, V.J.; Ho, R.; Yucer, N.; Qian, T.; et al. Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell 2017, 20, 831–843 e835. [Google Scholar] [CrossRef] [PubMed]
- Mantle, J.L.; Lee, K.H. A differentiating neural stem cell-derived astrocytic population mitigates the inflammatory effects of TNF-alpha and IL-6 in an iPSC-based blood-brain barrier model. Neurobiol. Dis. 2018, 119, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Donnes, P.; Sanchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolen, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. Stem Cells 2018, 36, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Xu, Z.; Farrell, A.M.; Bogorad, M.I.; Chu, C.; Walczak, P.; Cheng, L.; Mahairaki, V.; et al. Human iPSC-derived blood-brain barrier microvessels: Validation of barrier function and endothelial cell behavior. Biomaterials 2019, 190–191, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Sareen, D.; Gowing, G.; Sahabian, A.; Staggenborg, K.; Paradis, R.; Avalos, P.; Latter, J.; Ornelas, L.; Garcia, L.; Svendsen, C.N. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J. Comp. Neurol. 2014, 522, 2707–2728. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Shelley, B.C.; Hurley, A.M.; Onorati, M.; Castiglioni, V.; Patitucci, T.N.; Svendsen, S.P.; Mattis, V.B.; McGivern, J.V.; Schwab, A.J.; et al. EZ spheres: A stable and expandable culture system for the generation of pre-rosette multipotent stem cells from human ESCs and iPSCs. Stem Cell Res 2013, 10, 417–427. [Google Scholar] [CrossRef]
- DeStefano, J.G.; Jamieson, J.J.; Linville, R.M.; Searson, P.C. Benchmarking in vitro tissue-engineered blood-brain barrier models. Fluids Barriers CNS 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Büth, H.; Thielecke, H. A scaffold-free in vitro model for osteogenesis of human mesenchymal stem cells. Tissue Cell 2011, 43, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Bartosh, T.J.; Ylostalo, J.H.; Bazhanov, N.; Kuhlman, J.; Prockop, D.J. Dynamic compaction of human mesenchymal stem/precursor cells (MSC) into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6 and STC1). Stem Cells 2013, 31, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Sart, S.; Liu, Y.; Ma, T.; Li, Y. Microenvironment regulation of pluripotent stem cell-derived neural progenitor aggregates by human mesenchymal stem cell secretome. Tissue Eng. Part A 2014, 20, 2666–2679. [Google Scholar] [CrossRef]
- Hsieh, J.-Y.; Wang, H.-W.; Chang, S.-J.; Liao, K.-H.; Lee, I.-H.; Lin, W.-S.; Wu, C.-H.; Lin, W.-Y.; Cheng, S.-M. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE 2013, 8, e72604. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.P.; Foraker, J.E.; Ylostalo, J.; Prockop, D.J. Human stem/progenitor cells from bone marrow enhance glial differentiation of rat neural stem cells: A role for transforming growth factor β and Notch signaling. Stem Cells Dev. 2010, 20, 289–300. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, W.; Lou, Y.; Xie, A.; Lai, X.; Guo, F.; Deng, Z. Mesenchymal stem cells regulate the proliferation and differentiation of neural stem cells through Notch signaling. Cell Biol. Int. 2009, 33, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Barkho, B.Z.; Munoz, A.E.; Li, X.; Li, L.; Cunningham, L.A.; Zhao, X. Endogenous Matrix Metalloproteinase (MMP)-3 and MMP-9 Promote the Differentiation and Migration of Adult Neural Progenitor Cells in Response to Chemokines. Stem Cells 2008, 26, 3139–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, M.M.; Allen, M.; Bortolotto, V.; Lim, S.T.; Conant, K.; Grilli, M. The MMP-1/PAR-1 axis enhances proliferation and neuronal differentiation of adult hippocampal neural progenitor cells. Neural Plasticity 2015, 2015, 646595. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Antony, J.M.; Paquin, A.; Nutt, S.L.; Kaplan, D.R.; Miller, F.D. Endogenous microglia regulate development of embryonic cortical precursor cells. J. Neurosci. Res. 2011, 89, 286–298. [Google Scholar] [CrossRef]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.; Neumann, H. Role of microglia in neuronal degeneration and regeneration. Semin. Immunopathol. 2009, 31, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Melief, J.; Sneeboer, M.; Litjens, M.; Ormel, P.; Palmen, S.; Huitinga, I.; Kahn, R.; Hol, E.; de Witte, L. Characterizing primary human microglia: A comparative study with myeloid subsets and culture models. Glia 2016, 64, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.-C.; Zheng, H.; Kanekiyo, T.; Atagi, Y.; Jia, L.; Wang, D.; N’songo, A.; Can, D.; Xu, H. LRP1 modulates the microglial immune response via regulation of JNK and NF-κB signaling pathways. J. Neuroinflamm. 2016, 13, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, M.; Erny, D.; Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 2017, 18, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.-B.; Julius, D. The P2Y 12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005. [Google Scholar]
- Walker, D.G.; Lue, L.F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- Furube, E.; Kawai, S.; Inagaki, H.; Takagi, S.; Miyata, S. Brain Region-dependent Heterogeneity and Dose-dependent Difference in Transient Microglia Population Increase during Lipopolysaccharide-induced Inflammation. Sci. Rep. 2018, 8, 2203. [Google Scholar] [CrossRef]
- Pallas, M.; Camins, A. Molecular and biochemical features in Alzheimer’s disease. Curr. Pharm. Des. 2006, 12, 4389–4408. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, K.I.; Bachstetter, A.D.; Colonna, M.; Ginhoux, F.; Holmes, C.; Lamb, B.; Landreth, G.; Lee, D.C.; Low, D.; Lynch, M.A. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J. Neurochem. 2016, 138, 653–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellwig, S.; Masuch, A.; Nestel, S.; Katzmarski, N.; Meyer-Luehmann, M.; Biber, K. Forebrain microglia from wild-type but not adult 5xFAD mice prevent amyloid-β plaque formation in organotypic hippocampal slice cultures. Sci. Rep. 2015, 5, 14624. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.H.; Tavitian, B.; Consortium, I.N. Noninvasive molecular imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1393–1415. [Google Scholar] [CrossRef] [PubMed]
- Douvaras, P.; Sun, B.; Wang, M.; Kruglikov, I.; Lallos, G.; Zimmer, M.; Terrenoire, C.; Zhang, B.; Gandy, S.; Schadt, E. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Rep. 2017, 8, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.-H.; Aubourg, P.; Ransohoff, R.M. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- McQuade, A.; Coburn, M.; Tu, C.H.; Hasselmann, J.; Davtyan, H.; Blurton-Jones, M. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol. Neurodegener. 2018, 13, 67. [Google Scholar] [CrossRef]
- Pocock, J.M.; Piers, T.M. Modelling microglial function with induced pluripotent stem cells: An update. Nat. Rev. Neurosci. 2018, 19, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 2017, 94, 278–293.e279. [Google Scholar] [CrossRef]
- Takata, K.; Kozaki, T.; Lee, C.Z.W.; Thion, M.S.; Otsuka, M.; Lim, S.; Utami, K.H.; Fidan, K.; Park, D.S.; Malleret, B.; et al. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity 2017, 47, 183–198 e186. [Google Scholar] [CrossRef]
- Brownjohn, P.W.; Smith, J.; Solanki, R.; Lohmann, E.; Houlden, H.; Hardy, J.; Dietmann, S.; Livesey, F.J. Functional Studies of Missense TREM2 Mutations in Human Stem Cell-Derived Microglia. Stem Cell Rep. 2018, 10, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Ormel, P.R.; Vieira de Sa, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Sheridan, S.D.; Gracias, J.; Xuan, D.; Fu, T.; Perlis, R.H. Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors. Mol. Psychiatry 2017, 22, 170–177. [Google Scholar] [CrossRef]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Limatola, C.; Lauro, C.; Catalano, M.; Ciotti, M.T.; Bertollini, C.; Di Angelantonio, S.; Ragozzino, D.; Eusebi, F. Chemokine CX3CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J. Neuroimmunol. 2005, 166, 19–28. [Google Scholar] [CrossRef]
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Targeting microglia for the treatment of Alzheimer’s disease. Exp. Opin. Ther. Targets 2015, 19, 497–506. [Google Scholar] [CrossRef]
- Pankiewicz, J.E.; Baquero-Buitrago, J.; Sanchez, S.; Lopez-Contreras, J.; Kim, J.; Sullivan, P.M.; Holtzman, D.M.; Sadowski, M.J. APOE genotype differentially modulates effects of anti-Aβ, passive immunization in APP transgenic mice. Mol. Neurodegener. 2017, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Richard, J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Crotti, A.; Benner, C.; Kerman, B.E.; Gosselin, D.; Lagier-Tourenne, C.; Zuccato, C.; Cattaneo, E.; Gage, F.H.; Cleveland, D.W.; Glass, C.K. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat. Neurosci. 2014, 17, 513. [Google Scholar] [CrossRef] [PubMed]
- O’rourke, J.; Bogdanik, L.; Yáñez, A.; Lall, D.; Wolf, A.; Muhammad, A.; Ho, R.; Carmona, S.; Vit, J.; Zarrow, J. C9orf72 is required for proper macrophage and microglial function in mice. Science 2016, 351, 1324–1329. [Google Scholar] [CrossRef] [Green Version]
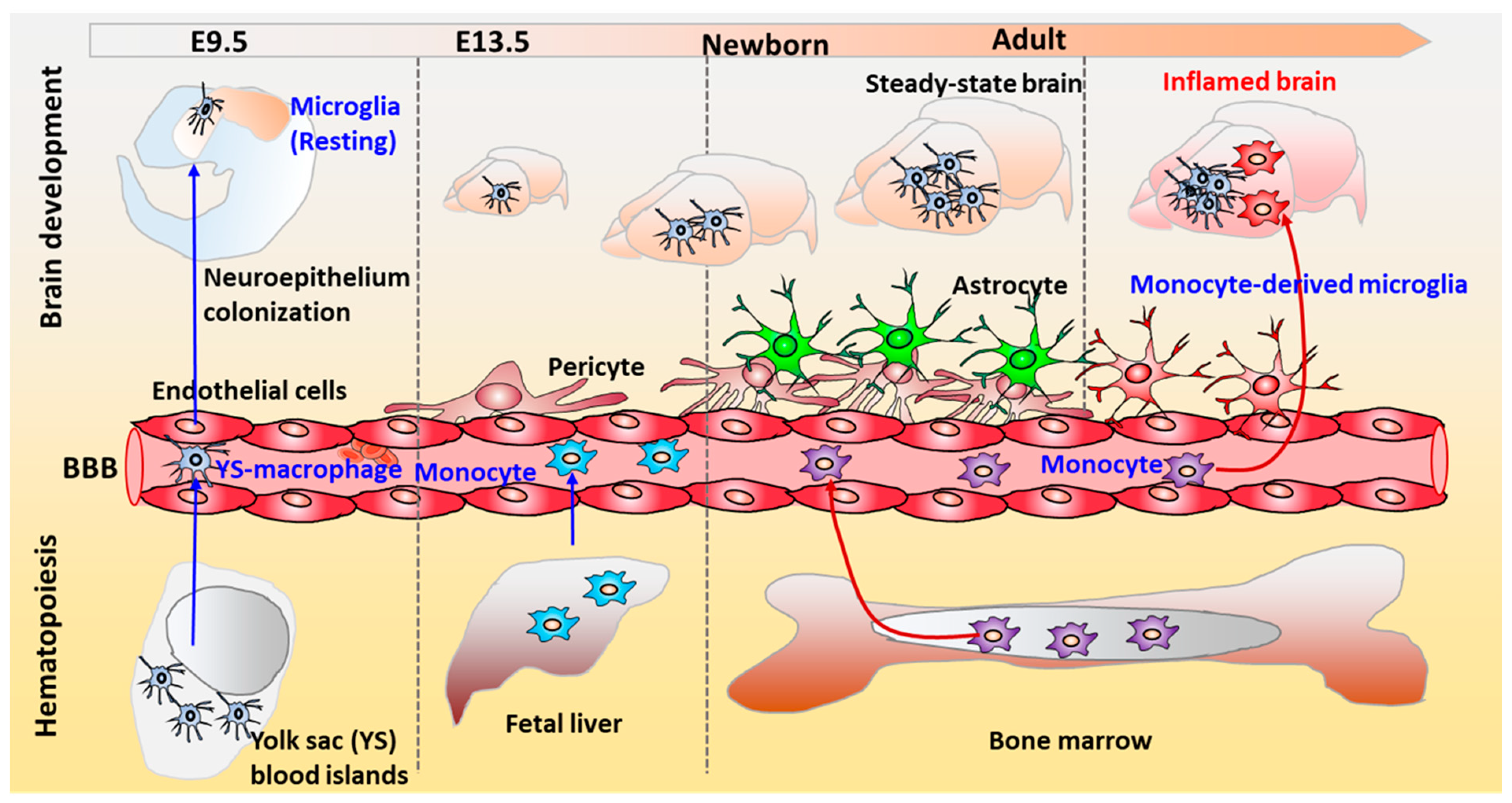
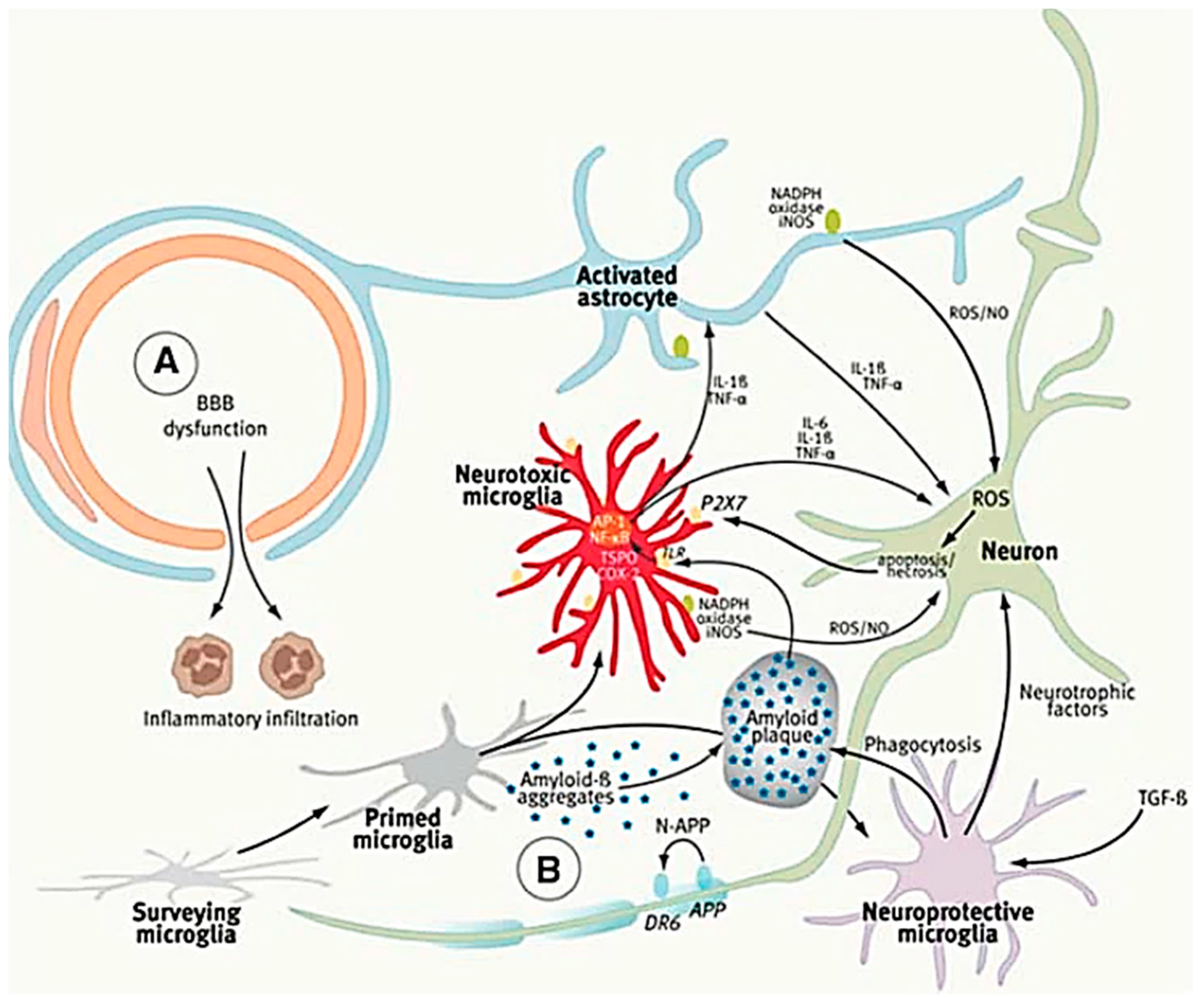
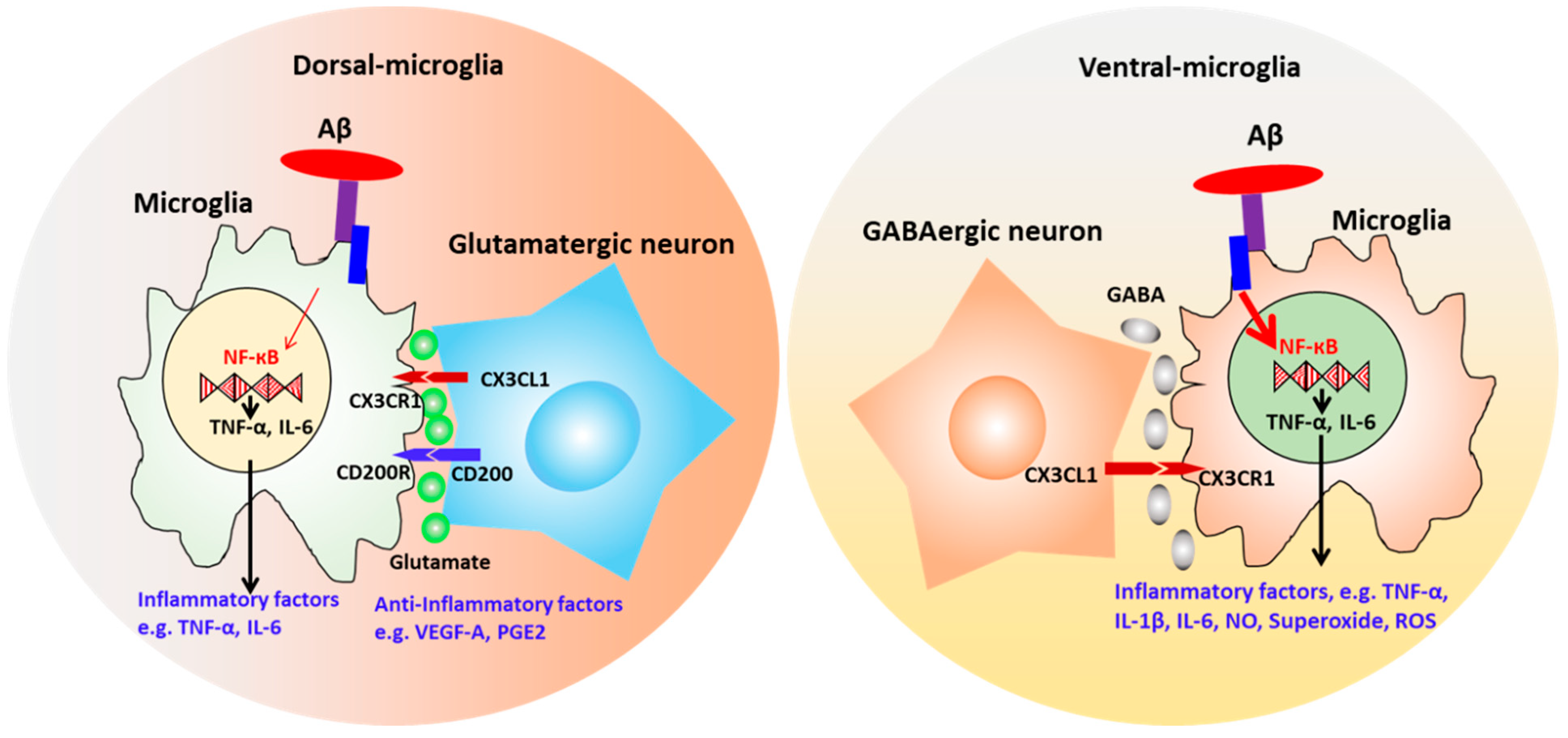
| Models | Characterization | Culture System | Improvement | Ref. |
|---|---|---|---|---|
| Monoculture hiPSC-brain microvascular endothelial cells (BMECs) | Expression of tight junction proteins occludin and claudin-5 and p-glycoprotein and BBB glucose transporter GLUT-1; TEER was about 1450 Ωcm2 with astrocytes coculture. | 2D neural and endothelial co-differentiation, providing a microenvironment resembling embryonic brain in vitro. | The first hPSC differentiation method that can reproducibly generate pure populations of EC with BBB properties. | Lippmann et al., 2012 [67] |
| Quadruple culture; hPSC-BMECs, pericytes, astrocytes, neurons | Expression of PECAM-1, GLUT-1, claudin-5 and occludin; TEER was about 5000 Ωcm2 with astrocytes coculture. | Adherent 2D transwell culture coated with collagen IV and fibronectin. | Retinoic acid (RA) enhanced BBB phenotypes in hPSC-BMECs. | Lippmann et al., 2014 [72] |
| Monoculture hiPSC-endothelial cells (ECs) | Expression of tight junction proteins, ZO-1, occludin, and claudin-5 and transporters proteins, PGG, LAT-1; Upregulated VECAD and TEER >2,000 Ωcm2 with the addition of retinoic acid. | Reproducible ECs induction protocol; Collagen IV and fibronectin coated surfaces. | The complex neurovascular environment should be employed. | Katt et al, 2016. [69] |
| Monoculture hiPSC-ECs | TEER >1,500 Ωcm2; Expression of BBB tight junction proteins ZO-1, Claudin-5, and Occludin, and BBB efflux transporters BCRP, MRP1, and PGP. | Adherent 2D transwell culture using derived BMECs. | Reproducibly consistent high TEER value. Evaluated cancer-targeting drug permeability. | Clark et al, 2016. [71] |
| Quadruple culture model hiPSC-ECs, hiPSC-NPCs, fetal brain astrocytes, pericytes | Robust BBB properties: TEER >2,500 Ωcm2; Upregulated BBB genes; ABCB1, SLC1A1, SLC2A1, and OCLN; Paracellular transport of small molecules were detected; In vitro recapitulation of transcellular passage of lipid-soluble agents. | Adherent 2D culture; Simultaneous co-culture effects; | Dynamic flow culture; Need to apply protocols for hiPSC-derived astrocytes and pericytes; BBB models from patient-specific hiPSCs. | Appelt-Menzel wt al, 2017. [38] |
| Triple culture hiPSC-ECs, hiPSC-derived neurons and astrocytes (1:3) | TEER ~ 886 Ωcm2; Slight increase in tight junction protein, occludin and cludin-5; Unchanged PGP efflux transporter activity. | EZ spheres; Isogenic BBB model. | A powerful tool for investigation of genetic disease modeling using patient-specific hiPSCs. | Canfield et al, 2017. [39] |
| Monoculture hiPSC-BMECs | Expressed GLUT-1, claudin-5, occludin, PECM-1 and VE-cadherin and consistently achieved TEER values exceeding 2500 Ωxcm2. | Adherent 2D transwell culture coated with collagen IV and fibronectin in E6 medium. | Reduced the differentiation time of iPSCs to BMECs from 13 to 8 days. | Hollmann et al., 2017 [73] |
| Monoculture hiPSC-BMECs | TEER > 3000 Ωcm2; BMECs phenotypes included tight junctions, low passive permeability, and polarized efflux transporters. | Adherent 2D transwell culture coated with collagen IV and fibronectin. | A facile, chemically defined method to differentiate hPSCs to BMECs via sequential Wnt and RA activation. | Qian et al., 2017 [74] |
| Monoculture HD hiPSC-BMECs | HD-BMECs had intrinsic impairments in angiogenic potential and drug efflux, and showed impaired paracellular and transcellular barrier properties. | Adherent 2D transwell culture coated with collagen IV and fibronectin. | Reduce disease burden and assess BBB penetration of drugs for HD. | Lim et al., 2017 [75] |
| Coculture hiPSC-BMECs, rat astrocytes | TEER levels peaked above 4000 Ωcm2 and were sustained above 2000 Ωcm2 up to 10 days. | Microfluidic platform. | A microfluidic BBB model mimicked in vivo BBB integrity and compound permeability. | Wang et al., 2017 [76] |
| Coculture MCT8 hiPSC-BMECs, hiPSC-NPCs | MCT8-deficient BMECs showed defects in thyroid hormone transport. | BMECs were cocultured with EZ sphere-derived neural cells in transwell. | A platform to test candidate drug transport across the diseased BBB. | Vatine et al., 2017 [77] |
| Triculture hiPSC-ECs, pericytes, astrocytes | The BBB model exhibited perfusable and selective microvasculature, permeability lower than conventional in vitro models, and similar to in vivo measurements in rat brain. | PDMS microfluidic system in fibrin gel. | A robust and physiologically relevant BBB microvascular model. | Campisi eti al., 2018 [65] |
| Coculture hiPSC-BMECs, NPC-astrocytes | TNF-α and IL-6 treatment impaired BBB integrity; Coculture with NPC-astrocytes improved TEER. | Transwell culture system. | The model mimicked cellular responses to inflammation at the BBB. | Mantle et al., 2018 [78] |
| Four cell types: hiPSC-BMECs, hiPSC-astrocytes, neurons, and pericytes | BMECs in coculture model showed high TEER and functional efflux; Whole genome expression profiling revealed upregulation of tight junction proteins. | Transwell culture coated with collagen IV and fibronectin. | Whole genome analysis about hiPSC-BBB model. | Delsing et al., 2018 [79] |
| Coculture hiPSC-ECs, hiPSC-NPCs | significant barrier integrity with tight junction protein expression, an effective permeability to sodium fluorescein and higher TEER value. | 3D printed electrospun PLGA scaffold. | BBB model reduced the penetration of Aβ oligomer into the neurons from hiPSC-NPCs. | Qi et al., 2018 [66] |
| Six cell types: hiPSC-microglia, oligodendrocyte, neurons, human primary BMECs, astrocytes, pericytes | Spheroids showed expression of tight junctions, adherens junctions, adherens junction-associated proteins and cell specific markers. | 3D cortex spheroid. | Organoid model formed a functional BBB. | Nzou et al., 2018 [20] |
| Coculture AD hiPSC-BMECs or healthy hiPSC-BMECs | Expression of tight junction proteins occludin and claudin-5 and p-glycoprotein and BBB glucose transporter GLUT-1. Adm BMECs showed no difference in TEER value and permeability compared to control. | Collagen type I microvessels in PDMS microfluidic chip. | Physiological BBB microvessel model to study barrier function. | Linville et al., 2019 [80] |
| Cell Source | Culture System | Yield (MG/PSC) | Phenotypic and Functional Characterization | Ref. |
|---|---|---|---|---|
| hiPSCs | Monolayer serum-free culture using IL-34/GM-CSF | 2.24 | iPSC-MGs expressed typical microglial markers, IBA1, CD11c (~95%), TMEM119, P2RY12 (~58%), CD11b (~94%) and CX3CR1 (~50%); iPSC-MGs showed phagocytosis of microspheres (~90%) as human primary microglia and macrophages; ADP-evoked intracellular Ca2+ transients were observed in iPSC-MGs and primary microglia but not in macrophages. | Douvaras et al, 2017 [117] |
| hESCs or hiPSCs | EBs using serum-free culture | 0.5–4.0 | Expressed specific markers of microglia, including TMEM119, P2RY12, and IBA1; responded to IFN-γ and LPS by upregulating IL-6, TNF-α at both protein and transcriptional levels. | Muffat et al, 2016 [118] |
| hiPSCs | Co-culture with astrocytes on monolayer | 2–3 | Human iPS-MGs expressed HLA-DR, CD45, TREM-2 and CX3CR1 in addition to CD11b and IBA1; MGs phagocytosed pHrodo red E. coli BioParticles (pHrodo) and produced reactive oxygen species (ROS) following stimulation with phorbol myristate acetate. | Pandya et al, 2017 [47] |
| hiPSCs H9 hESCs | FACS-sorted CD43+ cells, with M-CSF, IL-34, TGF-β1 | 125 CD43+ cells/PSC | Similar transcriptome and identical phagocytosis ability compared to iPSC-MG of previous protocols. “iPSC-microglia 2.0” engrafted well into xenotransplantation compatible MITRG mice. | McQuade wt al, 2018 [119] |
| hiPSCs | Co-culture with hiPSC-cortical neurons and IL-34 and GM-CSF | 40 | Expressed key surface protein markers; Positive for P2RY12, GPR34, <ERTK, C1QA, PROS1, GAS6, TMEM119 and TREM2; Phagocytic and release microglia-relevant cytokines and upregulate homeostatic function pathways. | Haenseler et al., 2017 [121] |
| hiPSCs | FACS-sorted CD43+ cells, with MCSF, IL-34, TGFβ1, insulin, CD200 and CX3CL1 | 30–40 | Positive for MERTK, ITGB5, CX3CR1, TGFβR1, PROS1, P2RY12, PU.1 and TREM2; Transcriptome comparable to adult and fetal human microglia; Secreted cytokines, respond to inflammatory stimuli; calcium transients, phagocytosisfor Aβ fibrils and tau oligomers; transplanted into transgenic mice and human brain organoids, resembled microglia in vivo. | Abud et al., 2017 [122] |
| hiPSCs | EBs in hypoxia with BMP4, activin A, FGF2, VEGF, CSF-1, and IL-3 | unknown | Positive for IBA1 and CX3CR1; Phagocytosis of beads and Aβ; FACS-sorted CD45+ CD11b+, coculture with hiPSC-neurons. | Takata et al., 2017 [123] |
| hPSC-macrophage precursors | EB, using GM-CSF and IL-34 | 30–40 | Positive for IBA1, CD45, TREM2; Whole-transcriptome showed similar signature to primary microglia; Mutant TREM2 caused to immature form of microglia without typical proteolysis. | Brownjohn et al., 2018 [124] |
| hiPSCs | Mesodermal progenitors developed into microglia-like cells within cerebral organoids | unknown | Positive for PU.1, CSF1R, CD68, IBA1, IRF8, TREM2, CXCR1, C1QA; Transcriptome analysis showed similar signature to primary microglia; Mediated phagocytosis and synaptic activities. | Ormel et al., 2018 [125] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Yan, Y.; Marzano, M.; Li, Y. Studying Heterotypic Cell–Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration. Cells 2019, 8, 299. https://doi.org/10.3390/cells8040299
Song L, Yan Y, Marzano M, Li Y. Studying Heterotypic Cell–Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration. Cells. 2019; 8(4):299. https://doi.org/10.3390/cells8040299
Chicago/Turabian StyleSong, Liqing, Yuanwei Yan, Mark Marzano, and Yan Li. 2019. "Studying Heterotypic Cell–Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration" Cells 8, no. 4: 299. https://doi.org/10.3390/cells8040299
APA StyleSong, L., Yan, Y., Marzano, M., & Li, Y. (2019). Studying Heterotypic Cell–Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration. Cells, 8(4), 299. https://doi.org/10.3390/cells8040299




