Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3
Abstract
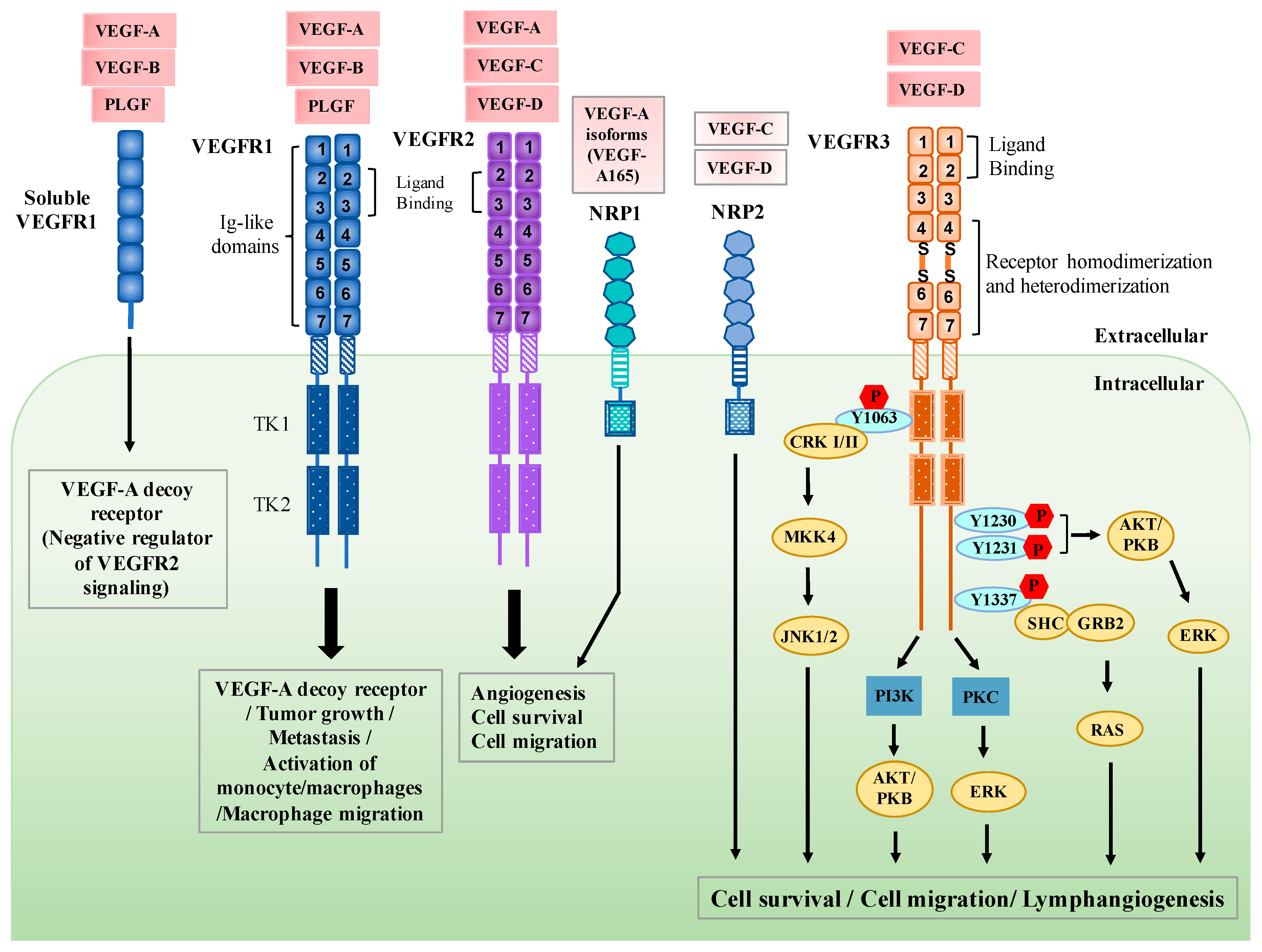
:1. Introduction
2. Regulation of VEGFR3 Signaling
3. Functional Roles of VEGFR3 in Lymphatic Endothelial Cells
4. Clinical Significance of VEGF-C/VEGFR3 Expression in Tumors
5. Expression and Function of VEGFR3 in Immune Cells
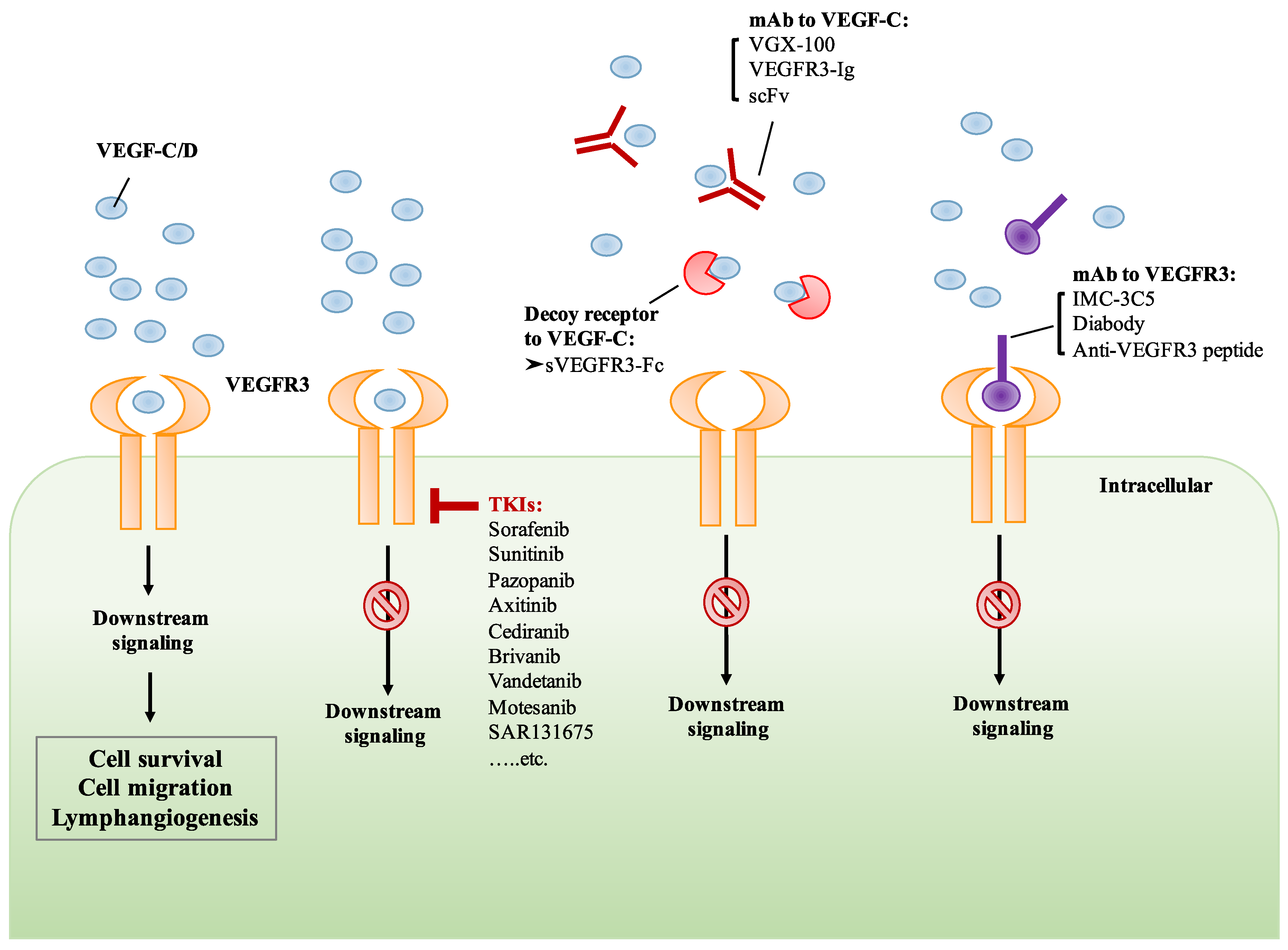
6. Development of Drugs That Target VEGF-C/VEGFR3 Signaling
6.1. Small Molecule TKIs of VEGFR3
6.2. Monoclonal Antibody Targeting VEGF-C/VEGFR3
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shibuya, M.; Yamaguchi, S.; Yamane, A.; Ikeda, T.; Tojo, A.; Matsushime, H.; Sato, M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990, 5, 519–524. [Google Scholar]
- Matthews, W.; Jordan, C.T.; Gavin, M.; Jenkins, N.A.; Copeland, N.G.; Lemischka, I.R. A receptor tyrosine kinase cdna isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc. Natl. Acad. Sci. USA 1991, 88, 9026–9030. [Google Scholar] [CrossRef] [PubMed]
- Pajusola, K.; Aprelikova, O.; Korhonen, J.; Kaipainen, A.; Pertovaara, L.; Alitalo, R.; Alitalo, K. Flt4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992, 52, 5738–5743. [Google Scholar] [PubMed]
- Aprelikova, O.; Pajusola, K.; Partanen, J.; Armstrong, E.; Alitalo, R.; Bailey, S.K.; McMahon, J.; Wasmuth, J.; Huebner, K.; Alitalo, K. Flt4, a novel class iii receptor tyrosine kinase in chromosome 5q33-qter. Cancer Res. 1992, 52, 746–748. [Google Scholar] [PubMed]
- Matthews, W.; Jordan, C.T.; Wiegand, G.W.; Pardoll, D.; Lemischka, I.R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 1991, 65, 1143–1152. [Google Scholar] [CrossRef]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by vegf receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef]
- Terman, B.I.; Carrion, M.E.; Kovacs, E.; Rasmussen, B.A.; Eddy, R.L.; Shows, T.B. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene 1991, 6, 1677–1683. [Google Scholar]
- Olofsson, B.; Korpelainen, E.; Pepper, M.S.; Mandriota, S.J.; Aase, K.; Kumar, V.; Gunji, Y.; Jeltsch, M.M.; Shibuya, M.; Alitalo, K.; et al. Vascular endothelial growth factor b (vegf-b) binds to vegf receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 11709–11714. [Google Scholar] [CrossRef]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to flt-1 but not to flk-1/kdr. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar]
- Sawano, A.; Takahashi, T.; Yamaguchi, S.; Aonuma, M.; Shibuya, M. Flt-1 but not kdr/flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996, 7, 213–221. [Google Scholar]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef] [PubMed]
- Waltenberger, J.; Claesson-Welsh, L.; Siegbahn, A.; Shibuya, M.; Heldin, C.H. Different signal transduction properties of kdr and flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 1994, 269, 26988–26995. [Google Scholar]
- Luttun, A.; Tjwa, M.; Moons, L.; Wu, Y.; Angelillo-Scherrer, A.; Liao, F.; Nagy, J.A.; Hooper, A.; Priller, J.; De Klerck, B.; et al. Revascularization of ischemic tissues by plgf treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-flt1. Nat. Med. 2002, 8, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.M.; Senior, R.M.; Shibuya, M. Mmp9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. Vegfr1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Iwai, S.; Hiratsuka, S.; Yamauchi, M.; Nakamura, K.; Iwakura, Y.; Shibuya, M. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood 2006, 108, 1849–1856. [Google Scholar] [CrossRef]
- Muramatsu, M.; Yamamoto, S.; Osawa, T.; Shibuya, M. Vascular endothelial growth factor receptor-1 signaling promotes mobilization of macrophage lineage cells from bone marrow and stimulates solid tumor growth. Cancer Res. 2010, 70, 8211–8221. [Google Scholar] [CrossRef] [PubMed]
- Niida, S.; Kondo, T.; Hiratsuka, S.; Hayashi, S.; Amizuka, N.; Noda, T.; Ikeda, K.; Shibuya, M. Vegf receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14016–14021. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. Vegf receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef]
- Zonta, Y.R.; Martinez, M.; Camargo, I.C.; Domeniconi, R.F.; Lupi Junior, L.A.; Pinheiro, P.F.; Reiter, R.J.; Martinez, F.E.; Chuffa, L.G. Melatonin reduces angiogenesis in serous papillary ovarian carcinoma of ethanol-preferring rats. Int. J. Mol. Sci. 2017, 18, 763. [Google Scholar] [CrossRef]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, vegf-c, is a ligand for the flt4 (vegfr-3) and kdr (vegfr-2) receptor tyrosine kinases. EMBO J. 1996, 15, 1751. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Makinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor d (vegf-d) is a ligand for the tyrosine kinases vegf receptor 2 (flk1) and vegf receptor 3 (flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef]
- Ghalamkarpour, A.; Morlot, S.; Raas-Rothschild, A.; Utkus, A.; Mulliken, J.B.; Boon, L.M.; Vikkula, M. Hereditary lymphedema type i associated with vegfr3 mutation: The first de novo case and atypical presentations. Clin. Genet. 2006, 70, 330–335. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor c is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef]
- Pajusola, K.; Aprelikova, O.; Pelicci, G.; Weich, H.; Claesson-Welsh, L.; Alitalo, K. Signalling properties of flt4, a proteolytically processed receptor tyrosine kinase related to two vegf receptors. Oncogene 1994, 9, 3545–3555. [Google Scholar] [PubMed]
- Jeltsch, M.; Karpanen, T.; Strandin, T.; Aho, K.; Lankinen, H.; Alitalo, K. Vascular endothelial growth factor (vegf)/vegf-c mosaic molecules reveal specificity determinants and feature novel receptor binding patterns. J. Biol. Chem. 2006, 281, 12187–12195. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, V.M.; Tvorogov, D.; Kisko, K.; Prota, A.E.; Jeltsch, M.; Anisimov, A.; Markovic-Mueller, S.; Stuttfeld, E.; Goldie, K.N.; Ballmer-Hofer, K.; et al. Structural and mechanistic insights into vegf receptor 3 ligand binding and activation. Proc. Natl. Acad. Sci. USA 2013, 110, 12960–12965. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular endothelial growth factor d is dispensable for development of the lymphatic system. Mol. Cell. Biol. 2005, 25, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Dettori, D.; Van Nuffelen, A.; Souffreau, J.; Marconcini, L.; Wallays, G.; Moons, L.; Bruyere, F.; Oliviero, S.; Noel, A.; et al. Vegf-d deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. J. Pathol. 2009, 219, 356–364. [Google Scholar] [CrossRef]
- Fournier, E.; Dubreuil, P.; Birnbaum, D.; Borg, J.P. Mutation at tyrosine residue 1337 abrogates ligand-dependent transforming capacity of the flt4 receptor. Oncogene 1995, 11, 921–931. [Google Scholar] [PubMed]
- Makinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the vegf-c/d receptor vegfr-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Galvagni, F.; Bardelli, M.; Bussolino, F.; Oliviero, S. Direct recruitment of crk and grb2 to vegfr-3 induces proliferation, migration, and survival of endothelial cells through the activation of erk, akt, and jnk pathways. Blood 2005, 106, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, A.; Makinen, T.; Alitalo, K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005, 19, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Herzog, Y.; Kalcheim, C.; Kahane, N.; Reshef, R.; Neufeld, G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev. 2001, 109, 115–119. [Google Scholar] [CrossRef]
- Whitaker, G.B.; Limberg, B.J.; Rosenbaum, J.S. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of vegf(165) and vegf(121). J. Biol. Chem. 2001, 276, 25520–25531. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Miao, H.Q.; Nomi, M.; Takashima, S.; Klagsbrun, M. Vegf165 mediates formation of complexes containing vegfr-2 and neuropilin-1 that enhance vegf165-receptor binding. J. Cell. Biochem. 2002, 85, 357–368. [Google Scholar] [CrossRef]
- Yuan, L.; Moyon, D.; Pardanaud, L.; Breant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129, 4797–4806. [Google Scholar]
- Kukk, E.; Lymboussaki, A.; Taira, S.; Kaipainen, A.; Jeltsch, M.; Joukov, V.; Alitalo, K. Vegf-c receptor binding and pattern of expression with vegfr-3 suggests a role in lymphatic vascular development. Development 1996, 122, 3829–3837. [Google Scholar]
- Wang, J.; Huang, Y.; Zhang, J.; Xing, B.; Xuan, W.; Wang, H.; Huang, H.; Yang, J.; Tang, J. Nrp-2 in tumor lymphangiogenesis and lymphatic metastasis. Cancer Lett. 2018, 418, 176–184. [Google Scholar] [CrossRef]
- Karpanen, T.; Heckman, C.A.; Keskitalo, S.; Jeltsch, M.; Ollila, H.; Neufeld, G.; Tamagnone, L.; Alitalo, K. Functional interaction of vegf-c and vegf-d with neuropilin receptors. FASEB J. 2006, 20, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, L.; Mak, J.; Pardanaud, L.; Caunt, M.; Kasman, I.; Larrivee, B.; Del Toro, R.; Suchting, S.; Medvinsky, A.; et al. Neuropilin-2 mediates vegf-c-induced lymphatic sprouting together with vegfr3. J. Cell. Biol. 2010, 188, 115–130. [Google Scholar] [CrossRef]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; van Hinsbergh, V.W.; Fang, G.H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef]
- Partanen, T.A.; Arola, J.; Saaristo, A.; Jussila, L.; Ora, A.; Miettinen, M.; Stacker, S.A.; Achen, M.G.; Alitalo, K. Vegf-c and vegf-d expression in neuroendocrine cells and their receptor, vegfr-3, in fenestrated blood vessels in human tissues. FASEB J. 2000, 14, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Achen, M.G.; Jussila, L.; Baldwin, M.E.; Alitalo, K. Lymphangiogenesis and cancer metastasis. Nat. Rev. Cancer 2002, 2, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Partanen, T.A.; Alitalo, K.; Miettinen, M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer 1999, 86, 2406–2412. [Google Scholar] [CrossRef]
- Paavonen, K.; Puolakkainen, P.; Jussila, L.; Jahkola, T.; Alitalo, K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol. 2000, 156, 1499–1504. [Google Scholar] [CrossRef]
- Valtola, R.; Salven, P.; Heikkila, P.; Taipale, J.; Joensuu, H.; Rehn, M.; Pihlajaniemi, T.; Weich, H.; deWaal, R.; Alitalo, K. Vegfr-3 and its ligand vegf-c are associated with angiogenesis in breast cancer. Am. J. Pathol. 1999, 154, 1381–1390. [Google Scholar] [CrossRef]
- Tammela, T.; Zarkada, G.; Wallgard, E.; Murtomaki, A.; Suchting, S.; Wirzenius, M.; Waltari, M.; Hellstrom, M.; Schomber, T.; Peltonen, R.; et al. Blocking vegfr-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008, 454, 656–660. [Google Scholar] [CrossRef]
- Le Bras, B.; Barallobre, M.J.; Homman-Ludiye, J.; Ny, A.; Wyns, S.; Tammela, T.; Haiko, P.; Karkkainen, M.J.; Yuan, L.; Muriel, M.P.; et al. Vegf-c is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat. Neurosci. 2006, 9, 340–348. [Google Scholar] [CrossRef]
- Orlandini, M.; Spreafico, A.; Bardelli, M.; Rocchigiani, M.; Salameh, A.; Nucciotti, S.; Capperucci, C.; Frediani, B.; Oliviero, S. Vascular endothelial growth factor-d activates vegfr-3 expressed in osteoblasts inducing their differentiation. J. Biol. Chem. 2006, 281, 17961–17967. [Google Scholar] [CrossRef]
- Schmeisser, A.; Christoph, M.; Augstein, A.; Marquetant, R.; Kasper, M.; Braun-Dullaeus, R.C.; Strasser, R.H. Apoptosis of human macrophages by flt-4 signaling: Implications for atherosclerotic plaque pathology. Cardiovasc. Res. 2006, 71, 774–784. [Google Scholar] [CrossRef]
- Batsi, O.; Giannopoulou, I.; Nesseris, I.; Valavanis, C.; Gakiopoulou, H.; Patsouris, E.S.; Arapandoni-Dadioti, P.; Lazaris, A.C. Immunohistochemical evaluation of cxcl12-cxcr4 axis and vegfr3 expression in primary urothelial cancer and its recurrence. Anticancer Res. 2014, 34, 3537–3542. [Google Scholar]
- Goussia, A.; Simou, N.; Zagouri, F.; Manousou, K.; Lazaridis, G.; Gogas, H.; Koutras, A.; Sotiropoulou, M.; Pentheroudakis, G.; Bafaloukos, D.; et al. Associations of angiogenesis-related proteins with specific prognostic factors, breast cancer subtypes and survival outcome in early-stage breast cancer patients. A hellenic cooperative oncology group (hecog) trial. PLoS ONE 2018, 13, e0200302. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.L.; Singh, R.K. Vegf-c-vegfr3/flt4 axis regulates mammary tumor growth and metastasis in an autocrine manner. Am. J. Cancer Res. 2015, 5, 616–628. [Google Scholar] [PubMed]
- Eroglu, A.; Ersoz, C.; Karasoy, D.; Sak, S. Vascular endothelial growth factor (vegf)-c, vegf-d, vegfr-3 and d2-40 expressions in primary breast cancer: Association with lymph node metastasis. Adv. Clin. Exp. Med. 2017, 26, 245–249. [Google Scholar]
- Takizawa, H.; Kondo, K.; Fujino, H.; Kenzaki, K.; Miyoshi, T.; Sakiyama, S.; Tangoku, A. The balance of vegf-c and vegfr-3 mrna is a predictor of lymph node metastasis in non-small cell lung cancer. Br. J. Cancer 2006, 95, 75–79. [Google Scholar] [CrossRef]
- Li, Y.; Weng, Y.; Zhong, L.; Chong, H.; Chen, S.; Sun, Y.; Li, W.; Shi, Q. Vegfr3 inhibition chemosensitizes lung adenocarcinoma a549 cells in the tumor-associated macrophage microenvironment through upregulation of p53 and pten. Oncol. Rep. 2017, 38, 2761–2773. [Google Scholar] [CrossRef]
- Lim, J.J.; Yang, K.; Taylor-Harding, B.; Wiedemeyer, W.R.; Buckanovich, R.J. Vegfr3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of brca1 and brca2. Neoplasia 2014, 16, 343–353.e2. [Google Scholar] [CrossRef]
- Decio, A.; Taraboletti, G.; Patton, V.; Alzani, R.; Perego, P.; Fruscio, R.; Jurgensmeier, J.M.; Giavazzi, R.; Belotti, D. Vascular endothelial growth factor c promotes ovarian carcinoma progression through paracrine and autocrine mechanisms. Am. J. Pathol. 2014, 184, 1050–1061. [Google Scholar] [CrossRef]
- Virman, J.; Bono, P.; Luukkaala, T.; Sunela, K.; Kujala, P.; Kellokumpu-Lehtinen, P.L. Vegfr3 and cd31 as prognostic factors in renal cell cancer. Anticancer Res. 2015, 35, 921–927. [Google Scholar] [PubMed]
- Bierer, S.; Herrmann, E.; Kopke, T.; Neumann, J.; Eltze, E.; Hertle, L.; Wulfing, C. Lymphangiogenesis in kidney cancer: Expression of vegf-c, vegf-d and vegfr-3 in clear cell and papillary renal cell carcinoma. Oncol. Rep. 2008, 20, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Diao, L.; Yang, Q.; Duo, J.; Liu, Y.X.; Liu, S.X.; Yao, X. expression of vegfr-2 and vegfr-3 in papillary renal cell carcinoma and their relationship with prognosis. Zhonghua Zhong Liu Za Zhi 2010, 32, 752–756. [Google Scholar] [PubMed]
- Wang, J.; Taylor, A.; Showeil, R.; Trivedi, P.; Horimoto, Y.; Bagwan, I.; Ewington, L.; Lam, E.W.; El-Bahrawy, M.A. Expression profiling and significance of vegf-a, vegfr2, vegfr3 and related proteins in endometrial carcinoma. Cytokine 2014, 68, 94–100. [Google Scholar] [CrossRef]
- Xin, X.; Zeng, X.; Feng, D.; Hua, T.; Liu, S.; Chi, S.; Hu, Q.; Wang, H. The suppressive role of calcium sensing receptor in endometrial cancer. Sci. Rep. 2018, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Huang, Q.; Zheng, W.; Huang, Y.; Hua, J.; Yang, S.; Zhuang, J.; Wang, J.; Chang, J.; Xu, J.; et al. Lps upregulated vegfr-3 expression promote migration and invasion in colorectal cancer via a mechanism of increased nf-kappab binding to the promoter of vegfr-3. Cell. Physiol. Biochem. 2016, 39, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Paiva, T.F., Jr.; de Jesus, V.H.; Marques, R.A.; da Costa, A.A.; de Macedo, M.P.; Peresi, P.M.; Damascena, A.; Rossi, B.M.; Begnami, M.D.; de Lima, V.C. Angiogenesis-related protein expression in bevacizumab-treated metastatic colorectal cancer: Notch1 detrimental to overall survival. BMC Cancer 2015, 15, 643. [Google Scholar] [CrossRef]
- Hong, K.D.; Lee, Y.; Kim, B.H.; Lee, S.I.; Moon, H.Y. Expression of gli1 correlates with expression of lymphangiogenesis proteins, vascular endothelial growth factor c and vascular endothelial growth factor receptor 3, in colorectal cancer. Am. Surg. 2013, 79, 198–204. [Google Scholar]
- Yu, J.W.; Wu, S.H.; Lu, R.Q.; Wu, J.G.; Ni, X.C.; Zhou, G.C.; Jiang, H.G.; Zheng, L.H.; Li, X.Q.; Du, G.Y.; et al. Expression and significances of contactin-1 in human gastric cancer. Gastroenterol. Res. Pract. 2013, 2013, 210205. [Google Scholar] [CrossRef]
- Dai, X.; Liu, D.; Liu, M.; Zhang, X.; Wang, W.; Jin, F.; Qian, Y.; Wang, X.; Zhao, J.; Wu, Y.; et al. Anti-metastatic efficacy of traditional chinese medicine (tcm) ginsenoside conjugated to a vefgr-3 antibody on human gastric cancer in an orthotopic mouse model. Anticancer Res. 2017, 37, 979–986. [Google Scholar]
- Zhang, H.; Qi, F.; Cao, Y.; Zu, X.; Chen, M.; Li, Z.; Qi, L. 5-aza-2′-deoxycytidine enhances maspin expression and inhibits proliferation, migration, and invasion of the bladder cancer t24 cell line. Cancer Biother. Radiopharm. 2013, 28, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Al-Shareef, H.; Hiraoka, S.I.; Tanaka, N.; Shogen, Y.; Lee, A.D.; Bakhshishayan, S.; Kogo, M. Use of nrp1, a novel biomarker, along with vegf-c, vegfr-3, ccr7 and sema3e, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncol. Rep. 2016, 36, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Misawa, Y.; Misawa, K.; Kawasaki, H.; Imai, A.; Mochizuki, D.; Ishikawa, R.; Endo, S.; Mima, M.; Kanazawa, T.; Iwashita, T.; et al. Evaluation of epigenetic inactivation of vascular endothelial growth factor receptors in head and neck squamous cell carcinoma. Tumour Biol. 2017, 39, 1010428317711657. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.; Schopp, M.; Thurau, K.; Haier, J.; Kohler, G.; Hummel, R. Immunohistochemical analysis on potential new molecular targets for esophageal cancer. Dis. Esophagus 2014, 27, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Moehler, M.; Frerichs, K.; Drescher, D.; Trinh, T.T.; Duenschede, F.; Borschitz, T.; Schimanski, K.; Biesterfeld, S.; Herzer, K.; et al. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol. Rep. 2008, 20, 845–850. [Google Scholar]
- Van Trappen, P.O.; Steele, D.; Lowe, D.G.; Baithun, S.; Beasley, N.; Thiele, W.; Weich, H.; Krishnan, J.; Shepherd, J.H.; Pepper, M.S.; et al. Expression of vascular endothelial growth factor (vegf)-c and vegf-d, and their receptor vegfr-3, during different stages of cervical carcinogenesis. J. Pathol. 2003, 201, 544–554. [Google Scholar] [CrossRef]
- Shi, X.; Chen, G.; Xing, H.; Weng, D.; Bai, X.; Ma, D. Vegf-c, vegfr-3, and cox-2 enhances growth and metastasis of human cervical carcinoma cell lines in vitro. Oncol. Rep. 2007, 18, 241–247. [Google Scholar] [CrossRef]
- Kaushal, V.; Mukunyadzi, P.; Dennis, R.A.; Siegel, E.R.; Johnson, D.E.; Kohli, M. Stage-specific characterization of the vascular endothelial growth factor axis in prostate cancer: Expression of lymphangiogenic markers is associated with advanced-stage disease. Clin. Cancer Res. 2005, 11, 584–593. [Google Scholar]
- Yang, Z.S.; Xu, Y.F.; Huang, F.F.; Ding, G.F. Associations of nm23h1, vegf-c, and vegf-3 receptor in human prostate cancer. Molecules 2014, 19, 6851–6862. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Munoz-Repeto, I.; Inglada-Perez, L.; de Cubas, A.A.; Mancikova, V.; Canamero, M.; Maliszewska, A.; Gomez, A.; Leton, R.; Leandro-Garcia, L.J.; et al. Influence of ret mutations on the expression of tyrosine kinases in medullary thyroid carcinoma. Endocr. Relat. Cancer 2013, 20, 611–619. [Google Scholar] [CrossRef]
- Mancikova, V.; Inglada-Perez, L.; Curras-Freixes, M.; de Cubas, A.A.; Gomez, A.; Leton, R.; Kersten, I.; Leandro-Garcia, L.J.; Comino-Mendez, I.; Apellaniz-Ruiz, M.; et al. Vegf, vegfr3, and pdgfrb protein expression is influenced by ras mutations in medullary thyroid carcinoma. Thyroid 2014, 24, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Kurenova, E.; Liao, J.; He, D.H.; Hunt, D.; Yemma, M.; Bshara, W.; Seshadri, M.; Cance, W.G. The fak scaffold inhibitor c4 disrupts fak-vegfr-3 signaling and inhibits pancreatic cancer growth. Oncotarget 2013, 4, 1632–1646. [Google Scholar] [CrossRef]
- Gogate, P.N.; Kurenova, E.V.; Ethirajan, M.; Liao, J.; Yemma, M.; Sen, A.; Pandey, R.K.; Cance, W.G. Targeting the c-terminal focal adhesion kinase scaffold in pancreatic cancer. Cancer Lett. 2014, 353, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Beierle, E.A.; Dai, W.; Langham, M.R., Jr.; Copeland, E.M., 3rd; Chen, M.K. Expression of vegf receptors in cocultured neuroblastoma cells. J. Surg. Res. 2004, 119, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Ma, X.; Megison, M.; Nabers, H.; Cance, W.G.; Kurenova, E.V.; Beierle, E.A. Inhibition of fak and vegfr-3 binding decreases tumorigenicity in neuroblastoma. Mol. Carcinog. 2015, 54, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; McCarthy, M.M.; Jilaveanu, L.; Flaherty, K.T.; Aziz, S.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. Quantitative expression of vegf, vegf-r1, vegf-r2, and vegf-r3 in melanoma tissue microarrays. Hum. Pathol. 2010, 41, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kurenova, E.; Ucar, D.; Liao, J.; Yemma, M.; Gogate, P.; Bshara, W.; Sunar, U.; Seshadri, M.; Hochwald, S.N.; Cance, W.G. A fak scaffold inhibitor disrupts fak and vegfr-3 signaling and blocks melanoma growth by targeting both tumor and endothelial cells. Cell Cycle 2014, 13, 2542–2553. [Google Scholar] [CrossRef]
- Jenny, B.; Harrison, J.A.; Baetens, D.; Tille, J.C.; Burkhardt, K.; Mottaz, H.; Kiss, J.Z.; Dietrich, P.Y.; De Tribolet, N.; Pizzolato, G.P.; et al. Expression and localization of vegf-c and vegfr-3 in glioblastomas and haemangioblastomas. J. Pathol. 2006, 209, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Ma, X.; Guan, Y.; Mizoguchi, M.; Nakamizo, A.; Amano, T.; Hata, N.; Kuga, D.; Sasaki, T. Expression of stem cell marker and receptor kinase genes in glioblastoma tissue quantified by real-time rt-pcr. Brain Tumor Pathol. 2011, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Min, K.; Kim, H.S.; Jung, W.W.; Park, Y.K. Expression of vascular endothelial growth factor-c and its receptor in osteosarcomas. Pathol. Res. Pract. 2008, 204, 575–582. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Li, X.; Xu, L.; Ma, W.; Chang, L.; Ju, F. Expression of vegf-c/vegfr-3 in human laryngeal squamous cell carcinomas and its significance for lymphatic metastasis. Asian Pac. J. Cancer Prev. 2012, 13, 27–31. [Google Scholar] [CrossRef]
- Yeh, Y.W.; Cheng, C.C.; Yang, S.T.; Tseng, C.F.; Chang, T.Y.; Tsai, S.Y.; Fu, E.; Chiang, C.P.; Liao, L.C.; Tsai, P.W.; et al. Targeting the vegf-c/vegfr3 axis suppresses slug-mediated cancer metastasis and stemness via inhibition of kras/yap1 signaling. Oncotarget 2017, 8, 5603–5618. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, S.; Kim, D.C.; Yoon, J.H.; Shin, S.H.; Min, W.S.; Kim, H.J. A vegfr-3 antagonist increases ifn-gamma expression on low functioning nk cells in acute myeloid leukemia. J. Clin. Immunol. 2013, 33, 826–837. [Google Scholar] [CrossRef]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; Sabine, A.; Petrova, T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell. Biol. 2011, 193, 607–618. [Google Scholar] [CrossRef]
- Witte, M.H.; Bernas, M.J.; Martin, C.P.; Witte, C.L. Lymphangiogenesis and lymphangiodysplasia: From molecular to clinical lymphology. Microsc. Res. Tech. 2001, 55, 122–145. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Ferrell, R.E.; Lawrence, E.C.; Kimak, M.A.; Levinson, K.L.; McTigue, M.A.; Alitalo, K.; Finegold, D.N. Missense mutations interfere with vegfr-3 signalling in primary lymphoedema. Nat. Genet. 2000, 25, 153–159. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Saaristo, A.; Jussila, L.; Karila, K.A.; Lawrence, E.C.; Pajusola, K.; Bueler, H.; Eichmann, A.; Kauppinen, R.; Kettunen, M.I.; et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 2001, 98, 12677–12682. [Google Scholar] [CrossRef]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular failure in mouse embryos deficient in vegf receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef]
- Hagerling, R.; Pollmann, C.; Andreas, M.; Schmidt, C.; Nurmi, H.; Adams, R.H.; Alitalo, K.; Andresen, V.; Schulte-Merker, S.; Kiefer, F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013, 32, 629–644. [Google Scholar] [CrossRef]
- Jeltsch, M.; Jha, S.K.; Tvorogov, D.; Anisimov, A.; Leppanen, V.M.; Holopainen, T.; Kivela, R.; Ortega, S.; Karpanen, T.; Alitalo, K. Ccbe1 enhances lymphangiogenesis via a disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-c activation. Circulation 2014, 129, 1962–1971. [Google Scholar] [CrossRef]
- Roukens, M.G.; Peterson-Maduro, J.; Padberg, Y.; Jeltsch, M.; Leppanen, V.M.; Bos, F.L.; Alitalo, K.; Schulte-Merker, S.; Schulte, D. Functional dissection of the ccbe1 protein: A crucial requirement for the collagen repeat domain. Circ. Res. 2015, 116, 1660–1669. [Google Scholar] [CrossRef]
- Jeltsch, M.; Kaipainen, A.; Joukov, V.; Meng, X.; Lakso, M.; Rauvala, H.; Swartz, M.; Fukumura, D.; Jain, R.K.; Alitalo, K. Hyperplasia of lymphatic vessels in vegf-c transgenic mice. Science 1997, 276, 1423–1425. [Google Scholar] [CrossRef]
- Kurebayashi, J.; Otsuki, T.; Kunisue, H.; Mikami, Y.; Tanaka, K.; Yamamoto, S.; Sonoo, H. Expression of vascular endothelial growth factor (vegf) family members in breast cancer. Jpn. J. Cancer Res. 1999, 90, 977–981. [Google Scholar] [CrossRef]
- Salven, P.; Lymboussaki, A.; Heikkila, P.; Jaaskela-Saari, H.; Enholm, B.; Aase, K.; von Euler, G.; Eriksson, U.; Alitalo, K.; Joensuu, H. Vascular endothelial growth factors vegf-b and vegf-c are expressed in human tumors. Am. J. Pathol. 1998, 153, 103–108. [Google Scholar] [CrossRef]
- Akagi, K.; Ikeda, Y.; Miyazaki, M.; Abe, T.; Kinoshita, J.; Maehara, Y.; Sugimachi, K. Vascular endothelial growth factor-c (vegf-c) expression in human colorectal cancer tissues. Br. J. Cancer 2000, 83, 887–891. [Google Scholar] [CrossRef]
- Niki, T.; Iba, S.; Tokunou, M.; Yamada, T.; Matsuno, Y.; Hirohashi, S. Expression of vascular endothelial growth factors a, b, c, and d and their relationships to lymph node status in lung adenocarcinoma. Clin. Cancer Res. 2000, 6, 2431–2439. [Google Scholar]
- Shushanov, S.; Bronstein, M.; Adelaide, J.; Jussila, L.; Tchipysheva, T.; Jacquemier, J.; Stavrovskaya, A.; Birnbaum, D.; Karamysheva, A. Vegfc and vegfr3 expression in human thyroid pathologies. Int. J. Cancer 2000, 86, 47–52. [Google Scholar] [CrossRef]
- Yonemura, Y.; Endo, Y.; Fujita, H.; Fushida, S.; Ninomiya, I.; Bandou, E.; Taniguchi, K.; Miwa, K.; Ohoyama, S.; Sugiyama, K.; et al. Role of vascular endothelial growth factor c expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res. 1999, 5, 1823–1829. [Google Scholar]
- Ohta, Y.; Shridhar, V.; Bright, R.K.; Kalemkerian, G.P.; Du, W.; Carbone, M.; Watanabe, Y.; Pass, H.I. Vegf and vegf type c play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br. J. Cancer 1999, 81, 54–61. [Google Scholar] [CrossRef]
- Eggert, A.; Ikegaki, N.; Kwiatkowski, J.; Zhao, H.; Brodeur, G.M.; Himelstein, B.P. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin. Cancer Res. 2000, 6, 1900–1908. [Google Scholar]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. Vegfs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by vegf-c promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef]
- Karpanen, T.; Alitalo, K. Lymphatic vessels as targets of tumor therapy? J. Exp. Med. 2001, 194, F37–F42. [Google Scholar] [CrossRef]
- Mattila, M.M.; Ruohola, J.K.; Karpanen, T.; Jackson, D.G.; Alitalo, K.; Harkonen, P.L. Vegf-c induced lymphangiogenesis is associated with lymph node metastasis in orthotopic mcf-7 tumors. Int. J. Cancer 2002, 98, 946–951. [Google Scholar] [CrossRef]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble vegf receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef]
- Friedlaender, M.H.; Baer, H. Immunologic tolerance: Role of the regional lymph node. Science 1972, 176, 312–314. [Google Scholar] [CrossRef]
- Friedlaender, M.H.; Chisari, F.V.; Baer, H. The role of the inflammatory response of skin and lymph nodes in the induction of sensitization to simple chemicals. J. Immunol. 1973, 111, 164–170. [Google Scholar]
- D’Alessio, S.; Correale, C.; Tacconi, C.; Gandelli, A.; Pietrogrande, G.; Vetrano, S.; Genua, M.; Arena, V.; Spinelli, A.; Peyrin-Biroulet, L.; et al. Vegf-c-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Investig. 2014, 124, 3863–3878. [Google Scholar] [CrossRef]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via vegfr-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef]
- Yao, L.C.; Baluk, P.; Feng, J.; McDonald, D.M. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am. J. Pathol. 2010, 176, 1525–1541. [Google Scholar] [CrossRef]
- Fiedler, E.; Helmbold, P.; Marsch, W.C. Increased vessel density in psoriasis: Involvement of lymphatic vessels in the papillary dermis. Br. J. Dermatol. 2008, 159, 258–261. [Google Scholar] [CrossRef]
- Hamrah, P.; Chen, L.; Zhang, Q.; Dana, M.R. Novel expression of vascular endothelial growth factor receptor (vegfr)-3 and vegf-c on corneal dendritic cells. Am. J. Pathol. 2003, 163, 57–68. [Google Scholar] [CrossRef]
- Fernandez Pujol, B.; Lucibello, F.C.; Zuzarte, M.; Lutjens, P.; Muller, R.; Havemann, K. Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J. Cell. Biol. 2001, 80, 99–110. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Birner, P.; Stockl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Kriehuber, E.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The role of the vegf-c/vegfr-3 axis in cancer progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ma, L.; Cao, X.; Xiao, J.; Chen, J.; Jiao, S.; Gao, Y.; Liu, C.; Duan, Z.; et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains tlr4-nf-kappab signaling and protects against endotoxin shock. Immunity 2014, 40, 501–514. [Google Scholar] [CrossRef]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007, 170, 774–786. [Google Scholar] [CrossRef]
- Lund, A.W.; Duraes, F.V.; Hirosue, S.; Raghavan, V.R.; Nembrini, C.; Thomas, S.N.; Issa, A.; Hugues, S.; Swartz, M.A. Vegf-c promotes immune tolerance in b16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012, 1, 191–199. [Google Scholar] [CrossRef]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 2004, 15, 603–612. [Google Scholar] [CrossRef]
- Ling, S.; Qi, C.; Li, W.; Xu, J.; Kuang, W. Crucial role of corneal lymphangiogenesis for allograft rejection in alkali-burned cornea bed. Clin. Exp. Ophthalmol. 2009, 37, 874–883. [Google Scholar] [CrossRef]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef]
- Pasquali, S.; van der Ploeg, A.P.; Mocellin, S.; Stretch, J.R.; Thompson, J.F.; Scolyer, R.A. Lymphatic biomarkers in primary melanomas as predictors of regional lymph node metastasis and patient outcomes. Pigment Cell Melanoma Res. 2013, 26, 326–337. [Google Scholar] [CrossRef]
- Bhargava, P.; Robinson, M.O. Development of second-generation vegfr tyrosine kinase inhibitors: Current status. Curr. Oncol. Rep. 2011, 13, 103–111. [Google Scholar] [CrossRef]
- Albiges, L.; Gizzi, M.; Carton, E.; Escudier, B. Axitinib in metastatic renal cell carcinoma. Expert Rev. Anticancer Ther. 2015, 15, 499–507. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Ellis, L.M.; Hicklin, D.J. Vegf-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase iii trial. J. Clin. Oncol. 2010, 28, 1061–1068. [Google Scholar] [CrossRef]
- Heckman, C.A.; Holopainen, T.; Wirzenius, M.; Keskitalo, S.; Jeltsch, M.; Yla-Herttuala, S.; Wedge, S.R.; Jurgensmeier, J.M.; Alitalo, K. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008, 68, 4754–4762. [Google Scholar] [CrossRef]
- Stark, D.P.; Cook, A.; Brown, J.M.; Brundage, M.D.; Embleton, A.C.; Kaplan, R.S.; Raja, F.A.; Swart, A.M.W.; Velikova, G.; Qian, W.; et al. Quality of life with cediranib in relapsed ovarian cancer: The icon6 phase 3 randomized clinical trial. Cancer 2017, 123, 2752–2761. [Google Scholar] [CrossRef]
- Kudo, M.; Han, G.; Finn, R.S.; Poon, R.T.; Blanc, J.F.; Yan, L.; Yang, J.; Lu, L.; Tak, W.Y.; Yu, X.; et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase iii trial. Hepatology 2014, 60, 1697–1707. [Google Scholar] [CrossRef]
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Matsumoto, S.; Kohno, T.; Tsuta, K.; et al. Vandetanib in patients with previously treated ret-rearranged advanced non-small-cell lung cancer (luret): An open-label, multicentre phase 2 trial. Lancet Respir. Med. 2017, 5, 42–50. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.K.; Ahn, M.J.; Kim, D.W.; Sun, J.M.; Keam, B.; Kim, T.M.; Heo, D.S.; Ahn, J.S.; Choi, Y.L.; et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring ret rearrangement: A phase ii clinical trial. Ann. Oncol. 2017, 28, 292–297. [Google Scholar]
- Kubota, K.; Yoshioka, H.; Oshita, F.; Hida, T.; Yoh, K.; Hayashi, H.; Kato, T.; Kaneda, H.; Yamada, K.; Tanaka, H.; et al. Phase iii, randomized, placebo-controlled, double-blind trial of motesanib (amg-706) in combination with paclitaxel and carboplatin in east asian patients with advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2017, 35, 3662–3670. [Google Scholar] [CrossRef]
- Alam, A.; Blanc, I.; Gueguen-Dorbes, G.; Duclos, O.; Bonnin, J.; Barron, P.; Laplace, M.C.; Morin, G.; Gaujarengues, F.; Dol, F.; et al. Sar131675, a potent and selective vegfr-3-tk inhibitor with antilymphangiogenic, antitumoral, and antimetastatic activities. Mol. Cancer Ther. 2012, 11, 1637–1649. [Google Scholar] [CrossRef]
- Hwang, S.D.; Song, J.H.; Kim, Y.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Hong, Y.A.; Chung, S.; Choi, B.S.; Kim, Y.S.; et al. Inhibition of lymphatic proliferation by the selective vegfr-3 inhibitor sar131675 ameliorates diabetic nephropathy in db/db mice. Cell Death Dis. 2019, 10, 219. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Saif, M.W.; Knost, J.A.; Chiorean, E.G.; Kambhampati, S.R.; Yu, D.; Pytowski, B.; Qin, A.; Kauh, J.S.; O’Neil, B.H. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody ly3022856/imc-3c5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother. Pharmacol. 2016, 78, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, X.; Lu, D.; Brennan, L.; Persaud, K.; Liu, M.; Miao, H.; Witte, L.; Zhu, Z. A recombinant, fully human, bispecific antibody neutralizes the biological activities mediated by both vascular endothelial growth factor receptors 2 and 3. Mol. Cancer Ther. 2005, 4, 427–434. [Google Scholar]
- Lin, J.; Lalani, A.S.; Harding, T.C.; Gonzalez, M.; Wu, W.W.; Luan, B.; Tu, G.H.; Koprivnikar, K.; VanRoey, M.J.; He, Y.; et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble vegfr-3 decoy receptor. Cancer Res. 2005, 65, 6901–6909. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, M.; Villa, A.; Ballmer-Hofer, K.; Neri, D.; Detmar, M. Phage-derived fully human monoclonal antibody fragments to human vascular endothelial growth factor-c block its interaction with vegf receptor-2 and 3. PLoS ONE 2010, 5, e11941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, B.; Shi, J.; Zhao, L.; Zhang, X.; Wang, C.; Hou, S.; Qian, W.; Kou, G.; Wang, H.; et al. Suppression of tumor growth and metastasis by simultaneously blocking vascular endothelial growth factor (vegf)-a and vegf-c with a receptor-immunoglobulin fusion protein. Cancer Res. 2010, 70, 2495–2503. [Google Scholar] [CrossRef]

| Tumor Type | Detection | Expression | Correlate to Lymph Angiogenesis | Correlate to Lymph Node Metastasis | Ref. |
|---|---|---|---|---|---|
| Urothelial cancer | IHC | Tumor cells | − | − | [53] |
| Breast cancer | IHC, RT-PCR, Western blot | Tumor cells | − | + | [54,55,56] |
| Lung cancer | IHC, qRT-PCR, Western blot | Tumor cells | − | + | [57,58] |
| Ovarian cancer | qRT-PCR, IHC, Western blot | Tumor cells | + | + | [59,60] |
| Renal cell cancer | IHC | Tumor cells | − | + | [61,62,63] |
| Endometrial cancer | IHC, qRT-PCR, Western blot | Tumor cells | − | + | [64,65] |
| Colorectal cancer | IHC, qRT-PCR, Western blot | Tumor cells | + | + | [66,67,68] |
| Gastric cancer | IHC, qRT-PCR | Tumor cells | + | + | [69,70] |
| Bladder cancer | Western blot | Tumor cells | − | − | [71] |
| Oral cancer | IHC | Tumor cells | + | + | [72] |
| Head and neck cancer | qRT-PCR, qMSP-PCR | Tumor cells | − | − | [73] |
| Esophageal cancer | IHC | Tumor cells | − | − | [74,75] |
| Cervical cancer | IHC, in situ hybridization, qRT-PCR, Western blot | Tumor cells | + | + | [76,77] |
| Prostate cancer | IHC, in situ hybridization, qRT-PCR | Tumor cells | + | + | [78,79] |
| Thyroid cancer | IHC | Tumor cells | − | − | [80,81] |
| Pancreatic cancer | Western blot | Tumor cells | + | + | [82,83] |
| Neuroblastoma | RT-PCR, Western blot | Tumor cells | − | − | [84,85] |
| Melanoma | Western blot, IHC | Tumor cells | + | − | [86,87] |
| Glioblastoma | In situ hybridization, qRT-PCR | Tumor cells | − | − | [88,89] |
| Osteosarcoma | IHC | Tumor cells | − | − | [90] |
| Laryngeal cancer | RT-PCR | Tumor cells | + | − | [91] |
| Basal cell carcinoma | qRT-PCR, Western blot | Tumor cells | − | + | [92] |
| Acute myeloid leukemia | RT-PCR, IHC | Acute myeloid leukemia (AML) natural killer (NK) cells | − | − | [93] |
| Agents | Agent Description | Developer | Current Status | Ref. |
|---|---|---|---|---|
| Sorafenib | Small molecule TKI (VEGFRs, PDGFRs, c-kit, RET) | Bayer and Onyx | FDA-approved | [138] |
| Sunitinib | Small molecule TKI (VEGFRs, PDGFRs, c-kit, Flt3, RET) | Pfizer Inc. | FDA-approved | [140] |
| Pazopanib | Small molecule TKI (VEGFRs, PDGFRs, c-kit) | GlaxoSmithKline | FDA-approved | [141] |
| Axitinib | Small molecule TKI (VEGFRs, PDGFRs, c-kit) | Pfizer Inc. | FDA-approved | [142,143] |
| Cediranib | Small molecule TKI (VEGFRs, PDGFRs, c-kit) | AstraZeneca | Phase III | [137] |
| Brivanib | Small molecule TKI (VEGFRs, PDGFRs, FGFRs) | Bristol-Myers Squibb | Phase III | [144] |
| Vandetanib | Small molecule TKI (VEGFRs, PDGFRs, EGFR, RET) | AstraZeneca | Phase II | [145,146] |
| Motesanib | Small molecule TKI (VEGFRs, PDGFRs, c-kit, RET) | Amgen | Phase III | [147] |
| SAR131675 | Small molecule TKI (more selective for VEGFR3 than VEGFR1/2) | Sanofi | Preclinical | [148,149] |
| Bevacizumab | Humanized anti-VEGF-A mAb | Genentech | FDA-approved | [150] |
| IMC-3C5 | Humanized anti-VEGFR3 mAb | ImClone Systems/Eli Lilly | Phase I | [153] |
| VGX-100 | Humanized anti-VEGF-C mAb | Circadian Technologies | Phase I | [154] |
| Diabody | Anti-VEGFR2/ VEGFR3 mAb | - | Preclinical | [155] |
| sVEGFR3-Fc | Soluble VEGFR3 decoy receptor | - | Preclinical | [156] |
| Single-chain fragment (scFv) | Anti-VEGF-C mAb fragment | - | Preclinical | [157] |
| VEGFR3-Ig | Anti-VEGF-C/A Receptor-Ig fusion protein | - | Preclinical | [158] |
| Anti-VEGFR3 peptide | Anti-VEGFR3 peptide | - | Preclinical | [92] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-C.; Pan, M.-R.; Hung, W.-C. Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3. Cells 2019, 8, 270. https://doi.org/10.3390/cells8030270
Hsu M-C, Pan M-R, Hung W-C. Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3. Cells. 2019; 8(3):270. https://doi.org/10.3390/cells8030270
Chicago/Turabian StyleHsu, Ming-Chuan, Mei-Ren Pan, and Wen-Chun Hung. 2019. "Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3" Cells 8, no. 3: 270. https://doi.org/10.3390/cells8030270
APA StyleHsu, M.-C., Pan, M.-R., & Hung, W.-C. (2019). Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3. Cells, 8(3), 270. https://doi.org/10.3390/cells8030270





