Structure Based Design and Molecular Docking Studies for Phosphorylated Tau Inhibitors in Alzheimer’s Disease
Abstract
1. Introduction
2. Methods
2.1. Homology Modeling
2.2. 3-Drefine
2.3. Simulations
2.4. Molecular Dynamics
2.5. Active Site Prediction
2.6. Pharmacophore Generation
2.7. Molecular Docking
2.8. ADMET Prediction
3. Results and Discussion
3.1. Homology Models Validation
3.2. Molecular Dynamics Simulations
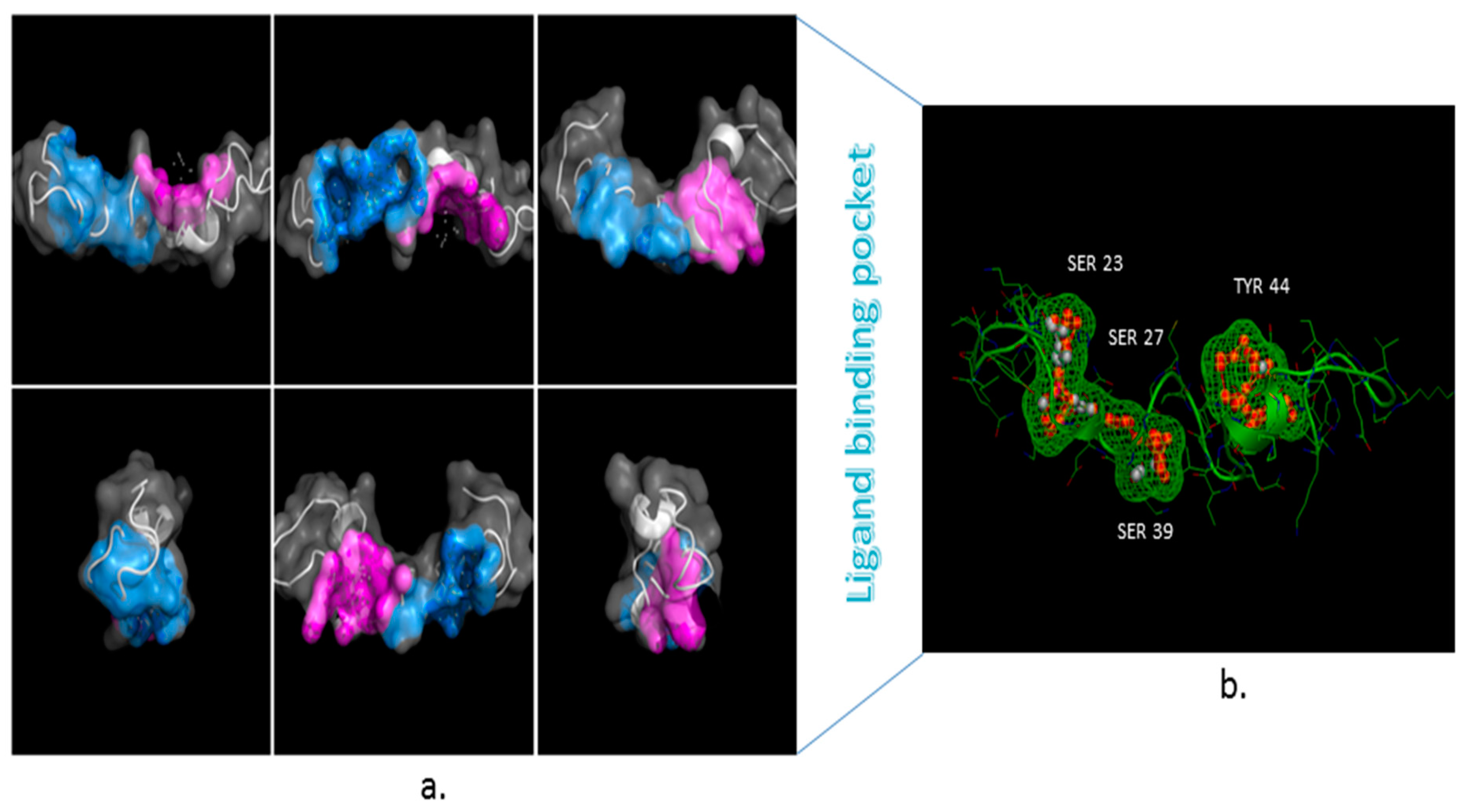
3.3. Ligand Binding Site
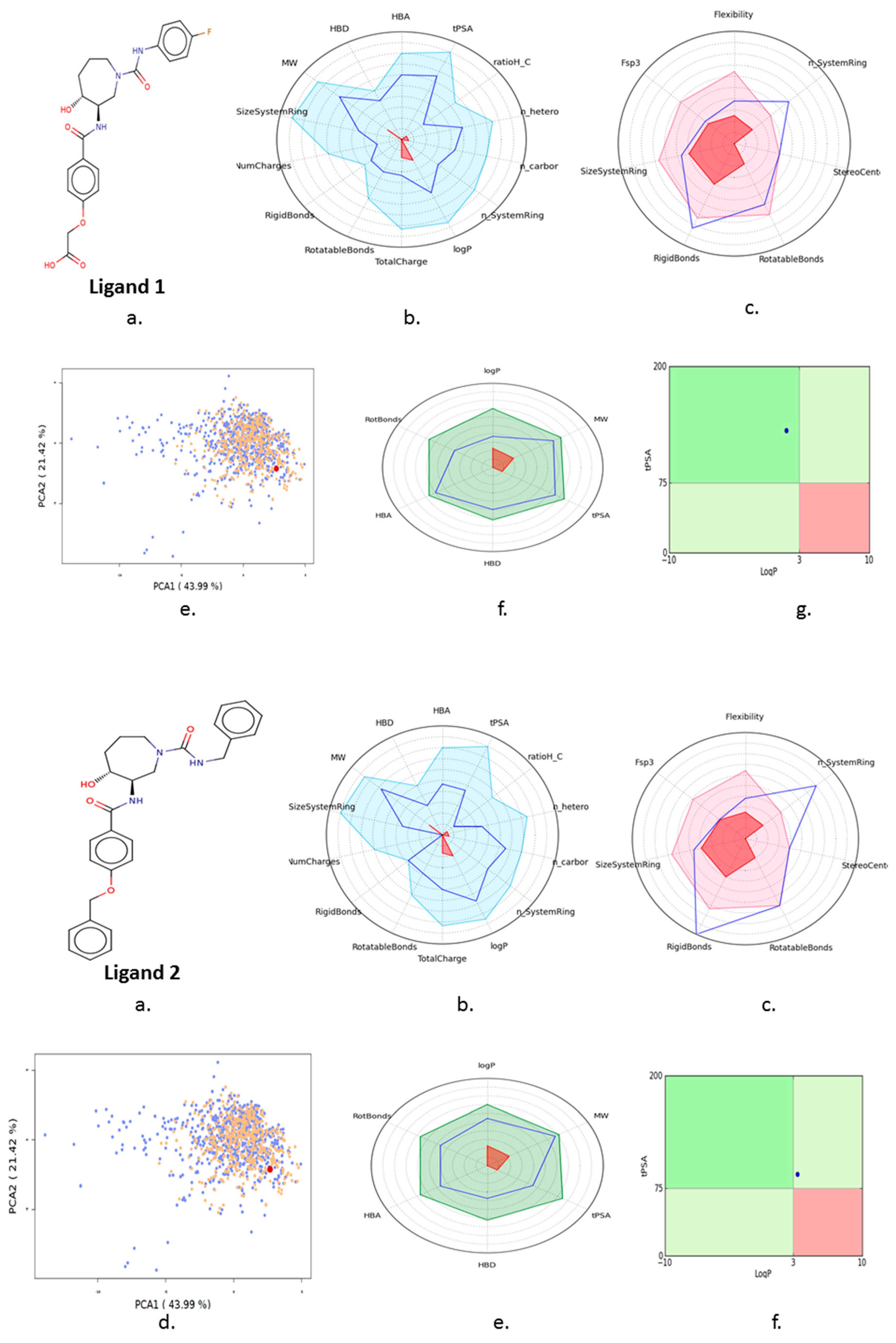
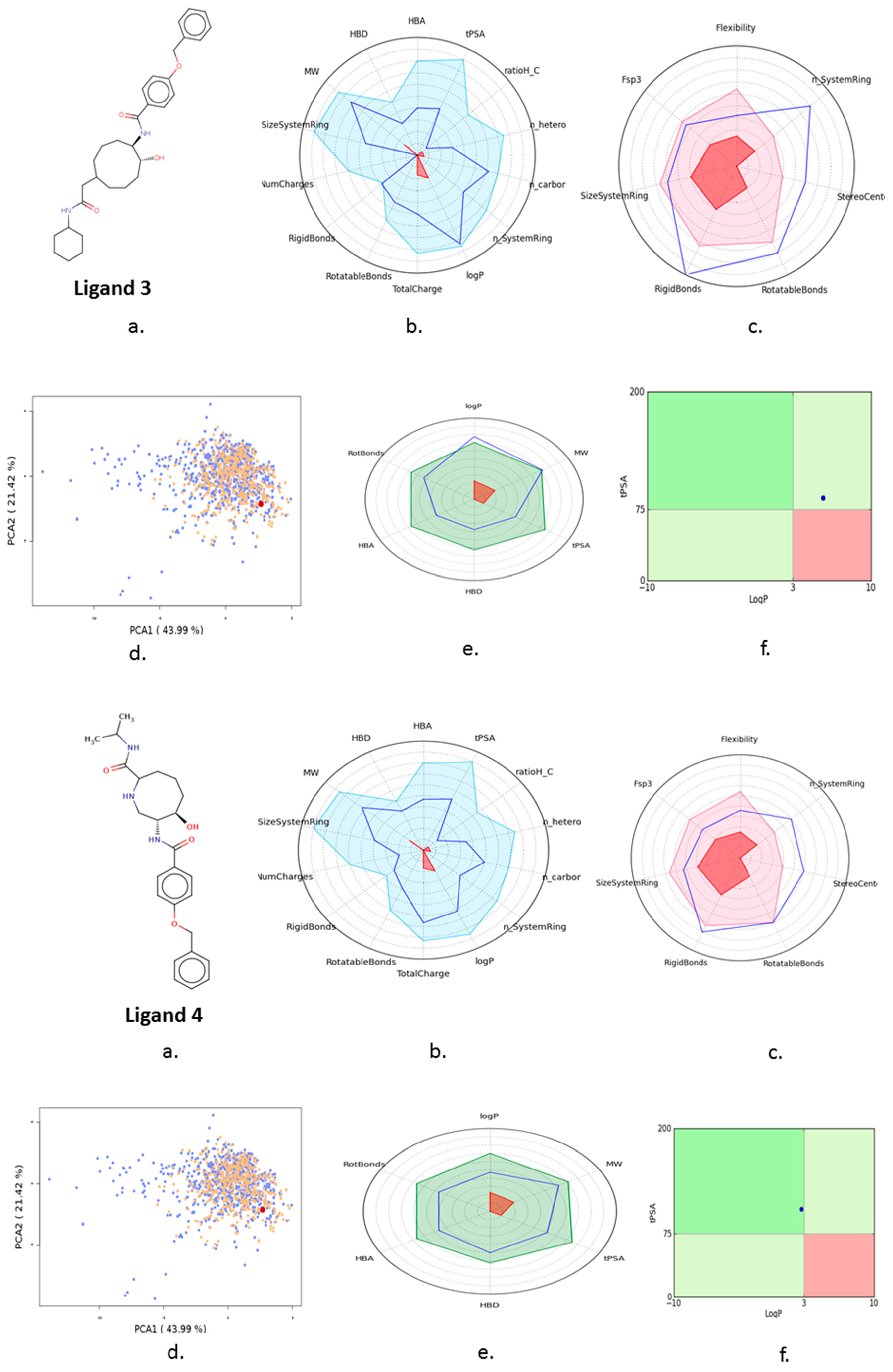
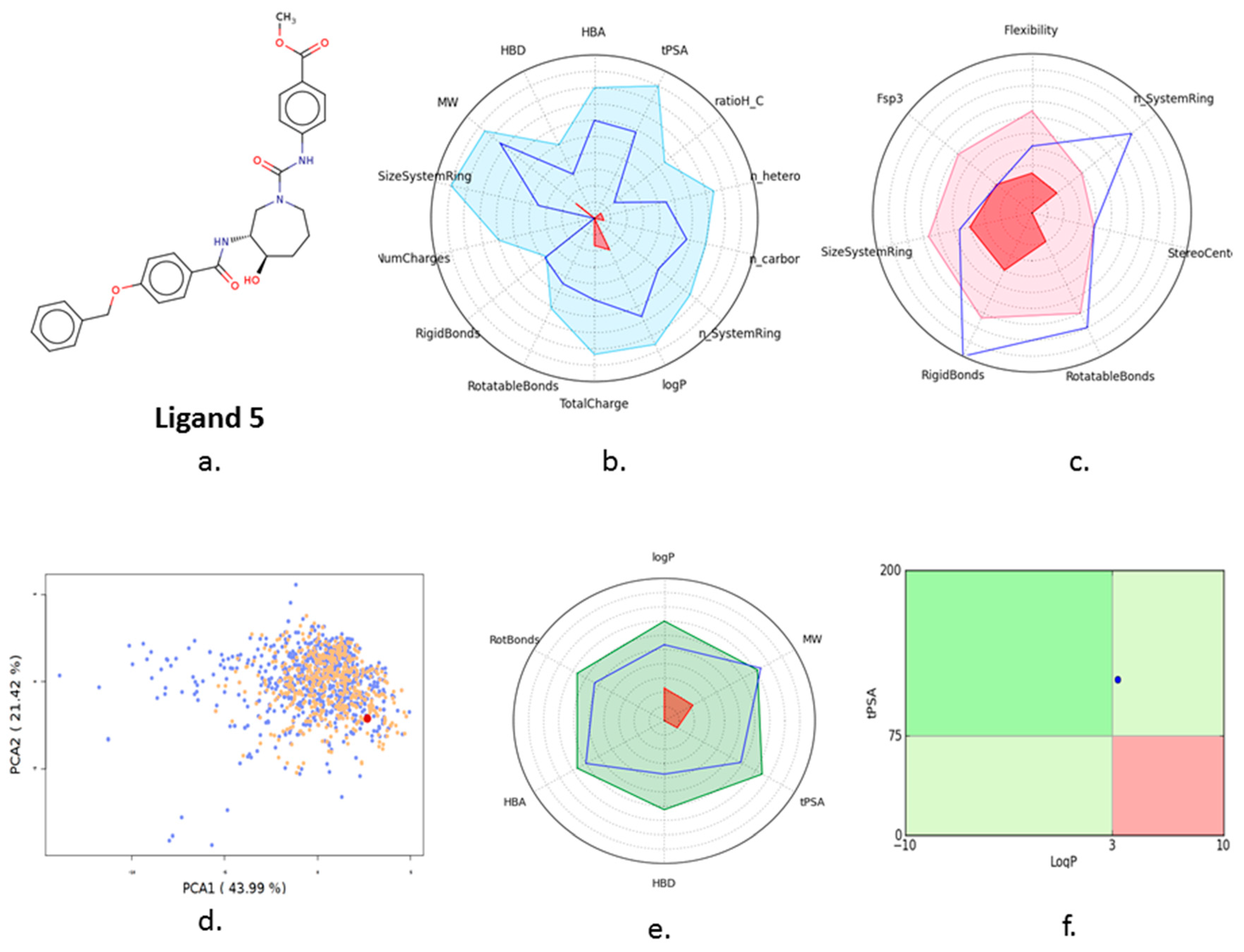
3.4. Ligand Screening
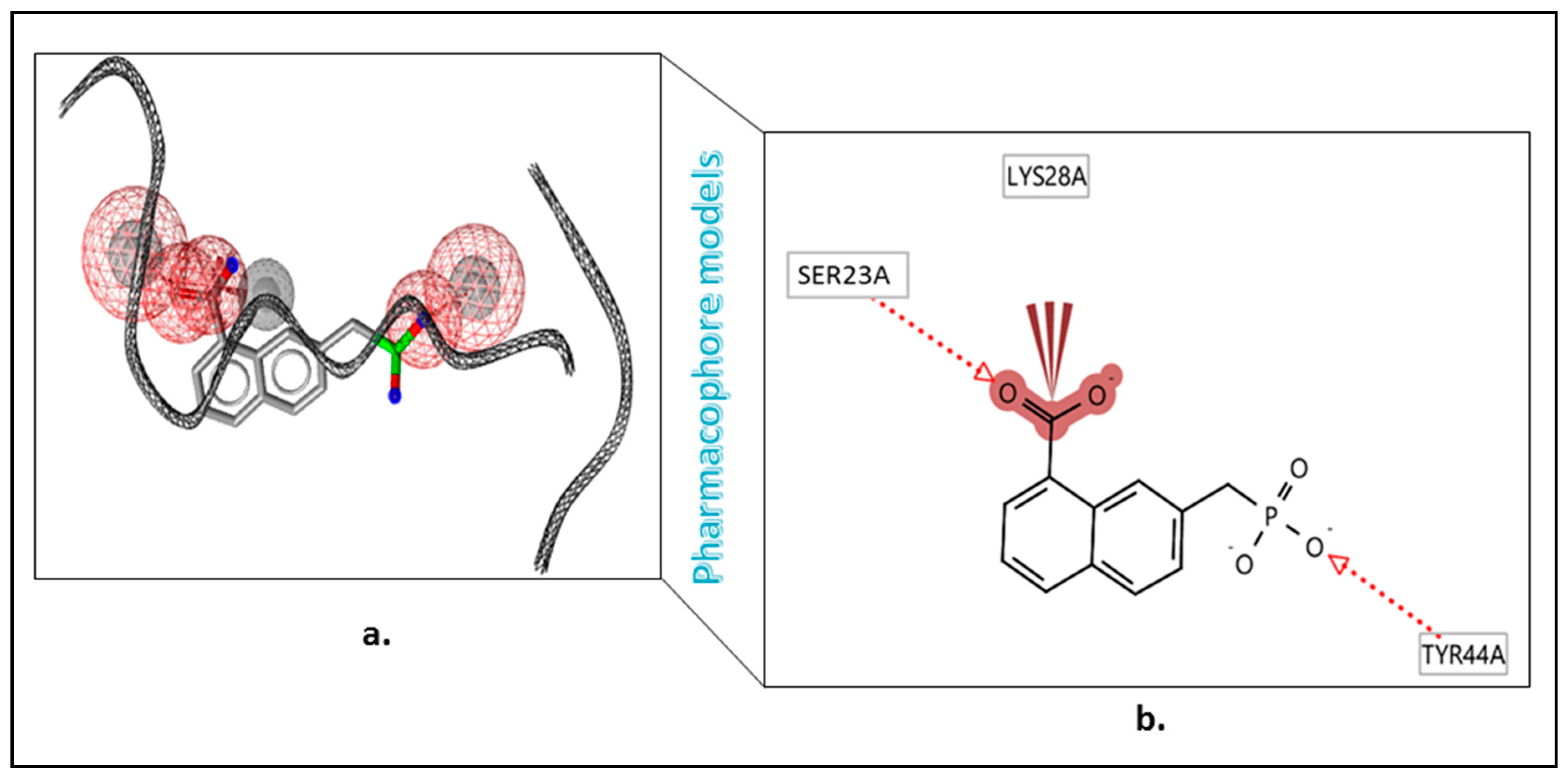
3.5. Pharmacophore Development
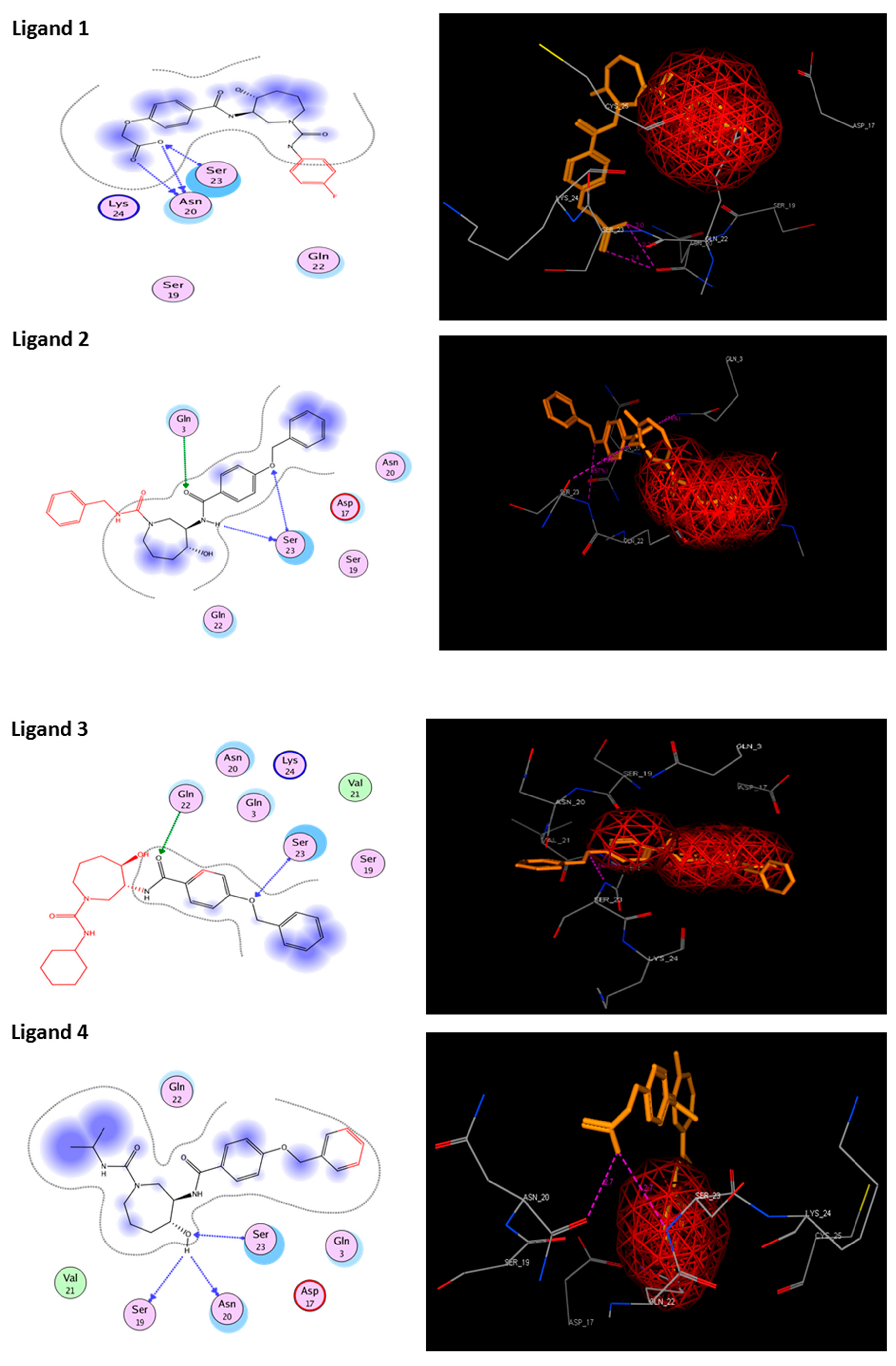
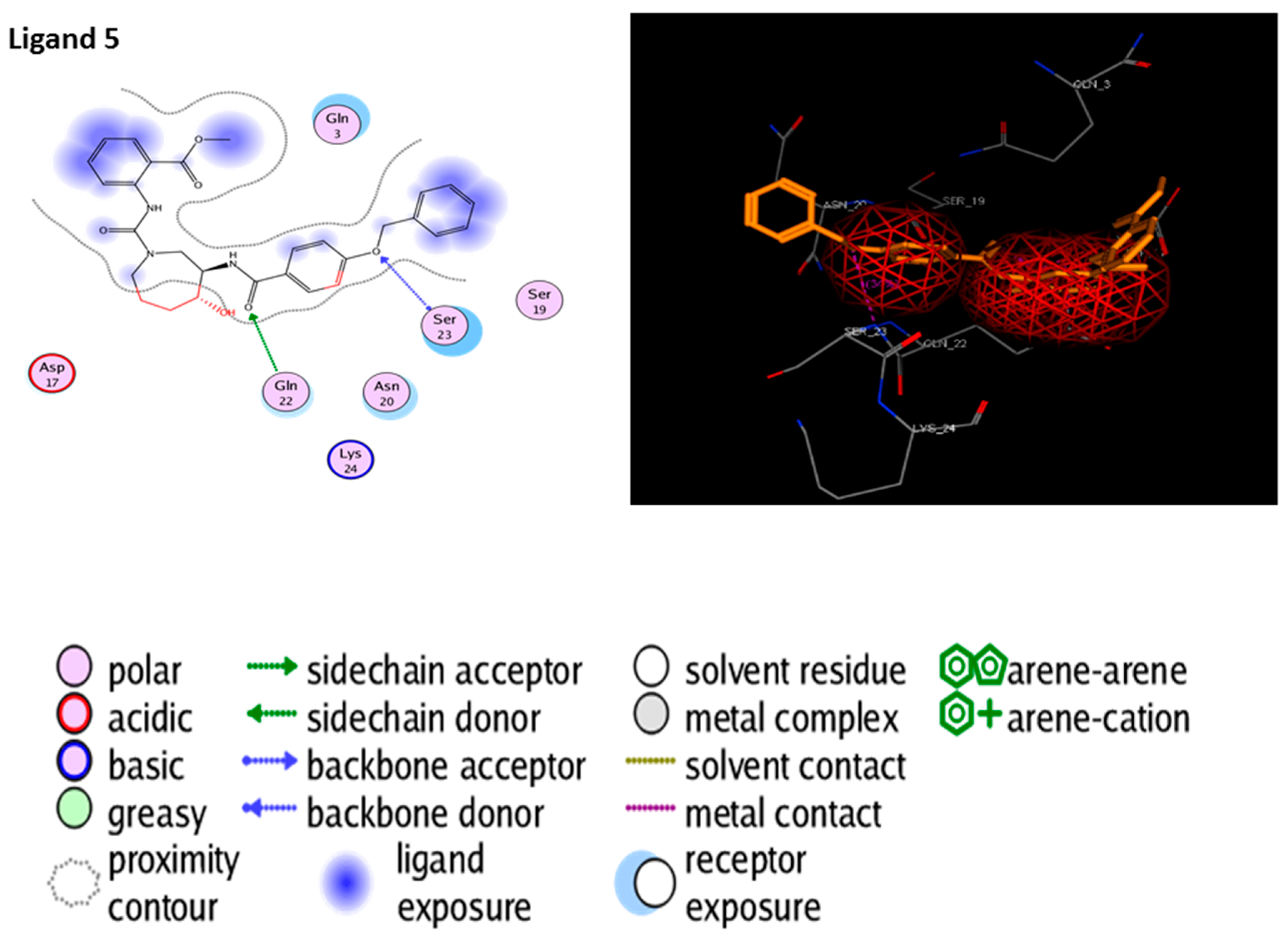
3.6. Molecular Docking
3.7. In Silico ADMET Analysis
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Alzheimer’s Disease Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2018; Available online: https://www.alz.org/documents_custom/2018-facts-andfigures.pdf (accessed on 3 May 2018).
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Pekkala, T.; Polvikoski, T.; van Gils, M.; Kivipelto, M.; Lötjönen, J.; Mattila, J.; Kero, M.; Myllykangas, L.; Mäkelä, M.; et al. Prediction models for dementia and neuropathology in the oldest old: The Vantaa 85+ cohort study. Alzheimers Res. Ther. 2019, 11, 11. [Google Scholar] [CrossRef]
- Musi, N.; Valentine, J.M.; Sickora, K.R.; Baeuerle, E.; Thompson, C.S.; Shen, Q.; Orr, M.E. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 2018, 17, e12840. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Serrano, A.; de Diego-Garcia, L.; Díaz-Hernandez, M. The Neurotoxic Role of Extracellular Tau Protein. Int. J. Mol. Sci. 2018, 19, 998. [Google Scholar] [CrossRef]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef] [PubMed]
- Simic, G.; Babic Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milosevic, N.; Bazadona, D.; Buee, L.; de Silva, R.; Di Giovanni, G.; et al. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A.; Reddy, A.P.; Reddy, P.H. Pharmacophore-based models for therapeutic drugs against phosphorylated tau in Alzheimer’s disease. Drug Discov. Today 2019, 24, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Metin-Armagan, D.; Gezen-Ak, D.; Dursun, E.; Atasoy, I.L.; Karabay, A.; Yılmazer, S.; Öztürk, M. Okadaic acid-induced tau hyperphosphorylation and the downregulation of Pin1 expression in primary cortical neurons. J. Chem. Neuroanat. 2018, 92, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, A.N.; Giannopoulos, P.F.; Pratico, D. The direct role of 5-lipoxygenase on tau pathology, synaptic integrity and cognition in a mouse model of tauopathy. Transl. Psychiatry 2017, 7, 1288. [Google Scholar] [CrossRef]
- Annadurai, N.; Agrawal, D.; Dzubak, P.; Hajdúch, M.; Das, V. Microtubule affinity-regulating kinases are potential druggable targets for Alzheimer’s disease. Cell. Mol. Life Sci. 2017, 74, 4159–4169. [Google Scholar] [CrossRef]
- Liu, D.; Lu, H.; Stein, E.; Zhou, Z.; Yang, Y.; Mattson, M.P. Brain regional synchronous activity predicts tauopathy in 3×TgAD mice. Neurobiol. Aging 2018, 70, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Iijima-Ando, K.; Sekiya, M.; Maruko-Otake, A.; Ohtake, Y.; Suzuki, E.; Lu, B.; Iijima, K.M. Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer’s disease-related tau phosphorylation via PAR-1. PLoS Genet. 2012, 8, e1002918. [Google Scholar] [CrossRef]
- Smit, F.X.; Luiken, J.A.; Bolhuis, P.G. Primary Fibril Nucleation of Aggregation Prone Tau Fragments PHF6 and PHF6. J. Phys. Chem. B 2017, 121, 3250–3261. [Google Scholar] [CrossRef]
- Inoue, M.; Konno, T.; Tainaka, K.; Nakata, E.; Yoshida, H.O.; Morii, T. Positional effects of phosphorylation on the stability and morphology of tau-related amyloid fibrils. Biochemistry 2012, 51, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Sekiya, M.; Taniguchi, T.; Iijima, K.M.; Wang, R.; Ando, K. Global analysis of phosphorylation of tau by the checkpoint kinases Chk1 and Chk2 in vitro. J. Proteome Res. 2013, 12, 2654–2665. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Prabakaran, S.; Cantrelle, F.X.; Chambraud, B.; Gunawardena, J.; Lippens, G.; Landrieu, I. Characterization of Neuronal Tau Protein as a Target of Extracellular Signal-regulated Kinase. J. Biol. Chem. 2016, 291, 7742–7753. [Google Scholar] [CrossRef]
- Hampel, H.; Prvulovic, D.; Teipel, S.; Jessen, F.; Luckhaus, C.; Frolich, L.; Riepe, M.W.; Dodel, R.; Leyhe, T.; Bertram, L.; et al. German Task Force on Alzheimer’s Disease (GTF-AD). The future of Alzheimer’s disease: The next 10 years. Prog. Neurobiol. 2011, 95, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Hilbush, B.S.; Morrison, J.H.; Young, W.G.; Sutcliffe, J.G.; Bloom, F.E. New prospects and strategies for drug target discovery in neurodegenerative disorders. NeuroRx 2005, 2, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.L.; Chen, C.C. Computational approaches for drug discovery. Drug Dev. Res. 2014, 75, 412–418. [Google Scholar] [CrossRef]
- Macalino, S.J.; Gosu, V.; Hong, S.; Choi, S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 2015, 16, 86–701. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Eramian, D.; Webb, B.; Shen, M.Y.; Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2008, 426, 145–159. [Google Scholar] [PubMed]
- Bhattacharya, A.; Tejero, R.; Montelione, G.T. Evaluating protein structures determined by structural genomics consortia. Proteins 2007, 66, 66778–66795. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nowotny, J.; Cao, R.; Cheng, J. 3Drefine: An interactive web server for efficient protein structure refinement. Nucleic Acids Res. 2016, 44, W406–W409. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, M.; Kolinski, A.; Kmiecik, S. CABS-flex: Server for fast simulation of protein structure fluctuations. Nucleic Acids Res. 2013, 41, W427–W431. [Google Scholar] [CrossRef] [PubMed]
- Hospital, A.; Andrio, P.; Fenollosa, C.; Cicin-Sain, D.; Orozco, M.; Gelpi, J.L. MDWeb and MDMoby: An integrated web-based platform for molecular dynamics simulations. Bioinformatics 2012, 28, 1278–1279. [Google Scholar] [CrossRef]
- Schmidtke, P.; Le Guilloux, V.; Maupetit, J.; Tufféry, P. fpocket: Online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res. 2010, 38, W582–W589. [Google Scholar] [CrossRef] [PubMed]
- Wolber, G.; Langer, T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015, 43, W200–W207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Berezovsky, I.N.; Zeldovich, K.B.; Shakhnovich, E.I. Positive and Negative Design in Stability and Thermal Adaptation of Natural Proteins. PLoS Comput. Biol. 2007, 3, e52. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.J.; Shukla, D.; Beauchamp, K.A.; Pande, V.S. To milliseconds and beyond: Challenges in the simulation of protein folding. Curr. Opin. Struct. Biol. 2013, 23, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, Y.; Wen, X.; Jorissen, R.N.; Gilson, M.K. BindingDB: A web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007, 35, D198–D201. [Google Scholar] [CrossRef]
- Deng, J.; Sanchez, T.; Neamati, N.; Briggs, J.M. Dynamic pharmacophore model optimization: Identification of novel HIV-1 integrase inhibitors. J. Med. Chem. 2006, 49, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Ferreira, E.; Tran, A.; Turner, E.C.; Belfiore, R.; Branca, C.; Oddo, S. Acute tau knockdown in the hippocampus of adult mice causes learning and memory deficits. Aging Cell 2018, e12775. [Google Scholar] [CrossRef] [PubMed]
- Maunz, A.; Gütlein, M.; Rautenberg, M.; Vorgrimmler, D.; Gebele, D.; Helma, C. lazar: A modular predictive toxicology framework. Front. Pharmacol. 2013, 9, 38. [Google Scholar] [CrossRef]

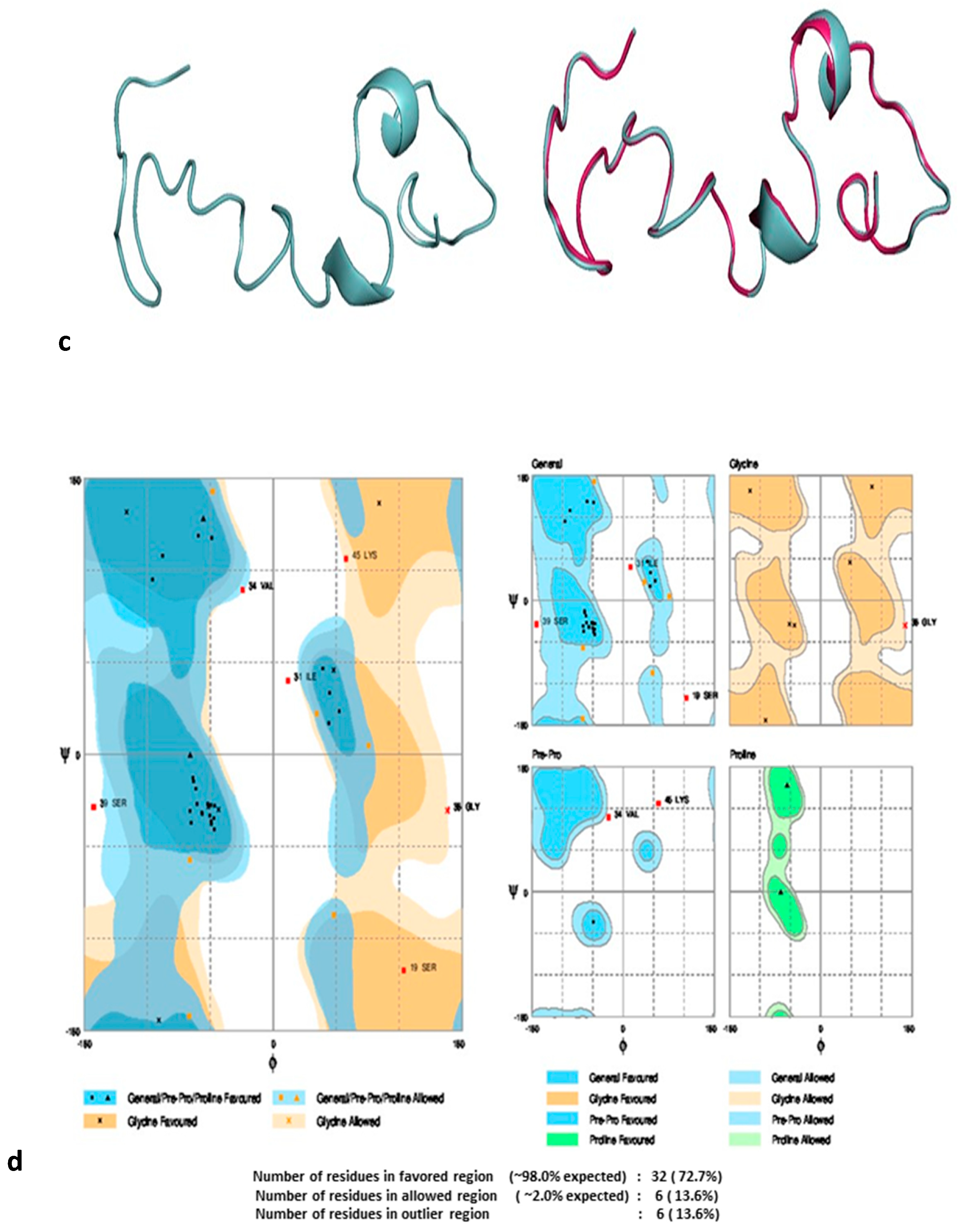
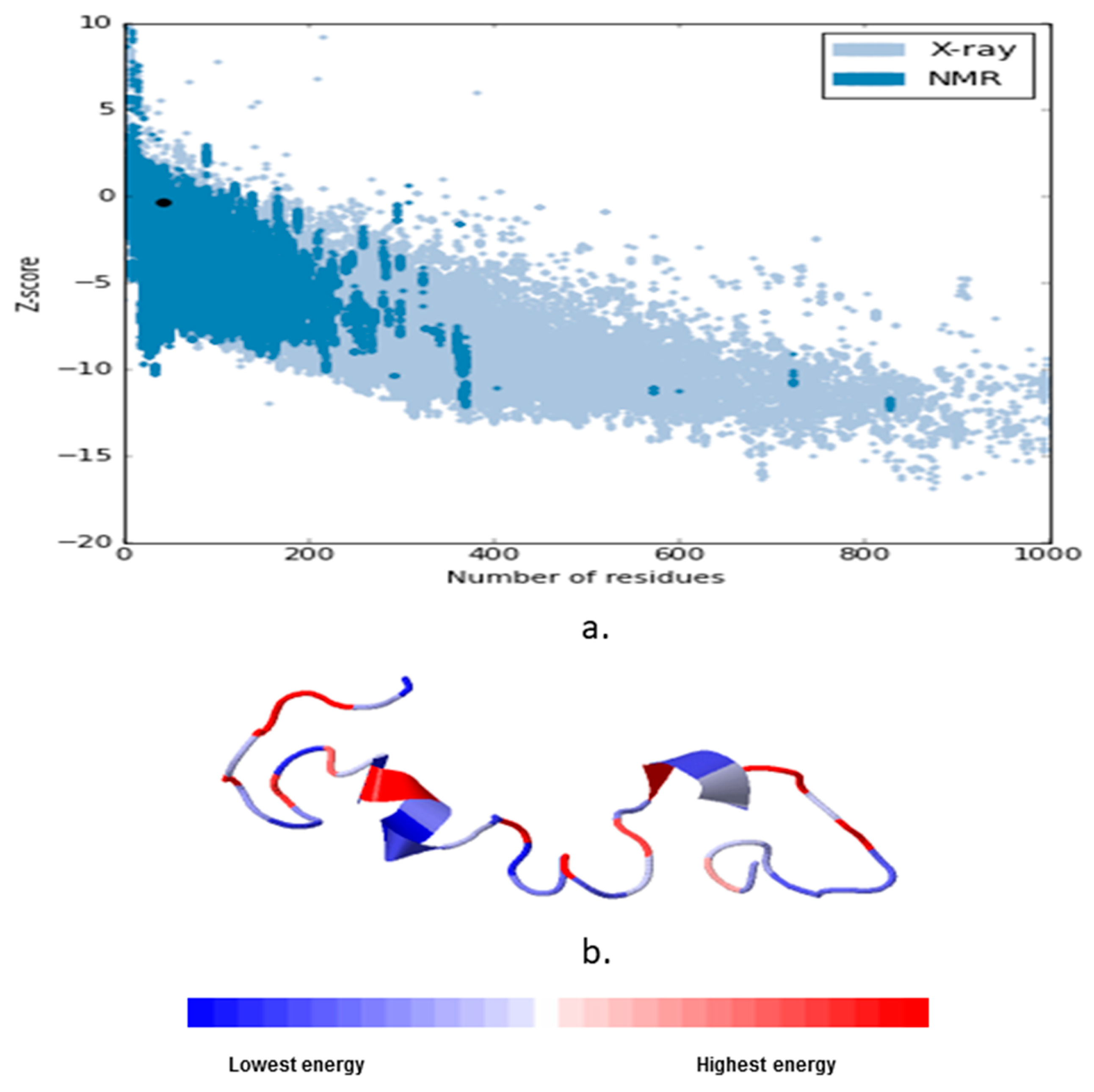
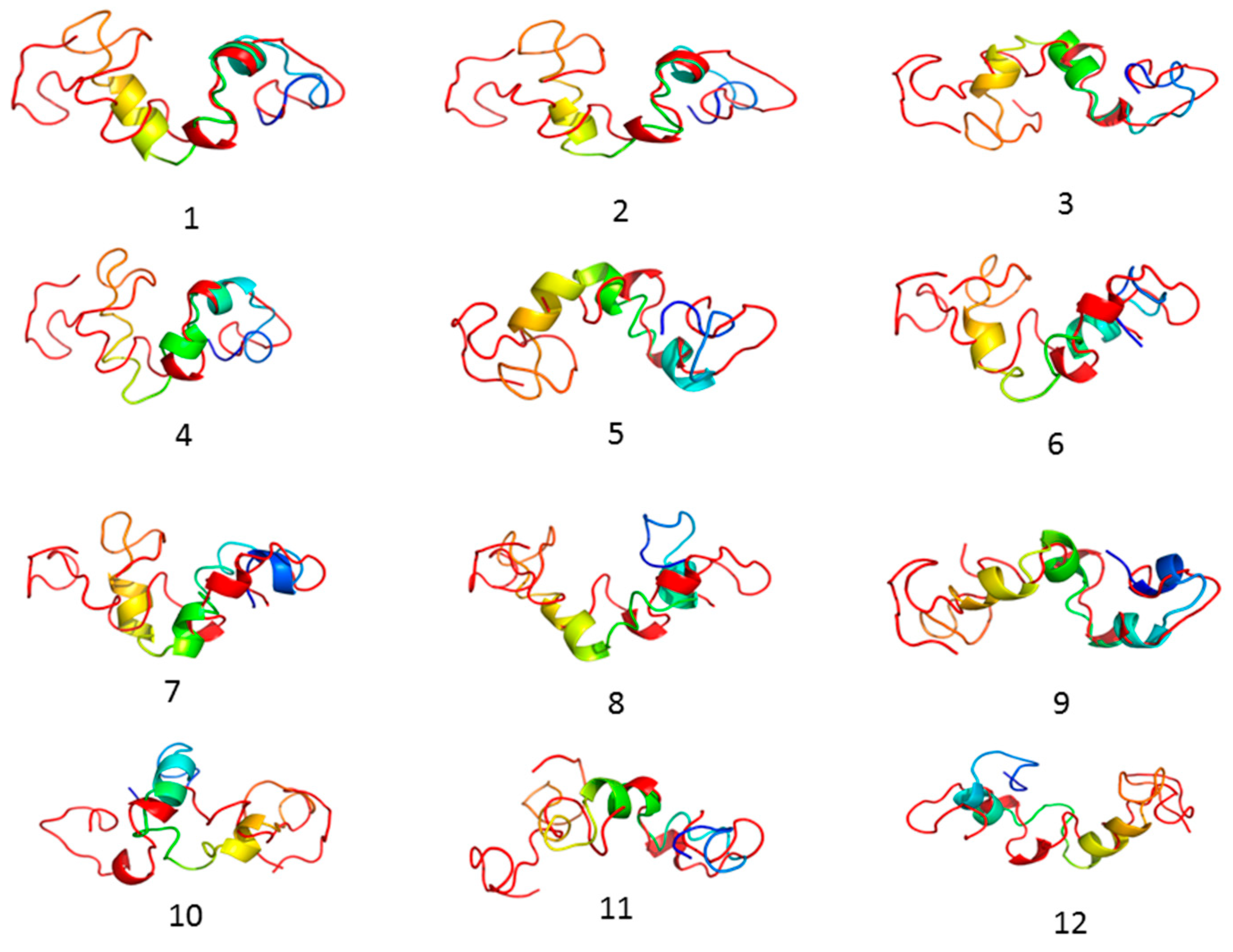
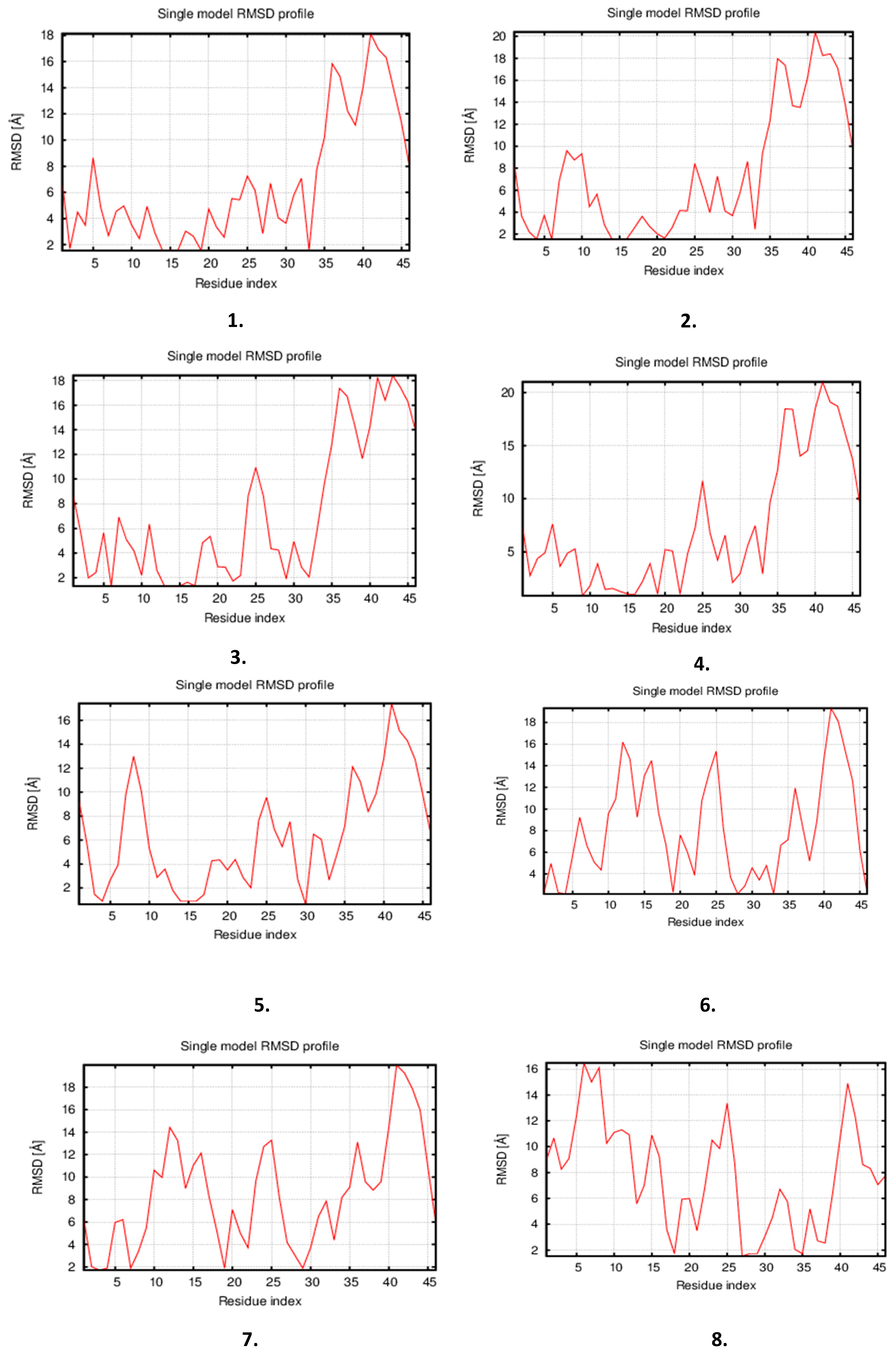


| S. No | Model | 3Drefine Score | GDT-TS | GDT-HA | RMSD (Å) | MolProbity |
|---|---|---|---|---|---|---|
| 1 | 2 | 4160.69 | 1.0000 | 1.0000 | 0.172 | −3264.862351 |
| 2 | 3 | 4111.35 | 1.0000 | 1.0000 | 0.194 | −3257.601994 |
| 3 | 1 | 4716.22 | 1.0000 | 1.0000 | 0.135 | −3230.220671 |
| 4 | 4 | 4056.68 | 1.0000 | 1.0000 | 0.222 | −3216.237507 |
| 5 | 5 | 4005.46 | 1.0000 | 1.0000 | 0.248 | −3202.466724 |
| Models | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.00 | 1.81 | 3.26 | 2.46 | 3.20 | 4.95 | 3.86 | 5.88 | 4.14 | 6.51 | 4.33 | 6.47 |
| 2 | 1.81 | 0.00 | 3.59 | 2.71 | 3.30 | 5.21 | 3.88 | 5.91 | 5.16 | 6.39 | 5.08 | 6.71 |
| 3 | 3.26 | 3.59 | 0.00 | 2.82 | 2.84 | 5.11 | 3.87 | 6.57 | 4.88 | 6.83 | 4.25 | 7.10 |
| 4 | 2.46 | 2.71 | 2.82 | 0.00 | 2.81 | 4.58 | 3.55 | 6.21 | 4.25 | 6.53 | 3.60 | 6.45 |
| 5 | 3.20 | 3.30 | 2.84 | 2.81 | 0.00 | 6.06 | 5.01 | 6.22 | 5.01 | 6.90 | 4.55 | 6.19 |
| 6 | 4.95 | 5.21 | 5.11 | 4.58 | 6.06 | 0.00 | 2.47 | 4.20 | 5.41 | 4.31 | 5.05 | 5.43 |
| 7 | 3.86 | 3.88 | 3.87 | 3.55 | 5.01 | 2.47 | 0.00 | 4.91 | 5.21 | 4.51 | 4.78 | 6.38 |
| 8 | 5.88 | 5.91 | 6.57 | 6.21 | 6.22 | 4.20 | 4.91 | 0.00 | 7.03 | 3.57 | 6.99 | 3.26 |
| 9 | 4.14 | 5.16 | 4.88 | 4.25 | 5.01 | 5.41 | 5.21 | 7.03 | 0.00 | 7.53 | 2.99 | 7.15 |
| 10 | 6.51 | 6.39 | 6.83 | 6.53 | 6.90 | 4.31 | 4.51 | 3.57 | 7.53 | 0.00 | 7.18 | 5.13 |
| 11 | 4.33 | 5.08 | 4.25 | 3.60 | 4.55 | 5.05 | 4.78 | 6.99 | 2.99 | 7.18 | 0.00 | 6.91 |
| 12 | 6.47 | 6.71 | 7.10 | 6.45 | 6.19 | 5.43 | 6.38 | 3.26 | 7.15 | 5.13 | 6.91 | 0.00 |
| Models | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSD | 6.94 | 7.74 | 7.21 | 7.70 | 7.26 | 8.81 | 8.69 | 8.53 | 6.79 | 9.47 | 7.33 | 8.10 |
| GDT_TS | 0.41 | 0.38 | 0.39 | 0.38 | 0.39 | 0.39 | 0.38 | 0.33 | 0.45 | 0.30 | 0.43 | 0.32 |
| S. No | Compound | 3-D Structure | Docking Score (S) | Binding Energy (kcal/mol−1) | Binding Affinity | Bonding Interaction | Bond Length (Å) | Bond Type |
|---|---|---|---|---|---|---|---|---|
| 1 | Ligand 1 |  | −11.39 | −110.32 | 2.6 | Asn 20 Ser 23 (S285) | 2.2 2.4 | H-acc H-don |
| 2 | Ligand 2 |  | −10.73 | −110.43 | 2.2 | Ser 23 (S285) Gln 3 | 2.2 2.5 | Ionic Ionic |
| 3 | Ligand 3 | 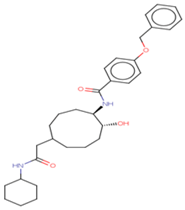 | −10.45 | −100.66 | 2.8 | Gln 22 Ser 23 | 1.8 2.2 | Ionic Ionic |
| 4 | Ligand 4 | 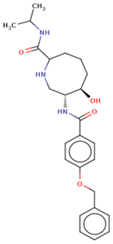 | −10.39 | −123.36 | 2.7 | Ser 19 Asn 20 Ser 23 (S285) | 2.7 2.7 2.2 | H-don H-acc Ionic |
| 5 | Ligand 5 | 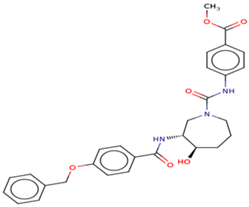 | −10.10 | −111.03 | 1.6 | Gly 22 Ser 23 (S285) | 2.0 2.3 | Ionic Ionic |
| S. No | Compound | Structure | Blood Brain Barrier Penetration (Human) Prediction | Probability Penetrating | Probability Non-Penetrating |
|---|---|---|---|---|---|
| 1 | Ligand 1 | 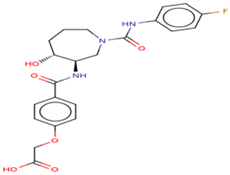 | Penetrating | 0.0944 | 0.0722 |
| 2 | Ligand 2 |  | Penetrating | 0.15 | 0.0667 |
| 3 | Ligand 3 | 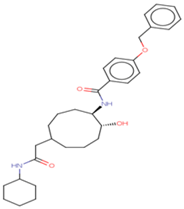 | Penetrating | 0.157 | 0.0933 |
| 4 | Ligand 4 | 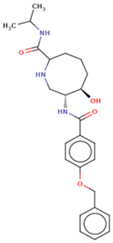 | Penetrating | 0.137 | 0.0738 |
| 5 | Ligand 5 | 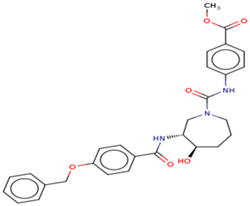 | Penetrating | 0.141 | 0.0539 |
| a. FAF-drugs4 | ||||||
|---|---|---|---|---|---|---|
| S. No | Property | Ligand 1 | Ligand 2 | Ligand 3 | Ligand 4 | Ligand 5 |
| 1 | Molecular formula | C22H24FN3O6 | C28H32N3O4 | C31H42N2O4 | C25H33N3O4 | C29H31N3O6 |
| 2 | Molecular weight | 445.44 | 473.56 | 506.68 | 439.55 | 517.57 |
| 3 | Number of HBA | 9 | 7 | 6 | 7 | 9 |
| 4 | Number of HBD | 4 | 3 | 3 | 4 | 3 |
| 5 | Mol Log P | 1.69 | 3.42 | 5.71 | 2.71 | 3.34 |
| 6 | Related topological surface area (tPSA) A2 | 131.03 | 90.90 | 87.66 | 104.27 | 117.20 |
| b. admetSAR | ||||||
| 1 | Human Intestinal Absorption | HIA+ | HIA+ | HIA+ | HIA+ | HIA+ |
| 2. | Human Ether-a-go-go-Related Gene Inhibition | Weak inhibitor | Weak inhibitor | Weak inhibitor | Weak inhibitor | Weak inhibitor |
| 3 | AMES Toxicity | Non AMES toxic | Non AMES toxic | Non AMES toxic | Non AMES toxic | Non AMES toxic |
| 4 | Carcinogens | Non-carcinogens | Non-carcinogens | Non-carcinogens | Non-carcinogens | Non-carcinogens |
| 5 | Fish Toxicity | High FHMT | High FHMT | High FHMT | High FHMT | High FHMT |
| 6 | Acute Oral Toxicity | III | III | III | III | III |
| 7 | Aqueous solubility | −3.3390 | −3.4553 | −3.9841 | −3.3390 | −3.5326 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeepkiran, J.A.; Reddy, P.H. Structure Based Design and Molecular Docking Studies for Phosphorylated Tau Inhibitors in Alzheimer’s Disease. Cells 2019, 8, 260. https://doi.org/10.3390/cells8030260
Pradeepkiran JA, Reddy PH. Structure Based Design and Molecular Docking Studies for Phosphorylated Tau Inhibitors in Alzheimer’s Disease. Cells. 2019; 8(3):260. https://doi.org/10.3390/cells8030260
Chicago/Turabian StylePradeepkiran, Jangampalli Adi, and P. Hemachandra Reddy. 2019. "Structure Based Design and Molecular Docking Studies for Phosphorylated Tau Inhibitors in Alzheimer’s Disease" Cells 8, no. 3: 260. https://doi.org/10.3390/cells8030260
APA StylePradeepkiran, J. A., & Reddy, P. H. (2019). Structure Based Design and Molecular Docking Studies for Phosphorylated Tau Inhibitors in Alzheimer’s Disease. Cells, 8(3), 260. https://doi.org/10.3390/cells8030260






