Activation of COUP-TFI by a Novel Diindolylmethane Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Chemicals, and Reagents
2.2. Transfection Assays
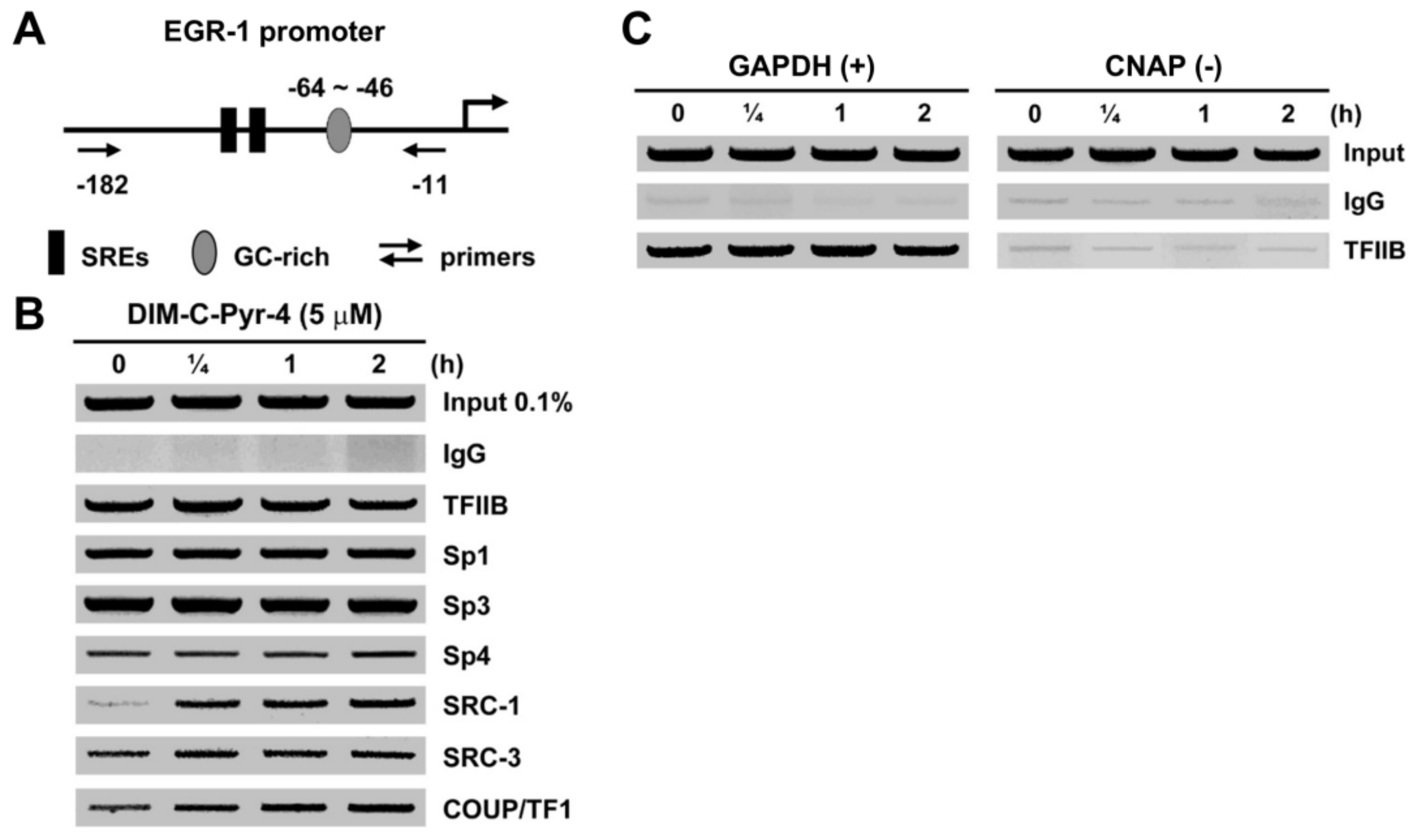
2.3. Chromatin Immunoprecipitation (ChIP) Assay
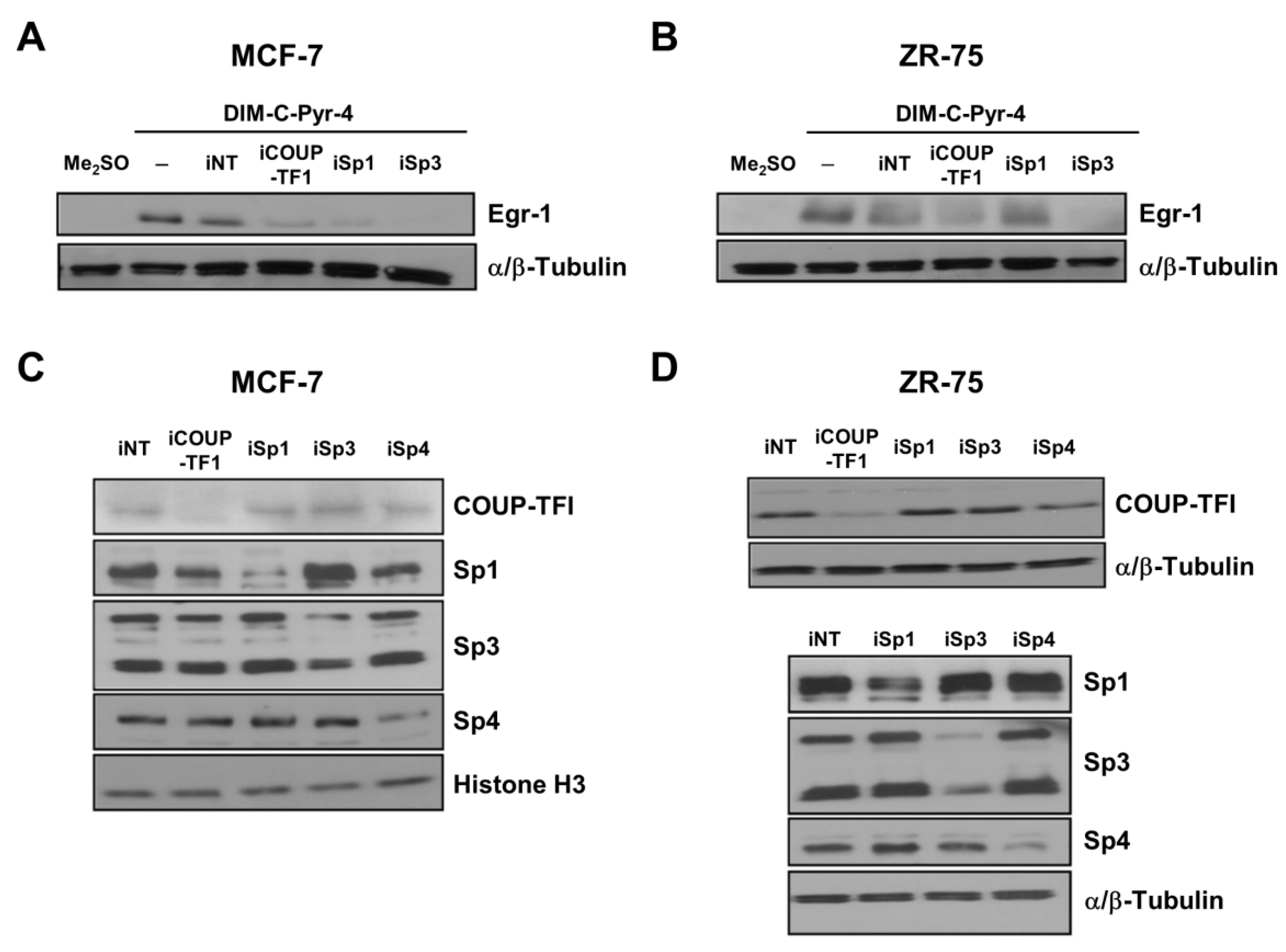
2.4. RNA Interference Studies
2.5. Induction of Egr-1 Protein/mRNA by DIM-C-Pyr-4
2.6. Clones and Reagents
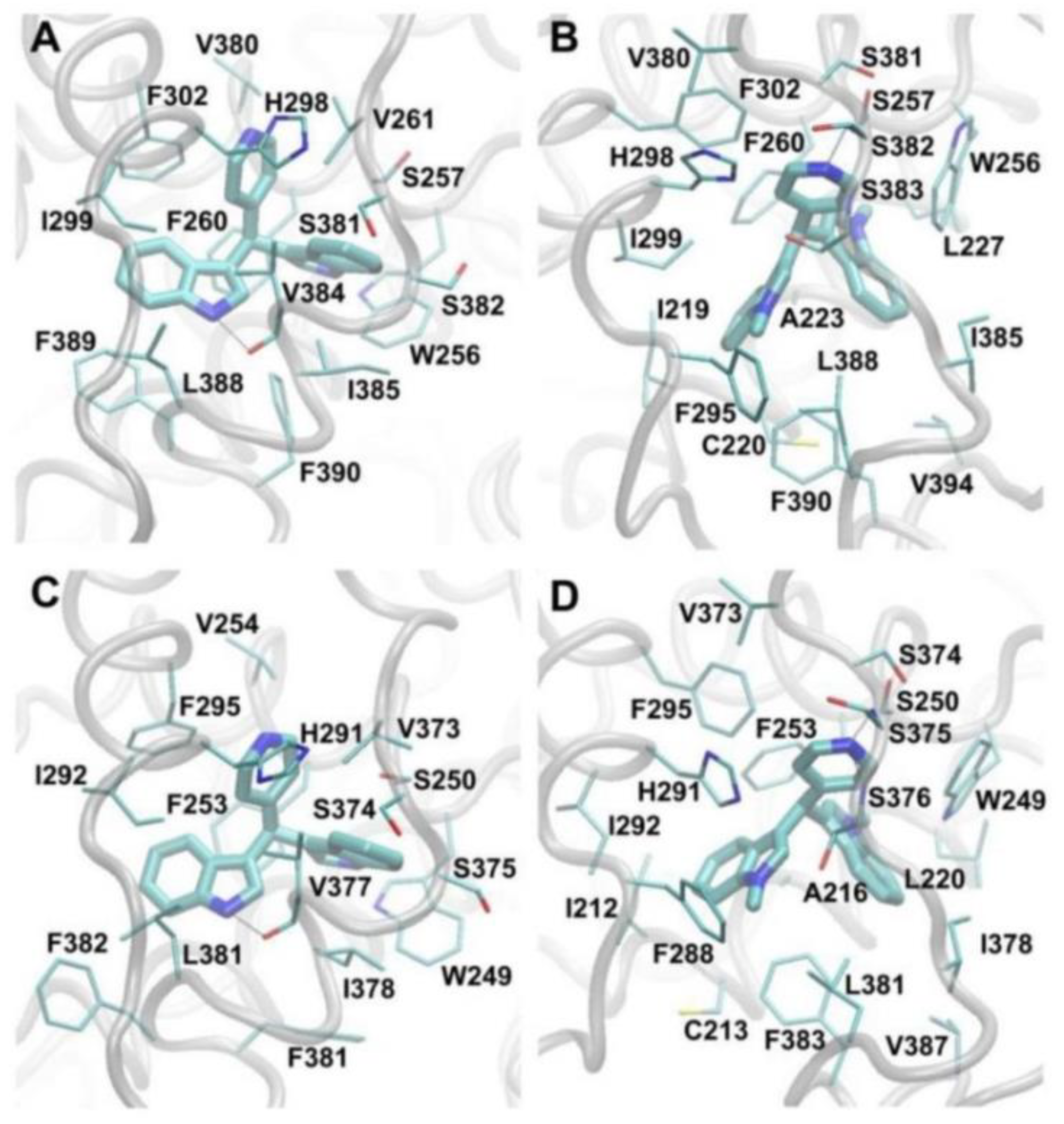
2.7. Computation Analysis of COUP-TFI and COUP-TFII Ligand Binding
2.8. Statistical Analysis
3. Results
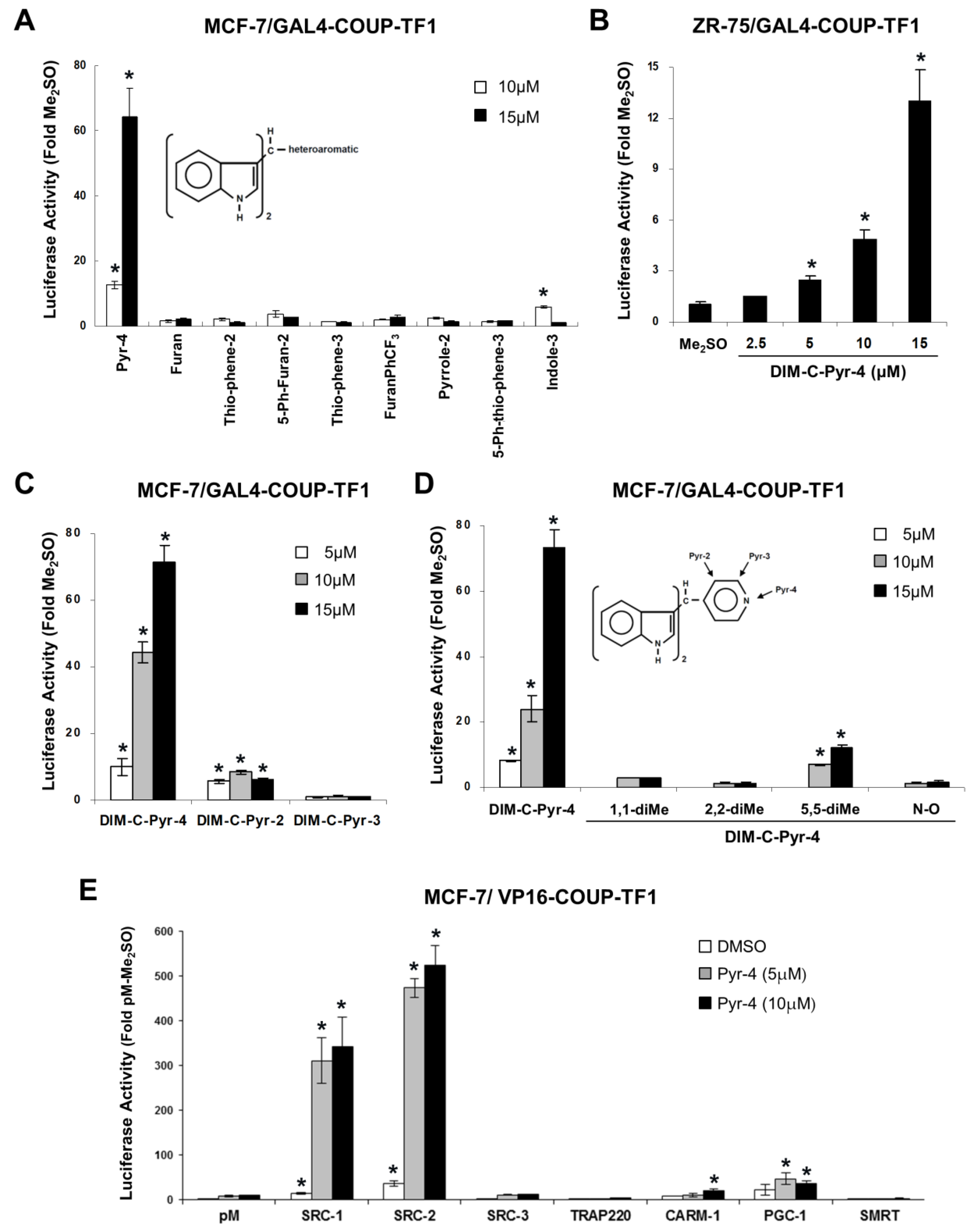
3.1. Structure-Dependent Activation of COUP-TFI and Interaction with Coactivators and Silencing Mediator of Retinoic Acid and Thyroid Hormone Receptor (SMRT)
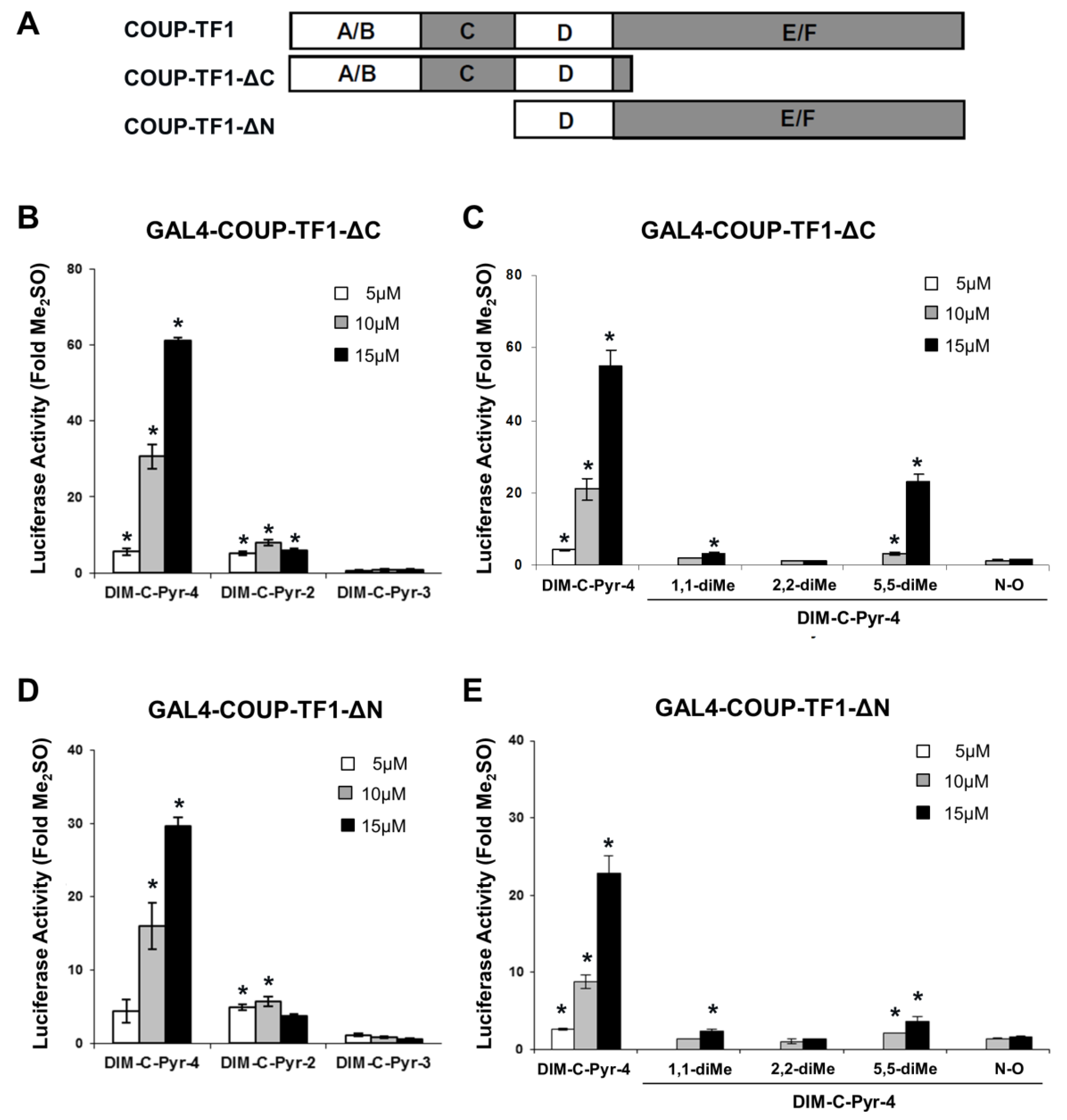
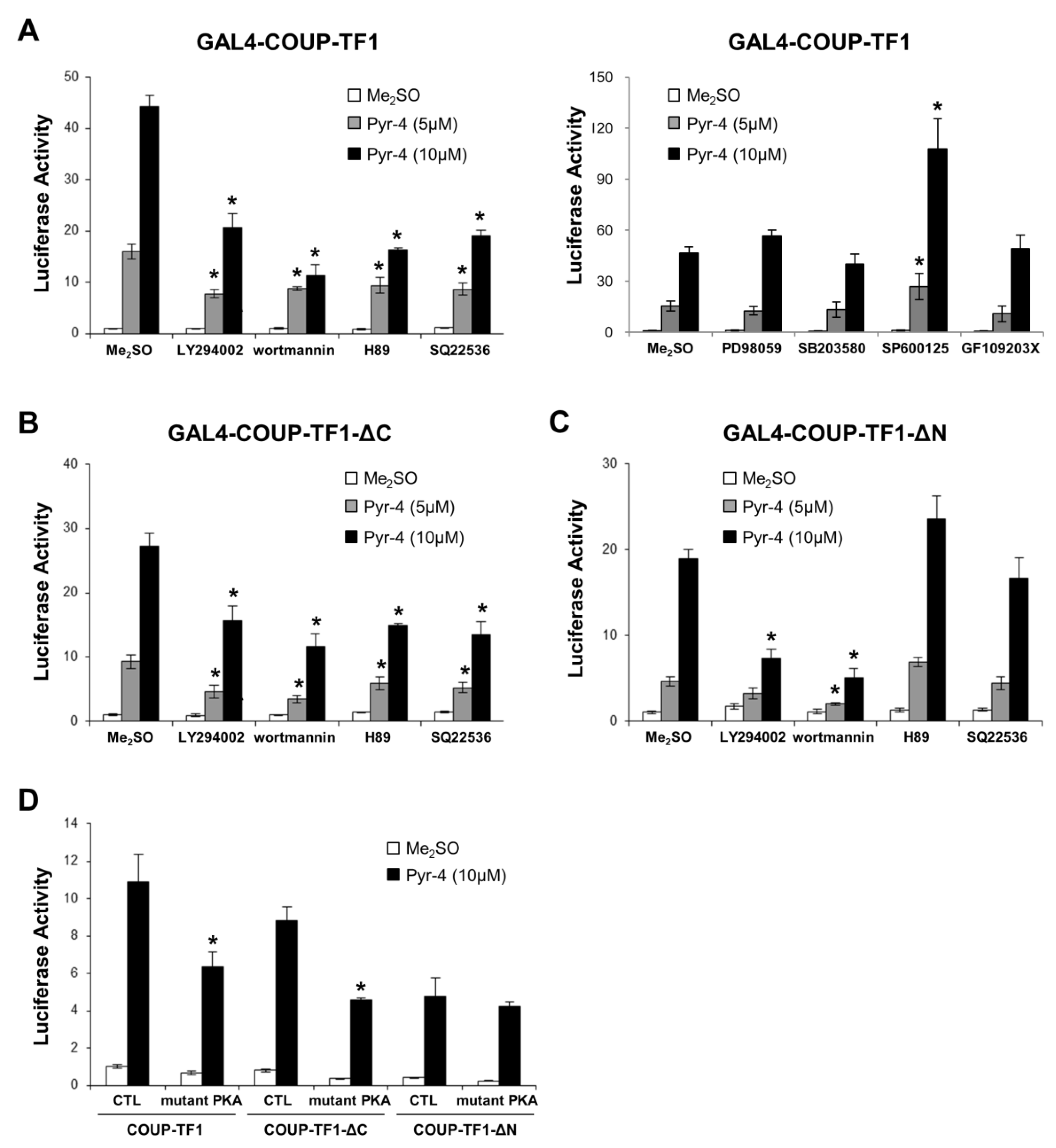
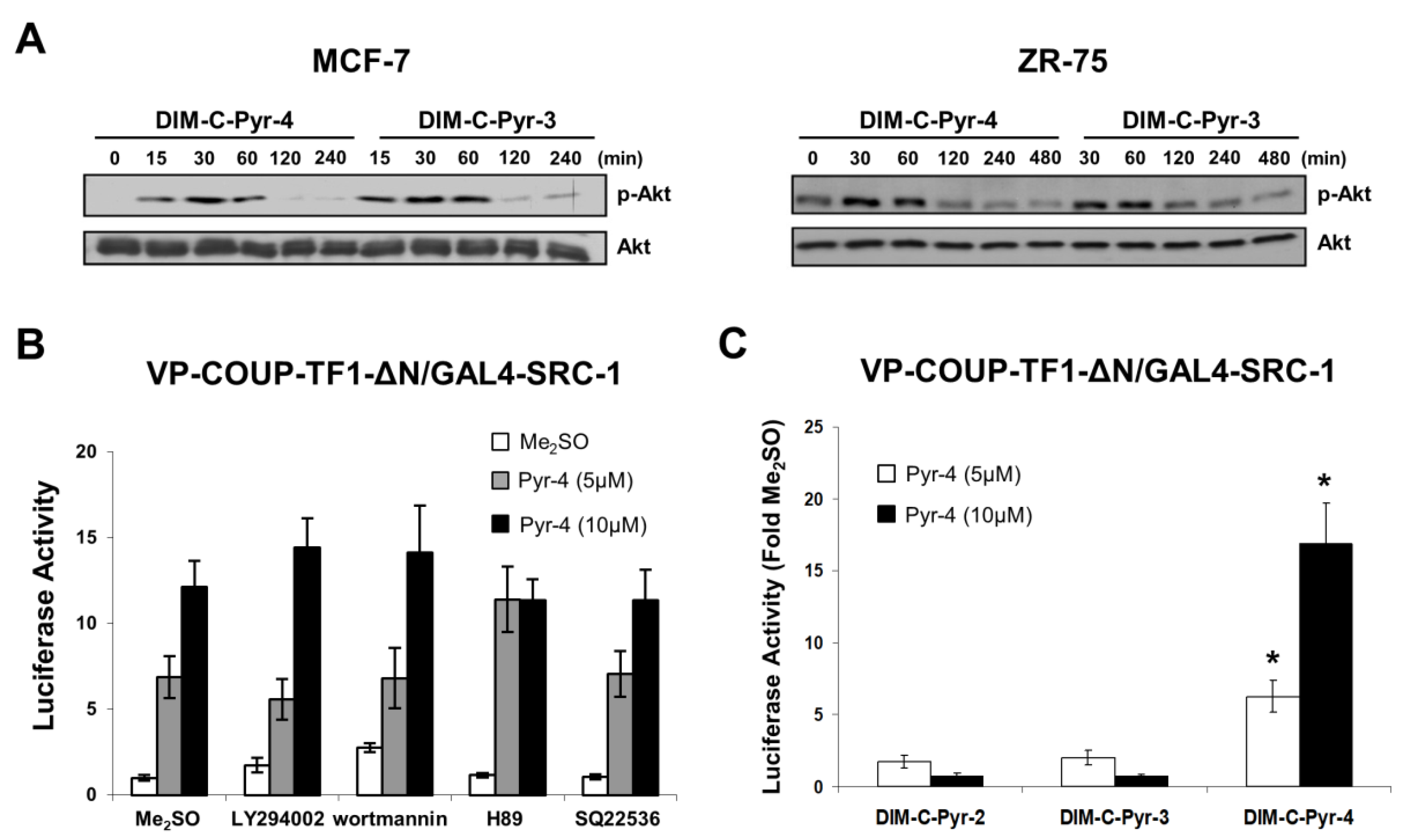
3.2. Activation of Variant GAL4-COUP-TFI Chimeras by DIM-C-Pyr-4 and Related Compounds
3.3. DIM-C-Pyr-4 Activation of Egr-1 Is Dependent on COUP-TFI and Sp Proteins
3.4. Molecular Modeling Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ing, N.H.; Beekman, J.M.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J. Biol. Chem. 1992, 267, 17617–17623. [Google Scholar] [PubMed]
- Pastorcic, M.; Wang, H.; Elbrecht, A.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Control of transcription initiation in vitro requires binding of a transcription factor to the distal promoter of the ovalbumin gene. Mol. Cell. Biol. 1986, 6, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Tsai, S.Y.; Cook, R.G.; Beattie, W.G.; Tsai, M.J.; O’Malley, B.W. COUP transcription factor is a member of the steroid receptor superfamily. Nature 1989, 340, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ladias, J.A.; Hadzopoulou-Cladaras, M.; Kardassis, D.; Cardot, P.; Cheng, J.; Zannis, V.; Cladaras, C. Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J. Biol. Chem. 1992, 267, 15849–15860. [Google Scholar] [PubMed]
- Cooney, A.J.; Lee, C.T.; Lin, S.C.; Tsai, S.Y.; Tsai, M.J. Physiological function of the orphans GCNF and COUP-TF. Trends Endocrinol. Metab. 2001, 12, 247–251. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Tsai, M.J. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): Coming of age. Endocr. Rev. 1997, 18, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dufau, M.L. Gene silencing by nuclear orphan receptors. Vitam. Horm. 2004, 68, 1–48. [Google Scholar] [PubMed]
- Cooney, A.J.; Tsai, S.Y.; O’Malley, B.W.; Tsai, M.J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol. Cell. Biol. 1992, 12, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, Y.; Toyoshima, K.; Yamamoto, T. Ear3/COUP-TF binds most tightly to a response element with tandem repeat separated by one nucleotide. Biochem. Biophys. Res. Commun. 1992, 183, 492–498. [Google Scholar] [CrossRef]
- Marcus, S.L.; Capone, J.P.; Rachubinski, R.A. Identification of COUP-TFII as a peroxisome proliferator response element binding factor using genetic selection in yeast: COUP-TFII activates transcription in yeast but antagonizes PPAR signaling in mammalian cells. Mol. Cell. Endocrinol. 1996, 120, 31–39. [Google Scholar] [CrossRef]
- Nishiyama, C.; Hi, R.; Osada, S.; Osumi, T. Functional interactions between nuclear receptors recognizing a common sequence element, the direct repeat motif spaced by one nucleotide (DR-1). J. Biochem. 1998, 123, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, N.; Teng, C.T. COUP-TF acts as a competitive repressor for estrogen receptor-mediated activation of the mouse lactoferrin gene. Mol. Cell. Biol. 1993, 13, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Mietus-Snyder, M.; Sladek, F.M.; Ginsburg, G.S.; Kuo, C.F.; Ladias, J.A.; Darnell, J.E., Jr.; Karathanasis, S.K. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol. Cell. Biol. 1992, 12, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Hirota, K.; Taniguchi-Yanai, K.; Shigematsu, Y.; Shimamoto, Y.; Saito, T.; Chowdhury, S.; Takiguchi, M.; Arakawa, M.; Nibu, Y.; et al. Regulated expression of human angiotensinogen gene by hepatocyte nuclear factor 4 and chicken ovalbumin upstream promoter-transcription factor. J. Biol. Chem. 1999, 274, 34605–34612. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, X.; Sigmund, C.D. Identification of a nuclear orphan receptor (Ear2) as a negative regulator of renin gene transcription. Circ. Res. 2003, 92, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shushan, E.; Sharir, H.; Pikarsky, E.; Bergman, Y. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Mol. Cell. Biol. 1995, 15, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Shah, M.; Richardson, B.; Handwerger, S. The ARP-1 orphan receptor represses steroid-mediated stimulation of human placental lactogen gene expression. J. Mol. Endocrinol. 1996, 16, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Zhang, X.K.; Salbert, G.; Hermann, T.; Lehmann, J.M.; Pfahl, M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol. Cell. Biol. 1992, 12, 4666–4676. [Google Scholar] [CrossRef] [PubMed]
- Wehrenberg, U.; Ivell, R.; Jansen, M.; von Goedecke, S.; Walther, N. Two orphan receptors binding to a common site are involved in the regulation of the oxytocin gene in the bovine ovary. Proc. Natl. Acad. Sci. USA 1994, 91, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Danilovich, N.; Sairam, M.R. Orphan receptor chicken ovalbumin upstream promoter transcription factors inhibit steroid factor-1, upstream stimulatory factor, and activator protein-1 activation of ovine follicle-stimulating hormone receptor expression via composite cis-elements. Biol. Reprod. 2002, 66, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Hoffmann, B.; Tran, P.B.; Graupner, G.; Pfahl, M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature 1992, 355, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Umesono, K.; Heyman, R.A.; Mangelsdorf, D.J.; Dyck, J.A.; Evans, R.M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc. Natl. Acad. Sci. USA 1992, 89, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Widom, R.L.; Rhee, M.; Karathanasis, S.K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol. Cell. Biol. 1992, 12, 3380–3389. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.J.; Parker, M.G. COUP-TF II homodimers are formed in preference to heterodimers with RXR alpha or TR beta in intact cells. Nucleic Acids Res. 1995, 23, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.J.; Dowhan, D.H.; Franke, K.; Burke, L.J.; Downes, M.; Muscat, G.E. Transcriptional repression by COUP-TF II is dependent on the C-terminal domain and involves the N-CoR variant, RIP13delta1. J. Steroid Biochem. Mol. Biol. 1997, 63, 165–174. [Google Scholar] [CrossRef]
- Shibata, H.; Nawaz, Z.; Tsai, S.Y.; O’Malley, B.W.; Tsai, M.J. Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT). Mol. Endocrinol. 1997, 11, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Pipaon, C.; Tsai, S.Y.; Tsai, M.J. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol. Cell. Biol. 1999, 19, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kobayashi, S.; Kurihara, I.; Suda, N.; Yokota, K.; Murai, A.; Ikeda, Y.; Saito, I.; Rainey, W.E.; Saruta, T. COUP-TF and transcriptional co-regulators in adrenal steroidogenesis. Endocr. Res. 2004, 30, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Wang, J.C.; Scott, D.K.; Granner, D.K. Transcription activation by the orphan nuclear receptor, chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI). Definition of the domain involved in the glucocorticoid response of the phosphoenolpyruvate carboxykinase gene. J. Biol. Chem. 2000, 275, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Grocock, C.A.; Charlton, H.M.; Pike, M.C. Role of the fetal pituitary in cryptorchidism induced by exogenous maternal oestrogen during pregnancy in mice. J. Reprod. Fertil. 1988, 83, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, I.; Shibata, H.; Kobayashi, S.; Suda, N.; Ikeda, Y.; Yokota, K.; Murai, A.; Saito, I.; Rainey, W.E.; Saruta, T. Ubc9 and Protein Inhibitor of Activated STAT 1 Activate Chicken Ovalbumin Upstream Promoter-Transcription Factor I-mediated Human CYP11B2 Gene Transcription. J. Biol. Chem. 2005, 280, 6721–6730. [Google Scholar] [CrossRef] [PubMed]
- Compe, E.; de Sousa, G.; Francois, K.; Roche, R.; Rahmani, R.; Torresani, J.; Raymondjean, M.; Planells, R. Spot 14 protein interacts and co-operates with chicken ovalbumin upstream promoter-transcription factor 1 in the transcription of the L-type pyruvate kinase gene through a specificity protein 1 (Sp1) binding site. Biochem. J. 2001, 358, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Horowitz, J.M.; Eling, T.E. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J. Biol. Chem. 2001, 276, 33384–33392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dufau, M.L. Repression of the luteinizing hormone receptor gene promoter by cross talk among EAR3/COUP-TFI, Sp1/Sp3, and TFIIB. Mol. Cell. Biol. 2003, 23, 6958–6972. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Morrow, D.; Stewart, J.; Spencer, K.; Porter, W.; Smith, R., 3rd; Phillips, T.; Abdelrahim, M.; Samudio, I.; Safe, S. A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes. Mol. Cancer Ther. 2004, 3, 247–260. [Google Scholar] [PubMed]
- Hong, J.; Samudio, I.; Liu, S.; Abdelrahim, M.; Safe, S. Peroxisome proliferator-activated receptor gamma-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology 2004, 145, 5774–5785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Abdelrahim, M.; Yoon, K.; Chintharlapalli, S.; Papineni, S.; Kim, K.; Wang, H.; Safe, S. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010, 70, 6824–6836. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Li, X.; Hedrick, E.; Jin, U.H.; Tjalkens, R.B.; Backos, D.S.; Li, L.; Zhang, Y.; Wu, Q.; Safe, S. Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol. Endocrinol. 2014, 28, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Kruse, S.W.; Suino-Powell, K.; Zhou, X.E.; Kretschman, J.E.; Reynolds, R.; Vonrhein, C.; Xu, Y.; Wang, L.; Tsai, S.Y.; Tsai, M.J.; et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008, 6, e227. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54. [Google Scholar] [CrossRef]
- Tamamis, P.; Morikis, D.; Floudas, C.A.; Archontis, G. Species specificity of the complement inhibitor compstatin investigated by all-atom molecular dynamics simulations. Proteins 2010, 78, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.A.; Wördehoff, M.M.; Hoyer, W.; Tamamis, P. Uncovering the Binding and Specificity of β-Wrapins for Amyloid-β and α-Synuclein. J. Phys. Chem. B 2016, 120, 12781–12794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jin, U.H.; Davidson, L.A.; Chapkin, R.S.; Jayaraman, A.; Tamamis, P.; Orr, A.; Allred, C.; Denison, M.S.; Soshilov, A.; et al. Editor’s Highlight: Microbial-Derived 1,4-Dihydroxy-2-naphthoic Acid and Related Compounds as Aryl Hydrocarbon Receptor Agonists/Antagonists: Structure-Activity Relationships and Receptor Modeling. Toxicol. Sci. 2017, 155, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.A.; Jayaraman, A.; Tamamis, P. Molecular Modeling of Chemoreceptor:Ligand Interactions. Methods Mol. Biol. 2018, 1729, 353–372. [Google Scholar] [PubMed]
- Jin, U.H.; Park, H.; Li, X.; Davidson, L.A.; Allred, C.; Patil, B.; Jayaprakasha, G.; Orr, A.A.; Mao, L.; Chapkin, R.S.; et al. Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. Toxicol. Sci. 2018, 164, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.A.; Shaykhalishahi, H.; Mirecka, E.; Jonnalagadda, S.V.R.; Hoyer, W.; Tamamis, P. Elucidating Multi-Targeted Anti-Amyloid Activity and Enhanced Islet Amyloid Polypeptide Binding of β-wrapins. Comput. Chem. Eng. 2018, 116, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.A.; Gonzalez-Rivera, J.C.; Wilson, M.; Bhikha, P.R.; Wang, D.; Contreras, L.M.; Tamamis, P. A high-throughput and rapid computational method for screening of RNA post-transcriptional modifications that can be recognized by target proteins. Methods 2018, 143, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Wilson, M.; Gorham, R.D., Jr.; Harrison, R.E.S.; Morikis, V.A.; Kieslich, C.A.; Orr, A.A.; Coley, A.V.; Tamamis, P.; Morikis, D. Virtual Screening of Chemical Compounds for Discovery of Complement C3 Ligands. ACS Omega 2018, 3, 6427–6438. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L., 3rd; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Krivov, G.G.; Shapovalov, M.V.; Dunbrack, R.L., Jr. Improved prediction of protein side-chain conformations with SCWRL4. Proteins 2009, 77, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Lannigan, D.A. Estrogen receptor phosphorylation. Steroids 2003, 68, 1–9. [Google Scholar] [CrossRef]
- Weigel, N.L. Steroid hormone receptors and their regulation by phosphorylation. Biochem. J. 1996, 319 Pt 3, 657–667. [Google Scholar] [CrossRef]
- Gianni, M.; Bauer, A.; Garattini, E.; Chambon, P.; Rochette-Egly, C. Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-induced RAR gamma degradation and transactivation. EMBO J. 2002, 21, 3760–3769. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M.; Kopf, E.; Bastien, J.; Oulad-Abdelghani, M.; Garattini, E.; Chambon, P.; Rochette-Egly, C. Down-regulation of the phosphatidylinositol 3-kinase/Akt pathway is involved in retinoic acid-induced phosphorylation, degradation, and transcriptional activity of retinoic acid receptor gamma 2. J. Biol. Chem. 2002, 277, 24859–24862. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Smith, C.L.; O’Malley, B.W. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr. Rev. 2005, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Kim, K. Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog. Nucleic Acid Res. Mol. Biol. 2004, 77, 1–36. [Google Scholar] [PubMed]
- Higgins, K.J.; Liu, S.; Abdelrahim, M.; Yoon, K.; Vanderlaag, K.; Porter, W.; Metz, R.P.; Safe, S. Vascular endothelial growth factor receptor-2 expression is induced by 17beta-estradiol in ZR-75 breast cancer cells by estrogen receptor alpha/Sp proteins. Endocrinology 2006, 147, 3285–3295. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cooney, A.J.; Kuratani, S.; DeMayo, F.J.; Tsai, S.Y.; Tsai, M.J. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: Evidence for a role in segmental patterning of the diencephalon. Proc. Natl. Acad. Sci. USA 1994, 91, 4451–4455. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Pereira, F.A.; DeMayo, F.J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997, 11, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A.; Qiu, Y.; Zhou, G.; Tsai, M.J.; Tsai, S.Y. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999, 13, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Zhou, C.; Lin, S.C.; Durand, B.; Tsai, S.Y.; Tsai, M.J. The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes. Dev. Biol. 2004, 266, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Borgen, E.; Rypdal, M.C.; Sosa, M.S.; Renolen, A.; Schlichting, E.; Lonning, P.E.; Synnestvedt, M.; Aguirre-Ghiso, J.A.; Naume, B. NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res. 2018, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Silver, B.F.; Driscoll, M.D.; Sathya, G.; Bambara, R.A.; Hilf, R. Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites, and inhibits estrogen-induced gene expression. J. Biol. Chem. 1997, 272, 31465–31474. [Google Scholar] [CrossRef] [PubMed]
- Le Dily, F.; Metivier, R.; Gueguen, M.M.; Le Peron, C.; Flouriot, G.; Tas, P.; Pakdel, F. COUP-TFI modulates estrogen signaling and influences proliferation, survival and migration of breast cancer cells. Breast Cancer Res. Treat. 2008, 110, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Lemon, B.D.; Freedman, L.P. Nuclear receptor cofactors as chromatin remodelers. Curr. Opin. Genet. Dev. 1999, 9, 499–504. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen receptor interaction with co-activators and co-repressors. Steroids 2000, 65, 227–251. [Google Scholar] [CrossRef]
- Smith, C.L.; O’Malley, B.W. Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004, 25, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.P. Coregulatory proteins in nuclear hormone receptor action. Vitam. Horm. 1999, 55, 165–218. [Google Scholar] [PubMed]
- Naar, A.M.; Lemon, B.D.; Tjian, R. Transcriptional coactivator complexes. Annu. Rev. Biochem. 2001, 70, 475–501. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.G.; Glass, C.K. Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 2001, 276, 36865–36868. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, K.; Chen, C.-C.; Orr, A.A.; Barreto, P.N.; Tamamis, P.; Safe, S. Activation of COUP-TFI by a Novel Diindolylmethane Derivative. Cells 2019, 8, 220. https://doi.org/10.3390/cells8030220
Yoon K, Chen C-C, Orr AA, Barreto PN, Tamamis P, Safe S. Activation of COUP-TFI by a Novel Diindolylmethane Derivative. Cells. 2019; 8(3):220. https://doi.org/10.3390/cells8030220
Chicago/Turabian StyleYoon, Kyungsil, Chien-Cheng Chen, Asuka A. Orr, Patricia N. Barreto, Phanourios Tamamis, and Stephen Safe. 2019. "Activation of COUP-TFI by a Novel Diindolylmethane Derivative" Cells 8, no. 3: 220. https://doi.org/10.3390/cells8030220
APA StyleYoon, K., Chen, C.-C., Orr, A. A., Barreto, P. N., Tamamis, P., & Safe, S. (2019). Activation of COUP-TFI by a Novel Diindolylmethane Derivative. Cells, 8(3), 220. https://doi.org/10.3390/cells8030220





