Abstract
Dbl (B-cell lymphoma)-related guanine nucleotide exchange factors (GEFs), the largest family of GEFs, are directly responsible for the activation of Rho family GTPases and essential for a number of cellular events such as proliferation, differentiation and movement. The members of the Ephexin (Eph-interacting exchange protein) family, a subgroup of Dbl GEFs, initially were named for their interaction with Eph receptors and sequence homology with Ephexin1. Although the first Ephexin was identified about two decades ago, their functions in physiological and pathological contexts and regulatory mechanisms remained elusive until recently. Ephexins are now considered as GEFs that can activate Rho GTPases such as RhoA, Rac, Cdc42, and RhoG. Moreover, Ephexins have been shown to have pivotal roles in neural development, tumorigenesis, and efferocytosis. In this review, we discuss the known and proposed functions of Ephexins in physiological and pathological contexts, as well as their regulatory mechanisms.
Keywords:
Ephexin; Ephexin1; Ephexin2; Ephexin3; Ephexin4; Ephexin5; guanine nucleotide exchange factor; GEF; Dbl family; Rho GTPase 1. Introduction
The Rho family of GTPases is a subgroup of the Ras superfamily. Like other small GTPases, the members of the Rho family function as molecular switches [1,2,3,4]. Rho GTPases are best known for their roles in the regulation of the actin cytoskeleton. Consequently, they are involved in diverse cytoskeleton-dependent processes such as cell adhesion, cell motility, cytokinesis, phagocytosis, morphogenesis, and axon guidance [1,2,5]. In addition, Rho GTPases also play essential roles in transcriptional regulation and cell transformation [1,2,5,6,7]. Because Rho GTPases are associated with these essential cellular processes, the activity of Rho GTPases should be spatiotemporally and tightly controlled. Accordingly, various regulatory molecules are involved to modulate the activity of Rho GTPases, but guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs) are at the center of the regulation.
The overall activity of a Rho GTPase in cells depends on its ratio of the GTP-bound to GDP-bound form. Importantly, this ratio is controlled by its direct binding to GEFs, GAPs, or GDIs. GEFs activate Rho GTPases by catalyzing the exchange of bound GDP for GTP, which results in activated Rho GTPases to interact with their downstream effectors. On the other hand, GAPs antagonize the function of GEFs by stimulating Rho GTPases to hydrolyze bound GTP to GDP. Additionally, inactive GDP-bound Rho GTPases are sequestered in the cytosol by RhoGDIs (Figure 1) [1,2,8,9,10].
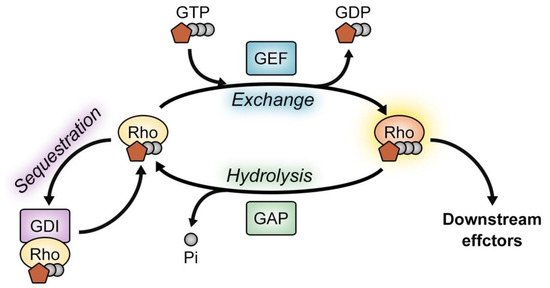
Figure 1.
Overview of Rho GTPase regulation. The activity of Rho GTPases is controlled by GEFs, GAPs and GDIs. GEFs facilitate the exchange of GTPase-bound GDP for GTP but GAPs inactivate the Rho GTPase by hydrolyzing GTP. Additionally, the sequestration of Rho GTPases by GDIs modulates the level of active Rho GTPases. GEF, guanine nucleotide factor; GAP, GTPase-activating protein; GDI, guanine nucleotide dissociation inhibitor.
Dbl, the first GEF of the Dbl family, was originally identified as an oncogene but its function as a GEF for Cdc42 was revealed later [11,12]. Since then, 82 RhoGEFs have been identified; 71 RhoGEFs comprise the Dbl family and the other 11 RhoGEFs belong to the Dock family [13]. An important characteristic of the Dbl family is the presence of the Dbl homology (DH) domain, which is composed of ~200 amino acid residues, and the pleckstrin homology (PH) domain, which is composed of ~100 residues [9,10,14]. The DH domain is mainly responsible for the catalytic activity of Dbl RhoGEFs and forms a minimal catalytic unit for the guanine nucleotide exchange reaction with the adjacent PH domain [9,14,15]. In addition to the tandem DH-PH domain, the members of the family also contain other domains that regulate their GEF activity, subcellular localization, or interaction with other molecules [9]. In contrast, the GEFs of the Dock family are considered as unconventional GEFs due to the absence of the typical DH domain. Thus, the Dock family, instead of the DH domain, is characterized by the presence of DHR1 (Dock homology region 1) and DHR2 (Dock homology region 2) whose roles are to bind phospholipids and to provide the guanine nucleotide exchange activity, respectively [15,16]. Intriguingly, the number of RhoGEFs far outnumbers that of Rho GTPases. Moreover, some RhoGEFs can modulate multiple Rho GTPases. These imply that the activity of Rho GTPases is modulated by intertwined networks [14,15].
Among the members of the Dbl family, five proteins from Ephexin1 to 5 belong to the Ephexin subfamily [13,17]. Their sequences are highly conserved among paralogs, and they possess the typical tandem DH-PH domain and an additional SH3 domain which is C-terminal to the PH domain except that the SH3 domain is absent in Ephexin5 [17,18]. Besides the high sequence homology, one of the important common features of the Ephexin family is that they are the direct downstream proteins of Eph receptors, the largest subfamily of receptor tyrosine kinases that is activated by Ephrins and involved in various cellular processes such as axon guidance, formation of tissue boundaries, long-term potentiation, angiogenesis, and cancer, through their association with Eph receptors [18,19,20,21,22,23]. Therefore, the GEF activity of Ephexins could be regulated by Ephrin/Eph receptor-mediated signaling, and diverse cellular processes induced by Ephexins occur through this module, Ephrin-Eph receptor-Ephexin (Figure 2).
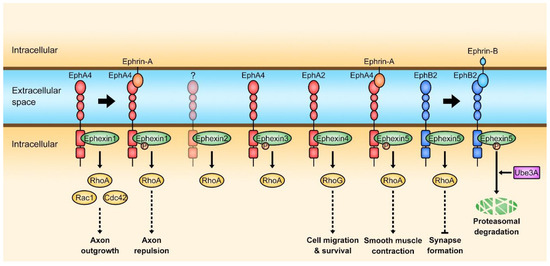
Figure 2.
Ephrin-Eph receptor-Ephexin signaling. The activation of EphA4 by Ephrin-A increases the GEF activity of Ephexin1 toward RhoA whereas the EphB2 activation by Ephrin-B induces ubiquitination of Ephexin5 resulting in proteasomal degradation. An Eph receptor for Ephexin2 has not been reported.
In particular, the Ephexin family plays essential roles in normal function of neurons and their development. It regulates the axon guidance of developing neurons [17,19,24,25,26,27,28,29,30] and synaptic homeostasis [31,32,33] via the reorganization of the actin cytoskeletons. Although a number of studies on the family have focused on its roles in the nervous system, several studies have also suggested that Ephexins have a variety of other functions. For example, it has been reported that they regulate angiogenesis [34], efferocytosis, a type of phagocytosis to clear apoptotic cells in the body [35,36,37], and vascular muscle contractility [18]. Moreover, aberrant expression or function of Ephexins are related to various types of cancer [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Thus, the roles of Ephexins are not limited to the nervous system. In this review, we highlight the molecular characteristics of Ephexins and their various roles in physiological and pathological contexts.
2. Ephexin Family
Since the first Ephexin, Ephexin1, was identified as an EphA4-interacting RhoGEF, Ephexin was named for Eph-interacting exchange protein [19]. Since then, four Ephexin1-homologous proteins have been identified and named sequentially from Ephexin2 to Ephexin5 [17]. These five Ephexins comprise a subfamily of the Dbl-related GEFs, the Ephexin family [13,17].
Ephexins function as GEFs for Rac, Cdc42, Rho and RhoG, and interact with Eph receptors to transduce signals from the receptors although an Eph receptor interacting with Ephexin2 has not been identified. Accordingly, they are engaged in a variety of Eph receptor-mediated cellular processes (Figure 2 and Table 1) [18,19,20,21,22]. In addition, the domain structure of Ephexins is highly conserved, which consists of a tandem DH-PH domain followed by a SH3 domain, but the length of their N-termini is irregular and the sequence identity is especially low among Ephexin1 orthologs, particularly drosophila and chicken Ephexin1 (dEpehxin1 and cEphexin1) (Figure 3). In the following sections, the members of the Ephexin family will be consecutively discussed in detail (also refer to the Table S1 to see all experimental systems for studies on Ephexins).

Table 1.
Overview of Ephexin family proteins.
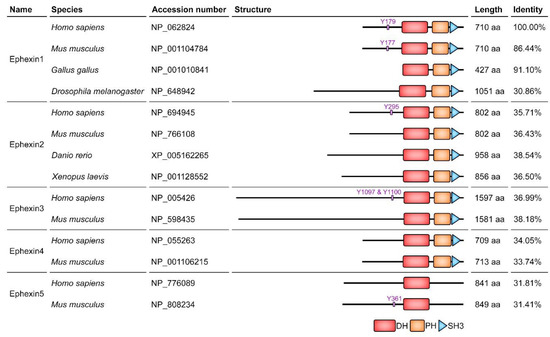
Figure 3.
Schematic diagram of the members of the Ephexin family and sequence identity among homologs. The phosphorylation sites involved in alleviating the auto-inhibition are shown. The domains of Ephexins were structured according to SMART, a domain prediction program, and the sequence identity among homologs was calculated by Clustal Omega. DH, Dbl homology; PH, Pleckstrin homology; SH3, Src homology 3.
2.1. Ephexin1
Ephexin1 was originally isolated from an adult mouse brain cDNA library in 2000 as Ngef. Ephexin1 is predominately expressed in the central nervous system, the brain and spinal cord, and its expression is developmentally regulated, that is, its mRNA levels gradually increase throughout embryonic development and peak at the P10 postnatal stage. In particular, Ephexin1 is highly expressed in the caudate nucleus associated with motor processes [19,54]. This expression timing and location of Ephexin1 is quite similar to those of EphA4, which suggests the implicated roles of Ephexin1 in the nervous system. Indeed, Ephexin1 plays crucial roles in axon guidance and synaptic homeostasis.
During development, the axon pathfinding is regulated by various molecules existing in the extracellular matrix or on the surrounding cell surfaces. These guidance cues determine the attraction and/or repulsion of the growth cone by regulating the actin cytoskeleton. Eph receptors and Ephrins are involved and their roles are well established in these processes [58]. In particular, Ephexin1 is a key regulator providing a linkage between Ephrin-Eph and the axon pathfinding through interaction of Ephexin1 with EphA4 [19]. Furthermore, Ephexin1 participates in mediating proper neuronal functions like synaptic homeostasis, maturation and myelination [33,59]. In the neuromuscular junction (NMJ) of fruit flies, presynaptic Eph receptors receive a retrograde signal from the postsynaptic terminal and activate dEphexin1, Cdc42, and calcium channels sequentially, leading to the enhanced presynaptic release for synaptic homeostasis [31]. In mouse NMJ, Ephexin1 expressed in the postsynaptic muscle regulates the actin cytoskeleton via RhoA. As a consequence, the membrane structure and postsynaptic AchR cluster stability are altered and thus NMJ maturation is mediated [32].
In addition, the importance of Ephexin1 in the nervous systems is also highlighted in various contexts. Optimal neuron innervation by axon guidance during embryonic development is one of the most well-characterized processes regulated by Ephexin1. Tissues from various model organisms like mice, chickens, and fruit flies have been used to confirm the roles of Ephexin1 in those developmental stages. Afferent innervation from spiral ganglion neurons (SGNs) is important for development of the proper auditory system during mouse embryogenesis and also regulated by the ephrin-EphA4-Ephexin1 axis [26]. Medial lateral motor column (LMC) neurons and dorsal limb mesenchyme express EphB1 and Ephrin-B, respectively, to induce the growth of medial LMC neurons towards the ventral part in chicken and mouse embryos [27]. dEphexin1 also mediates olfactory dendrite targeting [29] and cEphexin1 (chicken Ephexin1) takes an important role in successful retinal ganglion cell (RGC) projection to reach the optic tectum in chicken embryos [28].
The GEF activity of Ephexin1 is modulated by the activation state of EphA4: using cultured Ephexin1-/- neurons and RNA interference in the chick, it was found that Ephexin1 could function as a GEF for Rac1, Cdc42, and RhoA under the basal condition. However, when EphA4 is activated by Ephrins, the phosphorylation of Ephexin1 by Src provides the specificity of Ephexin1 towards RhoA [17,24]. Ahead of this modification, Cdk5 participates in the phosphorylation of Ephexin1 as a priming kinase. Activated EphA4 phosphorylates Cdk5, which causes Cdk5 to phosphorylate Ephexin1, results in further modification of Ephexin1 by Src [25]. Intriguingly, this phosphorylation of Ephexin1 relieves auto-inhibition generated by the inhibitory helix region to the N-terminus of the DH domain of Ephexin1. It is known that the activity of a number of Dbl family GEFs is auto-inhibited by interaction of the DH domain with an N-terminal helix region to exclude and prevent the activation of Rho GTPases. Similarly, Ephexin1 is also auto-inhibited and its activity is modulated by relief or strength of the auto-inhibition. The phosphorylation of tyrosine 179, located in the inhibitory helix region of Ephexin1, by Src disrupts interaction between the DH domain and the inhibitory helix region of Ephexin1, which makes the DH domain free to bind RhoA. Eventually, the phosphorylation increases the activity of Ephexin1 and the levels of RhoA activation [60].
It has been reported that Ephexin1 is positively correlated to pathophysiologic conditions like depression or recovery after CNS (central nervous system) injury, due to its roles in neuronal development and synaptic homeostasis, [61,62,63,64].
2.2. Ephexin2
Ephexin2 was identified in 2004 from a mouse liver cDNA library and initially was termed as Wgef, an abbreviation of weakly similar to Rho GEF 5 [55]. Studies on Ephexin2 have not extensively been done compared with Ephexin1. Thus, there is not much literature to refer to and data about Ephexin2 are also limited. However, its intrinsic role to function as a GEF for RhoA seems to be clear.
Ephexin2 is involved in convergent extension, a developmental step of anterior-posterior axis extension in Xenopus gastrulation through RhoA activation [65]. In addition, Ephexin2 participates in pronephric tubulogenesis of Xenopus and zebrafish. During this process, the GEF activity of Ephexin2 is modulated by Daam1 interacting with it [66]. Ephexin2 also plays a role in cancer. For instance, various cancer tissues and cell lines show elevated levels of Ephexin2, which results in the increased activity of RhoA causing higher cancer proliferation, migration, and invasion. The downregulated levels of Ephexin2 by miR-503 have an inhibitory effect on cancer metastasis in hepatocellular carcinoma model and miR-29b, which downregulates the levels of Ephexin2, is decreased in non-small cell lung cancer (NSCLC) [39,40]. Interestingly, the roles of Ephexin2 during pronephric tubulogenesis and in the cancer are relevant to the high expression levels of Ephexin2 in the kidney, as well as the liver and lung.
2.3. Ephexin3
Ephexin3, also called Tim (Transforming immortalized mammary), is ubiquitously expressed in many tissues, such as colon, kidney, trachea, prostate, liver, and pancreas, with tendency to be highly expressed in tissues containing epithelial cells [41]. Ephexin3 is able to activate RhoA in in vitro guanine nucleotide exchange assays using purified proteins and in Rho GTPase pull-down assays using COS-7 cells [20,41]. However, it promotes the formation of membrane ruffles and filopodia and induces a loss of actin stress fibers in NIH/3T3 and COS-7 cells, which seems to indicate that Ephexin3 activates Rac and Cdc42 [44]. However, the consecutive studies on Ephexin3 have shown that it functions as a GEF for RhoA rather than Rac and Cdc42.
The roles of Ephexin3 in physiological and pathological contexts are quite various. Genetic deletion of Ephexin3 causes defects in immature dendritic cell migration in vivo. In terms of cell migration, Ephexin3 is also involved in Src-induced podosome formation which is related to cell migration and adhesion. RNAi-mediated knockdown of Ephexin3 inhibits Src-dependent podosome formation whereas its overexpression increases the podosome formation through RhoA activation [45,67]. In addition, Ephexin3 is highly linked to cancer progression. Higher levels of Ephexin3 are observed in cervical, colorectal, and lung-derived cancers and patients with a high level of Ephexin3 and Src show shorter survival time [41,42,43,45,46]. Moreover, mutations in Ephexin3 is also correlated with cancer. Frequent mutations in Ephexin3 in lung cancer and familial lung cancer compared with the healthy control were detected through whole genome sequencing [68]. Furthermore, it is conceivable that the correlation of Ephexin3/Src with cancer malignancy comes from their function of promoting endothelial-mesenchymal transition (EndoMT) [47].
The activity of Ephexin3 is also auto-inhibited by intramolecular interaction caused by the N-terminal inhibitory helix and the DH domain, which is similar to that of Ephexin1. Therefore, the regulatory mechanism for the activity of Ephexin3 is also comparable to that of Ephexin1. The inhibitory helix of Ephexin3 binds to the DH domain, which prevents RhoA access to the DH domain of Ephexin3 and results in the auto-inhibition. Tyrosine phosphorylation at Y1097 and Y1100 by Src leads to disruption of the intramolecular interaction between the inhibitory helix and the DH domain, which results in RhoA activation [20,69,70].
2.4. Ephexin4
Most studies on Ephexin4, also known as Arhgef16, have focused on its role as a GEF for RhoG. However, the potential activity of Ephexin4 to regulate Cdc42 was also reported in a specific context. Tax-interacting-protein 1 (Tip-1) is a protein that interacts with HPV16 E6 oncoprotein to regulate E6-dependent cell motility. Intriguingly, Ephexin4 was identified as a novel binding partner of Tip-1 via a yeast two-hybrid screen. The interaction of Ephexin4 with Tip-1 alters its GEF activity toward Cdc42 [56,57].
The biological significance of Ephexin4 in various physiological and pathological conditions has been addressed. The potential involvement of Ephexin4 in preventing carcinogenesis was suggested from its down-regulation in kojic acid-stimulated A375 malignant melanoma cells [48]. In addition, it was reported that Ephexin4 could modulate cancer cell migration in a breast cancer model. Ephexin4 activates RhoG by interacting with EphA2, which promotes RhoG/ELMO2/Dock4 complex formation resulting in Rac activation [21]. Since elevated EphA2 expression is correlated with the aggressiveness of breast cancer, Ephexin4 links EphA2 to RhoG causing cancer invasion. Cancer cells are resistant to anoikis, a type of cell death caused by detachment from surrounding ECM, leading to more progression of cancer. It has been revealed that Ephexin4 takes an important part in preventing anoikis as its loss in HeLa cells promoted anoikis. Phosphorylation of EphA2 enhances its interaction with Ephexin4 and, in turn, activates RhoG and RhoG-dependent PI3K/Akt signaling. Because Akt phosphorylates EphA2, these proteins compose a positive feedback loop for anoikis resistance [50,51,52]. Ephexin4 also correlates with the development and progression of brain tumors [49,53]. In patients with oligodendroglial brain tumors, a missense mutation (2125G to 2125A) in Ephexin4 is commonly found, although extensive investigation is required to fully elucidate its contribution to the pathogenesis [49]. One recent report revealed that GLI2, glioma-associated oncogene and a downstream effector of Hedgehog signaling, binds to the promoter region of Ephexin4 and upregulates the transcript level of Ephexin4. Although there is an absence of studies on its mechanism, interaction between Ephexin4 and cytoskeleton-associated protein 5 (CKAP5) in this model is newly reported. This suggests a correlation between spindle regulation and glioma proliferation and migration [53]. Besides tumorigenesis, Ephexin4 is related to optic nerve regeneration process in zebrafish. After optic nerve injury, Wnt-related pathways are altered and the transcription levels of Daam1 and Ephexin4 decrease as a consequence [71].
A novel role of Ephexin4 in clearance of apoptotic cells was recently reported [35]. Interaction of Ephexin4 and Elmo1 was newly identified through a yeast two-hybrid screen. Elmo1 is known to regulate various cellular processes, such as cell motility, neurite outgrowth, and formation of cellular protrusions, all of which are mediated by the actin cytoskeleton remodeling. Elmo1 plays a central role during efferocytosis as a scaffold protein modulating the activity of its associating proteins [72]. Since Ephexin4 was recognized as a new binding partner of Elmo1, the effects of Ephexin4 on efferocytosis has been elucidated. Ephexin4 indeed promotes the engulfment of apoptotic cells in a RhoG- and Rac1-dependent manner [35]. Interestingly, co-expression of Elmo1 and Ephexin4 synergistically promotes efferocytosis resulting from relief of the auto-inhibition of Ephexin4. The SH3 domain of Ephexin4 mediates homotypic intermolecular interaction, which generates an auto-inhibitory effect by the blockade of RhoG access to the DH domain of Ephexin4. This auto-inhibitory interaction is relieved by Elmo1; the N- and C-termini of Elmo1 bind to the DH and SH3 domain of Ephexin4, respectively. Therefore, the binding of Elmo1 to Ephexin4 relieves the auto-inhibition of Ephexin4 by disrupting the homotypic intermolecular interaction, leading to the augmented GEF activity of Ephexin4 toward RhoG and the consequent increase in the ability of efferocytosis [36,37]. It is notable that the regulatory mechanism for the activity of Ephexin4 differs from that of other Ephexins.
2.5. Ephexin5
Another name for Ephexin5 is Vsm-RhoGEF, standing for vascular smooth muscle-specific RhoGEF. As its name implies, it was identified as a RhoGEF expressed in vascular smooth muscle cells from different tissues including the heart, liver, kidney, aorta, and spleen. Along with the identification of its expression pattern, its roles as a key downstream regulator of EphA4 signaling have been revealed. Ephexin5 interacts with EphA4 through its DH-PH domain, and Ephrin-A-stimulated EphA4 phosphorylates Ephexin5, which activates RhoA and induces the formation of stress fibers related to the vascular smooth muscle contractility [18].
Ephexin5 is highly expressed in the brain, especially in the hippocampus, and its functions in the brain have been well established [22]. It is known that Eph-Ephrin signaling is important for the proper excitatory synapse formation in the brain. EphB binding to Ephrin-B enhances the kinase activity of EphBs in the developing dendrites, which results in the dendritic spine outgrowth and excitatory synapse formation. In the hippocampus, Ephexin5 shows a similar expression pattern to that of EphB2 and it interacts with EphB2 preferentially in neurons. Ephexin5 is a RhoA-specific GEF and decreases the number of excitatory synapses by activating RhoA. Upon Ephrin-B stimulation, however, activated EphB2 phosphorylates tyrosine 361 of Ephexin5 (mouse Ephexin5), leading to its ubiquitination by Ube3A and following proteasomal degradation. Therefore, the negative regulation of spine outgrowth by Ephexin5 is a checkpoint to limit the EphB-mediated excitatory synapse formation [22,73]. In addition, one recent study reported that Ephexin5 is required for spinogenesis. Ephexin5 is temporally increased at the site of new spine formation in hippocampal neurons prior to its removal and new spine formation. Interestingly, the complete loss of Ephexin5 prevents neuronal activity from promoting spinogenesis. Taken together, these data indicate that Ephexin5 may serve as a beacon locating sites of new spine formation keeping them in check until incoming activity promotes spine formation at these sites [74].
Angiogenesis occurs through a series of complicated processes including sprouting, migration, tubulogenesis, and stabilization [75]. To accomplish these processes, the actin cytoskeleton in endothelial cells is tightly controlled by various Rho GTPases. Vascular endothelial growth factor (VEGF) is one of the most crucial regulators for angiogenesis. The interaction between VEGF and its corresponding receptor, VEGFR2, facilitates the development of blood vessels via the activation of Cdc42 and the inactivation of RhoJ in the endothelial cells [76,77]. Ephexin5 has an important role in angiogenesis because it mediates the VEGF-induced Rho GTPase activity modulation. During angiogenesis, Ephexin5 is highly expressed in retinal endothelial cells where Ephexin5 promotes actin polymerization by regulating the activity of Cdc42 and RhoJ [34,78]. By increasing Cdc42 activity and decreasing RhoJ activity, Ephexin5 promotes actin polymerization in endothelial cells. The significance of Ephexin5 in angiogenesis is further supported by delayed retinal vascular growth in Ephexin5-deficient mice [34]. Furthermore, the mutations or aberrant expression of Ephexin5 are associated with cancer and neuronal disorders such as epilepsy, seizures and Alzheimer’s disease [38,79,80].
3. Targeting Ephexins in Diseases
All members of the Ephexin family function as GEFs for RhoA, Rac, Cdc42 and RhoG, which is closely related to cell proliferation and cell migration. Due to their pivotal roles in cell proliferation and migration, dysregulation of their activity or expression could be associated with tumorigenesis and metastasis. Indeed, the increased expression levels of Eph receptors or Ephexins have been observed in many cancer types. Moreover, mutations or transcript variants of Ephexins are also closely related to cancer [44,49,68]. Therefore, targeting the signal module or a regulator for the signal module could be a good therapeutic approach for cancer treatment.
A few studies tried to modulate the activity of Ephexin3 using peptides mimicking the inhibitory helix or the most highly conserved surface of the DH domain interacting the inhibitory helix. However, modulating their activity is only tested in in silico or in vitro systems [20,69,70]. Thus, the efficacy of the peptides should be further investigated in more physiologically relevant conditions. Another approach to target the signal module is to inhibit the activity of Src because the activity of Ephexin1 or Ephexin3 is modulated through phosphorylation by Src. A few studies showed that Ephexin1 expression is altered after spinal cord injury and treatment of PP2, a selective Src family inhibitor, for the injury leads to functional locomotor recovery [62,63]. Although PP2 is not specific to only Src, this approach shows a possibility that targeting an Ephexin regulator in the signal module could be effective therapeutics.
Furthermore, Ephexin1 and Ephexin5 are associated with neuronal disorders such as depression, epilepsy, and Alzheimer’s disease [38,61,79,80]. EphA4-Ephexin1 signaling was increased in mice after social defeat stress [61]. Through whole exome sequencing on children with epilepsy, a missense mutation (1810C to 1810T) in Ephexin5 was identified and this substitution resulted in ~50% reduction in its GEF activity on RhoA [79]. In addition, the amyloid-β accumulated brain showed an elevated Ephexin5 level and reduced postsynaptic spine density due to the decreased level of EphB2 [80]. Treatment of mice with depression-like phenotype with an EphA4 inhibitor showed an antidepressant-like effect [61]. These studies imply that it is also a good strategy to target the signal module for treatment of those neuronal disorders. Therefore, a drug modulating the Eph receptor-Ephexin signaling would be a promising treatment such disorders.
4. Conclusions
In this review, the biological significance of Ephexins in various physiological and pathological contexts and molecular mechanisms by which their GEF activity is regulated have been discussed. Starting from studies in the 2000s, their roles not only in neurogenesis but also in other cellular events have been reported. In particular, recent studies have provided the relevance of Ephexins to tumorigenesis and efferocytosis, suggesting that the unappreciated roles of Ephexins still remain and need to be explored. Accordingly, extensive and in-depth studies of them would provide a better understanding of the diverse processes, including neuronal development and cancer.
Supplementary Materials
The supplementary materials are available online at http://www.mdpi.com/2073-4409/8/2/87/s1.
Author Contributions
K.K., and S.-A.L. wrote the original draft of the manuscript. K.K and D.P. conceived this review and D.P. reviewed and edited the manuscript.
Funding
This research was funded by the National Research Foundation of Korea funded by the Korea government (MSIP) (2012R1A5A1048236 and 2016R1A2B4009737) and by Aging Research Institute at GIST and The APC was funded by Aging Research Institute at GIST.
Acknowledgments
We thank all members in the laboratory for helpful comments and discussion about this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Aelst, L.; D’Souza-Schorey, C. Rho gtpases and signaling networks. Genes. Dev. 1997, 11, 2295–2322. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.L.; Hall, A. Rho gtpases and their effector proteins. Biochem. J. 2000, 348, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho family gtpases. Biochem. Soc. Trans. 2012, 40, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.M.; Fuentes, G.; Rausell, A.; Valencia, A. The ras protein superfamily: Evolutionary tree and role of conserved amino acids. J. Cell Biol. 2012, 196, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S.; Hall, A. Rho gtpases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Boettner, B.; Van Aelst, L. The role of rho gtpases in disease development. Gene 2002, 286, 155–174. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. Rho-gtpases and cancer. Nat. Rev. Cancer 2002, 2, 133–142. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Rossman, K.L.; Der, C.J.; Sondek, J. Gef means go: Turning on rho gtpases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005, 6, 167–180. [Google Scholar] [CrossRef]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. Gefs and gaps: Critical elements in the control of small g proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef]
- Eva, A.; Vecchio, G.; Rao, C.D.; Tronick, S.R.; Aaronson, S.A. The predicted dbl oncogene product defines a distinct class of transforming proteins. Proc. Natl. Acad. Sci. USA 1988, 85, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.J.; Eva, A.; Evans, T.; Aaronson, S.A.; Cerione, R.A. Catalysis of guanine nucleotide exchange on the cdc42hs protein by the dbl oncogene product. Nature 1991, 354, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.; Blangy, A. The evolutionary landscape of dbl-like rhogef families: Adapting eukaryotic cells to environmental signals. Genome Biol. Evol. 2017, 9, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dvorsky, R.; Ahmadian, M.R. Deciphering the molecular and functional basis of dbl family proteins: A novel systematic approach toward classification of selective activation of the rho family proteins. J. Biol. Chem. 2013, 288, 4486–4500. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, S.M.; Awadia, S.; Garcia-Mata, R. I’m coming to GEF you: Regulation of rhogefs during cell migration. Cell Adh. Migr. 2014, 8, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Gadea, G.; Blangy, A. Dock-family exchange factors in cell migration and disease. Eur. J. Cell Biol. 2014, 93, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.; Greer, P.L.; Lin, M.Z.; Poucher, H.; Eberhart, J.; Schmidt, S.; Wright, T.M.; Shamah, S.M.; O’Connell, S.; Cowan, C.W.; et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 2005, 46, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Ogita, H.; Kunimoto, S.; Kamioka, Y.; Sawa, H.; Masuda, M.; Mochizuki, N. Epha4-mediated rho activation via vsm-rhogef expressed specifically in vascular smooth muscle cells. Circ. Res. 2003, 93, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Shamah, S.M.; Lin, M.Z.; Goldberg, J.L.; Estrach, S.; Sahin, M.; Hu, L.; Bazalakova, M.; Neve, R.L.; Corfas, G.; Debant, A.; et al. Epha receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 2001, 105, 233–244. [Google Scholar] [CrossRef]
- Yohe, M.E.; Rossman, K.L.; Gardner, O.S.; Karnoub, A.E.; Snyder, J.T.; Gershburg, S.; Graves, L.M.; Der, C.J.; Sondek, J. Auto-inhibition of the dbl family protein tim by an n-terminal helical motif. J. Biol. Chem. 2007, 282, 13813–13823. [Google Scholar] [CrossRef]
- Hiramoto-Yamaki, N.; Takeuchi, S.; Ueda, S.; Harada, K.; Fujimoto, S.; Negishi, M.; Katoh, H. Ephexin4 and epha2 mediate cell migration through a rhog-dependent mechanism. J. Cell Biol. 2010, 190, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Margolis, S.S.; Salogiannis, J.; Lipton, D.M.; Mandel-Brehm, C.; Wills, Z.P.; Mardinly, A.R.; Hu, L.; Greer, P.L.; Bikoff, J.B.; Ho, H.Y.; et al. Ephb-mediated degradation of the rhoa gef ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 2010, 143, 442–455. [Google Scholar] [CrossRef]
- Kullander, K.; Klein, R. Mechanisms and functions of eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Nissen, U.V.; Dufour, A.; Sahin, M.; Greer, P.; Kullander, K.; Mrsic-Flogel, T.D.; Greenberg, M.E.; Kiehn, O.; Vanderhaeghen, P.; et al. Regulation of epha 4 kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in eph function. Neuron 2005, 47, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.Y.; Chen, Y.; Sahin, M.; Zhao, X.S.; Shi, L.; Bikoff, J.B.; Lai, K.O.; Yung, W.H.; Fu, A.K.; Greenberg, M.E.; et al. Cdk5 regulates epha4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 2007, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; Poirrier, A.L.; Lallemend, F.; Mateo Sanchez, S.; Neef, J.; Vanderhaeghen, P.; Soriano, E.; Peuckert, C.; Kullander, K.; Fritzsch, B.; et al. Ephrin-a5/epha4 signalling controls specific afferent targeting to cochlear hair cells. Nat. Commun. 2013, 4, 1438. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chang, M.Y.; Chou, S.Y.; Huang, C.C.; Chuang, J.Y.; Hsu, T.I.; Chang, H.F.; Wu, Y.H.; Wu, C.C.; Morales, D.; et al. Ephexin1 is required for eph-mediated limb trajectory of spinal motor axons. J. Neurosci. 2018, 38, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Fiore, L.; Medori, M.; Spelzini, G.; Carreno, C.O.; Carri, N.G.; Sanchez, V.; Scicolone, G. Regulation of axonal epha4 forward signaling is involved in the effect of epha3 on chicken retinal ganglion cell axon growth during retinotectal mapping. Exp. Eye Res. 2018, 178, 46–60. [Google Scholar] [CrossRef]
- Sardana, J.; Organisti, C.; Grunwald Kadow, I.C. Eph receptor effector ephexin mediates olfactory dendrite targeting in drosophila. Dev. Neurobiol. 2018, 78, 873–888. [Google Scholar] [CrossRef]
- Shah, K.; Rossie, S. Tale of the good and the bad cdk5: Remodeling of the actin cytoskeleton in the brain. Mol. Neurobiol. 2018, 55, 3426–3438. [Google Scholar] [CrossRef]
- Frank, C.A.; Pielage, J.; Davis, G.W. A presynaptic homeostatic signaling system composed of the eph receptor, ephexin, cdc42, and cav2.1 calcium channels. Neuron 2009, 61, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Butt, B.; Ip, F.C.; Dai, Y.; Jiang, L.; Yung, W.H.; Greenberg, M.E.; Fu, A.K.; Ip, N.Y. Ephexin1 is required for structural maturation and neurotransmission at the neuromuscular junction. Neuron 2010, 65, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fu, A.K.; Ip, N.Y. Multiple roles of the rho gef ephexin1 in synapse remodeling. Commun. Integr. Biol. 2010, 3, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Fukushima, Y.; Fukuhara, S.; Jakt, L.M.; Okada, M.; Shimizu, Y.; Hata, M.; Nishida, K.; Negi, A.; Hirashima, M.; et al. Arhgef15 promotes retinal angiogenesis by mediating vegf-induced cdc42 activation and potentiating rhoj inactivation in endothelial cells. PLoS ONE 2012, 7, e45858. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.; Kim, G.; Kim, K.; Pak, J.; Kim, K.; Ye, M.B.; Park, S.G.; Park, D. Arhgef16, a novel elmo1 binding partner, promotes clearance of apoptotic cells via rhog-dependent rac1 activation. Biochim. Biophys. Acta 2014, 1843, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.; Lee, S.A.; Moon, H.; Park, B.; Kim, D.; Joo, Y.E.; Park, D. Intermolecular steric inhibition of ephexin4 is relieved by elmo1. Sci. Rep. 2017, 7, 4404. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.; Moon, H.; Lee, S.A.; Kim, D.; Yang, S.; Lee, D.H.; Lee, G.; Park, D. The intermolecular interaction of ephexin4 leads to autoinhibition by impeding binding of rhog. Cells 2018, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Yasumoto, M.; Ogasawara, S.; Akiba, J.; Kitasato, Y.; Nakayama, M.; Naito, Y.; Ishida, Y.; Okabe, Y.; Yasunaga, M.; et al. Arhgef15 overexpression worsens the prognosis in patients with pancreatic ductal adenocarcinoma through enhancing the motility and proliferative activity of the cancer cells. Mol. Cancer 2016, 15, 32. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Y.; Peng, C.; Gu, P.; Wang, W. Mir-503 regulates metastatic function through rho guanine nucleotide exchanger factor 19 in hepatocellular carcinoma. J. Surg. Res. 2014, 188, 129–136. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Chen, S.; Pan, Z.; Zhou, Q.; Li, Y.Z.; Shuai, W.D.; Kuang, C.M.; Peng, Q.H.; Shi, W.; et al. Arhgef19 interacts with braf to activate mapk signaling during the tumorigenesis of non-small cell lung cancer. Int. J. Cancer 2018, 142, 1379–1391. [Google Scholar] [CrossRef]
- Xie, X.; Chang, S.W.; Tatsumoto, T.; Chan, A.M.; Miki, T. TIM, a dbl-related protein, regulates cell shape and cytoskeletal organization in a rho-dependent manner. Cell. Signal. 2005, 17, 461–471. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wu, W.; Wang, H.; Liao, K.; Zhang, W.; Xiong, G.; Wu, F.; Meng, G.; Yang, K. Co-expression of rho guanine nucleotide exchange factor 5 and src associates with poor prognosis of patients with resected non-small cell lung cancer. Oncol. Rep. 2013, 30, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Onodera, Y.; Kuroiwa, M.; Nomimura, S.; Kubo, Y.; Nam, J.M.; Kajiwara, K.; Nada, S.; Oneyama, C.; Sabe, H.; et al. The rho guanine nucleotide exchange factor arhgef5 promotes tumor malignancy via epithelial-mesenchymal transition. Oncogenesis 2016, 5, e258. [Google Scholar] [CrossRef] [PubMed]
- Debily, M.A.; Camarca, A.; Ciullo, M.; Mayer, C.; El Marhomy, S.; Ba, I.; Jalil, A.; Anzisi, A.; Guardiola, J.; Piatier-Tonneau, D. Expression and molecular characterization of alternative transcripts of the arhgef5/tim oncogene specific for human breast cancer. Hum. Mol. Genet. 2004, 13, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, M.; Oneyama, C.; Nada, S.; Okada, M. The guanine nucleotide exchange factor arhgef5 plays crucial roles in src-induced podosome formation. J. Cell Sci. 2011, 124, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wu, W.; Yang, K.; Tan, D.; Tang, M.; Liu, H.; Wu, T.; Zhang, S.; Wang, H. Rho guanine nucleotide exchange factor 5 increases lung cancer cell tumorigenesis via mmp-2 and cyclin d1 upregulation. Mol. Cancer Ther. 2015, 14, 1671–1679. [Google Scholar] [CrossRef]
- Mihira, H.; Suzuki, H.I.; Akatsu, Y.; Yoshimatsu, Y.; Igarashi, T.; Miyazono, K.; Watabe, T. Tgf-beta-induced mesenchymal transition of ms-1 endothelial cells requires smad-dependent cooperative activation of rho signals and mrtf-a. J. Biochem. 2012, 151, 145–156. [Google Scholar] [CrossRef]
- Cheng, S.L.; Liu, R.H.; Sheu, J.N.; Chen, S.T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of kojic acid on gene expression profiling of a375 human malignant melanoma cells. Biol. Pharm. Bull. 2006, 29, 655–669. [Google Scholar] [CrossRef]
- Bralten, L.B.; Nouwens, S.; Kockx, C.; Erdem, L.; Hoogenraad, C.C.; Kros, J.M.; Moorhouse, M.J.; Sillevis Smitt, P.A.; van der Spek, P.; van Ijcken, W.; et al. Absence of common somatic alterations in genes on 1p and 19q in oligodendrogliomas. PLoS ONE 2011, 6, e22000. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hiramoto-Yamaki, N.; Negishi, M.; Katoh, H. Ephexin4 and epha2 mediate resistance to anoikis through rhog and phosphatidylinositol 3-kinase. Exp. Cell Res. 2011, 317, 1701–1713. [Google Scholar] [CrossRef]
- Kawai, H.; Kobayashi, M.; Hiramoto-Yamaki, N.; Harada, K.; Negishi, M.; Katoh, H. Ephexin4-mediated promotion of cell migration and anoikis resistance is regulated by serine 897 phosphorylation of epha2. FEBS Open Bio 2013, 3, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Akada, M.; Harada, K.; Negishi, M.; Katoh, H. Ephb6 promotes anoikis by modulating epha2 signaling. Cell. Signal. 2014, 26, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, Y.; Xu, L.; Chen, L.; Cheng, M.; Shi, W.; Xiong, H.; Zalli, D.; Luo, S. Gli2 promotes cell proliferation and migration through transcriptional activation of arhgef16 in human glioma cells. J. Exp. Clin. Cancer Res. 2018, 37, 247. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.R.; Theodosiou, A.M.; Nesbit, M.A.; Campbell, L.; Tandle, A.T.; Saranath, D.; Davies, K.E. Characterization of ngef, a novel member of the dbl family of genes expressed predominantly in the caudate nucleus. Genomics 2000, 65, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Suzuki, H.; Yokoo, T.; Tada-Iida, K.; Kihara, R.; Miura, M.; Watanabe, K.; Sone, H.; Shimano, H.; Toyoshima, H.; et al. Wgef is a novel rhogef expressed in intestine, liver, heart, and kidney. Biochem. Biophys. Res. Commun. 2004, 324, 1053–1058. [Google Scholar] [CrossRef]
- Hampson, L.; He, X.T.; Oliver, A.W.; Hadfield, J.A.; Kemp, T.; Butler, J.; McGown, A.; Kitchener, H.C.; Hampson, I.N. Analogues of y27632 increase gap junction communication and suppress the formation of transformed nih3t3 colonies. Br. J. Cancer 2009, 101, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.W.; He, X.; Borthwick, K.; Donne, A.J.; Hampson, L.; Hampson, I.N. The hpv16 e6 binding protein tip-1 interacts with arhgef16, which activates cdc42. Br. J. Cancer 2011, 104, 324–331. [Google Scholar] [CrossRef]
- Egea, J.; Klein, R. Bidirectional eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Harboe, M.; Torvund-Jensen, J.; Kjaer-Sorensen, K.; Laursen, L.S. Ephrin-a1-epha4 signaling negatively regulates myelination in the central nervous system. Glia 2018, 66, 934–950. [Google Scholar] [CrossRef]
- Yohe, M.E.; Rossman, K.; Sondek, J. Role of the c-terminal sh3 domain and n-terminal tyrosine phosphorylation in regulation of tim and related dbl-family proteins. Biochemistry 2008, 47, 6827–6839. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Qu, Y.; Nakamura, M.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Shirayama, Y.; et al. Increased epha4-ephexin1 signaling in the medial prefrontal cortex plays a role in depression-like phenotype. Sci. Rep. 2017, 7, 7133. [Google Scholar] [CrossRef] [PubMed]
- Rosas, O.R.; Figueroa, J.D.; Torrado, A.I.; Rivera, M.; Santiago, J.M.; Konig-Toro, F.; Miranda, J.D. Expression and activation of ephexin is altered after spinal cord injury. Dev. Neurobiol. 2011, 71, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Rosas, O.R.; Torrado, A.I.; Santiago, J.M.; Rodriguez, A.E.; Salgado, I.K.; Miranda, J.D. Long-term treatment with pp2 after spinal cord injury resulted in functional locomotor recovery and increased spared tissue. Neural. Regen. Res. 2014, 9, 2164–2173. [Google Scholar] [PubMed]
- Fan, R.; Enkhjargal, B.; Camara, R.; Yan, F.; Gong, L.; Yao, S.; Tang, J.; Chen, Y.; Zhang, J.H. Critical role of epha4 in early brain injury after subarachnoid hemorrhage in rat. Exp. Neurol. 2017, 296, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tanegashima, K.; Zhao, H.; Dawid, I.B. Wgef activates rho in the wnt-pcp pathway and controls convergent extension in xenopus gastrulation. EMBO J. 2008, 27, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.K.; Canny, S.G.; Jang, C.W.; Cho, K.; Ji, H.; Wagner, D.S.; Jones, E.A.; Habas, R.; McCrea, P.D. Pronephric tubulogenesis requires daam1-mediated planar cell polarity signaling. J. Am. Soc. Nephrol. 2011, 22, 1654–1664. [Google Scholar] [CrossRef]
- Wang, Z.; Kumamoto, Y.; Wang, P.; Gan, X.; Lehmann, D.; Smrcka, A.V.; Cohn, L.; Iwasaki, A.; Li, L.; Wu, D. Regulation of immature dendritic cell migration by rhoa guanine nucleotide exchange factor arhgef5. J. Biol. Chem. 2009, 284, 28599–28606. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, M.; Ding, X.J.; Ma, Z.H.; Li, L.W.; Wang, P.; Chen, Y.; Huang, Y.C.; Cao, Y. Characterization of germline mutations in familial lung cancer from the chinese population. Gene 2018, 641, 94–104. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Tan, D.L.; Liu, H.X.; Lv, F.L.; Wu, W. The auto-inhibitory state of rho guanine nucleotide exchange factor arhgef5/tim can be relieved by targeting its sh3 domain with rationally designed peptide aptamers. Biochimie 2015, 111, 10–18. [Google Scholar] [CrossRef]
- Huang, O.; Wu, D.; Xie, F.; Lin, L.; Wang, X.; Jiang, M.; Li, Y.; Chen, W.; Shen, K.; Hu, X. Targeting rho guanine nucleotide exchange factor arhgef5/tim with auto-inhibitory peptides in human breast cancer. Amino Acids 2015, 47, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Morita, K.; Omizu, S.; Kato, S.; Koriyama, Y. Mechanisms of rhoa inactivation and cdc42 and rac1 activation during zebrafish optic nerve regeneration. Neurochem. Int. 2018, 112, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S.; Lorenz, U. Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Dalva, M.B. Ephecting excitatory synapse development. Cell 2010, 143, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.M.; Lambert, J.T.; Parajuli, L.K.; Vivas, O.; Park, D.K.; Stein, I.S.; Jahncke, J.N.; Greenberg, M.E.; Margolis, S.S.; Zito, K. A dual role for the rhogef ephexin5 in regulation of dendritic spine outgrowth. Mol. Cell Neurosci. 2017, 80, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Houle, F.; Jourdan, G.; Huot, J. Phosphorylation of tyrosine 1214 on vegfr2 is required for vegf-induced activation of cdc42 upstream of sapk2/p38. Oncogene 2004, 23, 434–445. [Google Scholar] [CrossRef]
- Fukushima, Y.; Okada, M.; Kataoka, H.; Hirashima, M.; Yoshida, Y.; Mann, F.; Gomi, F.; Nishida, K.; Nishikawa, S.; Uemura, A. Sema3e-plexind1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Invest. 2011, 121, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, R.; Iruela-Arispe, M.L.; Reyes-Cruz, G.; Vazquez-Prado, J. Endothelial rhogefs: A systematic analysis of their expression profiles in vegf-stimulated and tumor endothelial cells. Vascul. Pharmacol. 2015, 74, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Veeramah, K.R.; Johnstone, L.; Karafet, T.M.; Wolf, D.; Sprissler, R.; Salogiannis, J.; Barth-Maron, A.; Greenberg, M.E.; Stuhlmann, T.; Weinert, S.; et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia 2013, 54, 1270–1281. [Google Scholar] [CrossRef]
- Sell, G.L.; Schaffer, T.B.; Margolis, S.S. Reducing expression of synapse-restricting protein ephexin5 ameliorates alzheimer’s-like impairment in mice. J. Clin. Invest. 2017, 127, 1646–1650. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).