MicroRNAs at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration
Abstract
1. Introduction
2. Molecular Regulation of Bone Angiogenesis
3. The Role of MicroRNAs
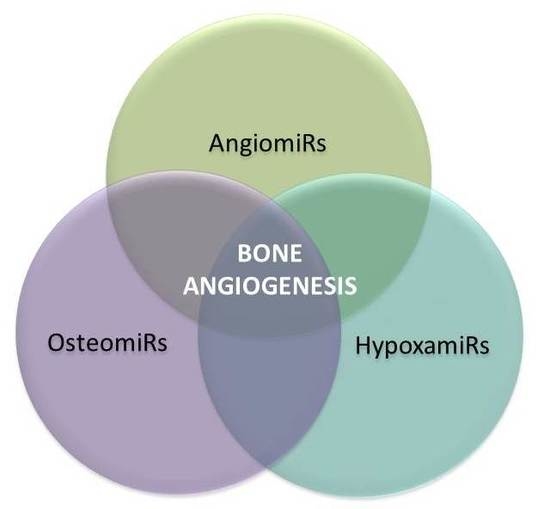
4. MicroRNAs in Bone Angiogenesis: OsteomiRs, AngiomiRs, and HypoxamiRs
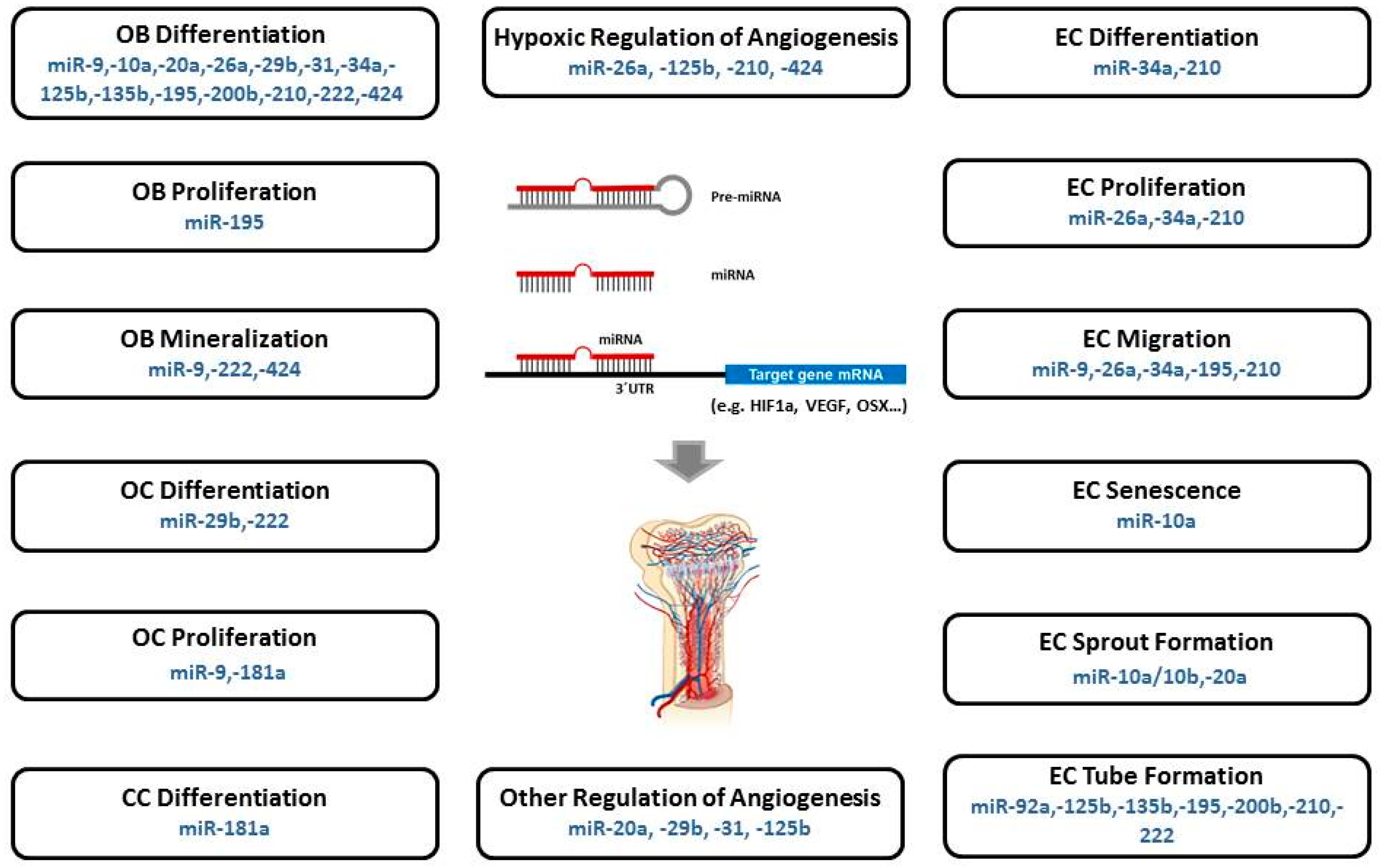
5. Specific MicroRNAs Implicated in Angiogenic-Osteogenic Coupling
5.1. MiR-9
5.2. MiR-10a
5.3. MiR-10a/10b
5.4. MiR-20a
5.5. MiR-26a/b
5.6. MiR-29b
5.7. MiR-31
5.8. MiR-34a
5.9. MiR-92a
5.10. MiR-125b
5.11. MiR-135b
5.12. MiR-181a
5.13. MiR-195
5.14. MiR-200b
5.15. MiR-210
5.16. MiR-222
5.17. MiR-424
6. Outlook and Future Directions: MiRNAs in Therapeutic Applications
Author Contributions
Funding
Conflicts of Interest
References
- Crane, G.; Ishaug, S.; Mikos, A. Bone tissue engineering. Nat. Med. 1995, 1, 1322–1324. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Hertig, A. Angiogenesis in the early human chorion and the primary placenta of the macaque monkey. Contrib. Embryol. 1935, 25, 37–81. [Google Scholar]
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2013, 8, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg HM Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [CrossRef] [PubMed]
- Berendesen, A.; Olsen, B. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Olsen, B.; Reginato, A.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef]
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef]
- Helms, J.; Schneider, R. Cranial skeletal biology. Nature 2003, 423, 326–331. [Google Scholar] [CrossRef]
- Maes, C.; Carmeliet, P.; Moermans, K.; Stockmans, I.; Smets, N. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF 164 and VEGF 188. Mech Dev. 2002, 111, 61–73. [Google Scholar] [CrossRef]
- Gerber, H.; Ferrara, N. Angiogenesis and Bone Growth. TCM 2000, 10, 223–228. [Google Scholar] [CrossRef]
- Brandi, M.; Collin-Osdoby, P. Vascular biology and the skeleton. J. Bone Miner. Res. 2006, 21, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Wu, C.; Rankin, E.B.; Giaccia, A.J. Regulation of Bone Marrow Angiogenesis by Osteoblasts during Bone Development and Homeostasis. Front. Endocrinol. 2013, 4, 85. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eerhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Carulli, C.; Innocenti, M.; Brandi, M.L. Bone vascularization in normal and disease conditions. Front. Endocrinol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Ding, W.-G.; Yan, W.; Wei, Z.-X.; Liu, J.-B. Difference in intraosseous blood vessel volume and number in osteoporotic model mice induced by spinal cord injury and sciatic nerve resection. J. Bone Miner. Metab. 2012, 30, 400–407. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, F.; Zhang, P.; Wang, H.; Qu, Z.; Jia, P.; Yao, Z.; Shen, G.; Li, G.; Zhao, G.; et al. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017, 8, e2760. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2014, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest. 2016, 126, 509–526. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Kobayashi, T.; Selig, M.; Torrekens, S.; Roth, S.; Mackem, S.; Carmeliet, G.; Kronenberg, H. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 2007, 117, 1616–1626. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.; Lecouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Ornitz, D.; Marie, P. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015, 29, 1463–1468. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Behonick, D.; Werb, Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kleinheinz, J.; Stratmann, U.; Joos, U.; Wiesmann, H.-P. VEGF-Activated Angiogenesis During Bone Regeneration. J. Oral Maxillofac. Surg. 2005, 63, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Coenegrachts, L.; Stockmans, I.; Daci, E.; Luttun, A.; Petryk, A.; Gopalakrishnan, R.; Moermans, K.; Smets, N.; Verfaillie, C.M.; et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J. Clin. Invest. 2006, 116, 16–18. [Google Scholar] [CrossRef]
- Kingsley, D. What do BMPs do in mammals? Clues from the mouse short-ear mutation. Trends Genet. 1994, 10, 16–21. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Tye, C.E.; Stein, G.S.; Lian, J.B. Non-coding RNAs: Epigenetic regulators of bone development and homeostasis. Bone 2015, 81, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Mirzamohammadi, F.; Kobayashi, T. MicroRNAs involved in bone formation. Cell Mol. Life Sci. 2014, 71, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G. miRNAs in Bone Development. Curr. Genom. 2015, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. MicroRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef]
- Bartel, D.P.; Lee, R.; Feinbaum, R. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function Genomics: The miRNA Genes. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; McLachlan, J.; Pasquinelli, A.E.; Balint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Grishok, A.; Pasquinelli, A.E.; Conte, D.; Li, N.; Parrish, S.; Ha, I.; Baillie, D.L.; Fire, A.; Ruvkun, G.; Mello, C. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001, 106, 23–34. [Google Scholar] [CrossRef]
- Knight, S.W.; Bass, B.L. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 2001, 293, 2269–2271. [Google Scholar] [CrossRef]
- Yang, J.S.; Lai, E.C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell 2011, 43, 892–903. [Google Scholar] [CrossRef]
- Lai, E.C. Micro RNAs are complementary to 3´UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Wang, Y.; Medvid, C.; Melton, R.; Jaenisch, R.; Blelloch, R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat. Genet. 2007, 39, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Förstemann, K.; Tomari, Y.; Du, T.; Vagin, V.V.; Denli, A.M.; Bratu, D.P.; Klattenhoff, C.; Theurkauf, W.E.; Zamore, P.D. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double stranded RNA-binding domain protein. PLoS Biol. 2005, 3, e236. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: A review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Melton, C.; Judson, R.L.; Blelloch, R. Opposing micro-RNA families regulate self-renewal in mouse embryonic stem cells. Nature 2010, 463, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Hipfner, D.R.; Stark, A.; Russell, R.B.; Cohen, S.M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 2003, 113, 25–36. [Google Scholar] [CrossRef]
- Hipfner, D.R.; Weigmann, K.; Cohen, S.M. The bantam gene regulates Drosophila growth. Genetics 2002, 161, 1527–1537. [Google Scholar] [PubMed]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ´antagomirs´. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Miska, E.A. How microRNAs control cell division, differentiation and death. Curr. Opin. Genet. Dev. 2005, 15, 563–568. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, L.F.; Bartel, D. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, I.; Miska, E.A. MicroRNA functions in animal development and human disease. Development 2005, 132, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Bianciardi, S.; Merlotti, D. MicroRNAs in bone diseases. Osteoporos. Int. 2017, 28, 1191–1213. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Kalomoiris, S.; Nolta, J.; Fierro, F. Concise Review: MicroRNA Function in Multipotent Mesenchymal Stromal Cells. Stem Cells 2014, 32, 1074–1082. [Google Scholar] [CrossRef]
- Peng, S.; Gao, D.; Gao, C.; Wei, P.; Niu, M.; Shuai, C. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (Review). Mol. Med. Rep. 2016, 14, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Deng, Y.; Gu, P.; Fan, X. MicroRNAs Regulate Bone Development and Regeneration. Int. J. Mol. Sci. 2015, 16, 8227–8253. [Google Scholar] [CrossRef]
- Ji, X.; Chen, X.; Yu, X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. Int. J. Mol. Sci. 2016, 17, 349. [Google Scholar] [CrossRef]
- Dong, S.; Yang, B.; Guo, H.; Kang, F. MicroRNAs regulate osteogenesis and chondrogenesis. Biochem. Biophys. Res. Commun. 2012, 418, 587–591. [Google Scholar] [CrossRef]
- Kiga, K.; Mimuro, H.; Suzuki, M.; Shinozaki-Ushiku, A.; Kobayashi, T.; Sanada, T.; Kim, M.; Ogawa, M.; Iwasaki, Y.W.; Kayo, H.; et al. Epigenetic silencing of miR-210 increases the proliferation of gastric epithelium during chronic Helicobacter pylori infection. Nat. Commun. 2014, 5, 4497. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, M.; Dou, C.; Cao, Z.; Dong, S. MicroRNAs in Bone Balance and Osteoporosis. Drug Dev. Res. 2015, 76, 235–245. [Google Scholar] [CrossRef]
- Nugent, M. MicroRNAs and Fracture Healing. Calcif. Tissue Int. 2017, 101, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tian, B.O.; Qu, X.; Liu, F.; Tang, T.; Qin, A.N.; Zhu, Z.; Dai, K. MicroRNAs play a role in chondrogenesis and osteoarthritis (Review). Int. J. Mol. Med. 2014, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Zhang, R.; Yao, J.; Jiang, C.; Guo, Y.; Cong, F.; Wang, W.; Tian, J.; Zhong, N.; Sun, J.; et al. MicroRNAs associated with osteoarthritis differently expressed in bone matrix gelatin (BMG) rat model. Int. J. Clin. Exp. Med. 2015, 8, 1009–1017. [Google Scholar] [PubMed]
- Seeliger, C.; Balmayor, E.; van Griensven, M. miRNAs Related to Skeletal Diseases. Stem Cells Dev. 2016, 25, 1261–1281. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Cheresh, D.A. Emerging Role of Micro-RNAs in the Regulation of Angiogenesis. Genes Cancer 2011, 2, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Olson, E.N. AngiomiRs—Key Regulators of Angiogenesis. Curr. Opin. Genet. Dev. 2009, 19, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M.; Olson, E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Weis, S.M.; Caheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.; Marchat, L.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Astudillo- De La Vega, H.; Echavarria-Zepeda, R.; Lopez-Camarillo, C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review). Int. J. Mol. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Suarez, Y.; Sessa, W.C. MicroRNAs As Novel Regulators of Angiogenesis. Circ. Res. 2009, 104, 442–454. [Google Scholar] [CrossRef]
- Yang, W.; Yang, D.; Na, S.; Sandusky, G.; Zhang, Q.; Zhao, G. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 2005, 280, 9330–9335. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, S.; Suarez, Y.; Skoura, A.; Offermann, S.; Miano, J.M.; Sessa, W.C. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnnaly, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Suarez, Y.; Wang, C.; Manes, T.D.; Pober, J.S. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: Feedback control of inflammation. J. Immunol. 2010, 184, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Suarez, Y.; Fernandez-Hernando, C.; Pober, J.; Sessa, W. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Gaetano, C.; Martelli, F. HypoxamiR Regulation and Function in Ischemic Cardiovascular Diseases. Antioxid. Redox Signal. 2014, 21, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Balasubramanian, S.; Rajasingh, S.; Patel, U.; Dhanasekaran, A.; Dawn, B.; Rajasingh, J. MicroRNA: A new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc. Med. 2016, 26, 407–419. [Google Scholar] [CrossRef]
- Suarez, Y.; Fernandez-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Würdinger, T.; Tannous, B.A.; Saydam, O.; Skog, J.; Grau, S.; Soutschek, J.; Weissleder, R.; Breakefield, X.O.; Krichevsky, A.M. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell 2008, 14, 382–393. [Google Scholar] [CrossRef]
- Anand, S.; Cheresh, D.A. MicroRNA-mediated Regulation of the Angiogenic Switch. Curr. Opin. Hematol. 2011, 18, 171–176. [Google Scholar] [CrossRef]
- Nallamshetty, S.; Chan, S.Y.; Loscalzo, J. Hypoxia: A master regulator of microRNA biogenesis and activity. Free Radic. Biol. Med. 2013, 64, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Madanecki, P.; Kapoor, N.; Bebok, Z.; Ochocka, R.; Collawn, J.F.; Bartoszewski, R. Regulation of angiogenesis by hypoxia: The role of microRNA. Cell. Mol. Biol. Lett. 2013, 18, 47–57. [Google Scholar] [CrossRef] [PubMed]
- el Azzouzi, H.; Leptidis, S.; Doevendans, P.A.; De Windt, L.J. HypoxamiRs: Regulators of cardiac hypoxia and energy metabolism. Trends Endocrinol. Metab. 2015, 26, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Martelli, F. MicroRNAs in Hypoxia Response. Antioxid. Redox Signal. 2014, 21, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Collet, G.; Skrzypek, K.; Grillon, C.; Matejuk, A.; El Hafni-Rahbi, B.; Fayel, N.L.; Kieda, C. Hypoxia control to normalize pathologic angiogenesis: Potential role for endothelial precursor cells and miRNAs regulation. Vascul. Pharmacol. 2012, 56, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Rezzonico, R.; Pottier, N.; Mari, B. Impact of MicroRNAs in the Cellular Response to Hypoxia. Int. Rev. Cell Mol. Biol. 2017, 333, 91–158. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, A.C.-K.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. MiRNA-Directed Regulation of VEGF and Other Angiogenic Factors under Hypoxia. PloS ONE 2006, 1, e116. [Google Scholar] [CrossRef]
- Loscalzo, J. The cellular response to hypoxia: Tuning the system with microRNAs. J. Clin. Invest. 2010, 120, 3815–3817. [Google Scholar] [CrossRef]
- Devlin, C.; Greco, S.; Martelli, F.; Ivan, M. MiR-210: More than a silent player in hypoxia. IUBMB Life 2011, 63, 94–100. [Google Scholar] [CrossRef]
- Chan, S.; Loscalzo, J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle 2010, 9, 1072–1083. [Google Scholar] [CrossRef]
- Ghosh, G.; Subramanian, I.V.; Adhikari, N.; Zhang, X.; Joshi, H.P.; Basi, D.; Chandrashekhar, Y.S.; Hall, J.L.; Roy, S.; Zeng, Y.; et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF- α isoforms and promotes angiogenesis. J. Clin. Invest. 2010, 120, 4141–4154. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Yanagisawa, K.; Tanaka, M.; Cao, K.; Matsuyama, Y.; Goto, H.; Takahashi, T. Identification of hypoxia-inducible factor-1alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008, 68, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tsai, M.; Hung, P.; Kao, S.; Liu, T.; Wu, K.; Chiou, S.; Lin, S.; Chang, K. miR31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010, 70, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Yuva-Aydemir, Y.; Simkin, A.; Gascon, E.; Gao, F.-B. MicroRNA-9: Functional evolution of a conserved small regulatory RNA. RNA Biol. 2011, 8, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Kan, Q.; Sun, Y.; Wang, S.; Zhang, G.; Peng, T.; Jia, Y. MiR-9 promotes the neural differentiation of mouse bone marrow mesenchymal stem cells via targeting zinc finger protein 521. Neurosci. Lett. 2012, 515, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, D.; Guo, H.; Miao, W.; Wu, G. MicroRNA-9 regulates osteoblast differentiation and angiogenesis via the AMPK signaling pathway. Mol. Cell Biochem. 2016, 411, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Kou, J.; Wang, Q.; Zheng, X.; Yu, T. MiR-9 promotes osteoblast differentiation of mesenchymal stem cells by inhibiting DKK1 gene expression. Mol. Biol. Rep. 2016, 43, 939–946. [Google Scholar] [CrossRef]
- Zhuang, G.; Wu, X.; Jiang, Z.; Kasman, I.; Yao, J.; Guan, Y.; Oeh, J.; Modrusan, Z.; Bais, C.; Sampath, D.; et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012, 31, 3513–3523. [Google Scholar] [CrossRef]
- Jones, S.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.; Brockbank, S.; Needham, M.; Read, S.; Newham, P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.; Zhang, Q.; Chen, W. Reduced miR-9 and miR-181a expression down-regulates Bim concentration and promote osteoclasts survival. Int. J. Clin. Exp. Pathol. 2014, 7, 2209–2218. [Google Scholar]
- Li, J.; Zhang, Y.; Zhao, Q.; Wang, J.; He, X. MicroRNA-10a Influences Osteoblast Differentiation and Angiogenesis by Regulating ß-Catenin Expression. Cell. Physiol. Biochem. 2015, 37, 2194–2208. [Google Scholar] [CrossRef]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Deng, S.; Ma, Q.; Zhang, T.; Jia, C.; Zhuo, D.; Yang, F.; Wei, J.; Wang, L.; Dykxhoorn, D.M.; et al. MicroRNA-10A* and MicroRNA-21 Modulate Endothelial Progenitor Cell Senescence Via Suppressing High-Mobility Group A2. Circ. Res. 2013, 112, 152–164. [Google Scholar] [CrossRef]
- Hassel, D.; Cheng, P.; White, M.P.; Ivey, K.N.; Kroll, J.; Augustin, H.G.; Katus, H.A.; Stainier, D.Y.R.; Srivastava, D. MicroRNA-10 Regulates the Angiogenic Behavior of Zebrafish and Human Endothelial Cells by Promoting Vascular Endothelial Growth Factor Signaling. Circ. Res. 2012, 111, 1421–1433. [Google Scholar] [CrossRef]
- Wang, X.; Ling, C.C.; Li, L.; Qin, Y.; Qi, J.; Liu, X.; You, B.; Shi, Y.; Zhang, J.; Xu, Q.J.H.; et al. MicroRNA-10a/10b represses a novel target gene mib1 to regulate angiogenesis. Cardiovasc. Res. 2016, 110, 140–150. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, W.; He, M.; Xie, W.; Lv, Q.; Li, G.; Wang, H.; Lu, G.; Hu, X.; Jiang, S.; et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011, 8, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Ress, Y.; Urbich, C.; Hofmann, W.-K.; Zeiher, A.M.; Dimmeler, S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef]
- Deng, H.-T.; Liu, H.-L.; Zhai, B.-B.; Zhang, K.; Xu, G.-C.; Peng, X.-M. Vascular endothelial growth factor suppresses TNFSF15 production in endothelial cells by stimulating miR-31 and miR-20a expression via activation of Akt and Erk signals. FEBS Open Bio. 2017, 7, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Luzi, E.; Marini, F.; Sala, S.C.; Tognarini, I.; Galli, G.; Brandi, M.L. Osteogenic Differentiation of Human Adipose Tissue–Derived Stem Cells Is Modulated by the miR-26a Targeting of the SMAD1 Transcription Factor. J. Bone Miner. Res. 2008, 23, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Luzi, E.; Marini, F.; Tognarini, I.; Galli, G.; Falchetti, A.; Brandi, M.L. The regulatory network menin-microRNA 26a as a possible target for RNA-based therapy of bone diseases. Nucleic. Acid Ther. 2012, 22, 103–108. [Google Scholar] [CrossRef]
- Su, X.; Liao, L.; Shuai, Y.; Jing, H.; Liu, S.; Zhou, H.; Liu, Y.; Jin, Y. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis. 2015, 6, e1851. [Google Scholar] [CrossRef] [PubMed]
- Trompeter, H.-I.; Dreesen, J.; Hermann, E.; Iwaniuk, K.M.; Hafner, M.; Renwick, N.; Tuschl, T.; Wernet, P. MicroRNAs miR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genom. 2013, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Icli, B.; Wara, A.K.M.; Moslehi, J.; Sun, X.; Plovie, E.; Cahill, M.; Marchini, J.F.; Schissler, A.; Padera, R.F.; Shi, J.; et al. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ. Res. 2013, 113, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hassan, M.Q.; Jafferji, M.; Aqeilan, R.I.; Garzon, R.; Croce, C.M.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Biological Functions of miR-29b Contribute to Positive Regulation of Osteoblast Differentiation. J. Biol. Chem. 2009, 284, 15676–15684. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Pitari, M.R.; Amodio, N.; Di Martino, T.M.; Conforti, F.; Leone, E.; Botta, C.; Paolino, F.M.; Giudice, T.D.E.L.; Iuliano, E.; et al. miR-29b Negatively Regulates Human Osteoclastic Cell Differentiation and Function: Implications for the Treatment of Multiple Myeloma-Related Bone Disease. J. Cell. Physiol. 2013, 228, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cai, H.-X.; Gao, S.; Yang, G.-L.; Deng, H.-T.; Xu, G.-C.; Han, J.; Zhang, Q.-Z.; Li, L.-Y. TNSF15 suppresses VEGF production in endothelial cells by stimulating miR-29b expression via activation of JNK-GATA3 Signals. Oncotarget 2016, 7, 69436–69449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, B.; Shen, L.; Dong, Y.; Lu, Q.; Sun, S.; Liu, S.; Ma, S.; Ma, P.X.; Chen, J. MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 2017, 397, 111–119. [Google Scholar] [CrossRef]
- Granchi, D.; Ochoa, G.; Leonardi, E.; Devescovi, V.; Baglìo, S.R.; Osaba, L.; Baldini, N.; Ciapetti, G. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng. Part. C Methods 2010, 16, 511–523. [Google Scholar] [CrossRef]
- Baglìo, S.R.; Devescovi, V.; Granchi, D.; Baldini, N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene 2013, 527, 321–331. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, S.; Zhou, H.; Bi, X.; Wang, Y.; Hu, Y.; Gu, P.; Fan, X. Effects of a miR-31, Runx2, and Satb2 regulatory loop on the osteogenic differentiation of bone mesenchymal stem cells. Stem Cells Dev. 2013, 22, 2278–2286. [Google Scholar] [CrossRef]
- Luo, J.; Lin, J.; Paranya, G.; Bischoff, J. Angiostatin Upregulates E-Selectin in Proliferating Endothelial Cells. Biochem. Biophys. Res. Commun. 1998, 911, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Holmstrom, K.; Qiu, W.; Ditzel, N.; Shi, K.; Hokland, L.; Kassem, M. MicroRNA-34a Inhibits Osteoblast Differentiation and In Vivo Bone Formation of Human Stromal Stem Cells. Stem Cells 2014, 32, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Chen, H.; Huang, P.; Qi, J.; Qian, N.; Deng, L.; Guo, L. Glucocorticoids impair bone formation of bone marrow stromal stem cells by reciprocally regulating microRNA-34a-5p. Osteoporos. Int. 2016, 27, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Jia, L.; Zheng, Y.; Jin, C.; Liu, Y.; Liu, H.; Zhou, Y. Mir-34a Promotes Osteogenic Differentiation of Human Adipose-Derived Stem Cells via the RBP2/NOTCH I/CYCLIN DI Coregulatory Network. Stem Cell Rep. 2016, 7, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Engin, F.; Yao, Z.; Yang, T.; Zhou, G.; Bertin, T.; Jiang, M.M.; Chen, Y.; Wang, L.; Zheng, H.; Sutton, R.E.; et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat. Med. 2008, 14, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Galindo, M.; Pratap, J.; Young, D.W.; Hovhannisyan, H.; Im, H.J.; Choi, J.Y.; Lian, J.B.; Stein, J.L.; Stein, G.S.; van Wijnen, A.J. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J. Biol. Chem. 2005, 280, 20274–20285. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Sun, B.; Zhang, R.; Li, C.; Yan, Z.; Chen, J. Regulatory Effect of MicroRNA-34a on Osteogenesis and Angiogenesis in Glucocorticoid-Induced Osteonecrosis of the Femoral Head. J. Orthop. Res. 2018, 36, 417–424. [Google Scholar] [CrossRef]
- Zhao, T.; Li, J.; Chen, A.F. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am. J. Endocrinol. Metab. 2010, 299, E110–E116. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.; Naqvi, A.; Yamamori, T.; Hoffman, T.; Jung, S.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Kumar, B.; Yadav, A.; Lang, J.; Teknos, T.N.; Kumar, P. Dysregulation of MicroRNA-34a Expression in Head and Neck Squamous Cell Carcinoma Promotes Tumor Growth and Tumor Angiogenesis. PLoS ONE 2012, 7, e37601. [Google Scholar] [CrossRef]
- Chai, Z.T.; Kong, J.; Zhu, X.D.; Zhang, Y.Y.; Lu, L.; Zhou, J.M.; Wang, L.R.; Zhang, K.Z.; Zhang, Q.B.; Ao, J.Y.; et al. MicroRNA-26a Inhibits Angiogenesis by Down-Regulating VEGFA through the PIK3C2α/Akt/HIF-1α Pathway in Hepatocellular Carcinoma. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Ito, H.; Yoshitomi, H.; Yamamoto, K.; Fukuda, A.; Yoshikawa, J.; Furu, M.; Ishikawa, M.; Shibuya, H.; Matsuda, S. Inhibition of miR-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J. Bone Miner. Res. 2014, 29, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Klink, G.; Glukhanyuk, E.; Lopatina, T.; Anastassia, E.; Akopyan, Z.; Tkachuk, V. miR-92a regulates angiogenic activity of adipose-derived mesenchymal stromal cells. Exp. Cell Res. 2015, 339, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.-M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Rauner, M.; Pacyna, N.; Hempel, U.; Bornstein, S.R.; Hofbauer, L.C. MiR-125b regulates calcification of vascular smooth muscle cells. Am. J. Pathol. 2011, 179, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Yagi, K.; Tokuzawa, Y.; Kanesaki-Yatsuka, Y.; Suda, T.; Katagiri, T.; Fukuda, T.; Maruyama, M.; Okuda, A.; Amemiya, T.; et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem. Biophys. Res. Commun. 2008, 368, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, P.; Liang, P.; Huang, X. The expression of miR-125b regulates angiogenesis during the recovery of heat-denatured HUVECs. Burns 2015, 41, 803–811. [Google Scholar] [CrossRef]
- Huang, K.; Fu, J.; Zhou, W.; Li, W.; Dong, S.; Yu, S.; Hu, Z.; Wang, H.; Xie, Z. MicroRNA-125b regulates osteogenic differentiation of mesenchymal stem cells by targeting Cbfb in vitro. Biochimie 2014, 102, 47–55. [Google Scholar] [CrossRef]
- Xihong, L.U.; Min, D.; Honghui, H.E.; Dehui, Z.; Wei, Z. miR-125b regulates osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting Smad4. J. Cent. South. Univ. (Med. Sci.) 2013, 38, 341–346. [Google Scholar] [CrossRef]
- Muramatsu, F.; Kidoya, H.; Naito, H.; Sakimoto, S.; Takakura, N. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene 2013, 32, 414–421. [Google Scholar] [CrossRef]
- Schaap-Oziemlak, A.M.; Raymakers, R.A.; Bergevoet, S.M.; Gilissen, C.; Jansen, B.J.H.; Adema, G.J.; Kögler, G.; le Sage, C.; Agami, R.; van der Reijden, B.A.; et al. MicroRNA hsa-miR-135b Regulates Mineralization in Osteogenic Differentiation of Human Unrestricted Somatic Stem Cells. Stem Cells Dev. 2010, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Santini, G.C.; De Veirman, K. ; Broek, I.V.; Leleu, X.; De, A.; Van Camp, B.; Vanderkerken, K.; Van Riet, I. Upregulation of miR-135b Is Involved in the Impaired Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. PLoS ONE 2013, 8, e79752. [Google Scholar] [CrossRef]
- Sumiyoshi, K.; Kubota, S.; Ohgawara, T.; Kawata, K.; Abd El Kader, T.; Nishida, T.; Ikeda, N.; Shimo, T.; Yamashiro, T.; Takigawa, M. Novel Role of miR-181a in Cartilage Metabolism. J. Cell. Biochem. 2013, 114, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Gabler, J.; Ruetze, M.; Kynast, K.L.; Grossner, T.; Diederichs, S.; Richter, W. Stage-Specific miRs in Chondrocyte Maturation: Differentiation-Dependent and Hypertrophy-Related miR Clusters and the miR-181 Family. Tissue Eng. Part. A 2015, 21, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Charbonneau, C.; Wei, L.; Chen, Q.; Terek, R.M. miR-181a Targets RGS16 to Promote Chondrosarcoma Growth, Angiogenesis, and Metastasis. Mol. Cancer Res. 2015, 13, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, L.; Chen, Q.; Terek, R.M. MicroRNA Regulates Vascular Endothelial Growth Factor Expression in Chondrosarcoma Cells. Clin. Orthop. Relat. Res. 2015, 473, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.; Silva, A.M.; Vasconcelos, D.M.; Almeida, C.R.; Caires, H.; Pinto, M.T.; Calin, A.; Santos, S.G.; Barbosa, M.A. miR-195 in human primary mesenchymal stromal/stem cells regulates proliferation, osteogenesis and paracrine effect on angiogenesis. Oncotarget 2015, 7, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, N.; Li, S.; Fang, J.; Chen, M.; Yang, J.; Jia, W.; Yuan, Y.; Zhuang, S. MicroRNA-195 Suppresses Angiogenesis and Metastasis of Hepatocellular Carcinoma by Inhibiting the Expression of VEGF, VAV2, and CDC42. Hepatology 2013, 58, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, C.; Tu, M.; Jiang, W.; Dai, Z.; Hu, Y.; Deng, Z.; Xiao, W. MicroRNA-200b acts as a tumor suppressor in osteosarcoma via targeting ZEB1. Onco Targets Ther. 2016, 9, 3101–3111. [Google Scholar]
- Fan, X.; Teng, Y.; Ye, Z.; Zhou, Y.; Tan, W.-S. The effect of gap junction-mediated transfer of miR-200b on osteogenesis and angiogenesis in a co-culture of MSCs and HUVECs. J. Cell Sci. 2018, 131, jcs216135. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, S.; Jeong, Y.; Yoon, J.; Baek, K. Regulation of Vascular Endothelial Growth Factor Signaling by miR-200b. Mol. Cells 2011, 32, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Tokuzawa, Y.; Ninomiya, Y.; Yagi, K.; Yatsuka-Kanesaki, Y.; Suda, T.; Fukuda, T.; Katagiri, T.; Kondoh, Y.; Amemiya, T.; et al. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett. 2009, 583, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-D.; Cai, F.; Liu, L.; Zhang, Y.; Yang, A.-L. microRNA-210 is involved in the regulation of postmenopausal osteoporosis through promotion of VEGF expression and osteoblast differentiation. Biol. Chem. 2015, 396, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, D.; Yang, S.; Sun, H.; Wu, B.; Zhou, D. Inhibition of miR-222-3p activity promoted osteogenic differentiation of hBMSCs by regulating Smad5-RUNX2 signal axis. Biochem. Biophys. Res. Commun. 2016, 470, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, S.; Chen, A.; Wan, Q.; Na, S.; Sudo, A.; Yokota, H.; Hamamura, K. Role of miR-222-3p in c-Src-Mediated Regulation of Osteoclastogenesis. Int. J. Mol. Sci. 2016, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Gao, J.; Yang, T.; Han, J.; Yan, K.; Qiu, X.; Zhou, Y.; Fan, Q.; Ma, B. MicroRNA Expression During Osteogenic Differentiation of Human Multipotent Mesenchymal Stromal Cells From Bone Marrow. J. Cell. Biochem. 2011, 112, 1844–1856. [Google Scholar] [CrossRef]
- Vimalraj, S.; Selvamurugan, N. MicroRNAs expression and their regulatory networks during mesenchymal stem cells differentiation toward osteoblasts. Int. J. Biol. Macromol. 2014, 66, 194–202. [Google Scholar] [CrossRef]
- Li, L.; Qi, Q.; Luo, J.; Huang, S.; Ling, Z.; Gao, M.; Zhou, Z.; Stiehler, M.; Zou, X. FOXO1-suppressed miR-424 regulates the proliferation and osteogenic differentiation of MSCs by targeting FGF2 under oxidative stress. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- de Pontual, L.; Yao, E.; Callier, P.; Faivre, L.; Drouin, V.; Cariou, S.; Van Haeringen, A.; Geneviève, D.; Goldenberg, A.; Oufadem, M.; Manouvrier, S.; Munnich, A.; et al. Germline deletion of the miR-17 ~ 92 cluster causes skeletal and growth defects in humans. Nat. Genet. 2011, 43, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Penzkofer, D.; Bonauer, A.; Fischer, A.; Tups, A.; Brandes, R.P.; Zeiher, A.M.; Dimmeler, S. Phenotypic Characterization of miR-92a - /- Mice Reveals an Important Function of miR-92a in Skeletal Development. PLoS ONE 2014, 9, e101153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ma, J.; Chen, S.; Chen, X.; Yu, X. MicroRNA-17-92 cluster regulates osteoblast proliferation and differentiation. Endocrine 2014, 45, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wu, P.; Zhang, Z.; Zhang, Z.; Liao, W.; Li, Y.; Kang, Y. MicroRNA-92a-3p Regulates Aggrecanase-1 and Aggrecanase-2 Expression in Chondrogenesis and IL-1β- Induced Catabolism in Human Articular Chondrocytes. Cell. Physiol. Biochem. 2017, 44, 38–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Y.; Zhang, Z.; Zhang, H.; Duan, X.; Liu, J.; Li, X.; Liao, W. Expression of microRNAs during chondrogenesis of human adipose-derived stem cells. Osteoarthr. Cartil. 2012, 20, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Zhang, Z.; Huang, Z.; Chen, W.; Huang, G.; Meng, F.; Zhang, Z.; Kang, Y. MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthr. Cartil. 2017, 25, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, M.; Qin, G.; Weintraub, N.L.; Tang, Y. MiR-92a regulates viability and angiogenesis of endothelial cells under oxidative stress. Biochem. Biophys. Res. Commun. 2015, 446, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Rippe, C.; Blimline, M.; Magerko, K.A.; Lawson, B.R.; LaRocca, T.; Donato, A.; Seals, D.R. MicroRNA Changes in Human Arterial Endothelial Cells with Senescence: Relation to Apoptosis, eNOS and Inflammation Catarina. Exp. Gerontol. 2012, 47, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, H.K.; Chung, S.; Kim, K.S.; Dutta, A. Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the down-regulation of putative targets during differentiation. J. Biol. Chem. 2005, 280, 16635–16641. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-F.; Yang, G.-H.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; et al. Altered MicroRNA Expression Profile in Exosomes during Osteogenic Differentiation of Human Bone Marrow- Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef] [PubMed]
- Fan, G. Hypoxic exosomes promote angiogenesis Platelets: Balancing the septic triad. Blood 2014, 124, 3669–3670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Z.-J.; Bian, Y.; Kulkarni, A.B. MicroRNA-135b acts as a tumor promoter by targeting the hypoxia-inducible factor pathway in genetically defined mouse model of head and neck squamous cell carcinoma. Cancer Lett. 2013, 331, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kazenwadel, J.; Michael, M.Z.; Harvey, N.L. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 2010, 116, 2395–2401. [Google Scholar] [CrossRef]
- Naguibneva, I.; Ameyar-Zazoua, M.; Polesskaya, A.; Ait-Si-Ali, S.; Groisman, R.; Souidi, M.; Cuvellier, S.; Harel-Bellan, A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat. Cell Biol. 2006, 8, 278–284. [Google Scholar] [CrossRef]
- Akiyama, T.; Bouillet, P.; Miyazaki, T.; Kadono, Y.; Chikuda, H.; Chung, U.; Fukuda, A.; Hikita, A.; Seto, H.; Okada, T.; et al. Regulation of osteoclast apoptosis by ubiquitination of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003, 22, 6653–6664. [Google Scholar] [CrossRef]
- Palmieri, A.; Pezzetti, F.; Brunelli, G.; Zollino, I.; Scapoli, L.; Martinelli, M.; Arlotti, M.; Carinci, F. Differences in osteoblast miRNA induced by cell binding domain of collagen and silicate-based synthetic bone. J. Biomed. Sci. 2007, 14, 777–782. [Google Scholar] [CrossRef]
- Christoffersen, N.R.; Silahtaroglu, A.; Ørom, U.L.F.A.; Kauppinen, S.; Lund, A.H. miR-200b mediates post-transcriptional repression of ZFHX1B. RNA 2007, 13, 1172–1178. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-lalic, D.; Verweij, F.J.; Lanzón, M.P.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.M.; Baldini, N.; et al. Human bone marrow- and adipose- mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Zhu, Y.; Liang, R.; Zhao, H.-B. Gap junction mediated miRNA intercellular transfer and gene regulation: A novel mechanism for intercellular genetic communication. Sci. Rep. 2016, 6, 19884. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-T.; Chen, H.-T.; Tsou, H.-K.; Tan, T.-W.; Fong, Y.-C.; Chen, P.-C.; Yang, W.-H.; Wang, S.-W.; Chen, J.-C.; Tang, C.-H. CCL5 promotes VEGF-dependent angiogenesis by downregulating miR-200b through PI3K/Akt signaling pathway in human chondrosarcoma cells. Oncotarget 2014, 5, 10718–10731. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Khanna, S.; Roy, S.; Sen, C.K. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. 2011, 286, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-L.; Guo, F.; Liu, F.; Gao, F.-L.; Zhang, P.-Q.; Niu, X.; Guo, S.-C.; Yin, J.-H.; Wang, Y.; Deng, Z.-F. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol. Cell Biochem. 2012, 370, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fasanaro, P.; Greco, S.; Lorenzi, M.; Pescatori, M.; Brioschi, M.; Kulshreshta, R.; Banfi, C.; Stubbs, A.; Calin, G.A.; Ivan, M.; et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J. Biol. Chem. 2009, 284, 35134–35143. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Huang, X. miR-210: Fine-Tuning the Hypoxic Response. Adv. Exp. Med. Biol. 2014, 772, 205–227. [Google Scholar] [CrossRef]
- Ivan, M.; Harris, A.L.; Martelli, F.; Kulshreshtha, R. Hypoxia response and microRNAs: No longer two separate worlds. J. Cell Mol. Med. 2008, 12, 1426–1431. [Google Scholar] [CrossRef]
- Kuijper, S.; Turner, C.J.; Adams, R.H. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc. Med. 2007, 17, 145–151. [Google Scholar] [CrossRef]
- Pandey, A.; Shao, H.; Marks, R.M.; Polverini, P.J.; Dixit, V.M. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science 1995, 268, 567–569. [Google Scholar] [CrossRef]
- Matsui, J.; Wakabayashi, T.; Asada, M.; Yoshimatsu, K. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J. Biol. Chem. 2004, 279, 18600–18607. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kleinheinz, J. Angiogenesis—The Key to Regeneration. In Tissue Engineering and Regenerative Medicine; Andrades, J.A., Ed.; InTechOpen: London, UK, 2013; pp. 453–473. [Google Scholar]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X.; Tang, T.; Dai, K.; Ge, R. Enhancement of bone formation by genetically-engineered bone marrow stromal cells expressing BMP-2, VEGF and angiopoietin-1. Biotechnol. Lett. 2009, 31, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiu, T.; Wu, X.; Wan, C.; Shi, W.; Wang, Y.; Chen, J.; Wan, M.; Clemens, T.L.; Cao, X. Sustained BMP Signaling in Osteoblasts Stimulates Bone Formation by Promoting Angiogenesis and Osteoblast Differentiation. J. Bone Miner. Res. 2009, 24, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, K. Effects of recombinant adeno-associated viral vectors on angiopoiesis and osteogenesis in cultured rabbit bone marrow stem cells via co-expressing hVEGF and hBMP genes: A preliminary study in vitro. Tissue Cell 2010, 42, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Hon, L.S.; Zhang, Z. The roles of binding site arrangement and combinatorial targeting in microRNA repression of gene expression. Genome Biol. 2007, 8, R166. [Google Scholar] [CrossRef]
- Grimson, A.; Farh, K.K.; Johnston, W.K.; Garrett-Engele, P.; Lim, L.P.; Bartel, D.P. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol. Cell 2007, 27, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef]
- Brennecke, J.; Stark, A.; Russell, R.; Cohen, S. Principles of microRNA-target recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef]
- Tsang, J.; Zhu, J.; van Oudenaarden, A. MicroRNAmediated feedback and feedforward loops are recurrent network motifs in mammals. Mol. Cell 2007, 26, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.; Burge, C.; Bartel, D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, H.; Liu, W.; Hu, R.; Huang, B.; Tan, Y.; Xu, K.; Sheng, Z.; Zhou, H.; Wu, X.; et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J. Clin. Invest. 2009, 119, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Mariner, P.; Johannesen, E.; Anseth, K. Manipulation of miRNA activity accelerates osteogenic differentiation of hMSCs in engineered 3D scaffolds. J. Tissue Eng. Regen Med. 2012, 6, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Li, N.; Lin, S.; Wang, B.; Lan, H.Y.; Li, G. miRNA-29b improves bone healing in mouse fracture model. Mol. Cell. Endocrinol. 2016, 430, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Liu, S.; Liu, W.; Zhang, H.; Zhou, T.; Wu, D.; Yang, P.; Shen, L.; Chen, J.; et al. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials 2013, 34, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, M.; Nakasa, T.; Kawanishi, Y.; Hachisuka, S.; Furuta, T.; Miyaki, S.; Adachi, N.; Ochi, M. Inhibition of microRNA-222 expression accelerates bone healing with enhancement of osteogenesis, chondrogenesis, and angiogenesis in a rat refractory fracture model. J. Orthop. Sci. 2016, 21, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Ørom, U.A.; Kauppinen, S.; Lund, A.H. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene 2006, 372, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Parsons, C.; Walker, L.; Zhang, W.C.; Slack, F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017, 127, 761–771. [Google Scholar] [CrossRef]
- Simonson, B.; Das, S. MicroRNA Therapeutics: The Next Magic Bullet? Mini Rev. Med. Chem. 2016, 15, 467–474. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs-microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- López-Camarillo, C.; Marchat, L.A.; Aréchaga-Ocampo, E.; Azuara-Liceaga, E.; Pérez-Plasencia, C.; Fuentes-Mera, L.; Fonseca-Sánchez, M.A.; Flores-Pérez, A. Functional Roles of microRNAs in Cancer: microRNomes and oncomiRs Connection; Oncogenomi.; In Tech Open Science: London, UK, 2013. [Google Scholar]
- Senanayake, U.; Das, S.; Vesely, P.; Alzoughbi, W.; Fröhlich, L.F.; Chowdhury, P.; Leuschner, I.; Hoefler, G.; Guertl, B. miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated and their common target ACVR2B is strongly expressed in renal childhood neoplasms. Carcinogenesis 2012, 33, 1014–1021. [Google Scholar] [CrossRef] [PubMed]

| MicroRNAs | Targets 1 | Regulatory Role | Effects | Study Models | Ref. |
|---|---|---|---|---|---|
| MiR-9 | VEGF, VE-CAD (CD144) | AMPK signaling pathway | Enhanced osteogenic diff. & mineral.; increased angiogenesis | MC3T3-E1 | [106] |
| DKK1 | COL1, OCN, BSP; ALP activity | OB diff. & mineralization | C2C12 cells | [107] | |
| SOCS5 | JAK-STAT signaling pathway | Promotion of EC migration & angiogenesis | Primary microvascular ECs, HUVECs | [108] | |
| Cbl | Bim ubiquitination, apoptosis | Promotion of OC survival | OC, OC precursor cells (RAW264.7) | [110] | |
| MiR-10a | β-catenin, LEF1; VEGF, VE-CAD (CD144), cyclin D1, MMP2 | Wnt signaling; angiogenesis-related gene expression | Inhibition of osteogenic diff. & blood vessel formation | MC3T3-E1 MUVECS | [111] |
| HMGA2 | β–galactosidase expr; p16Ink4a/p19Arf expression | EPC senescence & angiogenesis; self-renewal potential | lin−BM-MSCs | [113] | |
| MiR-10a/10b | MIB1 | Notch signaling | Regulating blood vessel outgrowth/tip cell behavior | HUVECs | [115] |
| MiR-20a | BMP2, BMP4, RUNX2 | Effects BMP/RUNX2 signaling positively; blocks OB inhibitors & PPARγ | Enhances osteogenic differentiation; suppresses adipogenesis | hBM-MSC | [116] |
| JAK1; p21, S1P receptor EDG | Downregulation of proangiogenic JAK 1 & cell cycle inhibitors | Inhibits EC sprout formation | HUVECs | [117] | |
| TNFSF15 | VEGF-AKT/ERK –miR20a/31 signaling | Stimulation of angiogenesis | HUVECs | [118] | |
| MiR-26a | VEGF, ANG1, RUNX2, BMP2 OCN, ALP; GSK3β | WNT signaling activation | Enhanced angiogenesis & bone regeneration | Primary hBM-MSC, MC3T3-E1 | [76,98] |
| VEGF | PIK3C2α/AKT/HIF-α/VEGFA pathway | Inhibition of angiogenesis; | HUVECs | [141] | |
| SMAD1 | BMP signaling inhibition | OB differentiation | hADSCs | [119] | |
| SMAD1 | BMP signaling | Inhibits EC growth, proliferation, migration; regulates early angiogenesis | HUVECs | [123] | |
| MiR-29b | TGF-β3, HDAC4, ACTVR2A, CTNNBIP1, DUSP2; COL1A1, 5A3, 4A2 | Silences neg. osteogenic regulators suppresses ECM protein synthesis | Promotes osteoblastogenesis at multiple stages | MC3T3 pre-OB | [124] |
| c-FOS | Reduced TRAP expr., lacunae generation, collagen degradation | Neg. regulator of human OC differentiation and activity | OC (CD14 +) | [125] | |
| TNFSF15 | TNFSF15-enhanced JNK-GATA3 signal. & VEGF inhibition | Suppression of VEGF secretion | Mouse EC line bEnd.3 | [126] | |
| AKT3 | Inhibition of tumor vascularization via VEGF & cancer cell activity via c-MYC | Anti-angiogenic and anti-tumorigenic role | HUVECs, Breast cancer cells | [127] | |
| MiR-31 | OSX | Downregulation of OSX | Influences osteogenic differentiation | hMSC; Osteosarcoma cell | [129] |
| Satb2 protein | Inhibition by RUNX2; Upregulation of Satb2 protein & osteogenic TF | Induces BM-MSC osteogenic differentiation | hBM-MSC | [130] | |
| E-selectin | Regulation of E-selectin expression | Inhibition of angiostatin-induced angiogenesis; TNF-mediated induction of endothelial adhesion | HUVECs | [84] | |
| TNFSF15 | VEGF-AKT/ERK –miR20a/31 signaling | Stimulation of angiogenesis | HUVECs | [118] | |
| MiR-34a | Jagged1 | Regulation of cell cycle regulator & proliferation proteins & Jagged1 | Inhibition of osteoblast differentiation | hMSC; mouse heterotopic bone formation model | [132] |
| JAGGED1 | Activation of Notch signaling | Induction of glucocorticoid-mediated osteogenic differentiation | hMSC | [133] | |
| RBP2 | Promotes mineral, ALP activity & RUNX2 expression; downreg. NOTCH1 & Cyclin D1 expr. | Promotion of osteogenic differentiation; enhanced heterotopic bone formation | hADSCs; mouse heterotopic bone formation model | [134] | |
| VEGF | Inhibitory effects of dexamethasone on EC viability & VEGF | Decreased blood vessel development | Rat Glucocorticoid- induced osteonecrosis | [137] | |
| SIRT1 | Increased SIRT1 expr. & FOXO1 acetylation regulating vascular EC homeostasis | Inhibition of EPC-mediated angiogenesis | Rat EPC | [138] | |
| E2F3a, survivin | Interference with VEGF secretion, EC proliferation & migration | Dysregulated tumor angiogenesis | HNSCC tumors & cells | [140] | |
| MiR-92a | ? | ? | Enhanced fracture healing & inhib. of neovascularization | Mice with femoral fracture | [142] |
| HGF, ANGPT1 | ITGA5, MEK4 | Inhibition of tube formation by HUVECs | hADSCs | [143] | |
| ? | integrin a5, sirtuin1, eNOS | Attenuates neointimal lesion by accelerating re-endothelialization | MiR-92a knockout mice | [144] | |
| MiR-125b | OSX | RUNX2, a-SMC, ALP, matrix mineralization | Calcification of vascular smooth muscle cells | HCASMCs | [145] |
| ErbB2 | ? | Inhibits OB diff by downreg. of cell proliferation | ST2 cells (mMSCs) | [146] | |
| VEGF, ERBB2 | Regulation of angiogenesis during wound healing | HUVECS | [147] | ||
| Cbf-beta | ALP, OCN, OPN | Inhibition of osteogenic differentiation | C3H10T1/2 | [148] | |
| SMAD4 | ALP, RUNX2 | Downregulation of osteogenic differentiation | hMSCs | [149] | |
| VE-Cadherin | Inhibition of blood vessel (tube) formation | HUVECs | [150] | ||
| MiR-135b | ? | ? | OB differentiation | hBM-SCs | [151] |
| HIF-1 | ? | Enhanced endothelial tube formation | Human MM cells; HUVECs | [152] | |
| SMAD5 | ? | Impaired osteogenic differentiation | hMSCs | [153] | |
| MiR-181a | ? | CCN1, aggrecan | Maintaining homeostasis of chondrocytes | Human HCS-2/8 cells | [154] |
| COL10A1 | Chondrocyte differentiation | hMSC | [155] | ||
| RGS16 | CXCR4 signaling; VEGF, MMP1 | Angiogenesis & metastasis in chondrosarcoma | Xenograft mice; JJ chondrosarc. cells | [156] | |
| ? | VEGF expression | Chondrosarcoma-associated angiogenesis | JJ chondrosarc. cell line | [157] | |
| Cbl | Bim ubiquitination, apoptosis | promote OC survival | OC, OC precursor cells (RAW264.7) | [110] | |
| MiR-195 | ? | VEGF | Osteogenic diff. & proliferation; control of angiogenesis | hMSC(MC3T3) chick chorio-allantoic membrane | [158] |
| ? | VEGF, VAV2 CDC42 | HCC-associated angiogenesis & metastasis; migration & capillary tube form. of ECs | QGY-7703, MHCC-97H HCC cells; HUVECs | [159] | |
| MiR-200b | ZEB1 | ZEB1-TF target genes | Inhibits proliferation, migration & invasion of osteosarcoma cells | OsteosarcomaU2OS, Saos2, HOS, MG63 | [160] |
| VEGF-A; ZEB2, ETS1, KDR,GATA2 | Decreases VEGF-A expression & TF-target genes | Inhibition of VEGF-A induced osteogenesis; Inhibition of TF-activated angiogenesis | Rat BM-MSC & HUVEC coculture | [161] | |
| VEGF, FLT-1, and KDR | VEGF-induced phosph. of ERK1/2 | Inhibition of angiogenesis; red. capillary formation | A549 cells, HUVECs | [162] | |
| MiR-210 | AcvR1b | Inhib. of TGFb/activin signaling | Promotes OB differentiation | ST2 stromal cells | [163] |
| VEGF | PPARgamma, ALP, OSX | Promoteion of OB diff., inhibition of adipocyte diff. | hBM-SCs, 17β-estradiol (E2)treated OB | [164] | |
| EFNA3 | VEGF-expression mediated angiogenesis | EC survival, diff., migration; stim. of tubulogen. & chemotaxis | HUVECs | [165] | |
| MiR-222 | SMAD 1, 5, 8 protein & phosphoryl. | Decreased SMAD5-RUNX2 signaling & OSX, ALP, and OC levels & mineral. | Neg. regulator of osteogenic differentiation | hBM-SC | [166] |
| c-Src, Dcstamp | RANKL-induced expression of TRAP & cathepsin K | Inhibitory regulator of c-Src-mediated osteoclastogenesis | RAW264.7 pre-OC cells | [167] | |
| c-KIT | Suppression of tube formation, wound healing, cell migration via SCF | Inhibitory regulation of in vitro angiogenesis | HUVEC | [168] | |
| MiR-424 | RUNX, CBFβ, BMP | Osteogenic diff. of hMSCs | Bone formation | hMSCs | [169] |
| MAPK, WNT & insulin signal. | OB differentiation of hMSCs | Bone formation | hMSCs | [170] | |
| FGF-2; via FOXO1 | Decrease of ALP, mineralization & osteog. markers | Enhances proliferation & osteogenic differentiation of hMSCs | Pigs, cellular oxidative stress model | [171] | |
| CUL2; via RUNX-1→ C/EBPα→ PU.1 | Stabilization of HIF-1α | Regulation of Angiogenesis | ECs, ischemic tissues | [101] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, L.F. MicroRNAs at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration. Cells 2019, 8, 121. https://doi.org/10.3390/cells8020121
Fröhlich LF. MicroRNAs at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration. Cells. 2019; 8(2):121. https://doi.org/10.3390/cells8020121
Chicago/Turabian StyleFröhlich, Leopold F. 2019. "MicroRNAs at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration" Cells 8, no. 2: 121. https://doi.org/10.3390/cells8020121
APA StyleFröhlich, L. F. (2019). MicroRNAs at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration. Cells, 8(2), 121. https://doi.org/10.3390/cells8020121