Host Vesicle Fusion Protein VAPB Contributes to the Nuclear Egress Stage of Herpes Simplex Virus Type-1 (HSV-1) Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Preparation of Fractions
2.3. Mass Spectrometry
2.4. Bioinformatics
2.5. siRNA Knockdown of VFPs
2.6. Rescue Experiments
2.7. Isolation of Nuclei and DNA Preparation for qPCR
2.8. qPCR
2.9. Western Blotting
2.10. Fluorescence In Situ Hybridization (FISH)
2.11. Fluorescence Microscopy
2.12. Electron Microscopy
2.13. Data Availability
3. Results
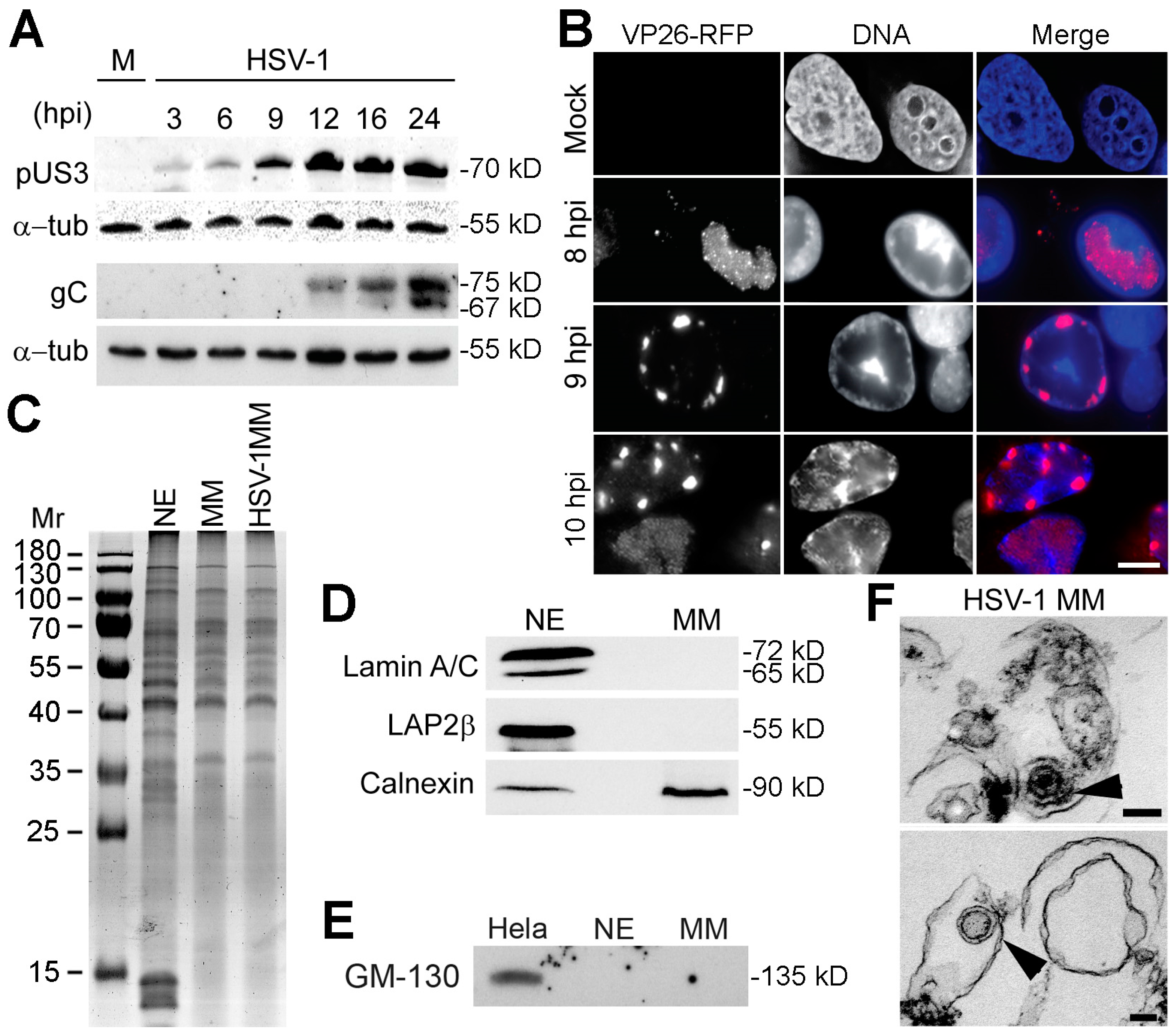
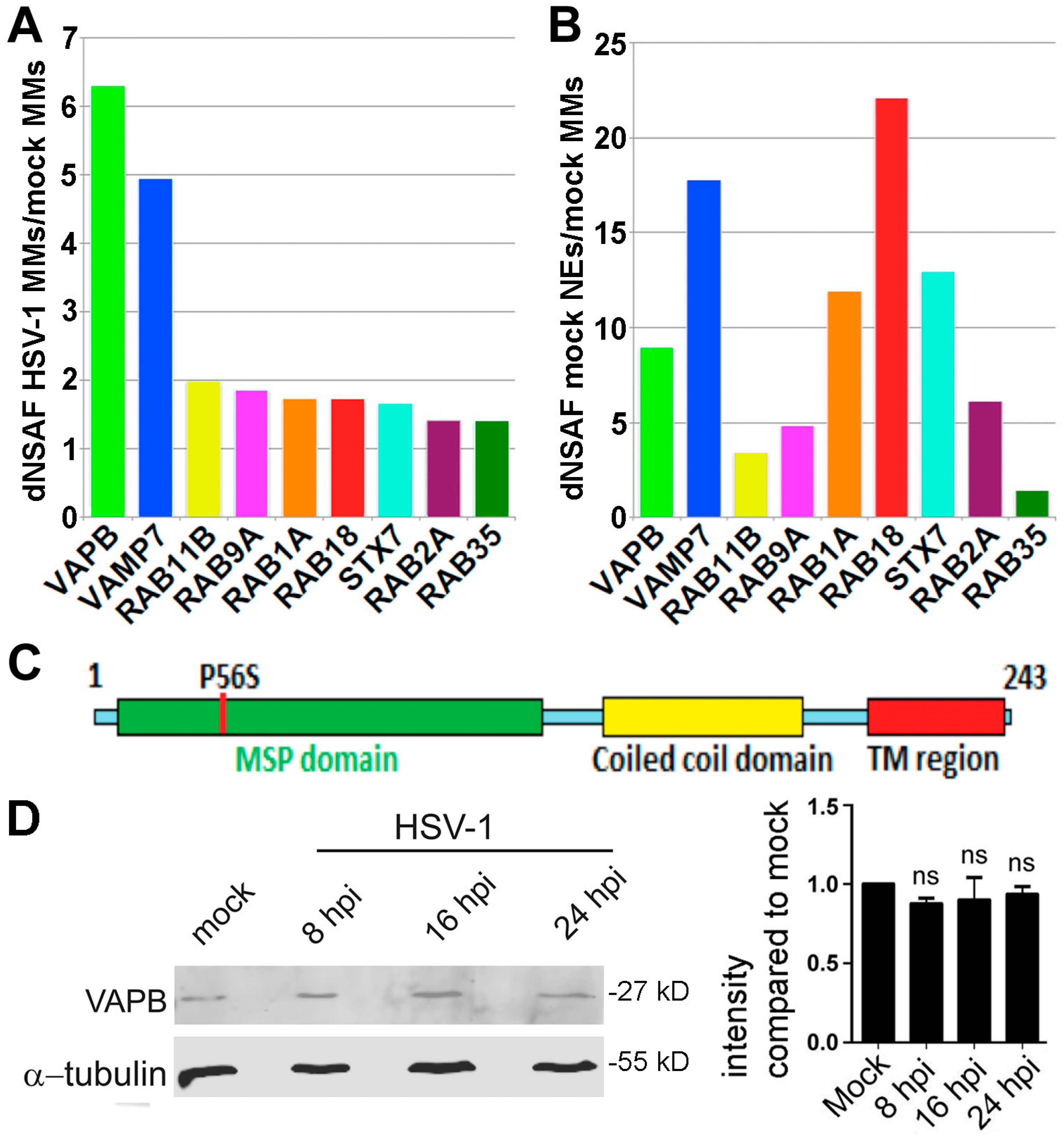
3.1. Identification of Cellular Proteins Potentially Involved in Viral Egress
3.2. Host and Viral Proteins Identified in HSV-1 Infected MMs
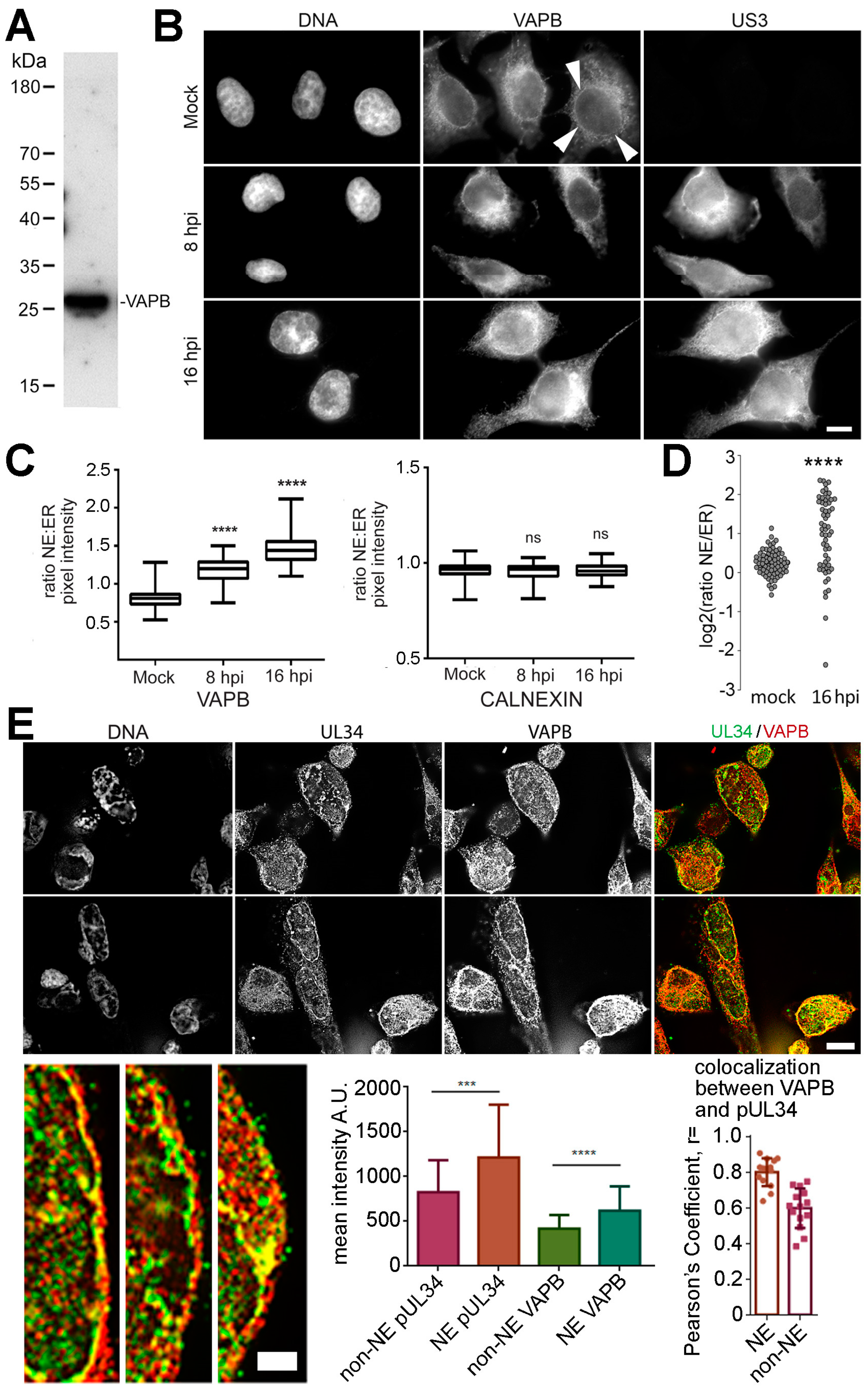
3.3. VAPB Expression and Localization During HSV-1 Infection
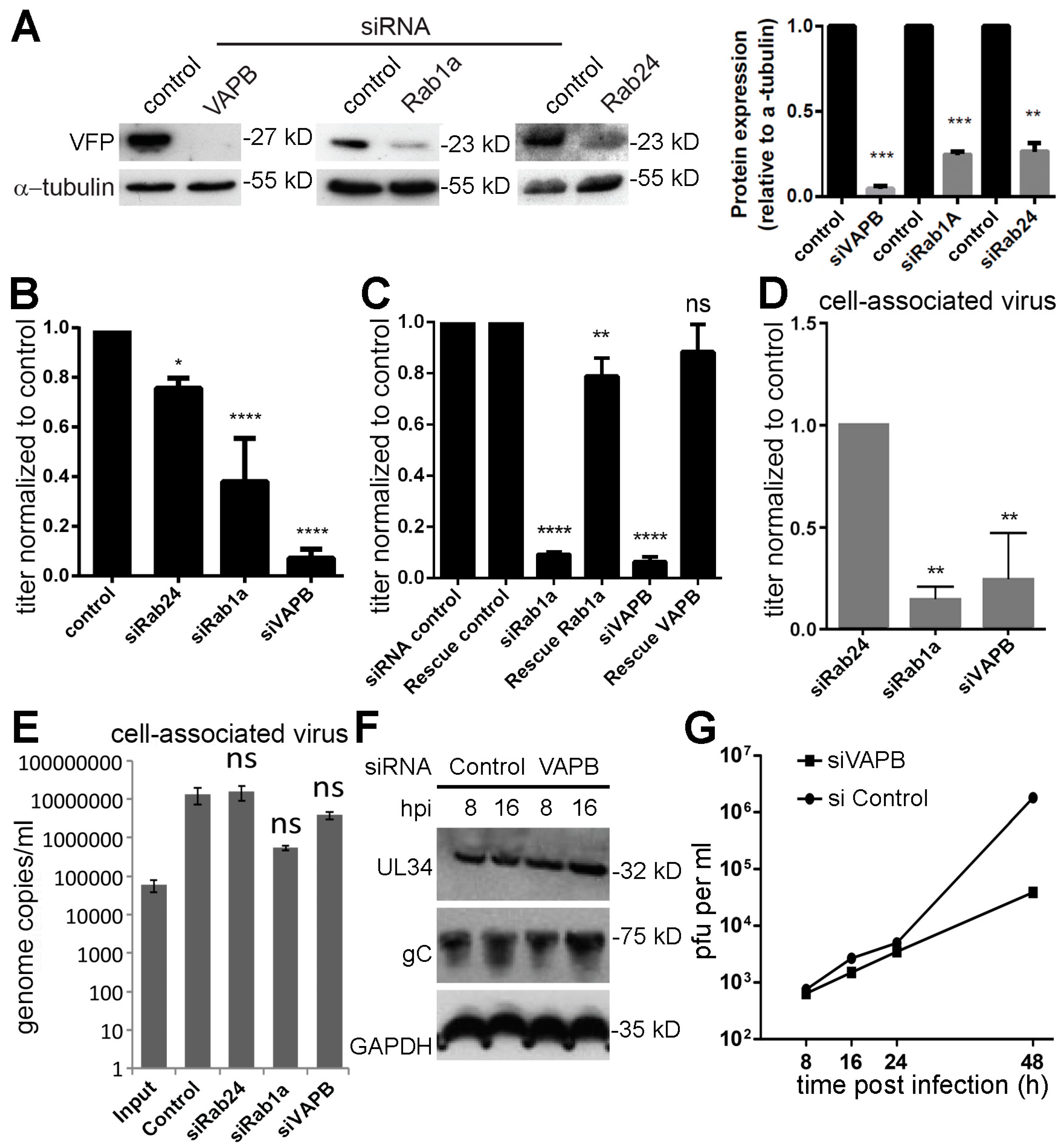
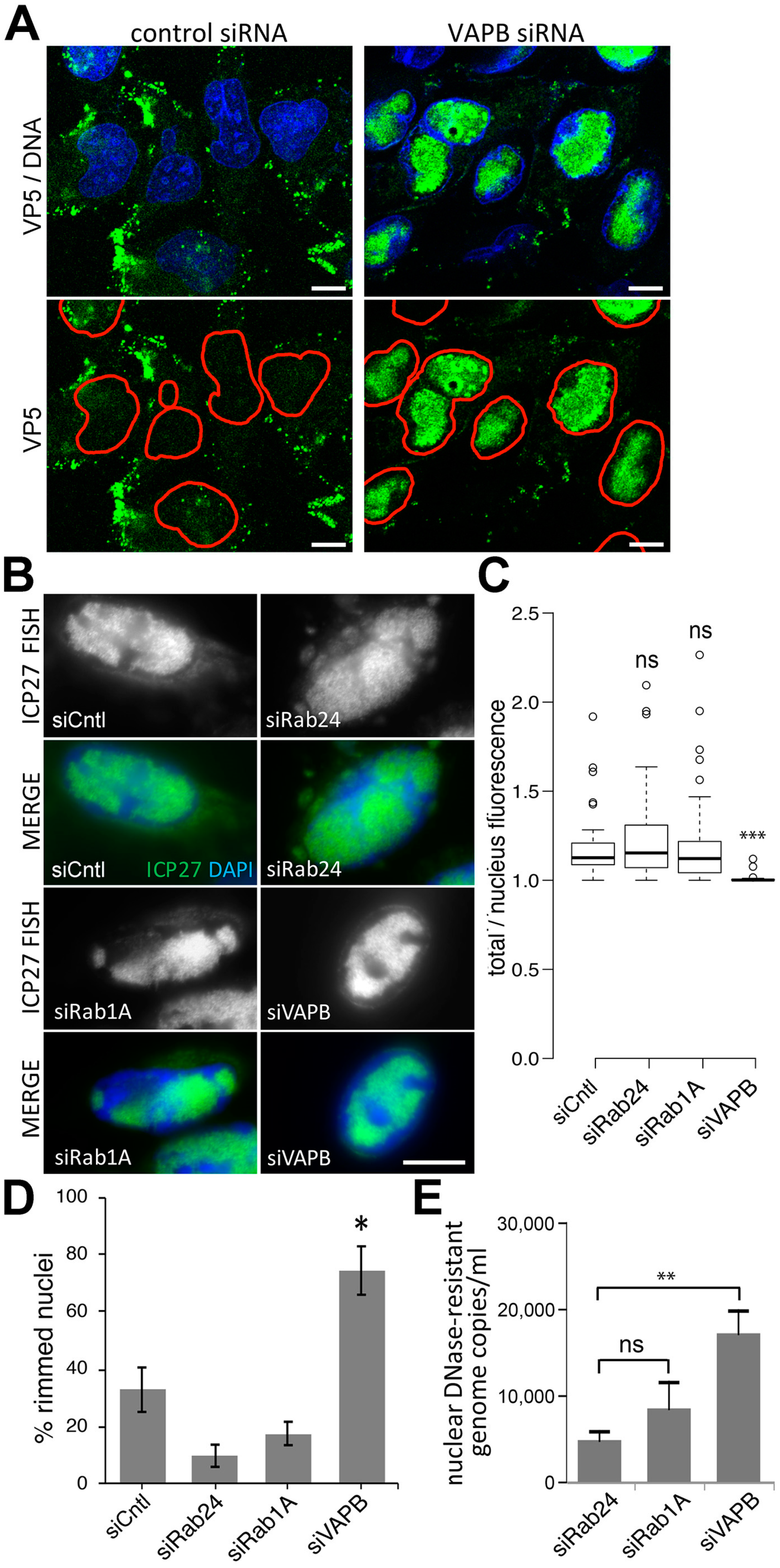
3.4. Knockdown of VAPB Yields Significant Reduction of HSV-1 Viral Titers
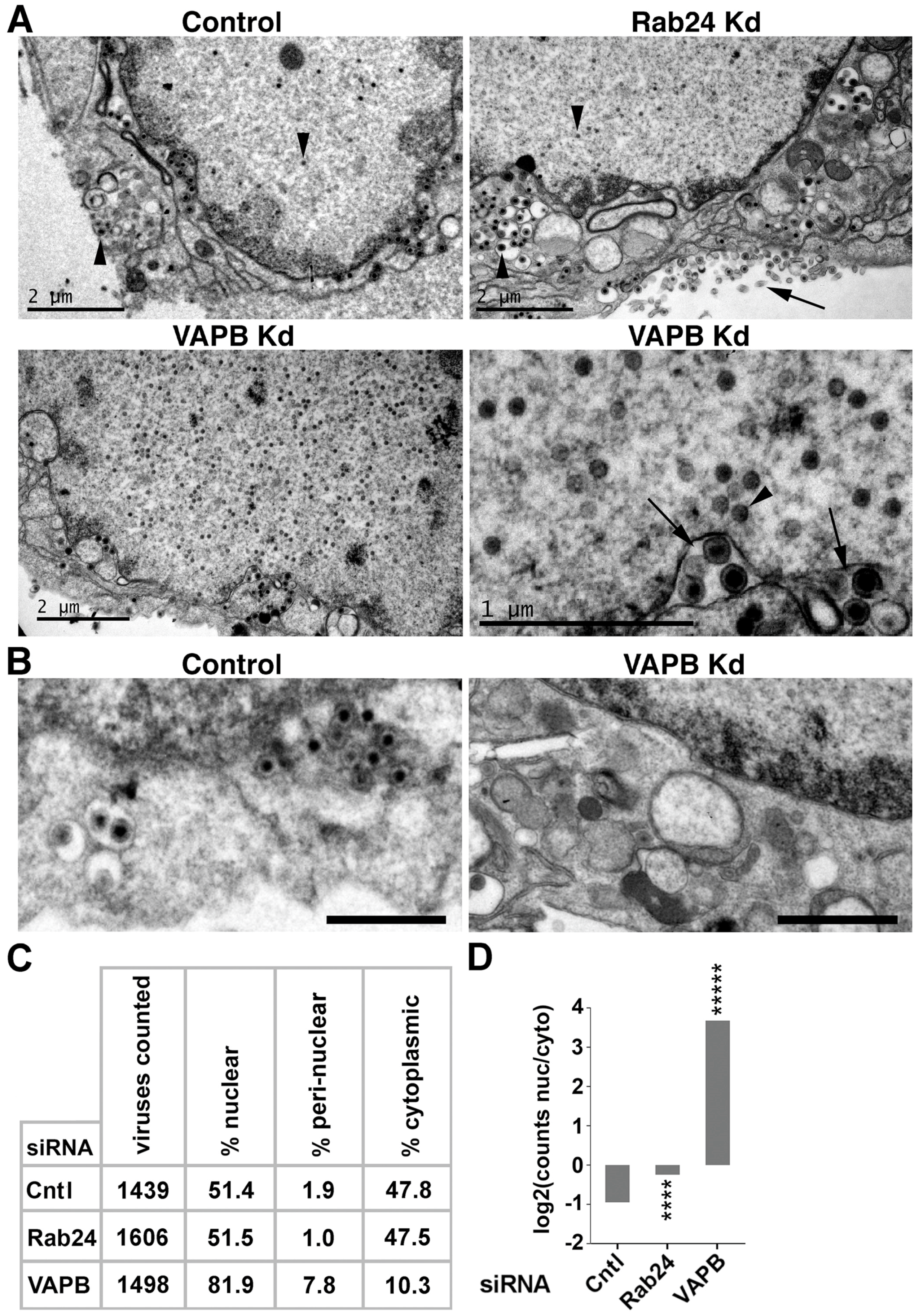
3.5. VAPB Knockdown Yields Reduced Cytoplasmic Virus Particles and Nuclear Particle Accumulation
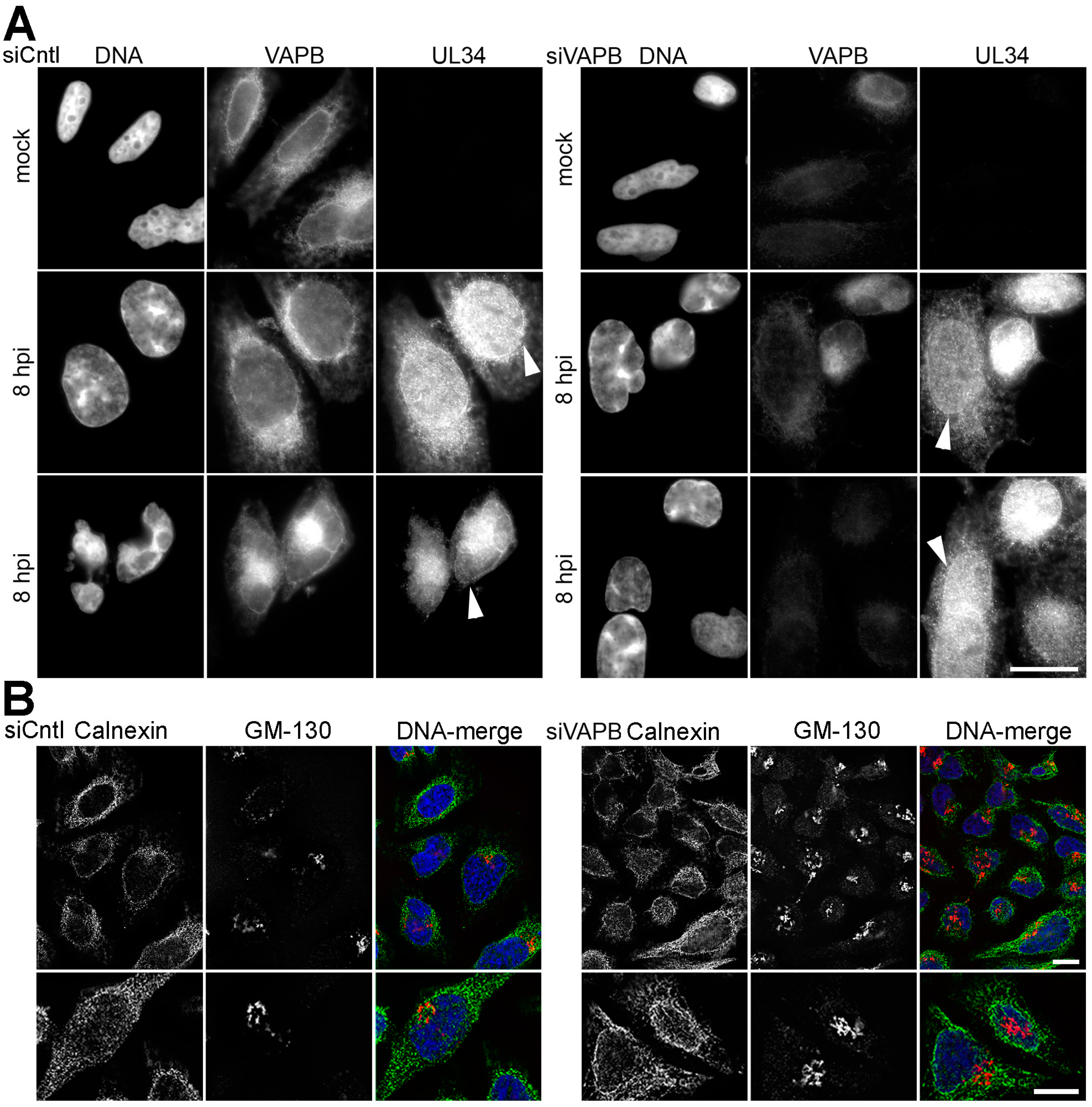
3.6. VAPB Knockdown Does Not Interfere with Virus Protein Accumulation at the NE
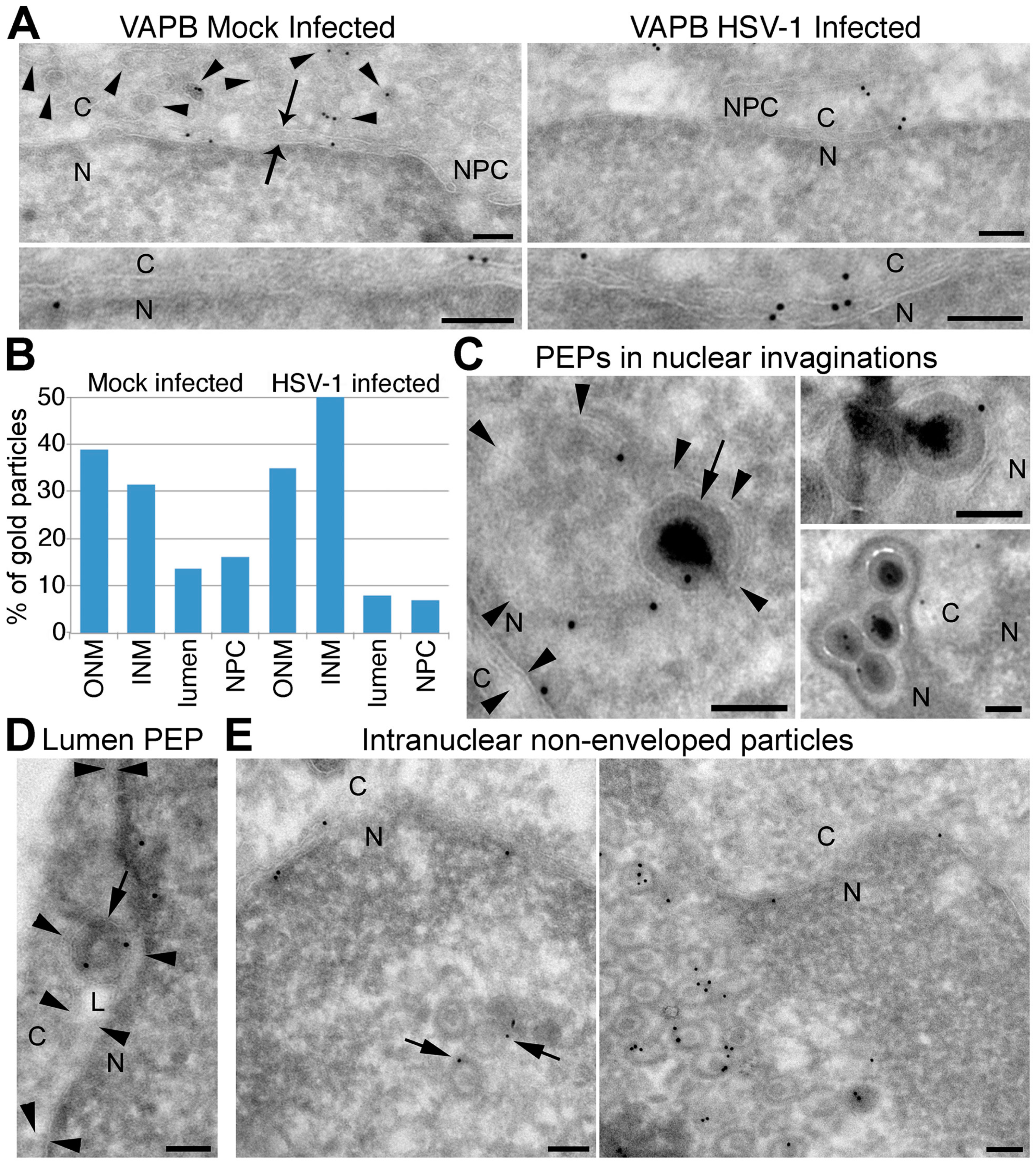
3.7. VAPB Is Present on the NE and in Association with Viral Particles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mettenleiter, T.C.; Klupp, B.G.; Granzow, H. Herpesvirus assembly: An update. Virus Res. 2009, 143, 222–234. [Google Scholar] [CrossRef]
- Johnson, D.C.; Baines, J.D. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 2011, 9, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Granzow, H.; Klupp, B.G.; Fuchs, W.; Veits, J.; Osterrieder, N.; Mettenleiter, T.C. Egress of alphaherpesviruses: Comparative ultrastructural study. J. Virol. 2001, 75, 3675–3684. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C.; Muller, F.; Granzow, H.; Klupp, B.G. The way out: What we know and do not know about herpesvirus nuclear egress. Cell. Microbiol. 2013, 15, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Skepper, J.N.; Whiteley, A.; Browne, H.; Minson, A. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 2001, 75, 5697–5702. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, S.L.; Roller, R.J. Roles for herpes simplex virus type 1 ul34 and us3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 2006, 347, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Auerochs, S.; Marschall, M. Cytomegaloviral proteins pul50 and pul53 are associated with the nuclear lamina and interact with cellular protein kinase c. J. Gen. Virol. 2007, 88, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Auerochs, S.; Sticht, H.; Marschall, M. Cytomegaloviral proteins that associate with the nuclear lamina: Components of a postulated nuclear egress complex. J. Gen. Virol. 2009, 90, 579–590. [Google Scholar] [CrossRef]
- Muranyi, W.; Haas, J.; Wagner, M.; Krohne, G.; Koszinowski, U.H. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 2002, 297, 854–857. [Google Scholar] [CrossRef]
- Bigalke, J.M.; Heuser, T.; Nicastro, D.; Heldwein, E.E. Membrane deformation and scission by the hsv-1 nuclear egress complex. Nat. Commun. 2014, 5, 4131. [Google Scholar] [CrossRef]
- Hagen, C.; Dent, K.C.; Zeev-Ben-Mordehai, T.; Grange, M.; Bosse, J.B.; Whittle, C.; Klupp, B.G.; Siebert, C.A.; Vasishtan, D.; Bauerlein, F.J.; et al. Structural basis of vesicle formation at the inner nuclear membrane. Cell 2015, 163, 1692–1701. [Google Scholar] [CrossRef]
- Klupp, B.G.; Granzow, H.; Mettenleiter, T.C. Effect of the pseudorabies virus us3 protein on nuclear membrane localization of the ul34 protein and virus egress from the nucleus. J. Gen. Virol. 2001, 82, 2363–2371. [Google Scholar] [CrossRef]
- Reynolds, A.E.; Wills, E.G.; Roller, R.J.; Ryckman, B.J.; Baines, J.D. Ultrastructural localization of the herpes simplex virus type 1 ul31, ul34, and us3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 2002, 76, 8939–8952. [Google Scholar] [CrossRef]
- Wagenaar, F.; Pol, J.M.; Peeters, B.; Gielkens, A.L.; de Wind, N.; Kimman, T.G. The us3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 1995, 76 Pt 7, 1851–1859. [Google Scholar] [CrossRef]
- Farnsworth, A.; Wisner, T.W.; Webb, M.; Roller, R.; Cohen, G.; Eisenberg, R.; Johnson, D.C. Herpes simplex virus glycoproteins gb and gh function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. USA 2007, 104, 10187–10192. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.C.; Wisner, T.W.; Hannah, B.P.; Eisenberg, R.J.; Cohen, G.H.; Johnson, D.C. Fusion between perinuclear virions and the outer nuclear membrane requires the fusogenic activity of herpes simplex virus gb. J. Virol. 2009, 83, 11847–11856. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Kumar, A.; Zheng, M.; Atherton, S.S.; Yu, F.S. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and tlr7 in human corneal epithelial cells. Immunology 2006, 117, 167–176. [Google Scholar] [CrossRef]
- Ni, L.; Wang, S.; Zheng, C. The nucleolus and herpesviral usurpation. J. Med. Microbiol. 2012, 61, 1637–1643. [Google Scholar] [CrossRef]
- Jahn, R.; Lang, T.; Sudhof, T.C. Membrane fusion. Cell 2003, 112, 519–533. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Klupp, B.G.; Granzow, H.; Fuchs, W.; Keil, G.M.; Finke, S.; Mettenleiter, T.C. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 7241–7246. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, Y.; Arii, J.; Liu, Z.; Shindo, K.; Oyama, M.; Kozuka-Hata, H.; Sagara, H.; Kato, A.; Kawaguchi, Y. Herpes simplex virus 1 recruits cd98 heavy chain and beta1 integrin to the nuclear membrane for viral de-envelopment. J. Virol. 2015, 89, 7799–7812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Wu, S.; Pan, S.; Zhou, C.; Ma, Y.; Ru, Y.; Dong, S.; He, B.; Zhang, C.; et al. P32 is a novel target for viral protein icp34.5 of herpes simplex virus type 1 and facilitates viral nuclear egress. J. Biol. Chem. 2014, 289, 35795–35805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kato, A.; Oyama, M.; Kozuka-Hata, H.; Arii, J.; Kawaguchi, Y. Role of host cell p32 in herpes simplex virus 1 de-envelopment during viral nuclear egress. J. Virol. 2015, 89, 8982–8998. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Schirmer, E.C.; Nishimoto, T.; Gerace, L. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J. Cell Biol. 2004, 167, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Soullam, B.; Worman, H.J. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J. Cell Biol. 1995, 130, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ungricht, R.; Klann, M.; Horvath, P.; Kutay, U. Diffusion and retention are major determinants of protein targeting to the inner nuclear membrane. J. Cell Biol. 2015, 209, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Zuleger, N.; Kelly, D.A.; Richardson, A.C.; Kerr, A.R.; Goldberg, M.W.; Goryachev, A.B.; Schirmer, E.C. System analysis shows distinct mechanisms and common principles of nuclear envelope protein dynamics. J. Cell Biol. 2011, 193, 109–123. [Google Scholar] [CrossRef]
- Korfali, N.; Wilkie, G.S.; Swanson, S.K.; Srsen, V.; Batrakou, D.G.; Fairley, E.A.; Malik, P.; Zuleger, N.; Goncharevich, A.; de Las Heras, J.; et al. The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol. Cell. Proteom. MCP 2010, 9, 2571–2585. [Google Scholar] [CrossRef]
- Florens, L.; Washburn, M.P. Proteomic analysis by multidimensional protein identification technology. Methods Mol. Biol. 2006, 328, 159–175. [Google Scholar]
- Washburn, M.P.; Wolters, D.; Yates, J.R.r. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 2001, 19, 242–247. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.H.; Tabb, D.L.; Sadygov, R.G.; MacCoss, M.J.; Venable, J.; Graumann, J.; Johnson, J.R.; Cociorva, D.; Yates, J.R., 3rd. Ms1, ms2, and sqt-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass. Spectrom. 2004, 18, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, Z.; Washburn, M.P.; Florens, L. Improving proteomics mass accuracy by dynamic offline lock mass. Anal. Chem. 2011, 83, 9344–9351. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.; McCormack, A.; Yates, J.r. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Tabb, D.L.; McDonald, W.H.; Yates, J.R.r. Dtaselect and contrast: Tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 2002, 1, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S. Amigo: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Chalhoub, A.; Schooley, A.; Zhang, W.; Ngsee, J.K. A mutation in vapb that causes amyotrophic lateral sclerosis also causes a nuclear envelope defect. J. Cell Sci. 2012, 125, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.; Tatham, M.H.; Groslambert, M.; Glass, M.; Orr, A.; Hay, R.T.; Everett, R.D. Analysis of the sumo2 proteome during hsv-1 infection. PLoS Pathog. 2015, 11, e1005059. [Google Scholar] [CrossRef]
- Weidmann, M.; Meyer-Konig, U.; Hufert, F.T. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time pcr. J. Clin. Microbiol. 2003, 41, 1565–1568. [Google Scholar] [CrossRef]
- Tokuyasu, K.T. A technique for ultracryotomy of cell suspensions and tissues. J. Cell Biol. 1973, 57, 551–565. [Google Scholar] [CrossRef]
- Padula, M.E.; Sydnor, M.L.; Wilson, D.W. Isolation and preliminary characterization of herpes simplex virus 1 primary enveloped virions from the perinuclear space. J. Virol. 2009, 83, 4757–4765. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.L.; Norrild, B. Herpes simplex virus type 1-infected human embryonic lung cells studied by optimized immunogold cryosection electron microscopy. J. Histochem. Cytochem. 1998, 46, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, M.R.; Di Lazzaro, C.; Pavan, A.; Pereira, L.; Campadelli-Fiume, G. Herpes simplex virus envelopment and maturation studied by fracture label. J. Virol. 1992, 66, 554–561. [Google Scholar] [PubMed]
- Zhang, Y.; Wen, Z.; Washburn, M.P.; Florens, L. Refinements to label free proteome quantitation: How to deal with peptides shared by multiple proteins. Anal. Chem. 2010, 82, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Ben Halevy, D.; Peretti, D.; Dahan, N. The vap protein family: From cellular functions to motor neuron disease. Trends Cell Biol. 2008, 18, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Gkogkas, C.; Wardrope, C.; Hannah, M.; Skehel, P. The als8-associated mutant vapb(p56s) is resistant to proteolysis in neurons. J. Neurochem. 2011, 117, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.; Roy, A.; Levine, T.P. A conserved er targeting motif in three families of lipid binding proteins and in opi1p binds vap. EMBO J. 2003, 22, 2025–2035. [Google Scholar] [CrossRef]
- Kim, S.; Leal, S.S.; Ben Halevy, D.; Gomes, C.M.; Lev, S. Structural requirements for vap-b oligomerization and their implication in amyotrophic lateral sclerosis-associated vap-b(p56s) neurotoxicity. J. Biol. Chem. 2010, 285, 13839–13849. [Google Scholar] [CrossRef]
- De Vos, K.J.; Morotz, G.M.; Stoica, R.; Tudor, E.L.; Lau, K.F.; Ackerley, S.; Warley, A.; Shaw, C.E.; Miller, C.C. Vapb interacts with the mitochondrial protein ptpip51 to regulate calcium homeostasis. Hum. Mol. Genet. 2012, 21, 1299–1311. [Google Scholar] [CrossRef]
- Kanekura, K.; Nishimoto, I.; Aiso, S.; Matsuoka, M. Characterization of amyotrophic lateral sclerosis-linked p56s mutation of vesicle-associated membrane protein-associated protein b (vapb/als8). J. Biol. Chem. 2006, 281, 30223–30233. [Google Scholar] [CrossRef]
- Nishimura, Y.; Hayashi, M.; Inada, H.; Tanaka, T. Molecular cloning and characterization of mammalian homologues of vesicle-associated membrane protein-associated (vamp-associated) proteins. Biochem. Biophys. Res. Commun. 1999, 254, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Stoica, R.; De Vos, K.J.; Paillusson, S.; Mueller, S.; Sancho, R.M.; Lau, K.F.; Vizcay-Barrena, G.; Lin, W.L.; Xu, Y.F.; Lewis, J.; et al. Er-mitochondria associations are regulated by the vapb-ptpip51 interaction and are disrupted by als/ftd-associated tdp-43. Nat. Commun. 2014, 5, 3996. [Google Scholar] [CrossRef] [PubMed]
- Soo, K.Y.; Halloran, M.; Sundaramoorthy, V.; Parakh, S.; Toth, R.P.; Southam, K.A.; McLean, C.A.; Lock, P.; King, A.; Farg, M.A.; et al. Rab1-dependent er-golgi transport dysfunction is a common pathogenic mechanism in sod1, tdp-43 and fus-associated als. Acta Neuropathol. 2015, 130, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Zenner, H.L.; Yoshimura, S.; Barr, F.A.; Crump, C.M. Analysis of rab gtpase-activating proteins indicates that rab1a/b and rab43 are important for herpes simplex virus 1 secondary envelopment. J. Virol. 2011, 85, 8012–8021. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, M.; Johns, H.L.; Sayers, C.L.; Gonzalez-Lopez, C.; Smith, G.L.; Elliott, G. Endocytic tubules regulated by rab gtpases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J. 2012, 31, 4204–4220. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Addison, C.; Cross, A.M.; Kennard, J.; Preston, V.G.; Rixon, F.J. Localization of the herpes simplex virus type 1 major capsid protein vp5 to the cell nucleus requires the abundant scaffolding protein vp22a. J. Gen. Virol. 1994, 75 Pt 5, 1091–1099. [Google Scholar] [CrossRef]
- Park, R.; Baines, J.D. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase c to the nuclear membrane and increased phosphorylation of lamin b. J. Virol. 2006, 80, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Matthews, D.A.; Wadd, S.; Scott, J.E.; Kean, J.; Graham, S.; Russell, W.C.; Clements, J.B. Interaction between herpes simplex virus type 1 ie63 protein and cellular protein p32. J. Virol. 2000, 74, 11322–11328. [Google Scholar] [CrossRef]
- Ito, Y.; Komada, H.; Kusagawa, S.; Tsurudome, M.; Matsumura, H.; Kawano, M.; Ohta, H.; Nishio, M. Fusion regulation proteins on the cell surface: Isolation and characterization of monoclonal antibodies which enhance giant polykaryocyte formation in newcastle disease virus-infected cell lines of human origin. J. Virol. 1992, 66, 5999–6007. [Google Scholar]
- Ohgimoto, S.; Tabata, N.; Suga, S.; Nishio, M.; Ohta, H.; Tsurudome, M.; Komada, H.; Kawano, M.; Watanabe, N.; Ito, Y. Molecular characterization of fusion regulatory protein-1 (frp-1) that induces multinucleated giant cell formation of monocytes and hiv gp160-mediated cell fusion. Frp-1 and 4f2/cd98 are identical molecules. J. Immunol. 1995, 155, 3585–3592. [Google Scholar]
- Ohta, H.; Tsurudome, M.; Matsumura, H.; Koga, Y.; Morikawa, S.; Kawano, M.; Kusugawa, S.; Komada, H.; Nishio, M.; Ito, Y. Molecular and biological characterization of fusion regulatory proteins (FRPs): Anti-frp mabs induced hiv-mediated cell fusion via an integrin system. EMBO J. 1994, 13, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Tsurudome, M.; Ohgimoto, S.; Kawano, M.; Nishio, M.; Komada, H.; Ito, M.; Sakakura, Y.; Ito, Y. An anti-fusion regulatory protein-1 monoclonal antibody suppresses human parainfluenza virus type 2-induced cell fusion. J. Gen. Virol. 1997, 78 Pt 1, 83–89. [Google Scholar] [CrossRef]
- Lee, C.P.; Liu, P.T.; Kung, H.N.; Su, M.T.; Chua, H.H.; Chang, Y.H.; Chang, C.W.; Tsai, C.H.; Liu, F.T.; Chen, M.R. The escrt machinery is recruited by the viral BFRF1 protein to the nucleus-associated membrane for the maturation of epstein-barr virus. PLoS Pathog. 2012, 8, e1002904. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.; Yu, K.L.; Teuling, E.; Akhmanova, A.; Jaarsma, D.; Hoogenraad, C.C. The als8 protein vapb interacts with the er-golgi recycling protein yif1a and regulates membrane delivery into dendrites. EMBO J. 2013, 32, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Peretti, D.; Dahan, N.; Shimoni, E.; Hirschberg, K.; Lev, S. Coordinated lipid transfer between the endoplasmic reticulum and the golgi complex requires the vap proteins and is essential for golgi-mediated transport. Mol. Biol. Cell 2008, 19, 3871–3884. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Hodgson, L.; Mantell, J.; Verkade, P.; Carlton, J.G. Escrt-iii controls nuclear envelope reformation. Nature 2015, 522, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, S.L.; Cowan, J.M.; Kerr, J.K.; Reynolds, A.E.; Baines, J.D.; Roller, R.J. Effects of charged cluster mutations on the function of herpes simplex virus type 1 ul34 protein. J. Virol. 2003, 77, 7601–7610. [Google Scholar] [CrossRef]
- Haugo, A.C.; Szpara, M.L.; Parsons, L.; Enquist, L.W.; Roller, R.J. Herpes simplex virus 1 pul34 plays a critical role in cell-to-cell spread of virus in addition to its role in virus replication. J. Virol. 2011, 85, 7203–7215. [Google Scholar] [CrossRef]
- Matsuzaki, F.; Shirane, M.; Matsumoto, M.; Nakayama, K.I. Protrudin serves as an adaptor molecule that connects kif5 and its cargoes in vesicular transport during process formation. Mol. Biol. Cell 2011, 22, 4602–4620. [Google Scholar] [CrossRef]
- Saita, S.; Shirane, M.; Natume, T.; Iemura, S.; Nakayama, K.I. Promotion of neurite extension by protrudin requires its interaction with vesicle-associated membrane protein-associated protein. J. Biol. Chem. 2009, 284, 13766–13777. [Google Scholar] [CrossRef]
- Hermjakob, H.; Montecchi-Palazzi, L.; Lewington, C.; Mudali, S.; Kerrien, S.; Orchard, S.; Vingron, M.; Roechert, B.; Roepstorff, P.; Valencia, A.; et al. Intact: An open source molecular interaction database. Nucleic Acids Res. 2004, 32, D452–D455. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, I.; Nishimura, Y.; Okamoto, T.; Aizaki, H.; Liu, M.; Mori, Y.; Abe, T.; Suzuki, T.; Lai, M.M.; Miyamura, T.; et al. Human vap-b is involved in hepatitis c virus replication through interaction with ns5a and ns5b. J. Virol. 2005, 79, 13473–13482. [Google Scholar] [CrossRef] [PubMed]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef] [PubMed]
| Protein | Unique Peptides | Spectral Counts | dNSAF Score | ID’d in Padula Study |
|---|---|---|---|---|
| US6 Glycoprotein D | 7 | 38 | 0.00303 | YES |
| UL27 Glycoprotein B | 14 | 62 | 0.00216 | NO |
| UL44 Glycoprotein C | 6 | 30 | 0.00185 | NO |
| Nuclear egress membrane protein pUL34 | 4 | 15 | 0.00171 | YES |
| Virion protein US2 | 4 | 14 | 0.00151 | NO |
| Membrane protein UL45 | 2 | 7 | 0.00128 | NO |
| UL49 Tegument protein VP22 | 4 | 11 | 0.00115 | YES |
| UL19/VP5 Major capsid protein | 18 | 46 | 0.00105 | YES |
| UL48 Tegument protein VP16 | 6 | 16 | 0.00103 | NO |
| UL50 Deoxyuridine triphosphatase | 3 | 12 | 0.00102 | NO |
| US8 Glycoprotein E | 8 | 16 | 0.00091 | NO |
| UL18/VP23 Capsid triplex subunit 2 | 4 | 9 | 0.00089 | YES |
| UL46 Tegument protein VP11/12 | 7 | 19 | 0.00083 | NO |
| UL47 Tegument protein VP13/14 | 7 | 14 | 0.00063 | NO |
| UL10 Glycoprotein M | 1 | 8 | 0.00053 | NO |
| UL42 DNA pol processivity subunit | 4 | 8 | 0.00052 | NO |
| US7 Glycoprotein I | 3 | 6 | 0.00048 | NO |
| UL40 Ribonucleotide reductase subunit 2 | 3 | 5 | 0.00046 | NO |
| Tegument protein UL7 | 3 | 4 | 0.00042 | NO |
| UL22 Glycoprotein H | 5 | 11 | 0.00041 | NO |
| pUL31 Nuclear egress lamina protein | 2 | 4 | 0.00041 | NO |
| UL29/ICP8 Single-stranded DNA binding protein | 7 | 15 | 0.00039 | NO |
| UL39 Ribonucleotide reductase subunit 1 | 8 | 14 | 0.00039 | NO |
| Tegument protein UL51 | 2 | 3 | 0.00039 | NO |
| ICP4 | 10 | 15 | 0.00036 | NO |
| US10 | 2 | 3 | 0.00030 | NO |
| UL12 Deoxyribonuclease | 4 | 6 | 0.00030 | NO |
| UL54 Multifunctional expression regulator | 3 | 4 | 0.00025 | NO |
| UL24 | 1 | 2 | 0.00023 | NO |
| US1/ICP22 | 2 | 3 | 0.00023 | NO |
| UL41 Tegument host shutoff protein | 2 | 3 | 0.00019 | NO |
| Tegument protein UL21 | 1 | 3 | 0.00018 | NO |
| UL26/VP24/VP21 Capsid maturation protease | 2 | 3 | 0.00015 | YES |
| UL39/VP19C Capsid triplex subunit 1 | 1 | 2 | 0.00014 | YES |
| Tegument protein UL25 | 2 | 2 | 0.00011 | NO |
| HSV-1 Infected MMs | Mock Infected MMs | Mock Infected NEs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Unique Peptides | Spectral Counts | dNSAF | HSV1 MMs:mockMMs Ratio | Unique Peptides | Spectral Counts | dNSAF | MockNEs:MockMMs Ratio | Unique Peptides | Spectral Counts | dNSAF |
| VAPB | 2 | 7 | 0.002286 | 6.3 | 1 | 3 | 0.000363 | 9.0 | 2 | 8 | 0.003261 |
| VAMP7 | 2 | 4 | 0.000702 | 4.9 | 1 | 2 | 0.000142 | 17.8 | 3 | 11 | 0.002523 |
| RAB11B | 7 | 49 | 0.007065 | 2.0 | 6 | 61 | 0.003560 | 3.4 | 7 | 65 | 0.012242 |
| RAB9A | 2 | 3 | 0.000469 | 1.9 | 1 | 4 | 0.000253 | 4.8 | 3 | 6 | 0.001226 |
| RAB1A | 4 | 35 | 0.005357 | 1.7 | 5 | 50 | 0.003093 | 11.9 | 5 | 184 | 0.036801 |
| RAB18 | 8 | 23 | 0.003509 | 1.7 | 7 | 33 | 0.002038 | 22.1 | 8 | 226 | 0.045044 |
| STX7 | 1 | 2 | 0.000241 | 1.7 | 1 | 3 | 0.000146 | 12.9 | 4 | 12 | 0.001888 |
| RAB2A | 4 | 17 | 0.002520 | 1.4 | 6 | 30 | 0.001800 | 6.1 | 9 | 57 | 0.011039 |
| RAB35 | 3 | 17 | 0.002658 | 1.4 | 4 | 30 | 0.001899 | 1.4 | 3 | 13 | 0.002655 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiz-Ros, N.; Czapiewski, R.; Epifano, I.; Stevenson, A.; Swanson, S.K.; Dixon, C.R.; Zamora, D.B.; McElwee, M.; Vijayakrishnan, S.; Richardson, C.A.; et al. Host Vesicle Fusion Protein VAPB Contributes to the Nuclear Egress Stage of Herpes Simplex Virus Type-1 (HSV-1) Replication. Cells 2019, 8, 120. https://doi.org/10.3390/cells8020120
Saiz-Ros N, Czapiewski R, Epifano I, Stevenson A, Swanson SK, Dixon CR, Zamora DB, McElwee M, Vijayakrishnan S, Richardson CA, et al. Host Vesicle Fusion Protein VAPB Contributes to the Nuclear Egress Stage of Herpes Simplex Virus Type-1 (HSV-1) Replication. Cells. 2019; 8(2):120. https://doi.org/10.3390/cells8020120
Chicago/Turabian StyleSaiz-Ros, Natalia, Rafal Czapiewski, Ilaria Epifano, Andrew Stevenson, Selene K. Swanson, Charles R. Dixon, Dario B. Zamora, Marion McElwee, Swetha Vijayakrishnan, Christine A. Richardson, and et al. 2019. "Host Vesicle Fusion Protein VAPB Contributes to the Nuclear Egress Stage of Herpes Simplex Virus Type-1 (HSV-1) Replication" Cells 8, no. 2: 120. https://doi.org/10.3390/cells8020120
APA StyleSaiz-Ros, N., Czapiewski, R., Epifano, I., Stevenson, A., Swanson, S. K., Dixon, C. R., Zamora, D. B., McElwee, M., Vijayakrishnan, S., Richardson, C. A., Dong, L., Kelly, D. A., Pytowski, L., Goldberg, M. W., Florens, L., Graham, S. V., & Schirmer, E. C. (2019). Host Vesicle Fusion Protein VAPB Contributes to the Nuclear Egress Stage of Herpes Simplex Virus Type-1 (HSV-1) Replication. Cells, 8(2), 120. https://doi.org/10.3390/cells8020120






