CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies
Abstract
1. Introduction
2. Immune-Modulatory Role of CD38 in T Lymphocytes: Implication for Treg Activities
3. CD38 and Regulatory B Cells
4. Immune-Regulatory Functions of CD38 on Innate Immunity: Focus on CD16−CD56bright NK Cells
5. CD38 Expression and Function in Non-Hemopoietic Immunoregulatory Cells
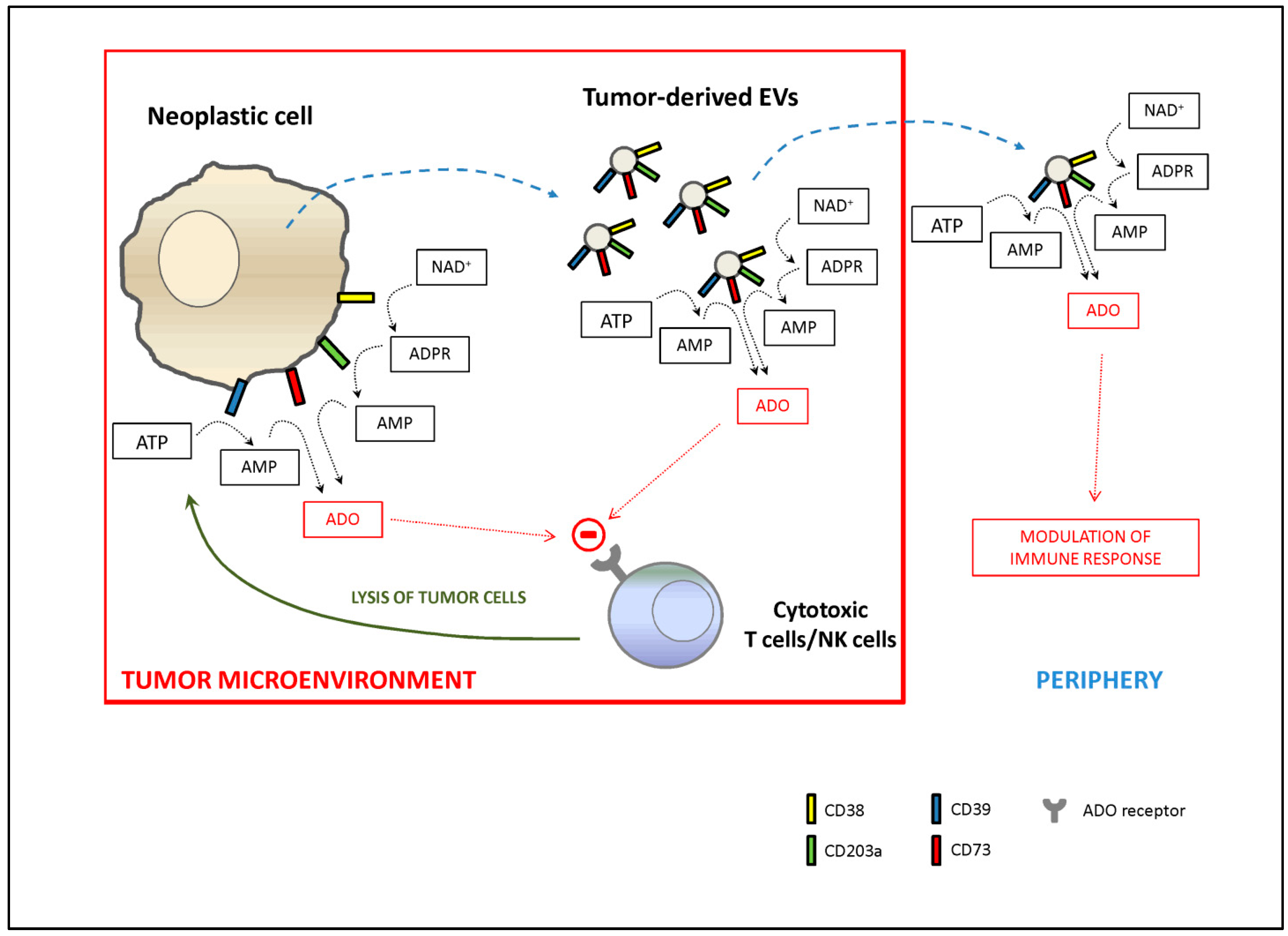
6. CD38 Expression on Extracellular Vesicles: Another Mechanism of Immune-Modulation?
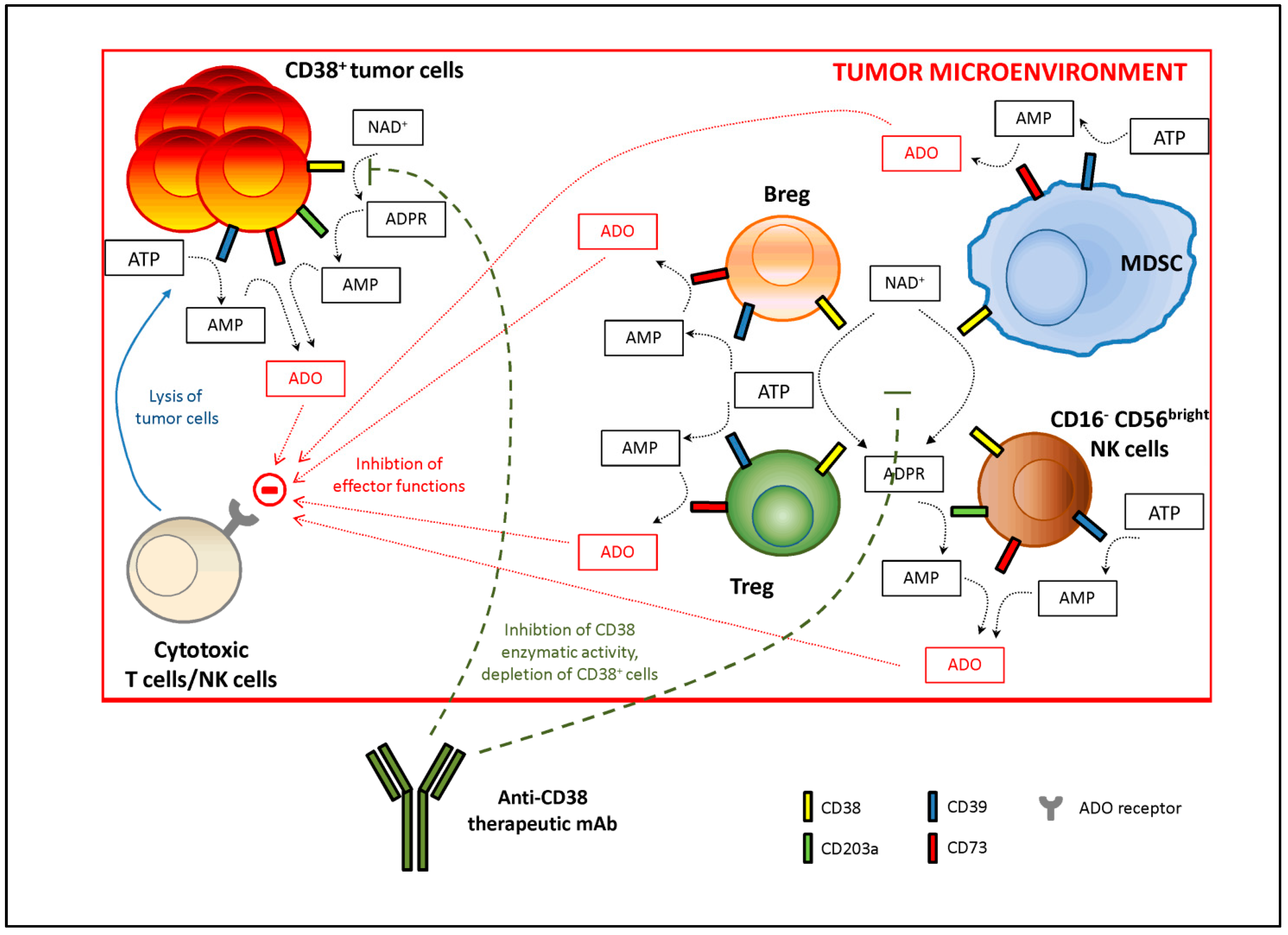
7. Therapeutic Approaches Based on Targeting CD38
8. Resistance to CD38 Antibodies: A Rationale for Multidrug Therapies
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhan, A.K.; Reinherz, E.L.; Poppema, S.; McCluskey, R.T.; Schlossman, S.F. Location of T cell and major histocompatibility complex antigens in the human thymus. J. Exp. Med. 1980, 152, 771–782. [Google Scholar] [CrossRef]
- Malavasi, F.; Funaro, A.; Alessio, M.; DeMonte, L.B.; Ausiello, C.M.; Dianzani, U.; Lanza, F.; Magrini, E.; Momo, M.; Roggero, S. CD38: A multi-lineage cell activation molecule with a split personality. Int. J. Clin. Lab. Res. 1992, 22, 73–80. [Google Scholar] [CrossRef]
- Alessio, M.; Roggero, S.; Funaro, A.; De Monte, L.B.; Peruzzi, L.; Geuna, M.; Malavasi, F. CD38 molecule: Structural and biochemical analysis on human T lymphocytes, thymocytes, and plasma cells. J. Immunol. 1990, 145, 878–884. [Google Scholar]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef]
- Erkers, T.; Stikvoort, A.; Uhlin, M. Lymphocytes in Placental Tissues: Immune Regulation and Translational Possibilities for Immunotherapy. Stem Cells Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Deaglio, S.; Dianzani, U.; Horenstein, A.L.; Fernandez, J.E.; van Kooten, C.; Bragardo, M.; Funaro, A.; Garbarino, G.; Di Virgilio, F.; Banchereau, J.; et al. Human CD38 ligand. A 120-KDA protein predominantly expressed on endothelial cells. J. Immunol. 1996, 156, 727–734. [Google Scholar]
- Funaro, A.; Horenstein, A.L.; Calosso, L.; Morra, M.; Tarocco, R.P.; Franco, L.; Flora, A.D.; Malavasi, F. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int. Immunol. 1996, 8, 1643–1650. [Google Scholar] [CrossRef]
- Lee, H.C.; Zocchi, E.; Guida, L.; Franco, L.; Benatti, U.; De Flora, A. Production and hydrolysis of cyclic ADP-ribose at the outer surface of human erythrocytes. Biochem. Biophys. Res. Commun. 1993, 191, 639–645. [Google Scholar] [CrossRef]
- Zocchi, E.; Franco, L.; Guida, L.; Benatti, U.; Bargellesi, A.; Malavasi, F.; Lee, H.C.; De Flora, A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem. Biophys. Res. Commun. 1993, 196, 1459–1465. [Google Scholar] [CrossRef]
- Kontani, K.; Nishina, H.; Ohoka, Y.; Takahashi, K.; Katada, T. NAD glycohydrolase specifically induced by retinoic acid in human leukemic HL-60 cells. Identification of the NAD glycohydrolase as leukocyte cell surface antigen CD38. J. Biol. Chem. 1993, 268, 16895–16898. [Google Scholar]
- Howard, M.; Grimaldi, J.C.; Bazan, J.F.; Lund, F.E.; Santos-Argumedo, L.; Parkhouse, R.M.; Walseth, T.F.; Lee, H.C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993, 262, 1056–1059. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Henttinen, T.; Samburski, S.S.; Spychala, J.; Jalkanen, S. The evidence for two opposite, ATP-generating and ATP-consuming, extracellular pathways on endothelial and lymphoid cells. Biochem. J. 2002, 367, 121–128. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Peola, S.; Borrione, P.; Matera, L.; Malavasi, F.; Pileri, A.; Massaia, M. Selective induction of CD73 expression in human lymphocytes by CD38 ligation: A novel pathway linking signal transducers with ecto-enzyme activities. J. Immunol. 1996, 157, 4354–4362. [Google Scholar] [PubMed]
- Horenstein, A.L.; Chillemi, A.; Zaccarello, G.; Bruzzone, S.; Quarona, V.; Zito, A.; Serra, S.; Malavasi, F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2013, 2, e26246. [Google Scholar] [CrossRef]
- Quarona, V.; Ferri, V.; Chillemi, A.; Bolzoni, M.; Mancini, C.; Zaccarello, G.; Roato, I.; Morandi, F.; Marimpietri, D.; Faccani, G.; et al. Unraveling the contribution of ectoenzymes to myeloma life and survival in the bone marrow niche. Ann. N. Y. Acad. Sci. 2015, 1335, 10–22. [Google Scholar] [CrossRef]
- Read, S.; Mauze, S.; Asseman, C.; Bean, A.; Coffman, R.; Powrie, F. CD38+ CD45RB(low) CD4+ T cells: A population of T cells with immune regulatory activities in vitro. Eur. J. Immunol. 1998, 28, 3435–3447. [Google Scholar] [CrossRef]
- Martins, T.C.; Aguas, A.P. A role for CD45RBlow CD38+ T cells and costimulatory pathways of T-cell activation in protection of non-obese diabetic (NOD) mice from diabetes. Immunology 1999, 96, 600–605. [Google Scholar] [CrossRef]
- Bahri, R.; Bollinger, A.; Bollinger, T.; Orinska, Z.; Bulfone-Paus, S. Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-gamma-mediated suppressor activities. PLoS ONE 2012, 7, e45234. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.G.; Reifsnyder, P.C.; Schott, W.H.; Lee, C.H.; Osborne, M.; Scheuplein, F.; Haag, F.; Koch-Nolte, F.; Serreze, D.V.; et al. Targeted disruption of CD38 accelerates autoimmune diabetes in NOD/Lt mice by enhancing autoimmunity in an ADP-ribosyltransferase 2-dependent fashion. J. Immunol. 2006, 176, 4590–4599. [Google Scholar] [CrossRef]
- Fedele, G.; Sanseverino, I.; D’Agostino, K.; Schiavoni, I.; Locht, C.; Horenstein, A.L.; Malavasi, F.; Ausiello, C.M. Unconventional, adenosine-producing suppressor T cells induced by dendritic cells exposed to BPZE1 pertussis vaccine. J. Leukoc. Biol. 2015, 98, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.T.; Wilson, M.D.; Rowan, W.C.; Soond, D.R.; Okkenhaug, K. The PI3K p110δ Regulates Expression of CD38 on Regulatory T Cells. PLoS ONE 2011, 6, e17359. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.R.; Sanchez-Diaz, R.; Bovolenta, E.R.; Barreiro, O.; Lasarte, S.; Matesanz-Marin, A.; Toribio, M.L.; Sanchez-Madrid, F.; Martin, P. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 2014, 55, 51–62. [Google Scholar] [CrossRef]
- Kim, B.J.; Choi, Y.M.; Rah, S.Y.; Park, D.R.; Park, S.A.; Chung, Y.J.; Park, S.M.; Park, J.K.; Jang, K.Y.; Kim, U.H. Seminal CD38 is a pivotal regulator for fetomaternal tolerance. Proc. Natl. Acad. Sci. USA 2015, 112, 1559–1564. [Google Scholar] [CrossRef]
- McMahon, C.M.; Luger, S.M. Relapsed T Cell ALL: Current Approaches and New Directions. Curr. Hematol. Malig. Rep. 2019, 14, 83–93. [Google Scholar] [CrossRef]
- Syed, Y.Y. Daratumumab: A Review in Combination Therapy for Transplant-Ineligible Newly Diagnosed Multiple Myeloma. Drugs 2019, 79, 447–454. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Feng, X.; Acharya, C.; An, G.; Wen, K.; Zhang, L.; Kalbasi, A.; Qiu, L.; Munshi, N.C.; Tai, Y.-T.; Anderson, K.C. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Reverse Immunosuppression in Multiple Myeloma. Blood 2016, 128, 2106. [Google Scholar] [CrossRef]
- Silverman, G.J.; Srikrishnan, R.; Germar, K.; Goodyear, C.S.; Andrews, K.A.; Ginzler, E.M.; Tsao, B.P. Genetic imprinting of autoantibody repertoires in systemic lupus erythematosus patients. Clin. Exp. Immunol. 2008, 153, 102–116. [Google Scholar] [CrossRef]
- Matsushita, T.; Yanaba, K.; Bouaziz, J.D.; Fujimoto, M.; Tedder, T.F. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 2008, 118, 3420–3430. [Google Scholar] [CrossRef]
- Evans, J.G.; Chavez-Rueda, K.A.; Eddaoudi, A.; Meyer-Bahlburg, A.; Rawlings, D.J.; Ehrenstein, M.R.; Mauri, C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J. Immunol. 2007, 178, 7868–7878. [Google Scholar] [CrossRef] [PubMed]
- Burlock, B.; Richardson, G.; García-Rodríguez, S.; Guerrero, S.; Zubiaur, M.; Sancho, J. The Role of CD38 on the Function of Regulatory B Cells in a Murine Model of Lupus. Int. J. Mol. Sci. 2018, 19, 2906. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, J.D.; Le Buanec, H.; Saussine, A.; Bensussan, A.; Bagot, M. IL-10 producing regulatory B cells in mice and humans: State of the art. Curr. Mol. Med. 2012, 12, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+) CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Figueiró, F.; Muller, L.; Funk, S.; Jackson, E.K.; Battastini, A.M.O.; Whiteside, T.L. Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg). Oncoimmunology 2016, 5, e1082703. [Google Scholar] [CrossRef]
- Domínguez-Pantoja, M.; López-Herrera, G.; Romero-Ramírez, H.; Santos-Argumedo, L.; Chávez-Rueda, A.K.; Hernández-Cueto, Á.; Flores-Muñoz, M.; Rodríguez-Alba, J.C. CD38 protein deficiency induces autoimmune characteristics and its activation enhances IL-10 production by regulatory B cells. Scand. J. Immunol. 2018, 87, e12664. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Bao, W.; Boulad, N.; Evangelista, J.; Haider, M.A.; Bussel, J.; Yazdanbakhsh, K. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012, 120, 3318–3325. [Google Scholar] [CrossRef]
- Das, A.; Ellis, G.; Pallant, C.; Lopes, A.R.; Khanna, P.; Peppa, D.; Chen, A.; Blair, P.; Dusheiko, G.; Gill, U.; et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 2012, 189, 3925–3935. [Google Scholar] [CrossRef]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013, 5, 173ra123. [Google Scholar] [CrossRef]
- Khoder, A.; Sarvaria, A.; Alsuliman, A.; Chew, C.; Sekine, T.; Cooper, N.; Mielke, S.; de Lavallade, H.; Muftuoglu, M.; Fernandez Curbelo, I.; et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014, 124, 2034–2045. [Google Scholar] [CrossRef]
- Vlkova, M.; Ticha, O.; Nechvatalova, J.; Kalina, T.; Litzman, J.; Mauri, C.; Blair, P.A. Regulatory B cells in CVID patients fail to suppress multifunctional IFN-γ+ TNF-α+ CD4+ T cells differentiation. Clin. Immunol. 2015, 160, 292–300. [Google Scholar] [CrossRef]
- Hayashi, M.; Yanaba, K.; Umezawa, Y.; Yoshihara, Y.; Kikuchi, S.; Ishiuji, Y.; Saeki, H.; Nakagawa, H. IL-10-producing regulatory B cells are decreased in patients with psoriasis. J. Derm. Sci. 2016, 81, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Simopoulou, T.; Varna, A.; Liaskos, C.; Katsiari, C.G.; Bogdanos, D.P.; Sakkas, L.I. Breg Cells Are Numerically Decreased and Functionally Impaired in Patients with Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tai, Y.T.; Ho, M.; Xing, L.; Chauhan, D.; Gang, A.; Qiu, L.; Anderson, K.C. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017, 7, e547. [Google Scholar] [CrossRef]
- Tsuda, B.; Miyamoto, A.; Yokoyama, K.; Ogiya, R.; Oshitanai, R.; Terao, M.; Morioka, T.; Niikura, N.; Okamura, T.; Miyako, H.; et al. B-cell populations are expanded in breast cancer patients compared with healthy controls. Breast Cancer 2018, 25, 284–291. [Google Scholar] [CrossRef]
- Guan, H.; Wan, Y.; Lan, J.; Wang, Q.; Wang, Z.; Li, Y.; Zheng, J.; Zhang, X.; Shen, Y.; Xie, F. PD-L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci. Rep. 2016, 6, 35651. [Google Scholar] [CrossRef]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Wiendl, H.; Marcenaro, E.; Kerlero de Rosbo, N.; Uccelli, A.; Laroni, A. Regulatory Functions of Natural Killer Cells in Multiple Sclerosis. Front. Immunol. 2016, 7, 606. [Google Scholar] [CrossRef]
- Tian, Z.; Gershwin, M.E.; Zhang, C. Regulatory NK cells in autoimmune disease. J. Autoimmun. 2012, 39, 206–215. [Google Scholar] [CrossRef]
- Laroni, A.; Armentani, E.; Kerlero de Rosbo, N.; Ivaldi, F.; Marcenaro, E.; Sivori, S.; Gandhi, R.; Weiner, H.L.; Moretta, A.; Mancardi, G.L.; et al. Dysregulation of regulatory CD56(bright) NK cells/T cells interactions in multiple sclerosis. J. Autoimmun. 2016, 72, 8–18. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Zhang, M.; Ma, H.; Xu, B.; Jiang, H.; Wang, J.; Li, G. CD56(bright)CD16(−) natural killer cells are shifted toward an IFN-gamma-promoting phenotype with reduced regulatory capacity in osteoarthritis. Hum. Immunol. 2019, 80, 871–877. [Google Scholar] [CrossRef]
- Laroni, A.; Gandhi, R.; Beynon, V.; Weiner, H.L. IL-27 imparts immunoregulatory function to human NK cell subsets. PLoS ONE 2011, 6, e26173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, W.; Chai, N.R.; Maric, D.; Bielekova, B. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J. Immunol. 2011, 187, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Horenstein, A.L.; Chillemi, A.; Quarona, V.; Chiesa, S.; Imperatori, A.; Zanellato, S.; Mortara, L.; Gattorno, M.; Pistoia, V.; et al. CD56brightCD16− NK Cells Produce Adenosine through a CD38-Mediated Pathway and Act as Regulatory Cells Inhibiting Autologous CD4+ T Cell Proliferation. J. Immunol. 2015, 195, 965–972. [Google Scholar] [CrossRef]
- de Jonge, K.; Ebering, A.; Nassiri, S.; Maby-El Hajjami, H.; Ouertatani-Sakouhi, H.; Baumgaertner, P.; Speiser, D.E. Circulating CD56(bright) NK cells inversely correlate with survival of melanoma patients. Sci. Rep. 2019, 9, 4487. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sattler, C.; Steinsdoerfer, M.; Offers, M.; Fischer, E.; Schierl, R.; Heseler, K.; Daubener, W.; Seissler, J. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transpl. 2011, 20, 1221–1230. [Google Scholar] [CrossRef]
- Consonni, F.M.; Porta, C.; Marino, A.; Pandolfo, C.; Mola, S.; Bleve, A.; Sica, A. Myeloid-Derived Suppressor Cells: Ductile Targets in Disease. Front. Immunol. 2019, 10, 949. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Li, J.; Fan, Z.; Yang, L.; Zhang, Z.; Zhang, C.; Yue, D.; Qin, G.; Zhang, T.; et al. Metformin-Induced Reduction of CD39 and CD73 Blocks Myeloid-Derived Suppressor Cell Activity in Patients with Ovarian Cancer. Cancer Res. 2018, 78, 1779–1791. [Google Scholar] [CrossRef]
- Karakasheva, T.A.; Waldron, T.J.; Eruslanov, E.; Kim, S.B.; Lee, J.S.; O’Brien, S.; Hicks, P.D.; Basu, D.; Singhal, S.; Malavasi, F.; et al. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Res. 2015, 75, 4074–4085. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Marongiu, F.; Ellis, E.C.; Dorko, K.; Mitamura, K.; Ranade, A.; Gramignoli, R.; Davila, J.; Strom, S.C. Production of hepatocyte-like cells from human amnion. Methods Mol. Biol. 2009, 481, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Strom, S.C.; Gramignoli, R. Human amnion epithelial cells expressing HLA-G as novel cell-based treatment for liver disease. Hum. Immunol. 2016, 77, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants with Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef]
- Tee, J.Y.; Vaghjiani, V.; Liu, Y.H.; Murthi, P.; Chan, J.; Manuelpillai, U. Immunogenicity and immunomodulatory properties of hepatocyte-like cells derived from human amniotic epithelial cells. Curr. Stem Cell Res. 2013, 8, 91–99. [Google Scholar]
- Kolanko, E.; Kopaczka, K.; Koryciak-Komarska, H.; Czech, E.; Szmytkowska, P.; Gramignoli, R.; Czekaj, P. Increased immunomodulatory capacity of human amniotic cells after activation by pro-inflammatory chemokines. Eur. J. Pharm. 2019, 859, 172545. [Google Scholar] [CrossRef]
- Li, H.; Niederkorn, J.Y.; Neelam, S.; Mayhew, E.; Word, R.A.; McCulley, J.P.; Alizadeh, H. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest. Ophthalmol. Vis. Sci. 2005, 46, 900–907. [Google Scholar] [CrossRef]
- Morandi, F.; Horenstein, A.L.; Quarona, V.; Faini, A.C.; Castella, B.; Srinivasan, R.C.; Strom, S.C.; Malavasi, F.; Gramignoli, R. Ectonucleotidase Expression on Human Amnion Epithelial Cells: Adenosinergic Pathways and Dichotomic Effects on Immune Effector Cell Populations. J. Immunol. 2019, 202, 724–735. [Google Scholar] [CrossRef]
- Caivano, A.; Laurenzana, I.; De Luca, L.; La Rocca, F.; Simeon, V.; Trino, S.; D’Auria, F.; Traficante, A.; Maietti, M.; Izzo, T.; et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumor Biol. 2015, 36, 9739–9752. [Google Scholar] [CrossRef]
- Morandi, F.; Marimpietri, D.; Horenstein, A.L.; Bolzoni, M.; Toscani, D.; Costa, F.; Castella, B.; Faini, A.C.; Massaia, M.; Pistoia, V.; et al. Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD(+). Oncoimmunology 2018, 7, e1458809. [Google Scholar] [CrossRef]
- Morandi, F.; Marimpietri, D.; Horenstein, A.L.; Corrias, M.V.; Malavasi, F. Microvesicles expressing adenosinergic ectoenzymes and their potential role in modulating bone marrow infiltration by neuroblastoma cells. Oncoimmunology 2019, 8, e1574198. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Kristensen, S.R.; Gregersen, H.; Teodorescu, E.M.; Christiansen, G.; Pedersen, S. Extracellular vesicle-associated procoagulant phospholipid and tissue factor activity in multiple myeloma. PLoS ONE 2019, 14, e0210835. [Google Scholar] [CrossRef] [PubMed]
- Zumaquero, E.; Munoz, P.; Cobo, M.; Lucena, G.; Pavon, E.J.; Martin, A.; Navarro, P.; Garcia-Perez, A.; Ariza-Veguillas, A.; Malavasi, F.; et al. Exosomes from human lymphoblastoid B cells express enzymatically active CD38 that is associated with signaling complexes containing CD81, Hsc-70 and Lyn. Exp. Cell Res. 2010, 316, 2692–2706. [Google Scholar] [CrossRef] [PubMed]
- Deaglio, S.; Mehta, K.; Malavasi, F. Human CD38: A (r)evolutionary story of enzymes and receptors. Leuk. Res. 2001, 25, 1–12. [Google Scholar] [CrossRef]
- Hoebeke, I.; De Smedt, M.; Stolz, F.; Pike-Overzet, K.; Staal, F.J.; Plum, J.; Leclercq, G. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(-)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia 2007, 21, 311–319. [Google Scholar] [CrossRef]
- Abramson, H.N. Monoclonal Antibodies for the Treatment of Multiple Myeloma: An Update. Int. J. Mol. Sci. 2018, 19, 3924. [Google Scholar] [CrossRef]
- Botta, C.; Ciliberto, D.; Rossi, M.; Staropoli, N.; Cuce, M.; Galeano, T.; Tagliaferri, P.; Tassone, P. Network meta-analysis of randomized trials in multiple myeloma: Efficacy and safety in relapsed/refractory patients. Blood Adv. 2017, 1, 455–466. [Google Scholar] [CrossRef]
- Sidiqi, M.H.; Gertz, M.A. Daratumumab for the treatment of AL amyloidosis. Leuk. Lymphoma. 2019, 60, 295–301. [Google Scholar] [CrossRef]
- Salas, M.Q.; Alahmari, A.; Howard Lipton, J. Successful Treatment of Refractory Red Cell Aplasia after Allogeneic Hematopoietic Cell Transplantation with Daratumumab. Eur. J. Haematol. 2019. [Google Scholar] [CrossRef]
- Shah, N.N.; Singavi, A.K.; Harrington, A. Daratumumab in Primary Effusion Lymphoma. N. Engl. J. Med. 2018, 379, 689–690. [Google Scholar] [CrossRef]
- Schuetz, C.; Hoenig, M.; Moshous, D.; Weinstock, C.; Castelle, M.; Bendavid, M.; Shimano, K.; Tolbert, V.; Schulz, A.S.; Dvorak, C.C. Daratumumab in life-threatening autoimmune hemolytic anemia following hematopoietic stem cell transplantation. Blood Adv. 2018, 2, 2550–2553. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, K.; Verkleij, C.; Bosman, P.; Zweegman, S.; Otten, H.; van de Donk, N. CD38-targeted therapy with daratumumab reduces autoantibody levels in multiple myeloma patients. J. Transl. Autoimmun. 2019, 100022. [Google Scholar] [CrossRef]
- Bride, K.; Vincent, T.; Im, S.-Y.; Aplenc, R.; Barrett, D.; Carroll, W.; Carson, R.; Dai, Y.; Devidas, M.; Dunsmore, K.; et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia (T-ALL). Blood 2018, 131, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Acharya, C.; An, G.; Zhong, M.; Feng, X.; Wang, L.; Dasilva, N.; Song, Z.; Yang, G.; Adrian, F.; et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia 2016, 30, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Zimmerman, T.M.; Hofmeister, C.C.; Talpaz, M.; Chanan-Khan, A.A.; Kaufman, J.L.; Laubach, J.P.; Chauhan, D.; Jakubowiak, A.J.; Reich, S.; et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood 2016, 127, 2693–2700. [Google Scholar] [CrossRef]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1228–1263. [Google Scholar] [CrossRef]
- Chillemi, A.; Quarona, V.; Zito, A.; Morandi, F.; Marimpietri, D.; Cuccioloni, M.; Robert, O.J.; Mark, C.S.; Bolzoni, M.; Toscani, D.; et al. Generation and Characterization of Microvesicles after Daratumumab Interaction with Myeloma Cells. Blood 2015, 126, 1849. [Google Scholar] [CrossRef]
- Nijhof, I.S.; Casneuf, T.; van Velzen, J.; van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.W.; van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Chillemi, A.; Quarona, V.; Zito, A.; Roato, I.; Morandi, F.; Marimpietri, D.; Bolzoni, M.; Toscani, D.; Oldham, R.J.; et al. NAD(+)-Metabolizing Ectoenzymes in Remodeling Tumor-Host Interactions: The Human Myeloma Model. Cells 2015, 4, 520–537. [Google Scholar] [CrossRef]
- Krejcik, J.; Frerichs, K.A.; Nijhof, I.S.; van Kessel, B.; van Velzen, J.F.; Bloem, A.C.; Broekmans, M.E.C.; Zweegman, S.; van Meerloo, J.; Musters, R.J.P.; et al. Monocytes and Granulocytes Reduce CD38 Expression Levels on Myeloma Cells in Patients Treated with Daratumumab. Clin. Cancer Res. 2017, 23, 7498–7511. [Google Scholar] [CrossRef]
- Nijhof, I.S.; Groen, R.W.; Lokhorst, H.M.; van Kessel, B.; Bloem, A.C.; van Velzen, J.; de Jong-Korlaar, R.; Yuan, H.; Noort, W.A.; Klein, S.K.; et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015, 29, 2039–2049. [Google Scholar] [CrossRef]
- Fedele, P.L.; Willis, S.N.; Liao, Y.; Low, M.S.; Rautela, J.; Segal, D.H.; Gong, J.N.; Huntington, N.D.; Shi, W.; Huang, D.C.S.; et al. IMiDs prime myeloma cells for daratumumab-mediated cytotoxicity through loss of Ikaros and Aiolos. Blood 2018, 132, 2166–2178. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Fotiou, D.; Roussou, M.; Migkou, M.; Ziogas, D.C.; Kanellias, N.; Terpos, E.; Dimopoulos, M.A. The addition of IMiDs for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood 2018, 131, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Joseph, N.S.; Kaufman, J.L.; Heffner, L.T.; Gupta, V.A.; Gleason, C.; Boise, L.H.; Lonial, S. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: Utility of re-treatment with daratumumab among refractory patients. Cancer 2019, 125, 2991–3000. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant global methylation patterns affect the molecular pathogenesis and prognosis of multiple myeloma. Blood 2011, 117, 553–562. [Google Scholar] [CrossRef]
- Kaiser, M.F.; Johnson, D.C.; Wu, P.; Walker, B.A.; Brioli, A.; Mirabella, F.; Wardell, C.P.; Melchor, L.; Davies, F.E.; Morgan, G.J. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood 2013, 122, 219–226. [Google Scholar] [CrossRef]
- Choudhry, P.; Mariano, M.C.; Geng, H.; Martin, T.G.; Wolf, J.L.; Wong, S.W.; Shah, N.; Wiita, A.P. DNA methyltransferase inhibitors upregulate CD38 protein expression and enhance daratumumab efficacy in multiple myeloma. Leukemia 2019. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morandi, F.; Airoldi, I.; Marimpietri, D.; Bracci, C.; Faini, A.C.; Gramignoli, R. CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies. Cells 2019, 8, 1527. https://doi.org/10.3390/cells8121527
Morandi F, Airoldi I, Marimpietri D, Bracci C, Faini AC, Gramignoli R. CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies. Cells. 2019; 8(12):1527. https://doi.org/10.3390/cells8121527
Chicago/Turabian StyleMorandi, Fabio, Irma Airoldi, Danilo Marimpietri, Cristiano Bracci, Angelo Corso Faini, and Roberto Gramignoli. 2019. "CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies" Cells 8, no. 12: 1527. https://doi.org/10.3390/cells8121527
APA StyleMorandi, F., Airoldi, I., Marimpietri, D., Bracci, C., Faini, A. C., & Gramignoli, R. (2019). CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies. Cells, 8(12), 1527. https://doi.org/10.3390/cells8121527





