Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction
Abstract
1. Introduction
2. Findings of the Relationship of Myelination Deficits, Impaired White Matter, and Cognition from Human Brain Imaging Studies
3. Histopathological Studies of Oligodendrocytes in Schizophrenia (SZ)
4. Evidence of Oligodendrocyte Deficits from Molecular Studies
5. The “Defective Maturation” Hypothesis of SZ
6. The Intercellular Interactions of Oligodendrocytes with Microglia and Neurons
7. The Role of Environmental Risk Factors in Oligodendrocyte Differentiation
8. The Impact of Genetic Schizophrenia Risk on the Oligodendroglial Linage
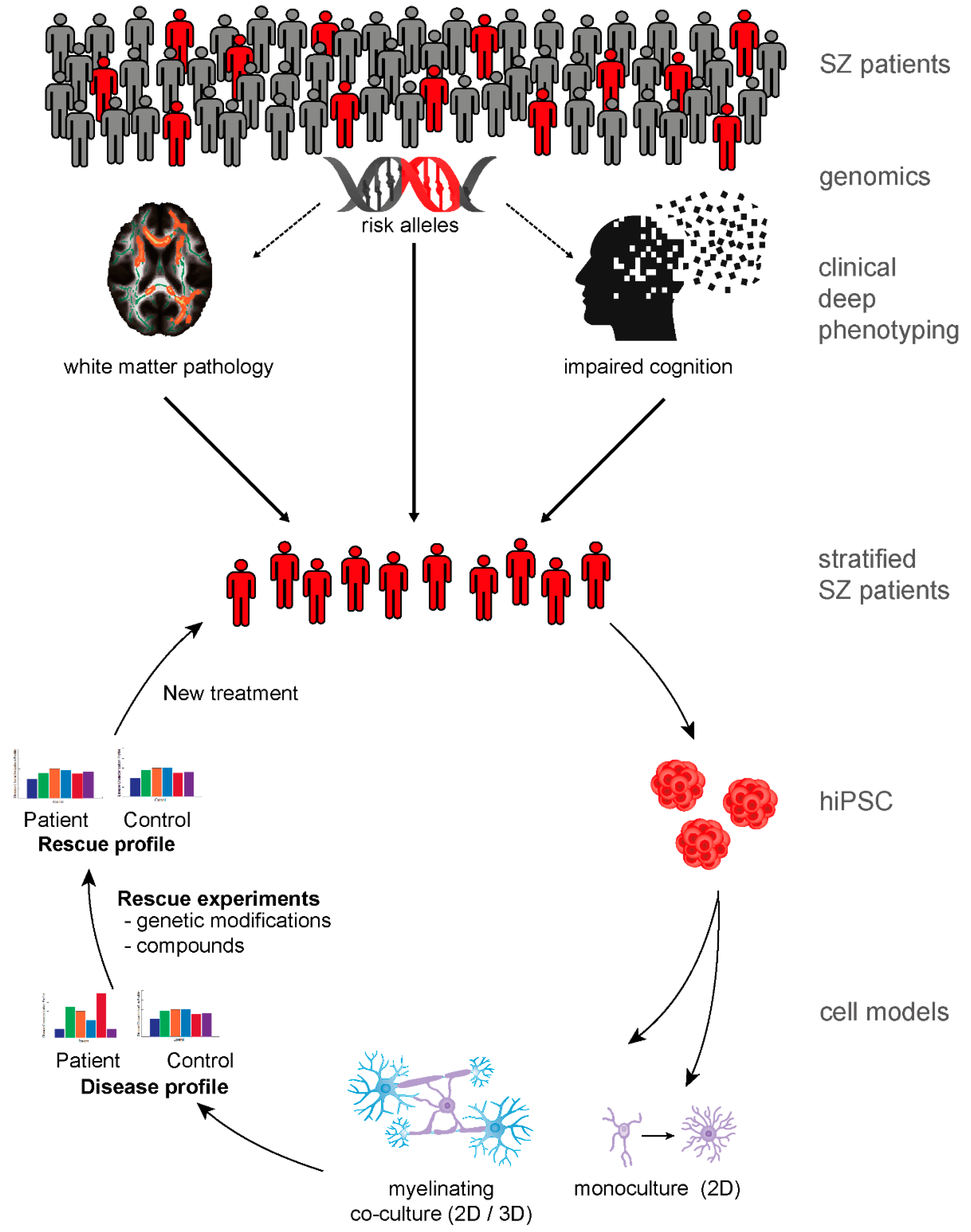
9. Patient-Derived Neurobiological Test Systems Indicate Oligodendroglial Contribution to SZ
10. The Road to New Therapies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 1996, 153, 321–330. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef]
- Goff, D.C.; Hill, M.; Barch, D. The treatment of cognitive impairment in schizophrenia. Pharm. Biochem. Behav. 2011, 99, 245–253. [Google Scholar] [CrossRef]
- Hasan, A.; Falkai, P.; Wobrock, T.; Lieberman, J.; Glenthoj, B.; Gattaz, W.F.; Thibaut, F.; Moller, H.J. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychia. 2012, 13, 318–378. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Schmitt, A.; Kaurani, L.; Senner, F.; Papiol, S.; Malchow, B.; Fischer, A.; Schulze, T.G.; Koutsouleris, N.; Falkai, P. Childhood Trauma in Schizophrenia: Current Findings and Research Perspectives. Front. Neurosci. 2019, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Malchow, B.; Hasan, A.; Falkai, P. The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 2014, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Hermoye, L.; Saint-Martin, C.; Cosnard, G.; Lee, S.K.; Kim, J.; Nassogne, M.C.; Menten, R.; Clapuyt, P.; Donohue, P.K.; Hua, K.; et al. Pediatric diffusion tensor imaging: Normal database and observation of the white matter maturation in early childhood. Neuroimage 2006, 29, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Duka, T.; Stimpson, C.D.; Schapiro, S.J.; Baze, W.B.; McArthur, M.J.; Fobbs, A.J.; Sousa, A.M.; Sestan, N.; Wildman, D.E.; et al. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 16480–16485. [Google Scholar] [CrossRef]
- Hoistad, M.; Segal, D.; Takahashi, N.; Sakurai, T.; Buxbaum, J.D.; Hof, P.R. Linking white and grey matter in schizophrenia: Oligodendrocyte and neuron pathology in the prefrontal cortex. Front. Neuroanat 2009, 3, 9. [Google Scholar] [CrossRef]
- Timmler, S.; Simons, M. Grey matter myelination. Glia 2019, 67, 2063–2070. [Google Scholar] [CrossRef]
- Alba-Ferrara, L.M.; de Erausquin, G.A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 2006, 63, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Argyelan, M.; Aggarwal, M.; Chandon, T.S.; Karlsgodt, K.H.; Mori, S.; Malhotra, A.K. The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage 2017, 147, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ellison-Wright, I.; Bullmore, E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009, 108, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Lui, S.; Liao, Y.; Du, M.Y.; Hu, N.; Thomas, J.A.; Gong, Q.Y. White matter deficits in first episode schizophrenia: An activation likelihood estimation meta-analysis. Prog. Neuro-Psychoph. 2013, 45, 100–106. [Google Scholar] [CrossRef]
- Vitolo, E.; Tatu, M.K.; Pignolo, C.; Cauda, F.; Costa, T.; Ando, A.; Zennaro, A. White matter and schizophrenia: A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiat. Res. Neuroim. 2017, 270, 8–21. [Google Scholar] [CrossRef]
- Kelly, S.; Jahanshad, N.; Zalesky, A.; Kochunov, P.; Agartz, I.; Alloza, C.; Andreassen, O.A.; Arango, C.; Banaj, N.; Bouix, S.; et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatr. 2018, 23, 1261–1269. [Google Scholar] [CrossRef]
- Kuroki, N.; Kubicki, M.; Nestor, P.G.; Salisbury, D.F.; Park, H.J.; Levitt, J.J.; Woolston, S.; Frumin, M.; Niznikiewicz, M.; Westin, C.F.; et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol. Psychiatry 2006, 60, 22–31. [Google Scholar] [CrossRef][Green Version]
- Lim, K.O.; Ardekani, B.A.; Nierenberg, J.; Butler, P.D.; Javitt, D.C.; Hoptman, M.J. Voxelwise correlational analyses of white matter integrity in multiple cognitive domains in schizophrenia. Am. J. Psychiatry 2006, 163, 2008–2010. [Google Scholar] [CrossRef]
- Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef]
- Voineskos, A.N.; Felsky, D.; Kovacevic, N.; Tiwari, A.K.; Zai, C.; Chakravarty, M.M.; Lobaugh, N.J.; Shenton, M.E.; Rajji, T.K.; Miranda, D.; et al. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex 2013, 23, 2044–2057. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.D.; Mandl, R.C.W.; Raghava, J.M.; Jessen, K.; Jepsen, J.R.M.; Fagerlund, B.; Glenthoj, L.B.; Wenneberg, C.; Krakauer, K.; Pantelis, C.; et al. Widespread higher fractional anisotropy associates to better cognitive functions in individuals at ultra-high risk for psychosis. Hum. Brain Mapp. 2019, 40, 5185–5201. [Google Scholar] [CrossRef] [PubMed]
- Vostrikov, V.M.; Uranova, N.A.; Orlovskaya, D.D. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007, 94, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Hof, P.R.; Haroutunian, V.; Friedrich, V.L., Jr.; Byne, W.; Buitron, C.; Perl, D.P.; Davis, K.L. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol. Psychiatry 2003, 53, 1075–1085. [Google Scholar] [CrossRef]
- Segal, D.; Schmitz, C.; Hof, P.R. Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol. 2009, 117, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Steyskal, C.; Bernstein, H.G.; Schneider-Axmann, T.; Parlapani, E.; Schaeffer, E.L.; Gattaz, W.F.; Bogerts, B.; Schmitz, C.; Falkai, P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 2009, 117, 395–407. [Google Scholar] [CrossRef]
- Falkai, P.; Malchow, B.; Wetzestein, K.; Nowastowski, V.; Bernstein, H.G.; Steiner, J.; Schneider-Axmann, T.; Kraus, T.; Hasan, A.; Bogerts, B.; et al. Decreased Oligodendrocyte and Neuron Number in Anterior Hippocampal Areas and the Entire Hippocampus in Schizophrenia: A Stereological Postmortem Study. Schizophr Bull. 2016, 42 Suppl. 1, S4–S12. [Google Scholar] [CrossRef]
- Falkai, P.; Steiner, J.; Malchow, B.; Shariati, J.; Knaus, A.; Bernstein, H.G.; Schneider-Axmann, T.; Kraus, T.; Hasan, A.; Bogerts, B.; et al. Oligodendrocyte and Interneuron Density in Hippocampal Subfields in Schizophrenia and Association of Oligodendrocyte Number with Cognitive Deficits. Front. Cell. Neurosci. 2016, 10, 78. [Google Scholar] [CrossRef]
- Arnett, H.A.; Fancy, S.P.; Alberta, J.A.; Zhao, C.; Plant, S.R.; Kaing, S.; Raine, C.S.; Rowitch, D.H.; Franklin, R.J.; Stiles, C.D. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 2004, 306, 2111–2115. [Google Scholar] [CrossRef]
- Falkai, P.; Rossner, M.J.; Schulze, T.G.; Hasan, A.; Brzozka, M.M.; Malchow, B.; Honer, W.G.; Schmitt, A. Kraepelin revisited: Schizophrenia from degeneration to failed regeneration. Mol. Psychiatry 2015, 20, 671–676. [Google Scholar] [CrossRef]
- Lavenex, P. Functional anatomy, development, and pathology of the hippocampus. In The Clinical Neurobiology of The Hippocampus: An Integrative View; Bartsch, T., Ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Falkai, P.; Bogerts, B. Cell loss in the hippocampus of schizophrenics. Eur. Arch. Psychiatry Neurol. Sci. 1986, 236, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Ivleva, E.I.; Wagner, A.D.; Stark, C.E.; Tamminga, C.A. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014, 159, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.W.; Rakic, P. Three-dimensional counting: An accurate and direct method to estimate numbers of cells in sectioned material. J. Comp. Neurol. 1988, 278, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Hof, P.R. Design-based stereology in neuroscience. Neuroscience 2005, 130, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Cassoli, J.S.; Guest, P.C.; Malchow, B.; Schmitt, A.; Falkai, P.; Martins-de-Souza, D. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: From structural findings to molecules. NPJ Schizophr. 2015, 1, 15034. [Google Scholar] [CrossRef] [PubMed]
- Saia-Cereda, V.M.; Cassoli, J.S.; Schmitt, A.; Falkai, P.; Nascimento, J.M.; Martins-de-Souza, D. Proteomics of the corpus callosum unravel pivotal players in the dysfunction of cell signaling, structure, and myelination in schizophrenia brains. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 601–612. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Gattaz, W.F.; Schmitt, A.; Maccarrone, G.; Hunyadi-Gulyás, E.; Eberlin, M.N.; Souza, G.H.; Marangoni, S.; Novello, J.C.; Turck, C.W.; et al. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J. Psychiatr. Res. 2009, 43, 978–986. [Google Scholar] [CrossRef]
- Parlapani, E.; Schmitt, A.; Erdmann, A.; Bernstein, H.G.; Breunig, B.; Gruber, O.; Petroianu, G.; von Wilmsdorff, M.; Schneider-Axmann, T.; Honer, W.; et al. Association between myelin basic protein expression and left entorhinal cortex pre-alpha cell layer disorganization in schizophrenia. Brain Res. 2009, 1301, 126–134. [Google Scholar] [CrossRef]
- Habib, N.; Li, Y.; Heidenreich, M.; Swiech, L.; Avraham-Davidi, I.; Trombetta, J.J.; Hession, C.; Zhang, F.; Regev, A. Div-Seq: Single-nucleus RNA-Seq reveals dynamics of rare adult newborn neurons. Science 2016, 353, 925–928. [Google Scholar] [CrossRef]
- Miron, V.E.; Kuhlmann, T.; Antel, J.P. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim. Biophys. Acta 2011, 1812, 184–193. [Google Scholar] [CrossRef]
- Jakel, S.; Agirre, E.; Mendanha Falcao, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.L.; Kuhlmann, T.; Miron, V.E.; Leong, S.Y.; Fang, J.; Gris, P.; Kennedy, T.E.; Almazan, G.; Antel, J. Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am. J. Pathol. 2013, 183, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Mauney, S.A.; Pietersen, C.Y.; Sonntag, K.C.; Woo, T.W. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr. Res. 2015, 169, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Remington, L.; Maruschak, B.; Owens, T.; Bruck, W. Nogo-A is a reliable oligodendroglial marker in adult human and mouse CNS and in demyelinated lesions. J. Neuropathol. Exp. Neurol. 2007, 66, 238–246. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Miron, V.; Cui, Q.; Wegner, C.; Antel, J.; Bruck, W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef]
- van Kesteren, C.F.; Gremmels, H.; de Witte, L.D.; Hol, E.M.; Van Gool, A.R.; Falkai, P.G.; Kahn, R.S.; Sommer, I.E. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, e1075. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M. Neuroinflammation and white matter pathology in schizophrenia: Systematic review. Schizophr. Res. 2015, 161, 102–112. [Google Scholar] [CrossRef]
- Uranova, N.A.; Vostrikov, V.M.; Vikhreva, O.V.; Zimina, I.S.; Kolomeets, N.S.; Orlovskaya, D.D. The role of oligodendrocyte pathology in schizophrenia. Int. J. Neuropsychoph. 2007, 10, 537–545. [Google Scholar] [CrossRef]
- Vikhreva, O.V.; Rakhmanova, V.I.; Orlovskaya, D.D.; Uranova, N.A. Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: A post-mortem morphometric study. Schizophr. Res. 2016, 177, 28–36. [Google Scholar] [CrossRef]
- Guest, P.C.; Iwata, K.; Kato, T.A.; Steiner, J.; Schmitt, A.; Turck, C.W.; Martins-de-Souza, D. MK-801 treatment affects glycolysis in oligodendrocytes more than in astrocytes and neuronal cells: Insights for schizophrenia. Front. Cell. Neurosci. 2015, 9, 180. [Google Scholar] [CrossRef]
- Falkai, P.; Schmitt, A.; Cannon, T.D. Pathophysiology of Schizophrenia. In Schizophrenia; Herrman, H., Gaebel, W., Eds.; Wiley-Blackwell: Singapore, 2011; pp. 31–65. [Google Scholar]
- Schmitt, A.; Malchow, B.; Keeser, D.; Falkai, P.; Hasan, A. Neurobiology of schizophrenia: New findings from the structure to the molecules. Nervenarzt 2015, 86, 324–326, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Curley, A.A.; Glausier, J.R.; Volk, D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Stedehouder, J.; Kushner, S.A. Myelination of parvalbumin interneurons: A parsimonious locus of pathophysiological convergence in schizophrenia. Mol. Psychiatry 2017, 22, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Micheva, K.D.; Wolman, D.; Mensh, B.D.; Pax, E.; Buchanan, J.; Smith, S.J.; Bock, D.D. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 2016, 5. [Google Scholar] [CrossRef]
- Lin, S.C.; Bergles, D.E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef]
- Cardin, J.A.; Carlen, M.; Meletis, K.; Knoblich, U.; Zhang, F.; Deisseroth, K.; Tsai, L.H.; Moore, C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009, 459, 663–667. [Google Scholar] [CrossRef]
- Sohal, V.S.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702. [Google Scholar] [CrossRef]
- Hormuzdi, S.G.; Pais, I.; LeBeau, F.E.N.; Towers, S.K.; Rozov, A.; Buhl, E.H.; Whittington, M.A.; Monyer, H. Impaired Electrical Signaling Disrupts Gamma Frequency Oscillations in Connexin 36-Deficient Mice. Neuron 2001, 31, 487–495. [Google Scholar] [CrossRef]
- Traub, R.D.; Kopell, N.; Bibbig, A.; Buhl, E.H.; LeBeau, F.E.N.; Whittington, M.A. Gap Junctions between Interneuron Dendrites Can Enhance Synchrony of Gamma Oscillations in Distributed Networks. J. Neurosci. 2001, 21, 9478–9486. [Google Scholar] [CrossRef]
- Hu, H.; Gan, J.; Jonas, P. Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef]
- Senkowski, D.; Gallinat, J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol. Psychiatry 2015, 77, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, G.; Cho, R.Y.; Lewis, D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatry 2015, 77, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Chen, C.Y.; Cohen, B.M.; Spencer, K.M.; Levy, D.L.; Ongur, D.; Smoller, J.W. Genomewide association analyses of electrophysiological endophenotypes for schizophrenia and psychotic bipolar disorders: A preliminary report. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168B, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Dricks, S. Effects of neonatal stress on gamma oscillations in hippocampus. Sci. Rep. 2016, 6, 29007. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Simons, M.; Cantuti-Castelvetri, L.; Falkai, P. A new role for oligodendrocytes and myelination in schizophrenia and affective disorders? Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Stedehouder, J.; Couey, J.J.; Brizee, D.; Hosseini, B.; Slotman, J.A.; Dirven, C.M.F.; Shpak, G.; Houtsmuller, A.B.; Kushner, S.A. Fast-spiking Parvalbumin Interneurons are Frequently Myelinated in the Cerebral Cortex of Mice and Humans. Cereb Cortex 2017, 27, 5001–5013. [Google Scholar] [CrossRef] [PubMed]
- Battefeld, A.; Klooster, J.; Kole, M.H. Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat. Commun. 2016, 7, 11298. [Google Scholar] [CrossRef]
- Snaidero, N.; Simons, M. The logistics of myelin biogenesis in the central nervous system. Glia 2017, 65, 1021–1031. [Google Scholar] [CrossRef]
- Funfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Mobius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwan, A.C.; Zhang, S.; Phoumthipphavong, V.; Flannery, J.G.; Masmanidis, S.C.; Taniguchi, H.; Huang, Z.J.; Zhang, F.; Boyden, E.S.; et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 2012, 488, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hozumi, Y.; Kaneko, K.; Fujii, S. Modulatory Effects of Perineuronal Oligodendrocytes on Neuronal Activity in the Rat Hippocampus. Neurochem Res. 2018, 43, 27–40. [Google Scholar] [CrossRef]
- Toritsuka, M.; Makinodan, M.; Kishimoto, T. Social Experience-Dependent Myelination: An Implication for Psychiatric Disorders. Neural Plast. 2015, 2015, 465345. [Google Scholar] [CrossRef] [PubMed]
- Varty, G.B.; Powell, S.B.; Lehmann-Masten, V.; Buell, M.R.; Geyer, M.A. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav. Brain Res. 2006, 169, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef]
- Liu, J.; Dupree, J.L.; Gacias, M.; Frawley, R.; Sikder, T.; Naik, P.; Casaccia, P. Clemastine Enhances Myelination in the Prefrontal Cortex and Rescues Behavioral Changes in Socially Isolated Mice. J. Neurosci. 2016, 36, 957–962. [Google Scholar] [CrossRef]
- Varese, F.; Barkus, E.; Bentall, R.P. Dissociation mediates the relationship between childhood trauma and hallucination-proneness. Psychol. Med. 2012, 42, 1025–1036. [Google Scholar] [CrossRef]
- Bonoldi, I.; Simeone, E.; Rocchetti, M.; Codjoe, L.; Rossi, G.; Gambi, F.; Balottin, U.; Caverzasi, E.; Politi, P.; Fusar-Poli, P. Prevalence of self-reported childhood abuse in psychosis: A meta-analysis of retrospective studies. Psychiatry Res. 2013, 210, 8–15. [Google Scholar] [CrossRef]
- Najm, F.J.; Madhavan, M.; Zaremba, A.; Shick, E.; Karl, R.T.; Factor, D.C.; Miller, T.E.; Nevin, Z.S.; Kantor, C.; Sargent, A.; et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 2015, 522, 216–220. [Google Scholar] [CrossRef]
- Pardinas, A.F.; Holmans, P.; Pocklington, A.J.; Escott-Price, V.; Ripke, S.; Carrera, N.; Legge, S.E.; Bishop, S.; Cameron, D.; Hamshere, M.L.; et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 2018. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Shields, J. A polygenic theory of schizophrenia. Proc. Natl. Acad. Sci. USA 1967, 58, 199–205. [Google Scholar] [CrossRef]
- Tansey, K.E.; Hill, M.J. Enrichment of schizophrenia heritability in both neuronal and glia cell regulatory elements. Transl. Psychiatry 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.E.; Holmans, P.A.; Lee, P.H.; O’Dushlaine, C.T.; Kirby, A.W.; Smoller, J.W.; Ongur, D.; Cohen, B.M. Pathway analyses implicate glial cells in schizophrenia. PLoS ONE 2014, 9, e89441. [Google Scholar] [CrossRef] [PubMed]
- Skene, N.G.; Bryois, J.; Bakken, T.E.; Breen, G.; Crowley, J.J.; Gaspar, H.A.; Giusti-Rodriguez, P.; Hodge, R.D.; Miller, J.A.; Munoz-Manchado, A.B.; et al. Genetic identification of brain cell types underlying schizophrenia. Nat. Genet. 2018, 50, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, F.G.; Wobrock, T.; Gruber, O.; Scherk, H.; Berner, D.; Kaizl, I.; Kierer, A.; Muller, S.; Oest, M.; Meyer, T.; et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 133–143. [Google Scholar] [CrossRef]
- Papiol, S.; Popovic, D.; Keeser, D.; Hasan, A.; Schneider-Axmann, T.; Degenhardt, F.; Rossner, M.J.; Bickeboller, H.; Schmitt, A.; Falkai, P.; et al. Polygenic risk has an impact on the structural plasticity of hippocampal subfields during aerobic exercise combined with cognitive remediation in multi-episode schizophrenia. Transl. Psychiatry 2017, 7, e1159. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic Exercise Improves Cognitive Functioning in People with Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr Bull. 2017, 43, 546–556. [Google Scholar] [CrossRef]
- Papiol, S.; Keeser, D.; Hasan, A.; Schneider-Axmann, T.; Raabe, F.; Degenhardt, F.; Rossner, M.J.; Bickeböller, H.; Cantuti-Castelvetri, L.; Simons, M.; et al. Polygenic burden associated to oligodendrocyte precursor cells and radial glia influences the hippocampal volume changes induced by aerobic exercise in schizophrenia patients. Transl. Psychiatry 2019, 9. [Google Scholar] [CrossRef]
- Soliman, M.A.; Aboharb, F.; Zeltner, N.; Studer, L. Pluripotent stem cells in neuropsychiatric disorders. Mol. Psychiatry 2017, 22, 1241–1249. [Google Scholar] [CrossRef]
- Raabe, F.J.; Galinski, S.; Papiol, S.; Falkai, P.G.; Schmitt, A.; Rossner, M.J. Studying and modulating schizophrenia-associated dysfunctions of oligodendrocytes with patient-specific cell systems. NPJ Schizophr. 2018, 4, 23. [Google Scholar] [CrossRef]
- Brennand, K.J.; Simone, A.; Jou, J.; Gelboin-Burkhart, C.; Tran, N.; Sangar, S.; Li, Y.; Mu, Y.; Chen, G.; Yu, D.; et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011, 473, 221–225. [Google Scholar] [CrossRef]
- Prytkova, I.; Brennand, K.J. Prospects for Modeling Abnormal Neuronal Function in Schizophrenia Using Human Induced Pluripotent Stem Cells. Front. Cell. Neurosci. 2017, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Hasan, A.; Gruber, O.; Falkai, P. Schizophrenia as a disorder of disconnectivity. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261 (Suppl. 2), S150–S154. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ku, L.; Mei, R.; Liu, G.; Xu, C.; Wen, Z.; Zhao, X.; Wang, F.; Xiao, L.; Feng, Y. Novel schizophrenia risk factor pathways regulate FEZ1 to advance oligodendroglia development. Transl. Psychiatry 2017, 7, 1293. [Google Scholar] [CrossRef]
- de Vrij, F.M.; Bouwkamp, C.G.; Gunhanlar, N.; Shpak, G.; Lendemeijer, B.; Baghdadi, M.; Gopalakrishna, S.; Ghazvini, M.; Li, T.M.; Quadri, M.; et al. Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Windrem, M.S.; Osipovitch, M.; Liu, Z.; Bates, J.; Chandler-Militello, D.; Zou, L.; Munir, J.; Schanz, S.; McCoy, K.; Miller, R.H.; et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 2017, 21, 195–208. [Google Scholar] [CrossRef] [PubMed]
- McPhie, D.L.; Nehme, R.; Ravichandran, C.; Babb, S.M.; Ghosh, S.D.; Staskus, A.; Kalinowski, A.; Kaur, R.; Douvaras, P.; Du, F.; et al. Oligodendrocyte differentiation of induced pluripotent stem cells derived from subjects with schizophrenias implicate abnormalities in development. Transl. Psychiatry 2018, 8, 230. [Google Scholar] [CrossRef]
- Goldman, S.A.; Kuypers, N.J. How to make an oligodendrocyte. Development 2015, 142, 3983–3995. [Google Scholar] [CrossRef] [PubMed]
- Chanoumidou, K.; Mozafari, S.; Baron-Van Evercooren, A.; Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 2019. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Mozafari, S.; Glatza, M.; Starost, L.; Velychko, S.; Hallmann, A.L.; Cui, Q.L.; Schambach, A.; Kim, K.P.; Bachelin, C.; et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl. Acad. Sci. USA 2017, 114, E2243–E2252. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Leon, J.A.; Kumar, M.; Boon, R.; Chau, D.; One, J.; Wolfs, E.; Eggermont, K.; Berckmans, P.; Gunhanlar, N.; de Vrij, F.; et al. SOX10 Single Transcription Factor-Based Fast and Efficient Generation of Oligodendrocytes from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, S.O.; Sitnikov, S.; Kamen, Y.; Evans, K.A.; Kronenberg-Versteeg, D.; Dietmann, S.; de Faria, O., Jr.; Agathou, S.; Karadottir, R.T. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 2019. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; Zhang, J.; Rolls, E.T.; Yang, W.; Palaniyappan, L.; Zhang, L.; Cheng, W.; Yao, Y.; Liu, Z.; et al. Brain-Wide Analysis of Functional Connectivity in First-Episode and Chronic Stages of Schizophrenia. Schizophr Bull. 2017, 43, 436–448. [Google Scholar] [CrossRef]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef]
- Haroutunian, V.; Katsel, P.; Dracheva, S.; Stewart, D.G.; Davis, K.L. Variations in oligodendrocyte-related gene expression across multiple cortical regions: Implications for the pathophysiology of schizophrenia. Int. J. Neuropsychoph. 2007, 10, 565–573. [Google Scholar] [CrossRef]
- Raabe, F.J.; Spengler, D. Epigenetic Risk Factors in PTSD and Depression. Front. Psychiatry 2013, 4, 80. [Google Scholar] [CrossRef]
| In vivo brain imaging studies | |
| Histopathology (postmortem) | |
| Transcriptomic studies | |
| Proteomic studies | |
| hiPSC studies |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raabe, F.J.; Slapakova, L.; Rossner, M.J.; Cantuti-Castelvetri, L.; Simons, M.; Falkai, P.G.; Schmitt, A. Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction. Cells 2019, 8, 1496. https://doi.org/10.3390/cells8121496
Raabe FJ, Slapakova L, Rossner MJ, Cantuti-Castelvetri L, Simons M, Falkai PG, Schmitt A. Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction. Cells. 2019; 8(12):1496. https://doi.org/10.3390/cells8121496
Chicago/Turabian StyleRaabe, Florian J., Lenka Slapakova, Moritz J. Rossner, Ludovico Cantuti-Castelvetri, Mikael Simons, Peter G. Falkai, and Andrea Schmitt. 2019. "Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction" Cells 8, no. 12: 1496. https://doi.org/10.3390/cells8121496
APA StyleRaabe, F. J., Slapakova, L., Rossner, M. J., Cantuti-Castelvetri, L., Simons, M., Falkai, P. G., & Schmitt, A. (2019). Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction. Cells, 8(12), 1496. https://doi.org/10.3390/cells8121496





