RETRACTED: Acetylshikonin Sensitizes Hepatocellular Carcinoma Cells to Apoptosis through ROS-Mediated Caspase Activation
Abstract
1. Introduction
2. Methods
2.1. Reagents and Cell Culture
2.2. Cell Viability
2.3. Western Blotting
2.4. Apoptosis Assessment by DAPI Staining and TUNEL Assay
2.5. ROS Production Assessment
2.6. DNA Comet Assay
2.7. siRNA Transfection
2.8. Orthotopic Transplantation Tumor Model of HCC Preparation and Treatment
2.9. Immunohistochemistry
2.10. Kidney and Liver Function Tests
2.11. Statistical Analyses
3. Results
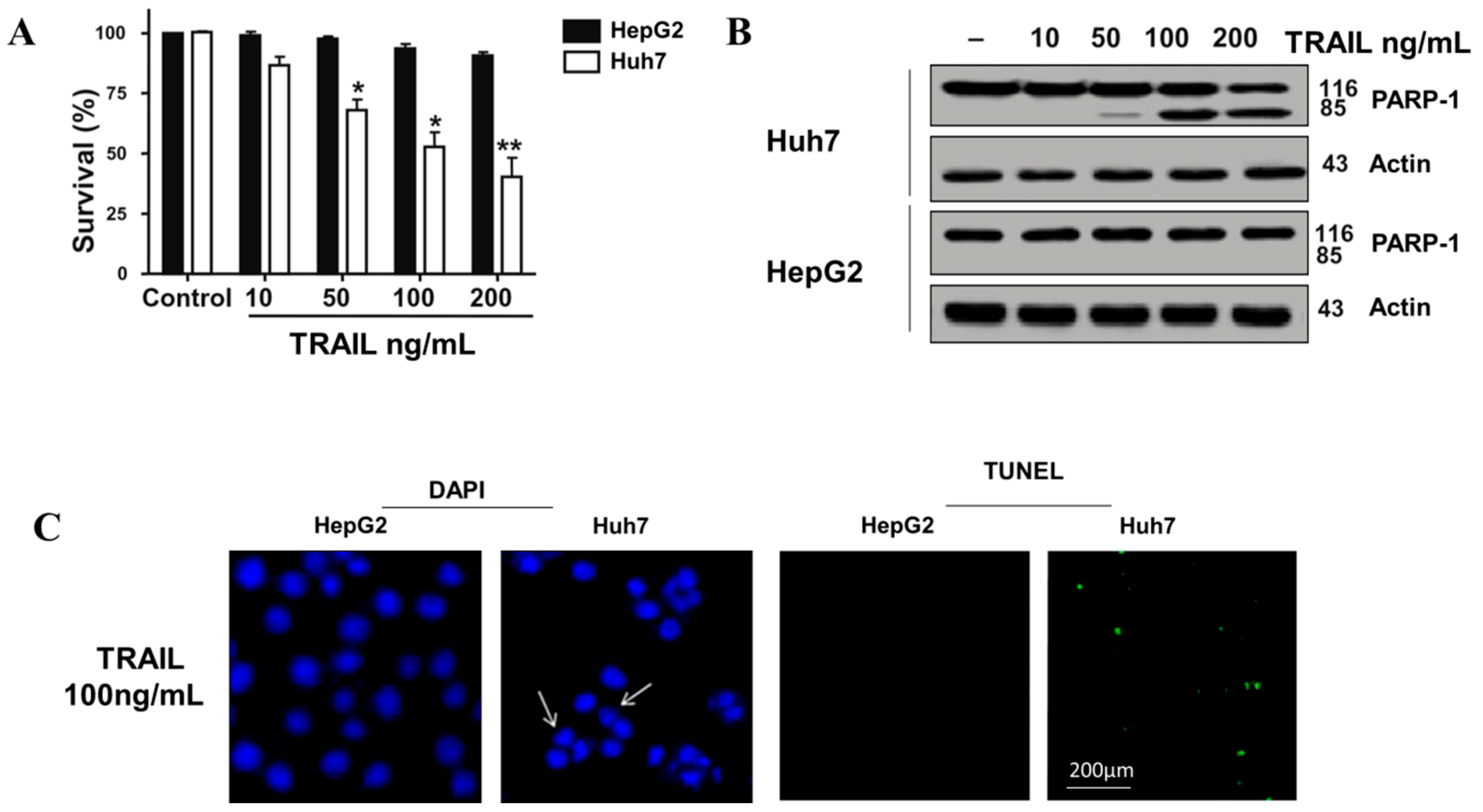
3.1. TRAIL Resistance in Human HepG2 Cell
3.2. The Synergetic Effects of ASH and TRAIL on HCC Cells Apoptosis
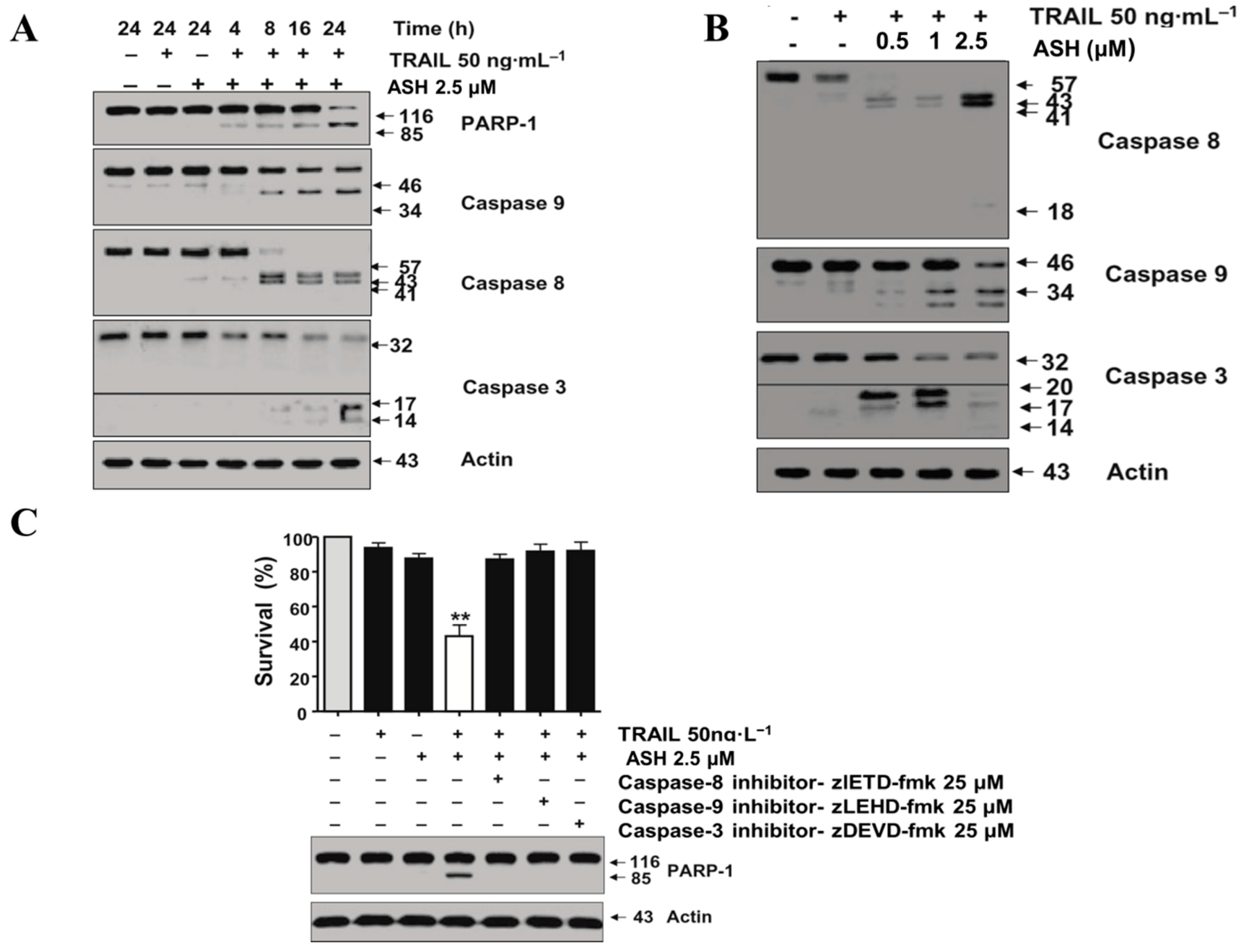
3.3. Combination Therapy with TRAIL and ASH Activates Caspases Signaling Pathway
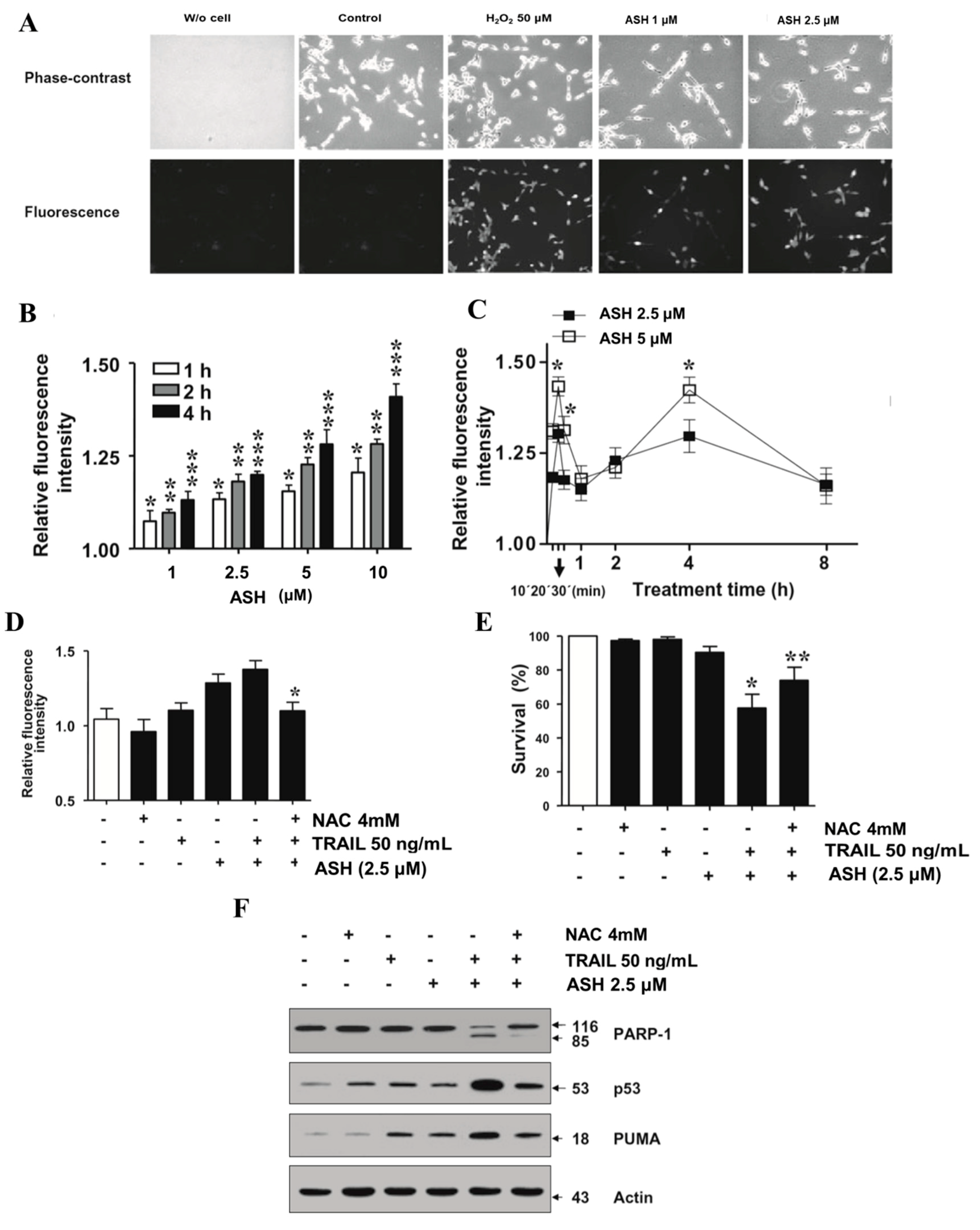
3.4. ASH Promotes ROS Production in HepG2 Cells
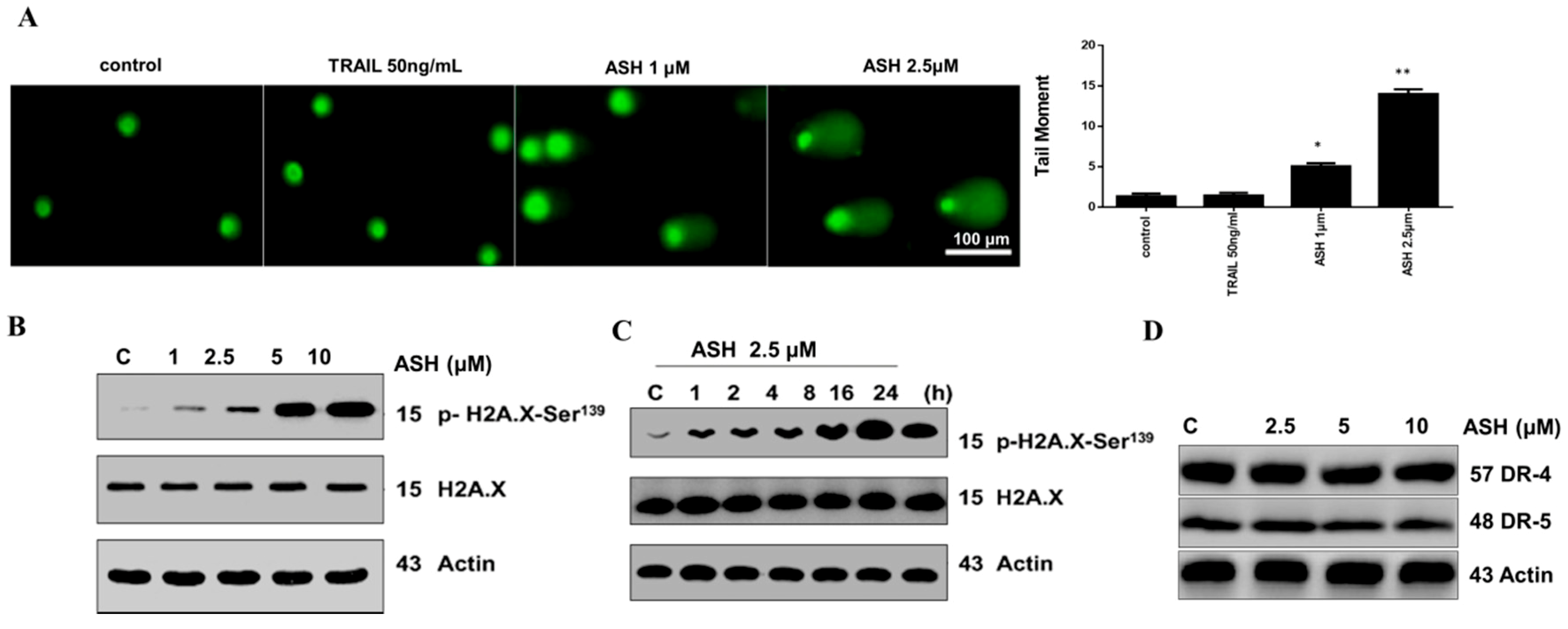
3.5. ASH Caused DNA Damage in HepG2 Cells
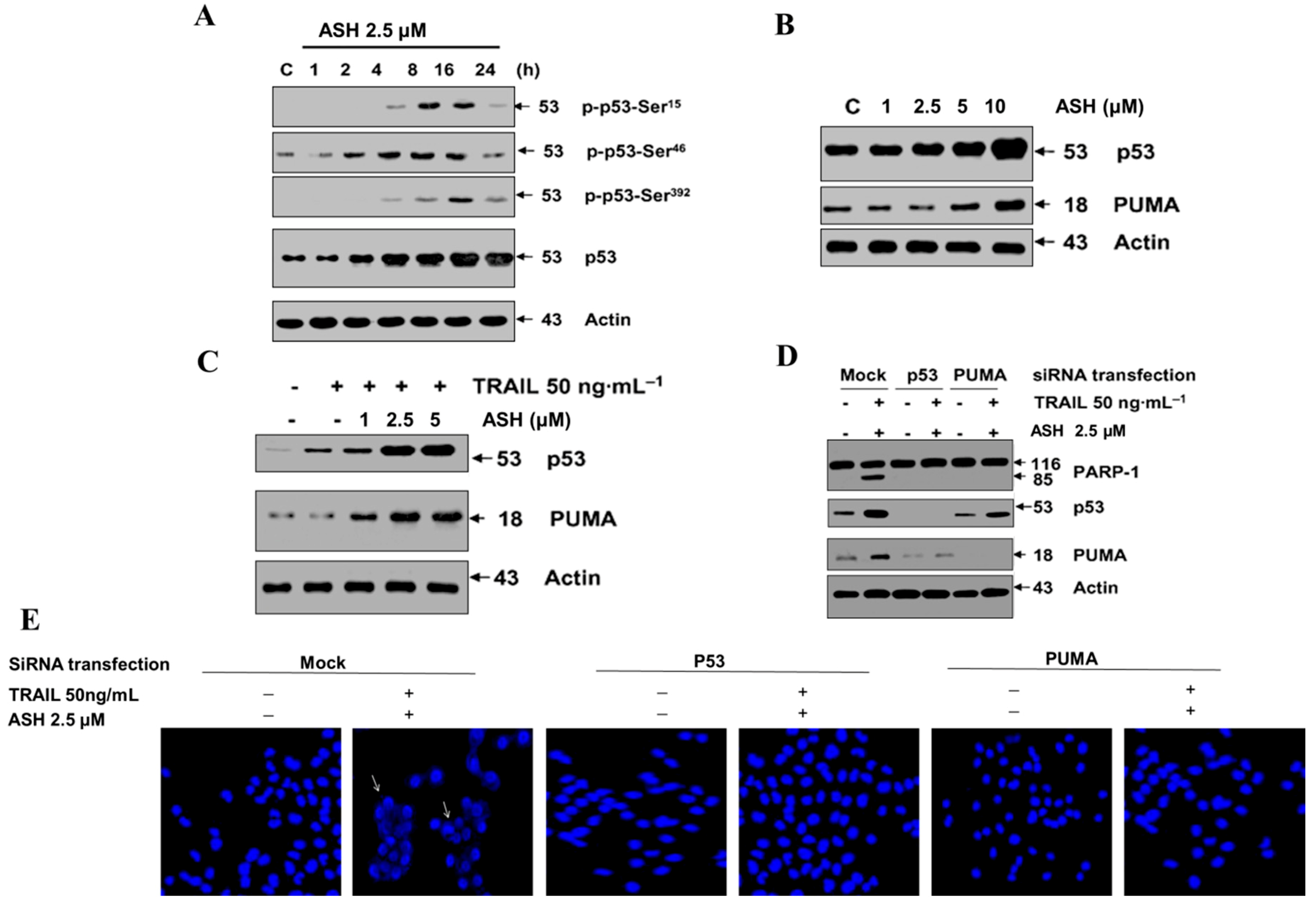
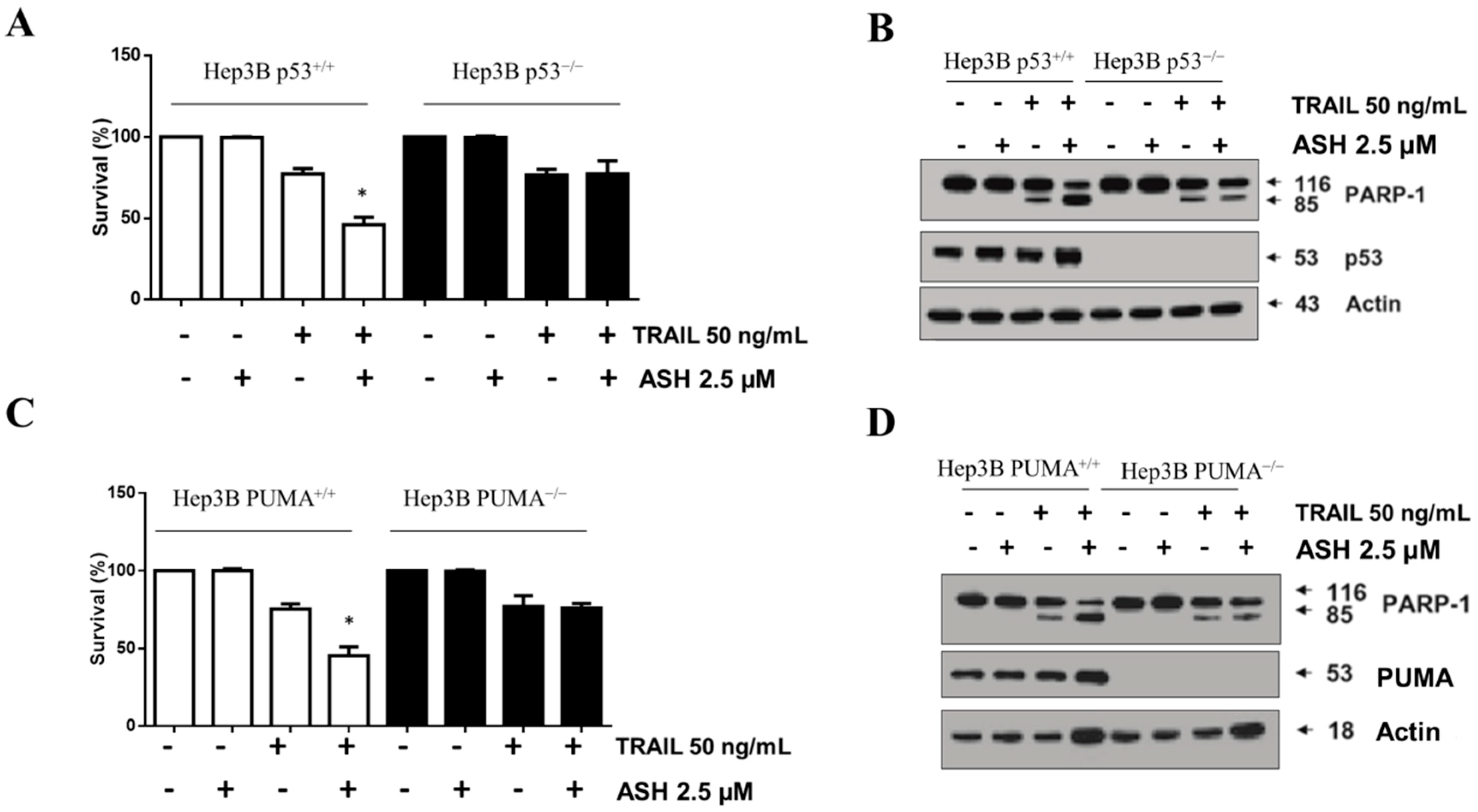
3.6. Involvement of p53 and PUMA in ASH-Induced TRAIL Sensitization
3.7. Bax Contributed to ASH and TRAIL Induced Apoptosis
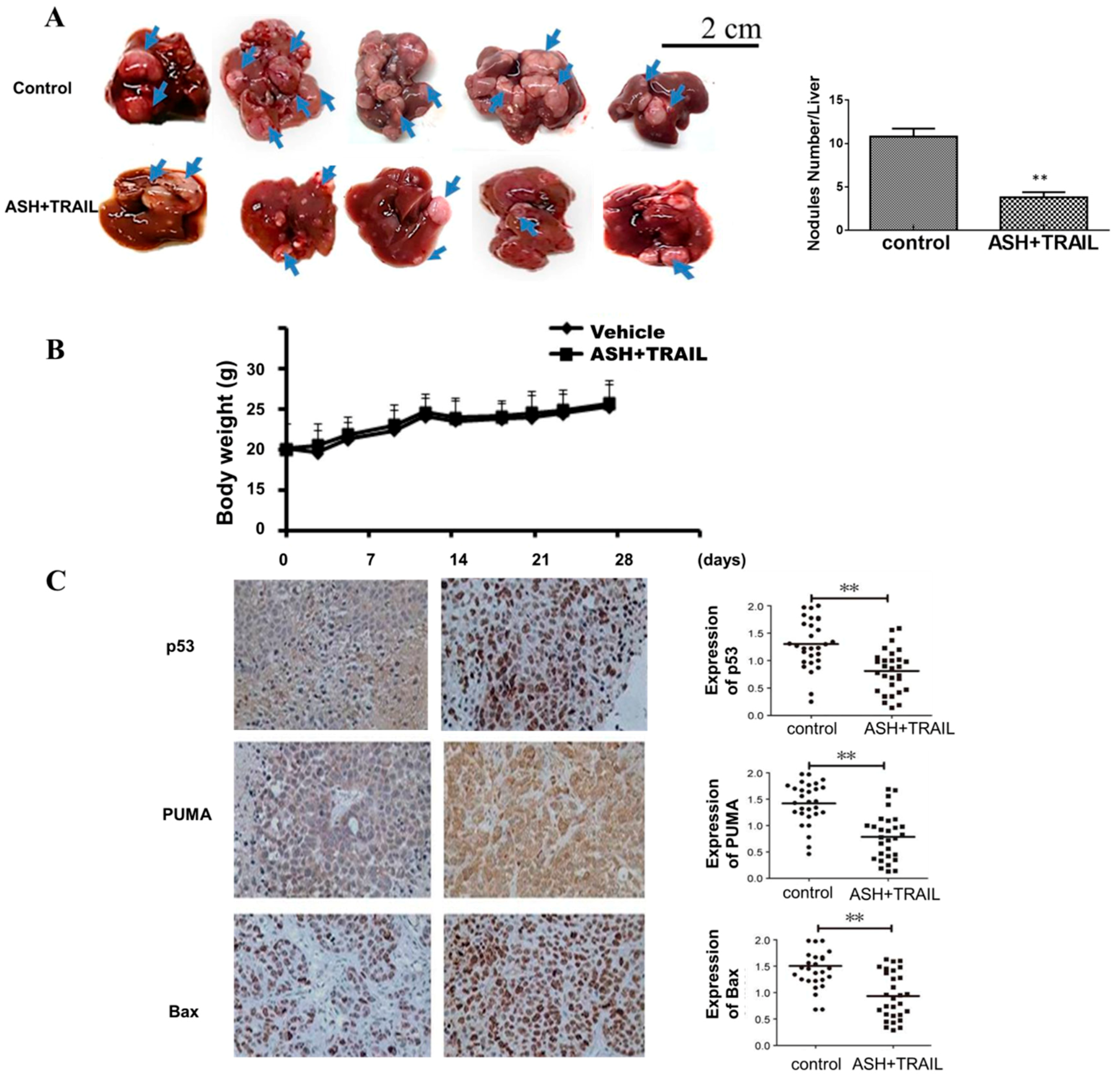
3.8. ASH and TRAIL Inhibits Tumor Growth in Orthotopically Transplanted Mouse HCC
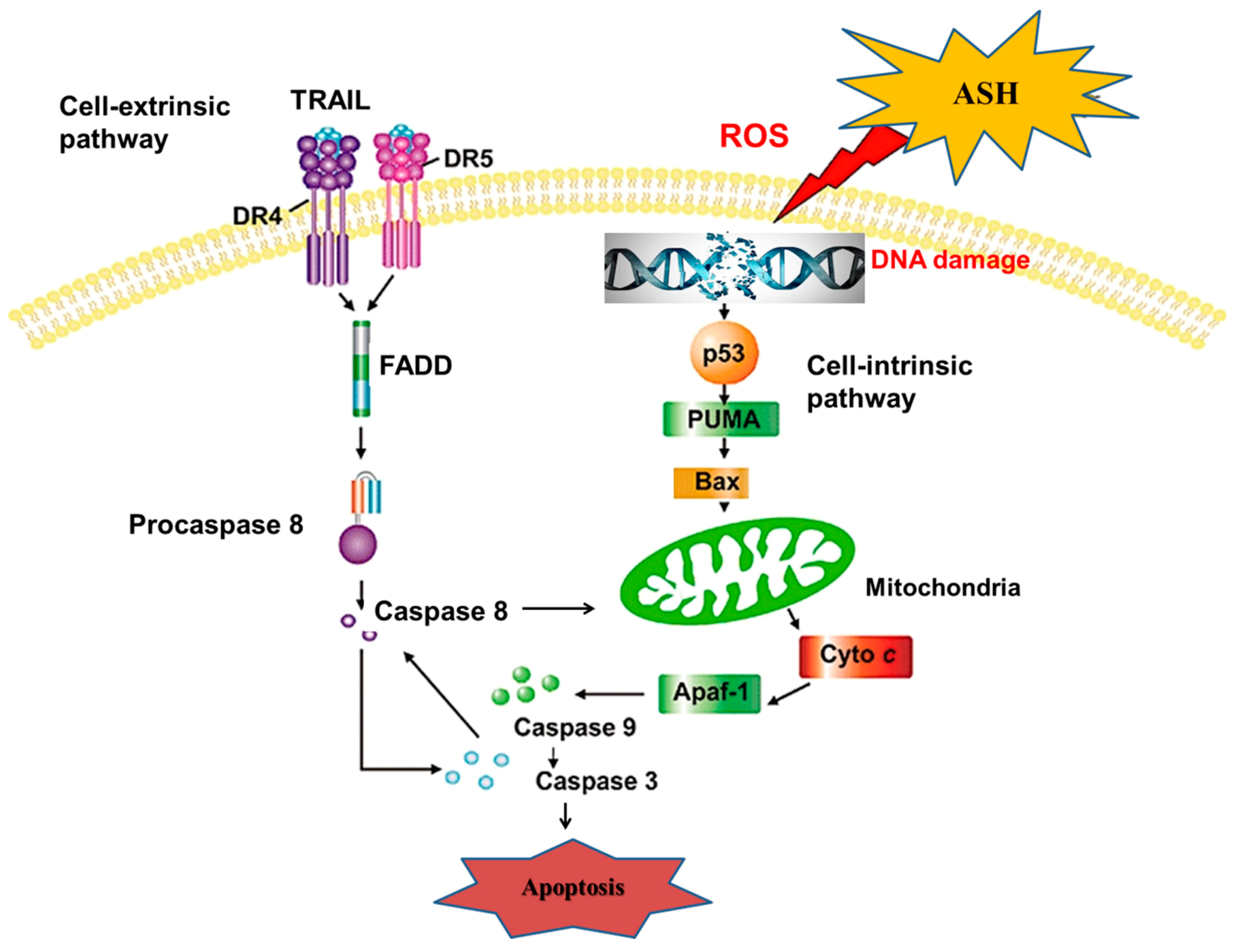
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gunjur, A. Short vs. long course adjuvant chemotherapy for colon cancer. Lancet Oncol. 2018, 19, e236. [Google Scholar] [CrossRef]
- Ilson, D.H. Adjuvant therapy in colon cancer: Less is more. Lancet Oncol. 2018, 19, 442–443. [Google Scholar] [CrossRef]
- Cervantes, A. Exploring better strategies for EGFR antibodies in colon cancer. Lancet Oncol. 2014, 15, 549–550. [Google Scholar] [CrossRef]
- Hong, M.; Wang, N.; Tan, H.Y.; Tsao, S.W.; Feng, Y. MicroRNAs and Chinese Medicinal Herbs: New Possibilities in Cancer Therapy. Cancers 2015, 7, 1643–1657. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.F.; Zhou, L.; Liao, F.; Wang, J. Effects of ebv-miR-BART7 on tumorigenicity, metastasis, and TRAIL sensitivity of non-small cell lung cancer. J. Cell. Biochem. 2019, 120, 10057–10068. [Google Scholar] [CrossRef] [PubMed]
- Radke, D.I.; Ling, Q.; Hasler, R.; Alp, G.; Ungefroren, H.; Trauzold, A. Downregulation of TRAIL-Receptor 1 Increases TGFbeta Type II Receptor Expression and TGFbeta Signalling Via MicroRNA-370-3p in Pancreatic Cancer Cells. Cancers 2018, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yang, Z.; Wang, X.; Tao, R.; Zhou, Y. TRAIL Enhances Shikonin Induced Apoptosis through ROS/JNK Signaling in Cholangiocarcinoma Cells. Cell. Physiol. Biochem. 2017, 42, 1073–1086. [Google Scholar] [CrossRef]
- Wen, X.; Li, J.; Cai, D.; Yue, L.; Wang, Q.; Zhou, L.; Fan, L.; Sun, J.; Wu, Y. Anticancer Efficacy of Targeted Shikonin Liposomes Modified with RGD in Breast Cancer Cells. Molecules 2018, 23, 268. [Google Scholar] [CrossRef]
- Wei, Y.; Li, M.; Cui, S.; Wang, D.; Zhang, C.Y.; Zen, K.; Li, L. Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules 2016, 21, 777. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, S.; Chen, A.; Zhang, Y.; Liu, M.; Wang, X. Evaluation of the inhibition risk of shikonin on human and rat UDP-glucuronosyltransferases (UGT) through the cocktail approach. Toxicol. Lett. 2019, 312, 214–221. [Google Scholar] [CrossRef]
- Andujar, I.; Rios, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Pavela, R.; Kolarcik, V.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Dall’Acqua, S.; Benelli, G. Identification of Onosma visianii Roots Extract and Purified Shikonin Derivatives as Potential Acaricidal Agents against Tetranychus urticae. Molecules 2017, 22, 1002. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.P.; Zhang, X.Y.; Zhang, S.D. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi 1991, 11, 598–599. [Google Scholar] [PubMed]
- Figat, R.; Zgadzaj, A.; Geschke, S.; Sieczka, P.; Pietrosiuk, A.; Sommer, S.; Skrzypczak, A. Cytotoxicity and antigenotoxicity evaluation of acetylshikonin and shikonin. Drug Chem. Toxicol. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, J.H.; Park, H.R.; Choi, Y.W. Acetylshikonin inhibits growth of oral squamous cell carcinoma by inducing apoptosis. Arch. Oral Biol. 2016, 70, 149–157. [Google Scholar] [CrossRef]
- Park, S.H.; Phuc, N.M.; Lee, J.; Wu, Z.; Kim, J.; Kim, H.; Kim, N.D.; Lee, T.; Song, K.S.; Liu, K.H. Identification of acetylshikonin as the novel CYP2J2 inhibitor with anti-cancer activity in HepG2 cells. Phytomedicine 2017, 24, 134–140. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Li, S.S.; Sun, Z.; Lin, B.; Lang, Y.Y.; He, J.Y.; Cao, X.; Yan, T.; Wang, L.; et al. Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res. 2008, 68, 8871–8880. [Google Scholar] [CrossRef]
- Magalhaes-Novais, S.; Bermejo-Millo, J.C.; Loureiro, R.; Mesquita, K.A.; Domingues, M.R.; Maciel, E.; Melo, T.; Baldeiras, I.; Erickson, J.R.; Holy, J.; et al. Cell quality control mechanisms maintain stemness and differentiation potential of P19 embryonic carcinoma cells. Autophagy 2019, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Huang, Y.; Liu, Y.; Zhao, Y.; He, X.; Zhang, L.; Wang, F.; Zhang, Y. Ipatasertib, a novel Akt inhibitor, induces transcription factor FoxO3a and NF-kappaB directly regulates PUMA-dependent apoptosis. Cell Death Dis. 2018, 9, 911. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef]
- Xiong, W.; Luo, G.; Zhou, L.; Zeng, Y.; Yang, W. In vitro and in vivo antitumor effects of acetylshikonin isolated from Arnebia euchroma (Royle) Johnst (Ruanzicao) cell suspension cultures. Chin. Med. 2009, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, W.L.; Liang, W.C.; Law, W.K.; Tsz-Ming, I.D.; Ng, T.B.; Miu-Yee, W.M.; Chi-Cheong, W.D. Acetylshikonin, a Novel AChE Inhibitor, Inhibits Apoptosis via Upregulation of Heme Oxygenase-1 Expression in SH-SY5Y Cells. Evid. Based Complement Altern. Med. 2013, 2013, 937370. [Google Scholar] [CrossRef] [PubMed]
- Moeglin, E.; Desplancq, D.; Conic, S.; Oulad-Abdelghani, M.; Stoessel, A.; Chiper, M.; Vigneron, M.; Didier, P.; Tora, L.; Weiss, E. Uniform Widespread Nuclear Phosphorylation of Histone H2AX Is an Indicator of Lethal DNA Replication Stress. Cancers 2019, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nagano, H.; Sakon, M.; Wada, H.; Eguchi, H.; Kondo, M.; Damdinsuren, B.; Ota, H.; Nakamura, M.; Wada, H.; et al. Partial contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway to antitumor effects of interferon-alpha/5-fluorouracil against Hepatocellular Carcinoma. Clin. Cancer Res. 2004, 10, 7884–7895. [Google Scholar] [CrossRef] [PubMed]
- Su, B.C.; Pan, C.Y.; Chen, J.Y. Antimicrobial Peptide TP4 Induces ROS-Mediated Necrosis by Triggering Mitochondrial Dysfunction in Wild-Type and Mutant p53 Glioblastoma Cells. Cancers 2019, 11, 71. [Google Scholar] [CrossRef]
- Weyemi, U.; Paul, B.D.; Bhattacharya, D.; Malla, A.P.; Boufraqech, M.; Harraz, M.M.; Bonner, W.M.; Snyder, S.H. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 7471–7476. [Google Scholar] [CrossRef]
- Saint-Germain, E.; Mignacca, L.; Huot, G.; Acevedo, M.; Moineau-Vallee, K.; Calabrese, V.; Bourdeau, V.; Rowell, M.C.; Ilangumaran, S.; Lessard, F.; et al. Phosphorylation of SOCS1 Inhibits the SOCS1-p53 Tumor Suppressor Axis. Cancer Res. 2019, 79, 3306–3319. [Google Scholar] [CrossRef]
- Pulikkan, J.A.; Hegde, M.; Ahmad, H.M.; Belaghzal, H.; Illendula, A.; Yu, J.; O’Hagan, K.; Ou, J.; Muller-Tidow, C.; Wolfe, S.A.; et al. CBFbeta-SMMHC Inhibition Triggers Apoptosis by Disrupting MYC Chromatin Dynamics in Acute Myeloid Leukemia. Cell 2018, 174, 1325. [Google Scholar] [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862. [Google Scholar] [CrossRef]
- Panner, A.; Parsa, A.T.; Pieper, R.O. Use of APO2L/TRAIL with mTOR inhibitors in the treatment of glioblastoma multiforme. Expert Rev. Anticancer Ther. 2006, 6, 1313–1322. [Google Scholar] [CrossRef]
- Wang, G.; Zhan, Y.; Wang, H.; Li, W. ABT-263 sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating the Bcl-2 family of anti-apoptotic protein. Cancer Chemother. Pharm. 2012, 69, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.; Lu, P.; Voelkel-Johnson, C. The histone deacetylase inhibitors depsipeptide and MS-275, enhance TRAIL gene therapy of LNCaP prostate cancer cells without adverse effects in normal prostate epithelial cells. Cancer Gene Ther. 2007, 14, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ganten, T.M.; Koschny, R.; Haas, T.L.; Sykora, J.; Li-Weber, M.; Herzer, K.; Walczak, H. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology 2005, 42, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.P.; Hatch, V.; Horvitz, H.R. Programmed elimination of cells by caspase-independent cell extrusion in C. elegans. Nature 2012, 488, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Hua, A.B.; Justiniano, R.; Perer, J.; Park, S.L.; Li, H.; Cabello, C.M.; Wondrak, G.T. Repurposing the Electron Transfer Reactant Phenazine Methosulfate (PMS) for the Apoptotic Elimination of Malignant Melanoma Cells through Induction of Lethal Oxidative and Mitochondriotoxic Stress. Cancers 2019, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]


| Gene Name | Gene Bank ID | siRNA Sequences |
|---|---|---|
| P53 | AB082923 | F: 5′-CUCCAUCUGGGAAUGACUU-3′ R: 5′-UAUCCAAUCUGAACUGGAC-3′ |
| PUMA | AF354654 | F: 5′-GUGCCAUUGCGAAUGAAUA-3′ R: 5′-ACUUCAUUCUCAAGUGACC-3′ |
| Serum Chemistry | Control | ASH + TRAIL | p-Value |
|---|---|---|---|
| Blood urea nitrogen (mg/dL) | 54.7 ± 4.97 | 49.8 ± 2.57 | 0.221 |
| Serum creatinine (µmol/L) | 105.48 ± 8.52 | 112.60 ± 7.58 | 0.647 |
| Alanine transaminase (U/L) | 59.78 ± 9.39 | 67.48 ± 12.81 | 0.569 |
| Aspartate transaminase (U/L) | 323.94 ± 71.4 | 380.15 ± 87.3 | 0.309 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Li, J.; Li, S.; M.Almutairi, M. RETRACTED: Acetylshikonin Sensitizes Hepatocellular Carcinoma Cells to Apoptosis through ROS-Mediated Caspase Activation. Cells 2019, 8, 1466. https://doi.org/10.3390/cells8111466
Hong M, Li J, Li S, M.Almutairi M. RETRACTED: Acetylshikonin Sensitizes Hepatocellular Carcinoma Cells to Apoptosis through ROS-Mediated Caspase Activation. Cells. 2019; 8(11):1466. https://doi.org/10.3390/cells8111466
Chicago/Turabian StyleHong, Ming, Jinke Li, Siying Li, and Mohammed M.Almutairi. 2019. "RETRACTED: Acetylshikonin Sensitizes Hepatocellular Carcinoma Cells to Apoptosis through ROS-Mediated Caspase Activation" Cells 8, no. 11: 1466. https://doi.org/10.3390/cells8111466
APA StyleHong, M., Li, J., Li, S., & M.Almutairi, M. (2019). RETRACTED: Acetylshikonin Sensitizes Hepatocellular Carcinoma Cells to Apoptosis through ROS-Mediated Caspase Activation. Cells, 8(11), 1466. https://doi.org/10.3390/cells8111466




