Targeting the Stress-Induced Protein NUPR1 to Treat Pancreatic Adenocarcinoma
Abstract
1. Introduction
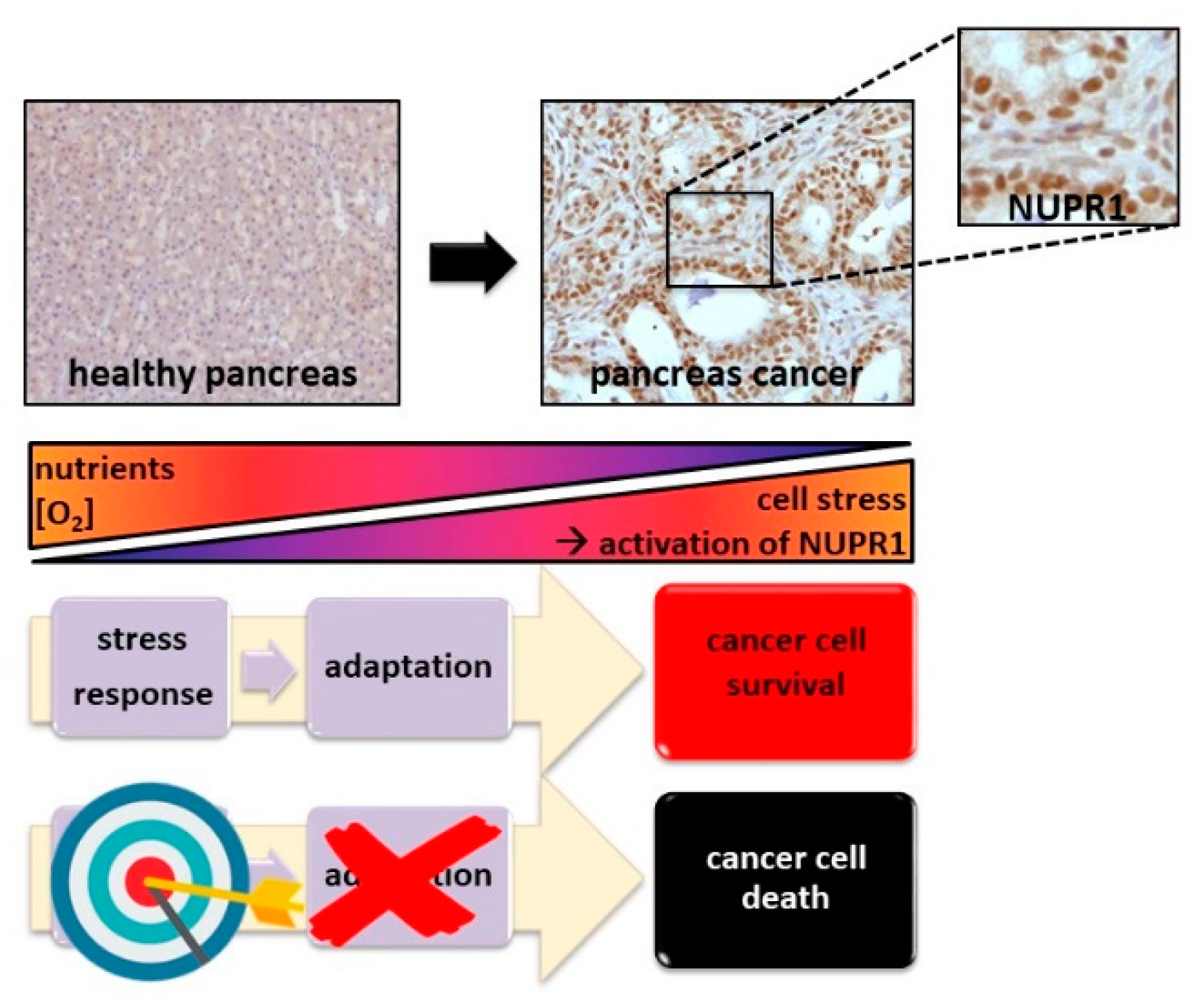
2. Why Is the Stress Response Essential to Cancer Cells and Why Could It Be an Exploitable Therapeutic Route?
3. The Intrinsically Disordered Stress Protein NUPR1 in PDAC
4. Screening Small Compounds as Anticancer Agents against NUPR1
5. ZZW-115 Is a Strongly Improved Trifluoperazine-Derived Compound with a New Mechanism of Action
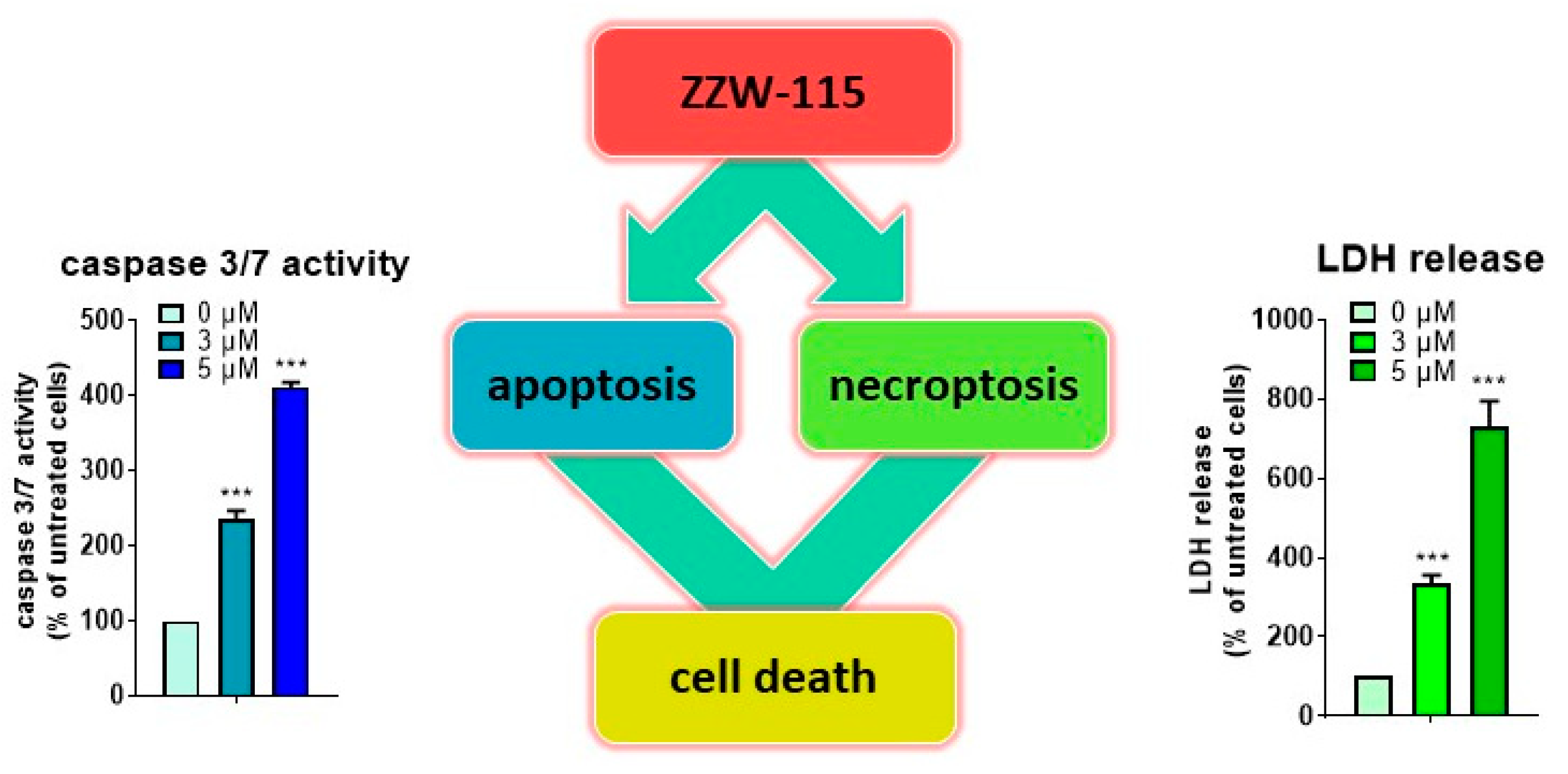
6. ZZW-115 Induces Tumor Cell Death by Necroptosis and Apoptosis
7. ZZW-115 Is Active in Some Type of Cancers
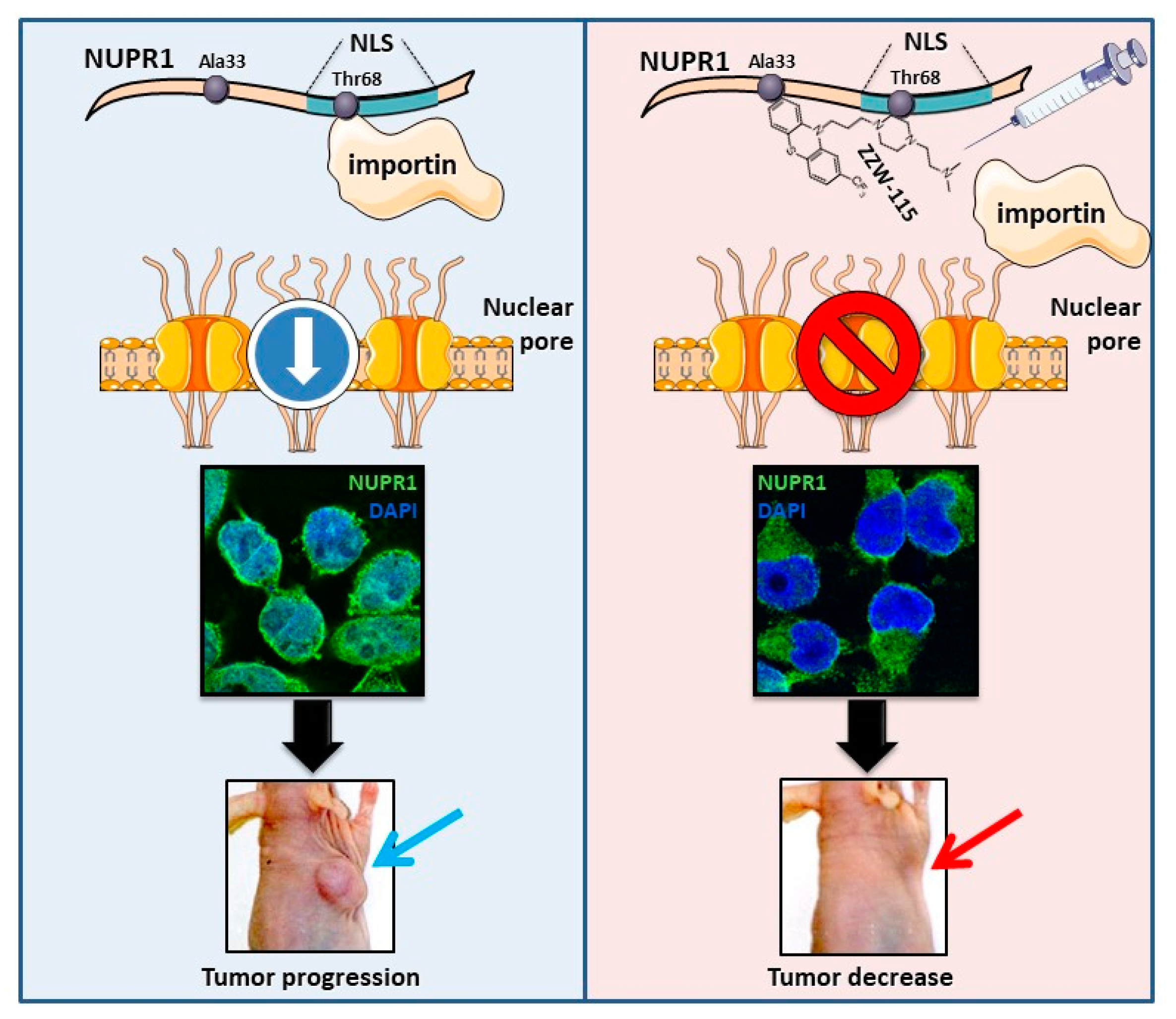
8. ZZW-115 Inhibits the Nuclear Translocation of NUPR1 by Competing with Importins
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef]
- Iovanna, J.L.; Marks, D.L.; Fernandez-Zapico, M.E.; Urrutia, R. Mechanistic insights into self-reinforcing processes driving abnormal histogenesis during the development of pancreatic cancer. Am. J. Pathol. 2013, 182, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, S. Therapeutic targeting of cellular stress responses in cancer. Thorac. Cancer 2018, 9, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Rah, B.; Nayak, D.; Rasool, R.; Chakraborty, S.; Katoch, A.; Amin, H.; Goswami, A. Reprogramming of Molecular Switching Events in UPR Driven ER Stress: Scope for Development of Anticancer Therapeutics. Curr. Mol. Med. 2016, 16, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Cano, C.E.; Iovanna, J.L. Stress proteins and pancreatic cancer metastasis. Sci. World J. 2010, 10, 1958–1966. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef]
- Jego, G.; Hazoume, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef]
- Mallo, G.V.; Fiedler, F.; Calvo, E.L.; Ortiz, E.M.; Vasseur, S.; Keim, V.; Morisset, J.; Iovanna, J.L. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J. Biol. Chem. 1997, 272, 32360–32369. [Google Scholar] [CrossRef]
- Garcia-Montero, A.; Vasseur, S.; Mallo, G.V.; Soubeyran, P.; Dagorn, J.C.; Iovanna, J.L. Expression of the stress-induced p8 mRNA is transiently activated after culture medium change. Eur J. Cell Biol. 2001, 80, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Goruppi, S.; Iovanna, J.L. Stress-inducible protein p8 is involved in several physiological and pathological processes. J. Biol. Chem. 2010, 285, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Malicet, C.; Dagorn, J.C.; Neira, J.L.; Iovanna, J.L. p8 and prothymosin alpha: Unity is strength. Cell Cycle 2006, 5, 829–830. [Google Scholar] [CrossRef][Green Version]
- Malicet, C.; Giroux, V.; Vasseur, S.; Dagorn, J.C.; Neira, J.L.; Iovanna, J.L. Regulation of apoptosis by the p8/prothymosin alpha complex. Proc. Natl. Acad. Sci. USA 2006, 103, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.; Garcia, M.N.; Hamidi, T.; Cano, C.; Calvo, E.; Lomberk, G.; Urrutia, R.; Iovanna, J.L. Genetic inactivation of the pancreatitis-inducible gene Nupr1 impairs PanIN formation by modulating Kras(G12D)-induced senescence. Cell Death Differ. 2014, 21, 1633–1641. [Google Scholar] [CrossRef]
- Sandi, M.J.; Hamidi, T.; Malicet, C.; Cano, C.; Loncle, C.; Pierres, A.; Dagorn, J.C.; Iovanna, J.L. p8 expression controls pancreatic cancer cell migration, invasion, adhesion, and tumorigenesis. J. Cell. Physiol. 2011, 226, 3442–3451. [Google Scholar] [CrossRef]
- Ree, A.H.; Pacheco, M.M.; Tvermyr, M.; Fodstad, O.; Brentani, M.M. Expression of a novel factor, com1, in early tumor progression of breast cancer. Clin. Cancer Res. 2000, 6, 1778–1783. [Google Scholar]
- Cano, C.E.; Hamidi, T.; Garcia, M.N.; Grasso, D.; Loncle, C.; Garcia, S.; Calvo, E.; Lomberk, G.; Dusetti, N.; Bartholin, L.; et al. Genetic inactivation of Nupr1 acts as a dominant suppressor event in a two-hit model of pancreatic carcinogenesis. Gut 2014, 63, 984–995. [Google Scholar] [CrossRef]
- Hamidi, T.; Algul, H.; Cano, C.E.; Sandi, M.J.; Molejon, M.I.; Riemann, M.; Calvo, E.L.; Lomberk, G.; Dagorn, J.C.; Weih, F.; et al. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J. Clin. Investig. 2012, 122, 2092–2103. [Google Scholar] [CrossRef]
- Giroux, V.; Malicet, C.; Barthet, M.; Gironella, M.; Archange, C.; Dagorn, J.C.; Vasseur, S.; Iovanna, J.L. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin. Cancer Res. 2006, 12, 235–241. [Google Scholar] [CrossRef]
- Palam, L.R.; Gore, J.; Craven, K.E.; Wilson, J.L.; Korc, M. Integrated stress response is critical for gemcitabine resistance in pancreatic ductal adenocarcinoma. Cell Death Dis. 2015, 6, e1913. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhang, Z.; Bai, Z.; Ma, X.; Guo, W.; Wang, Y. Enhancement of gemcitabine sensitivity in pancreatic cancer by co-regulation of dCK and p8 expression. Oncol. Rep. 2011, 25, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, S.; Hoffmeister, A.; Garcia, S.; Bagnis, C.; Dagorn, J.C.; Iovanna, J.L. p8 is critical for tumour development induced by rasV12 mutated protein and E1A oncogene. EMBO Rep. 2002, 3, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Emma, M.R.; Iovanna, J.L.; Bachvarov, D.; Puleio, R.; Loria, G.R.; Augello, G.; Candido, S.; Libra, M.; Gulino, A.; Cancila, V.; et al. NUPR1, a new target in liver cancer: Implication in controlling cell growth, migration, invasion and sorafenib resistance. Cell Death Dis. 2016, 7, e2269. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, W.; Hu, J.; Feng, K.; Pan, Y.; Zhang, L.; Feng, Y. Lentivirus-mediated RNAi knockdown of NUPR1 inhibits human nonsmall cell lung cancer growth in vitro and in vivo. Anat. Rec. (Hoboken) 2012, 295, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Jin, D.I.; Yoon, S.; Baek, S.Y.; Kim, B.S.; Oh, S.O. Expression and roles of NUPR1 in cholangiocarcinoma cells. Anat. Cell Biol. 2012, 45, 17–25. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Liu, Y.; Lian, Z.; Dong, B.; Yao, Y.; Xu, Y. Knockdown of NUPR1 inhibits the proliferation of glioblastoma cells via ERK1/2, p38 MAPK and caspase-3. J. Neurooncol. 2017, 132, 15–26. [Google Scholar] [CrossRef]
- Zeng, C.; Yi, B.; Li, X.; Chen, J. [Knockdown of nuclear protein 1 (NUPR1) gene inhibits proliferation and promotes apoptosis of human multiple myeloma U266 cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017, 33, 1240–1246. [Google Scholar]
- Zeng, C.; Li, X.; Li, A.; Yi, B.; Peng, X.; Huang, X.; Chen, J. Knockdown of NUPR1 inhibits the growth of U266 and RPMI8226 multiple myeloma cell lines via activating PTEN and caspase activation-dependent apoptosis. Oncol. Rep. 2018. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, J.; Lin, J.; Lin, R.; Chen, K.; Kong, J.; Shui, X. Long non-coding RNA FEZF1-AS1 promotes osteosarcoma progression by regulating miR-4443/NUPR1 axis. Oncol. Res. 2018, 26, 1335–1343. [Google Scholar] [CrossRef]
- Aguado-Llera, D.; Hamidi, T.; Domenech, R.; Pantoja-Uceda, D.; Gironella, M.; Santoro, J.; Velazquez-Campoy, A.; Neira, J.L.; Iovanna, J.L. Deciphering the binding between Nupr1 and MSL1 and their DNA-repairing activity. PLoS ONE 2013, 8, e78101. [Google Scholar] [CrossRef] [PubMed]
- Encinar, J.A.; Mallo, G.V.; Mizyrycki, C.; Giono, L.; Gonzalez-Ros, J.M.; Rico, M.; Canepa, E.; Moreno, S.; Neira, J.L.; Iovanna, J.L. Human p8 is a HMG-I/Y-like protein with DNA binding activity enhanced by phosphorylation. J. Biol. Chem. 2001, 276, 2742–2751. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.L.; Rizzuti, B.; Iovanna, J.L. Determinants of the pKa values of ionizable residues in an intrinsically disordered protein. Arch. Biochem. Biophys. 2016, 598, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Santofimia-Castano, P.; Rizzuti, B.; Abian, O.; Velazquez-Campoy, A.; Iovanna, J.L.; Neira, J.L. Amphipathic helical peptides hamper protein-protein interactions of the intrinsically disordered chromatin nuclear protein 1 (NUPR1). Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Santofimia-Castano, P.; Rizzuti, B.; Pey, A.L.; Soubeyran, P.; Vidal, M.; Urrutia, R.; Iovanna, J.L.; Neira, J.L. Intrinsically disordered chromatin protein NUPR1 binds to the C-terminal region of Polycomb RING1B. Proc. Natl. Acad. Sci. USA 2017, 114, 6332–6341. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, V.S.; Fred, L.M.; Gerhart, K.P.; Metallo, S.J. Characterization of the Binding of Small Molecules to Intrinsically Disordered Proteins. Methods Enzymol. 2018, 611, 677–702. [Google Scholar] [CrossRef]
- Neira, J.L.; Bintz, J.; Arruebo, M.; Rizzuti, B.; Bonacci, T.; Vega, S.; Lanas, A.; Velazquez-Campoy, A.; Iovanna, J.L.; Abian, O. Identification of a Drug Targeting an Intrinsically Disordered Protein Involved in Pancreatic Adenocarcinoma. Sci. Rep. 2017, 7, 39732. [Google Scholar] [CrossRef]
- Santofimia-Castano, P.; Xia, Y.; Lan, W.; Zhou, Z.; Huang, C.; Peng, L.; Soubeyran, P.; Velazquez-Campoy, A.; Abian, O.; Rizzuti, B.; et al. Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis. J. Clin. Investig. 2019, 130. [Google Scholar] [CrossRef]
- Santofimia-Castano, P.; Lan, W.; Bintz, J.; Gayet, O.; Carrier, A.; Lomberk, G.; Neira, J.L.; Gonzalez, A.; Urrutia, R.; Soubeyran, P.; et al. Inactivation of NUPR1 promotes cell death by coupling ER-stress responses with necrosis. Sci. Rep. 2018, 8, 16999. [Google Scholar] [CrossRef]
- Valacco, M.P.; Varone, C.; Malicet, C.; Canepa, E.; Iovanna, J.L.; Moreno, S. Cell growth-dependent subcellular localization of p8. J. Cell. Biochem. 2006, 97, 1066–1079. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santofimia-Castaño, P.; Xia, Y.; Peng, L.; Velázquez-Campoy, A.; Abián, O.; Lan, W.; Lomberk, G.; Urrutia, R.; Rizzuti, B.; Soubeyran, P.; et al. Targeting the Stress-Induced Protein NUPR1 to Treat Pancreatic Adenocarcinoma. Cells 2019, 8, 1453. https://doi.org/10.3390/cells8111453
Santofimia-Castaño P, Xia Y, Peng L, Velázquez-Campoy A, Abián O, Lan W, Lomberk G, Urrutia R, Rizzuti B, Soubeyran P, et al. Targeting the Stress-Induced Protein NUPR1 to Treat Pancreatic Adenocarcinoma. Cells. 2019; 8(11):1453. https://doi.org/10.3390/cells8111453
Chicago/Turabian StyleSantofimia-Castaño, Patricia, Yi Xia, Ling Peng, Adrián Velázquez-Campoy, Olga Abián, Wenjun Lan, Gwen Lomberk, Raul Urrutia, Bruno Rizzuti, Philippe Soubeyran, and et al. 2019. "Targeting the Stress-Induced Protein NUPR1 to Treat Pancreatic Adenocarcinoma" Cells 8, no. 11: 1453. https://doi.org/10.3390/cells8111453
APA StyleSantofimia-Castaño, P., Xia, Y., Peng, L., Velázquez-Campoy, A., Abián, O., Lan, W., Lomberk, G., Urrutia, R., Rizzuti, B., Soubeyran, P., Neira, J. L., & Iovanna, J. (2019). Targeting the Stress-Induced Protein NUPR1 to Treat Pancreatic Adenocarcinoma. Cells, 8(11), 1453. https://doi.org/10.3390/cells8111453







