Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
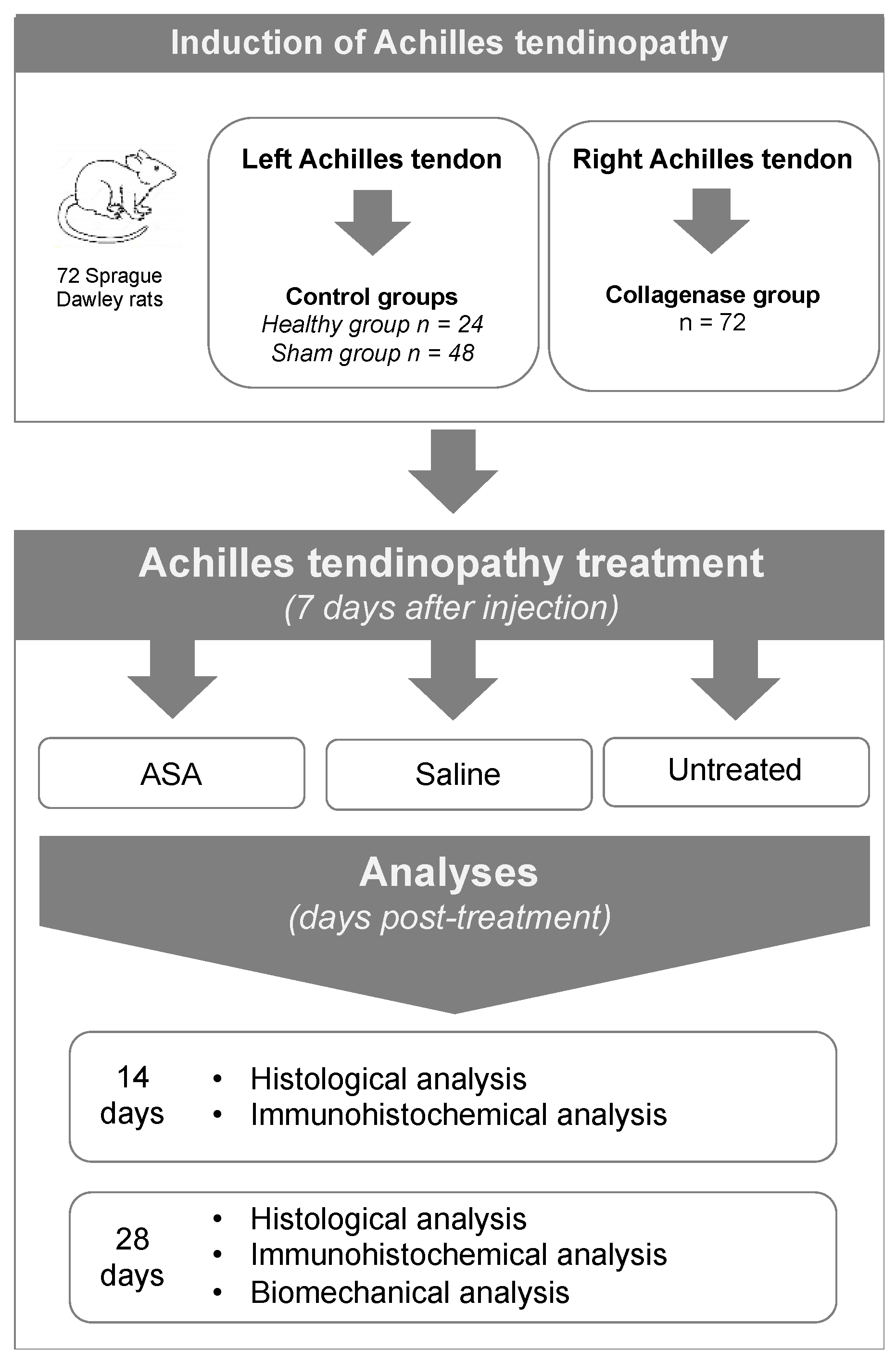
2.2. Study Design
2.3. In Vivo Procedures
2.3.1. Achilles Tendinopathy Induction
2.3.2. Amniotic Suspension Allograft (ASA) Preparation
2.3.3. Achilles Tendinopathy Treatment with ASA
2.3.4. Animal Care and Euthanasia
2.4. Histological and Immunohistochemical (IHC) Analyses
2.5. Biomechanical Testing
2.6. Statistical Analyses
3. Results
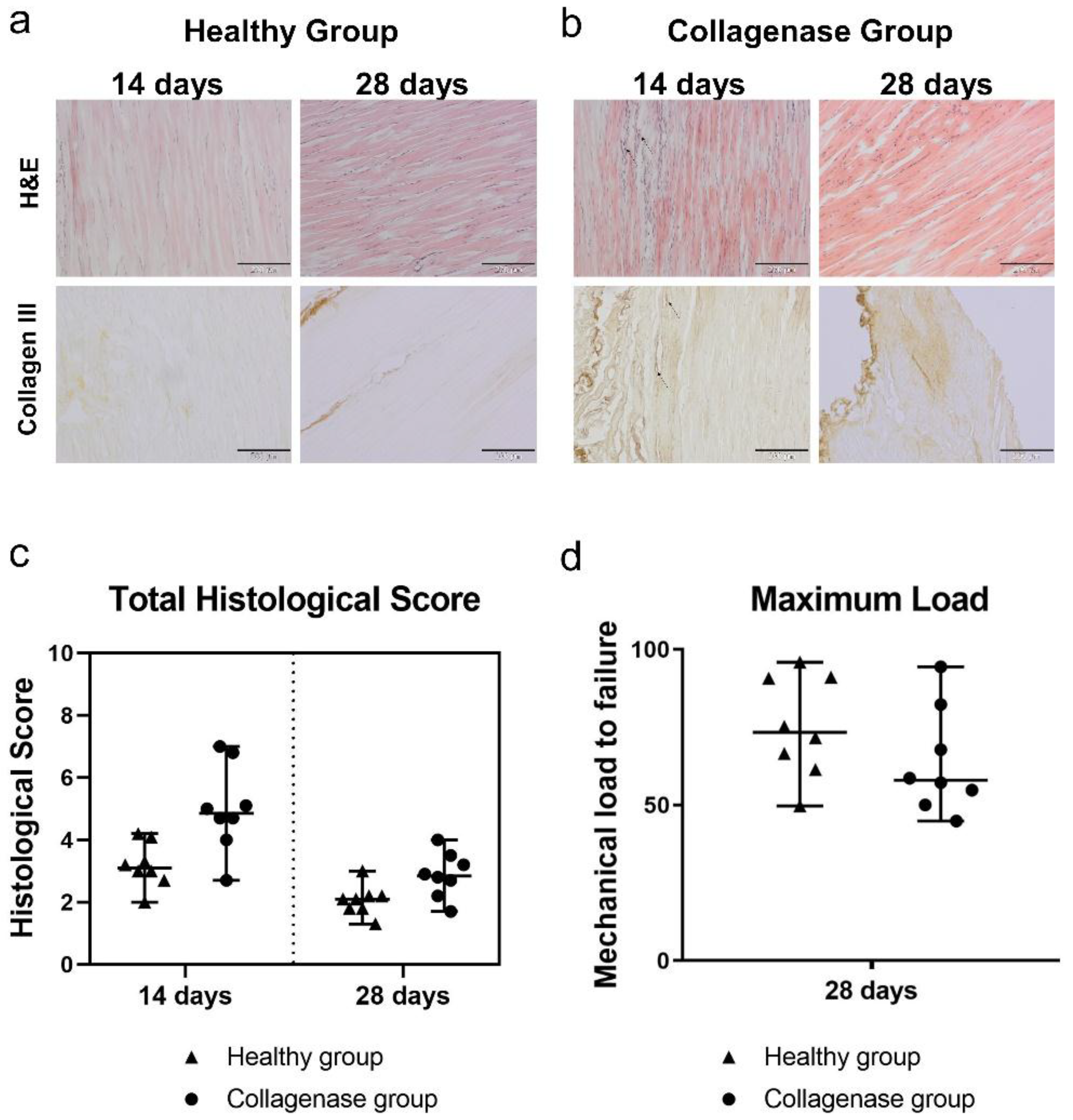
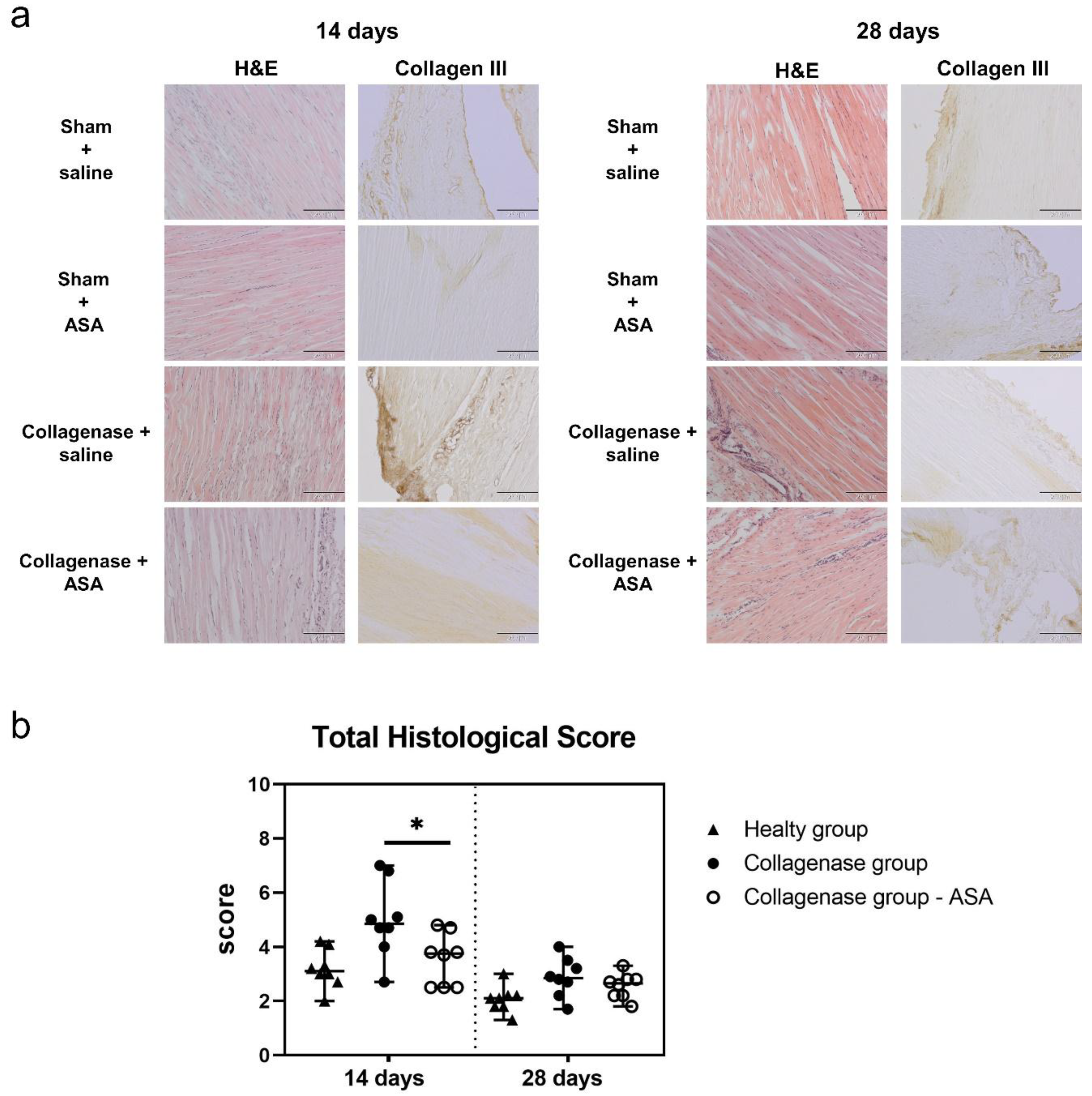
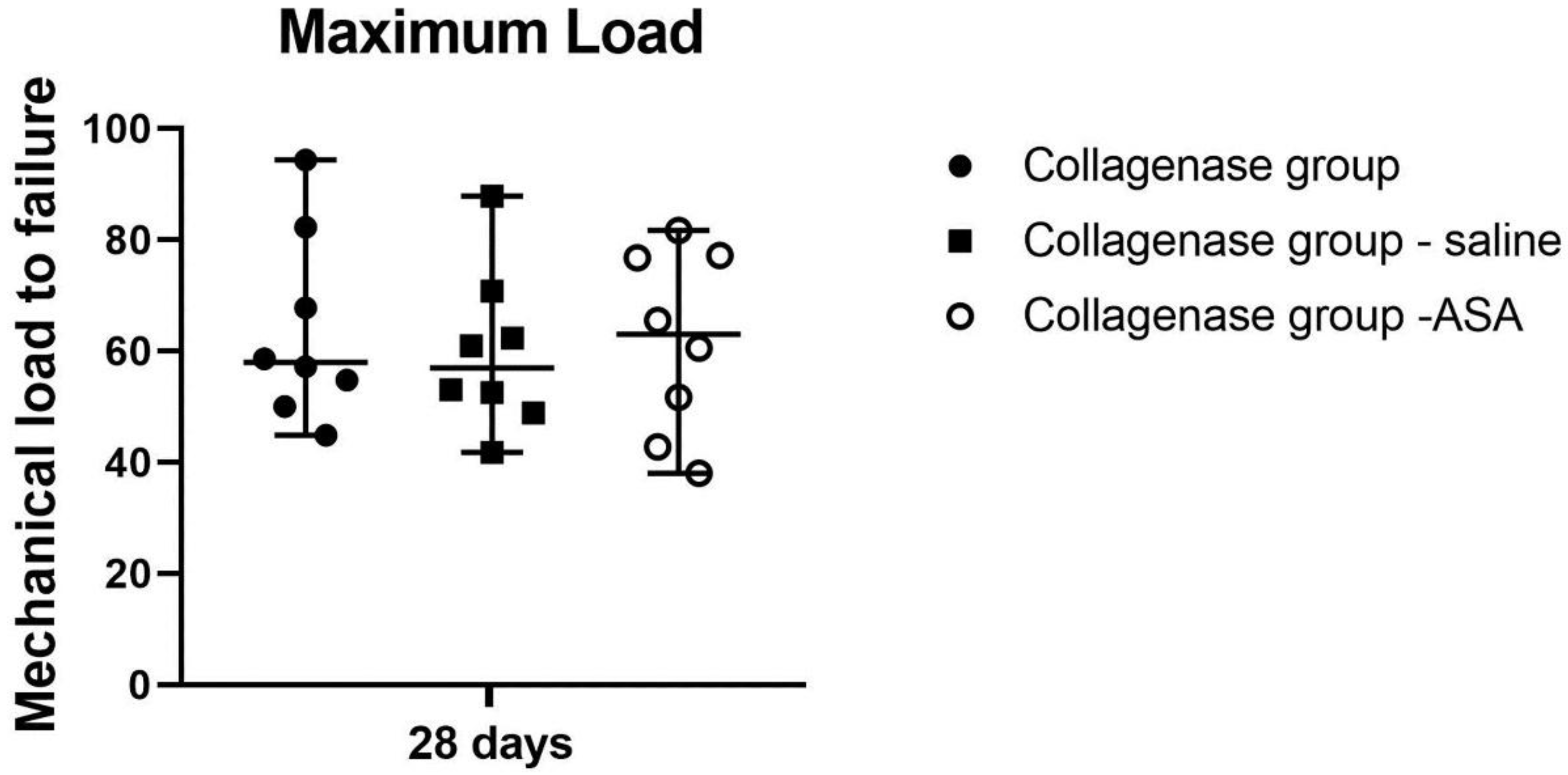
3.1. Histopathological Findings and Score Analyses
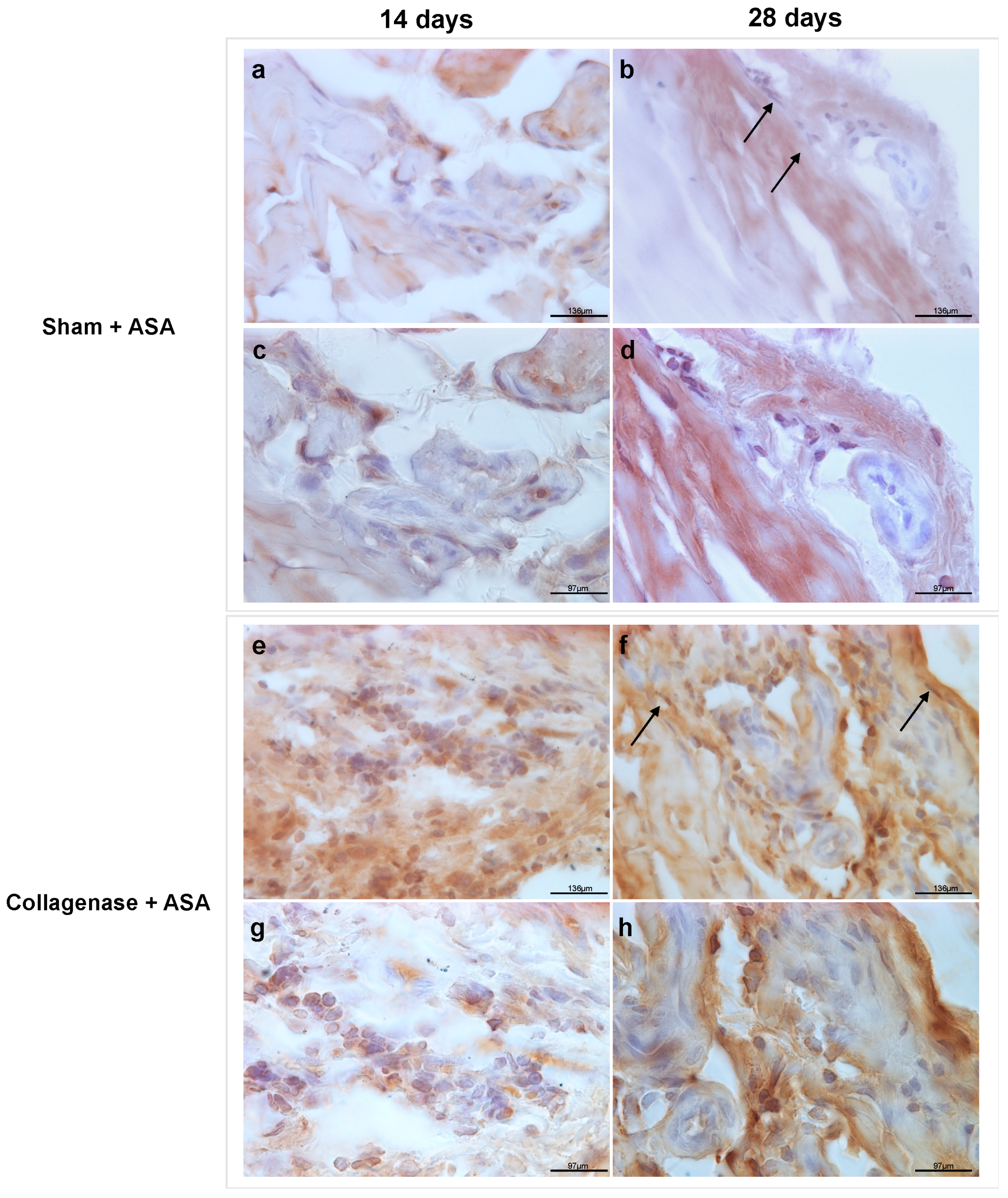
3.2. Qualitative Observations Of Retention of ASA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sussman, W.I.; Mautner, K.; Malanga, G. The role of rehabilitation after regenerative and orthobiologic procedures for the treatment of tendinopathy: A systematic review. Regen. Med. 2018, 13, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Sun, H.B. Mesenchymal stem cells in tendon repair and regeneration: Basic understanding and translational challenges. Ann. N. Y. Acad. Sci. 2016, 1383, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, K.; Villarreal, A.; Gottschalk, A.; Tokish, J.M.; Choate, W.S. Treatment of Muscle Injuries with Platelet-Rich Plasma: A Review of the Literature. Curr. Rev. Musculoskelet. Med. 2018, 11, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Vigano’, M.; Lanfranchi, L.; Alfieri Montrasio, U.; de Girolamo, L. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: Results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.A.; Jones, I.A.; Danilkovich, A.; Vangsness, C.T. The Placenta: Applications in Orthopaedic Sports Medicine. Am. J. Sports Med. 2017, 46, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Nunley, P.D.; Kerr, E.J.; Utter, P.A.; Cavanaugh, D.A.; Frank, K.A.; Moody, D.; McManus, B.; Stone, M.B. Preliminary Results of Bioactive Amniotic Suspension with Allograft for Achieving One and Two-Level Lumbar Interbody Fusion. Int. J. Spine Surg. 2016, 10, 12. [Google Scholar] [CrossRef]
- Vines, J.B.; Aliprantis, A.O.; Gomoll, A.H.; Farr, J. Cryopreserved Amniotic Suspension for the Treatment of Knee Osteoarthritis. J. Knee Surg. 2016, 29, 443–450. [Google Scholar] [CrossRef]
- Delo, D.M.; De Coppi, P.; Bartsch, G., Jr.; Atala, A. Amniotic Fluid and Placental Stem Cells. Methods Enzymol. 2006, 419, 426. [Google Scholar]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Cole, B.J. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am. J. Sports Med. 2016, 44, 2425–2434. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, D.H.-K.; Hwang, D.G.; Kim, W.-S.; Zhang, F. Identification of Antiangiogenic and Antiinflammatory Proteins in Human Amniotic Membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef]
- Heckmann, N.; Auran, R.; Mirzayan, R. Application of Amniotic Tissue in Orthopedic Surgery. Am. J. Orthop. 2016, 421–425. [Google Scholar]
- Koh, J.W.; Shin, Y.J.; Oh, J.Y.; Kim, M.K.; Ko, J.H.; Hwang, J.M.; Wee, W.R.; Lee, J.H. The expression of TIMPs in cryo-preserved and freeze-dried amniotic membrane. Curr. Eye Res. 2007, 32, 611–616. [Google Scholar] [CrossRef] [PubMed]
- McQuilling, J.P.; Vines, J.B.; Kimmerling, K.A.; Mowry, K.C. Proteomic Comparison of Amnion and Chorion and Evaluation of the Effects of Processing on Placental Membranes. Wounds Compend. Clin. Res. Pract. 2017, 29, E38–E42. [Google Scholar]
- Najafbeygi, A.; Fatemi, M.J.; Lebaschi, A.H.; Mousavi, S.J.; Husseini, S.A.; Niazi, M. Effect of Basic Fibroblast Growth Factor on Achilles Tendon Healing in Rabbit. World J. Plast. Surg. 2017, 6, 26–32. [Google Scholar]
- Barboni, B.; Russo, V.; Curini, V.; Mauro, A.; Martelli, A.; Muttini, A.; Bernabò, N.; Valbonetti, L.; Marchisio, M.; Di Giacinto, O.; et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012, 21, 2377–2395. [Google Scholar] [CrossRef]
- Hanselman, A.E.; Tidwell, J.E.; Santrock, R.D. Cryopreserved Human Amniotic Membrane Injection for Plantar Fasciitis. Foot Ankle Int. 2015, 36, 151–158. [Google Scholar] [CrossRef]
- McQuilling, J.P.; Sanders, M.; Poland, L.; Sanders, M.; Giacomo, B.; Waldrop, N.E.; Mowry, K.C. Dehydrated Amnion/Chorion Improves Achilles Tendon Repair in a Diabetic Animal Model. Wounds A Compend. Clin. Res. Pract. 2019, 31, 19–25. [Google Scholar]
- Özgenel, G.Y.; Şamli, B.; Özcan, M. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. J. Hand Surg. Am. 2001, 26, 332–339. [Google Scholar] [CrossRef]
- Kimmerling, K.A.; McQuilling, J.P.; Staples, M.C.; Mowry, K.C. Tenocyte Cell Density, Migration, and Extracellular Matrix Deposition with Amniotic Suspension Allograft. J. Orthop. Res. 2018, 37, 412–420. [Google Scholar] [CrossRef]
- Kueckelhaus, M.; Philip, J.; Kamel, R.A.; Canseco, J.A.; Hackl, F.; Kiwanuka, E.; Kim, M.J.; Wilkie, R.; Caterson, E.J.; Junker, J.P.E.; et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty 2014, 14, e29. [Google Scholar]
- Philip, J.; Hackl, F.; Canseco, J.A.; Kamel, R.A.; Kiwanuka, E.; Diaz-Siso, J.R.; Caterson, E.J.; Junker, J.P.E.; Eriksson, E. Amnion-Derived Multipotent Progenitor Cells. Eplasty 2013, 13, 225–234. [Google Scholar]
- Gellhorn, A.C.; Han, A. The Use of Dehydrated Human Amnion/Chorion Membrane Allograft Injection for the Treatment of Tendinopathy or Arthritis: A Case Series Involving 40 Patients. PM R 2017, 9, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Orfei, C.P.; Lovati, A.B.; Viganò, M.; Stanco, D.; Bottagisio, M.; Di Giancamillo, A.; Setti, S.; De Girolamo, L. Dose-related and time-dependent development of collagenase-induced tendinopathy in rats. PLoS ONE 2016, 11, e0161590. [Google Scholar]
- Chen, L.; Liu, J.P.; Tang, K.L.; Wang, Q.; Wang, G.D.; Cai, X.H.; Liu, X.M. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell. Physiol. Biochem. 2014, 34, 2153–2168. [Google Scholar] [CrossRef] [PubMed]
- Kamineni, S.; Butterfield, T.; Sinai, A. Percutaneous ultrasonic debridement of tendinopathy—A pilot Achilles rabbit model. J. Orthop. Surg. Res. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Dirks, R.C.; Warden, S.J. Models for the study of tendinopathy. J. Musculoskelet. Neuronal Interact. 2011, 11, 141–149. [Google Scholar]
- Hast, M.W.; Zuskov, A.; Soslowsky, L.J. The role of animal models in tendon research. Bone Jt. Res. 2014, 3, 193–202. [Google Scholar] [CrossRef]
- Abbott, A. The Renaissance Rat. Nature 2004, 428, 464. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Screen, H.R.C. Fatigue loading of tendon. Int. J. Exp. Pathol. 2013, 94, 260–270. [Google Scholar] [CrossRef]
- Lake, S.P.; Ansorge, H.L.; Soslowsky, L.J. Animal models of tendinopathy. Disabil. Rehabil. 2008, 30, 1530–1541. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Maffulli, N.; Rolf, C.; Smith, R.K.W. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sport 2011, 21, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Casalechi, H.L.; Leal-Junior, E.C.P.; Xavier, M.; Silva, J.A.; De Carvalho, P.D.T.C.; Aimbire, F.; Albertini, R. Low-level laser therapy in experimental model of collagenase-induced tendinitis in rats: Effects in acute and chronic inflammatory phases. Lasers Med. Sci. 2013, 28, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.L.; Leal-Junior, E.C.P.; Arnold, G.; Magnenet, V.; Rahouadj, R.; Wang, X.; Demeurie, F.; Magdalou, J.; De Carvalho, M.H.C.; Lopes-Martins, R.Á.B. Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: Analyses of biochemical and biomechanical aspects. J. Orthop. Res. 2012, 30, 1945–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, U.G.; Ronga, M.; Maffulli, N. Achilles Tendinopathy. Sports Med. Arthrosc. 2018, 26, 112–126. [Google Scholar] [CrossRef]
- Yee Lui, P.P. Stem cell technology for tendon regeneration: Current status, challenges, and future research directions. Stem Cells Cloning Adv. Appl. 2015, 8, 163–174. [Google Scholar] [CrossRef]
- Zhang, K.; Asai, S.; Hast, M.W.; Liu, M.; Usami, Y.; Iwamoto, M.; Soslowsky, L.J.; Enomoto-Iwamoto, M. Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix Biol. 2016, 52–54, 315–324. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chieh, H.F.; Lin, C.J.; Jou, I.M.; Sun, Y.N.; Kuo, L.C.; Wu, P.T.; Su, F.C. Characteristics of Sonography in a Rat Achilles Tendinopathy Model: Possible Non-invasive Predictors of Biomechanics. Sci. Rep. 2017, 7, 5100. [Google Scholar] [CrossRef]
- Machova Urdzikova, L.; Sedlacek, R.; Suchy, T.; Amemori, T.; Ruzicka, J.; Lesny, P.; Havlas, V.; Sykova, E.; Jendelova, P. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed. Eng. Online 2014, 13, 42. [Google Scholar] [CrossRef]
- Shah, V.; Bendele, A.; Dines, J.S.; Kestler, H.K.; Hollinger, J.O.; Chahine, N.O.; Hee, C.K. Dose-response effect of an intra-tendon application of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in a rat Achilles tendinopathy model. J. Orthop. Res. 2013, 31, 413–420. [Google Scholar] [CrossRef]
- Demirkan, F.; Colakoglu, N.; Herek, O.; Erkula, G. The use of amniotic membrane in flexor tendon repair: An experimental model. Arch. Orthop. Trauma Surg. 2002, 122, 396–399. [Google Scholar] [CrossRef]
| Right Achilles Tendon (n = 72) | 14 Days Post-Treatment | 28 Days Post-Treatment | ||
|---|---|---|---|---|
| Induction of the pathology (day 0) | Treatment of the pathology (day 7) | Histological analysis (n) | Histological analysis (n) | Biomechanical analysis (n) |
| Collagenase group | Untreated | 8 | 8 | 8 |
| Collagenase group | ASA | 8 | 8 | 8 |
| Collagenase group | Saline | 8 | 8 | 8 |
| Score | Fiber Structure and Arrangement | Cell Density | Cell Appearance | Inflammatory Cell Inflammation | Neovascularization | Fatty Deposits |
|---|---|---|---|---|---|---|
| 0 | Normal: continuous, parallel collagen fibers | Normal | Spindle-shape cells | <10% | Normal presence of vascular bundles | Absence of lipid vacuoles |
| 1 | Slightly abnormal: partially disorganized and fragmented fibers | Slightly increased | Slightly rounded cells | 10–20% | Slight increase of vascular bundles | Slight increase of lipid vacuoles |
| 2 | Abnormal: moderately disorganized, fragmented, crossed, and wavy fibers | Moderately increased | Moderately rounded cells | 20–30% | Moderate increase of vascular bundles | Moderate increase of lipid vacuoles |
| 3 | Markedly abnormal: total disorganized and non-identifiable fiber pattern | Markedly increased | Markedly rounded cells | >30% | Marked increase of vascular bundles | Marked increase of lipid vacuoles |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Girolamo, L.; Morlin Ambra, L.F.; Perucca Orfei, C.; McQuilling, J.P.; Kimmerling, K.A.; Mowry, K.C.; Johnson, K.A.; Phan, A.T.; Whited, J.L.; Gomoll, A.H. Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy. Cells 2019, 8, 1411. https://doi.org/10.3390/cells8111411
de Girolamo L, Morlin Ambra LF, Perucca Orfei C, McQuilling JP, Kimmerling KA, Mowry KC, Johnson KA, Phan AT, Whited JL, Gomoll AH. Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy. Cells. 2019; 8(11):1411. https://doi.org/10.3390/cells8111411
Chicago/Turabian Stylede Girolamo, Laura, Luiz Felipe Morlin Ambra, Carlotta Perucca Orfei, John P. McQuilling, Kelly A. Kimmerling, Katie C. Mowry, Kimberly A. Johnson, Amy T. Phan, Jessica L. Whited, and Andreas H. Gomoll. 2019. "Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy" Cells 8, no. 11: 1411. https://doi.org/10.3390/cells8111411
APA Stylede Girolamo, L., Morlin Ambra, L. F., Perucca Orfei, C., McQuilling, J. P., Kimmerling, K. A., Mowry, K. C., Johnson, K. A., Phan, A. T., Whited, J. L., & Gomoll, A. H. (2019). Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy. Cells, 8(11), 1411. https://doi.org/10.3390/cells8111411






