Inhibition of GSK3β Reduces Ectopic Lipid Accumulation and Induces Autophagy by The AMPK Pathway in Goat Muscle Satellite Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Muscle Satellite Cells’ Isolation and Adipogenic Differentiation
2.3. Immunofluorescence Assa
2.4. Cell Viability Assay
2.5. Caspase-3 and Caspase-8 Activity Assay
2.6. Oil Red O (ORO) Staining
2.7. Triglyceride (TG) Content Assay
2.8. Total RNA Isolation, cDNA Synthesis and Qpcr Analysis
2.9. Western Blotting
3. Results
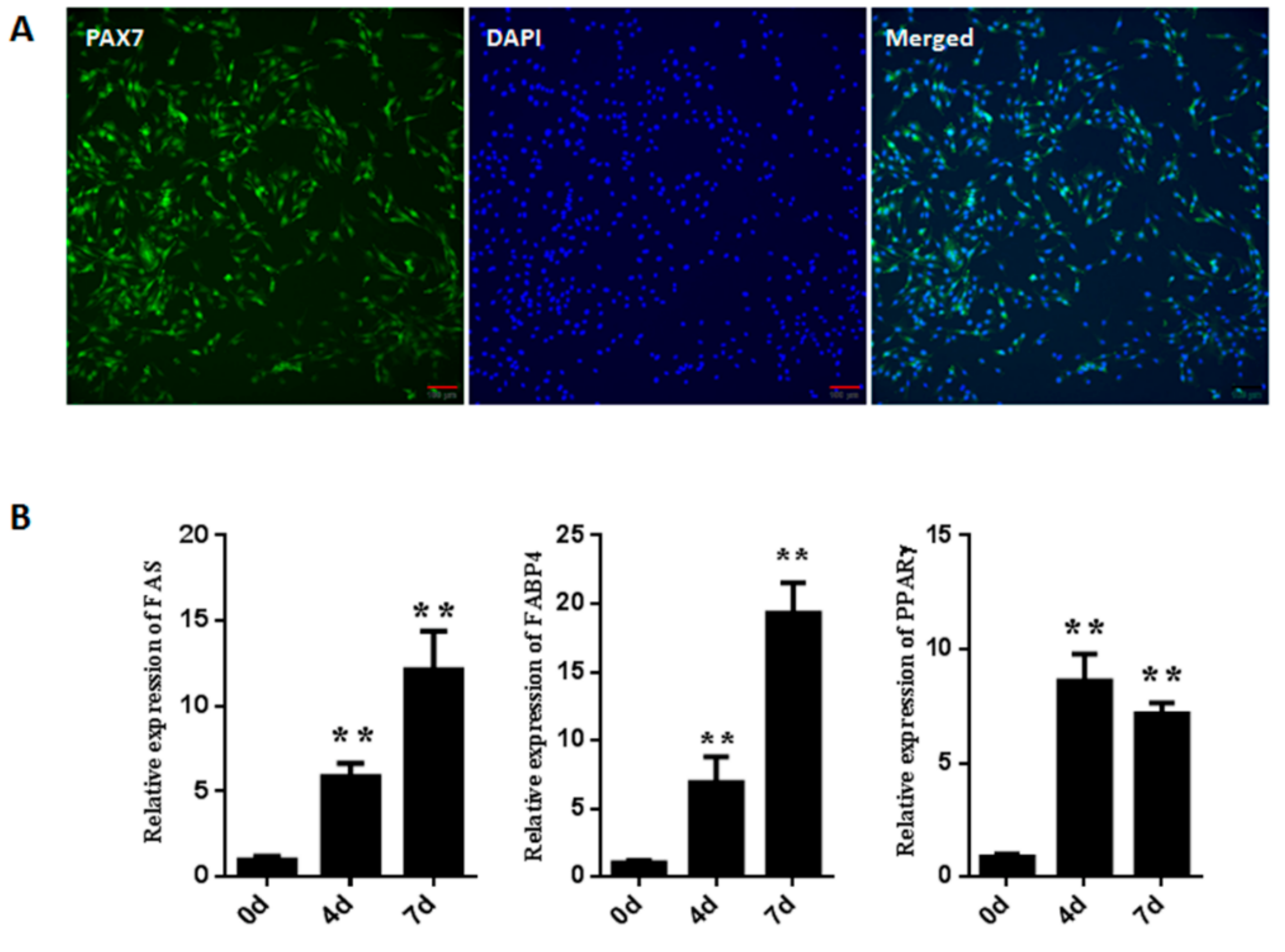
3.1. Isolation and Identification of Goat Muscle Satellite Cells
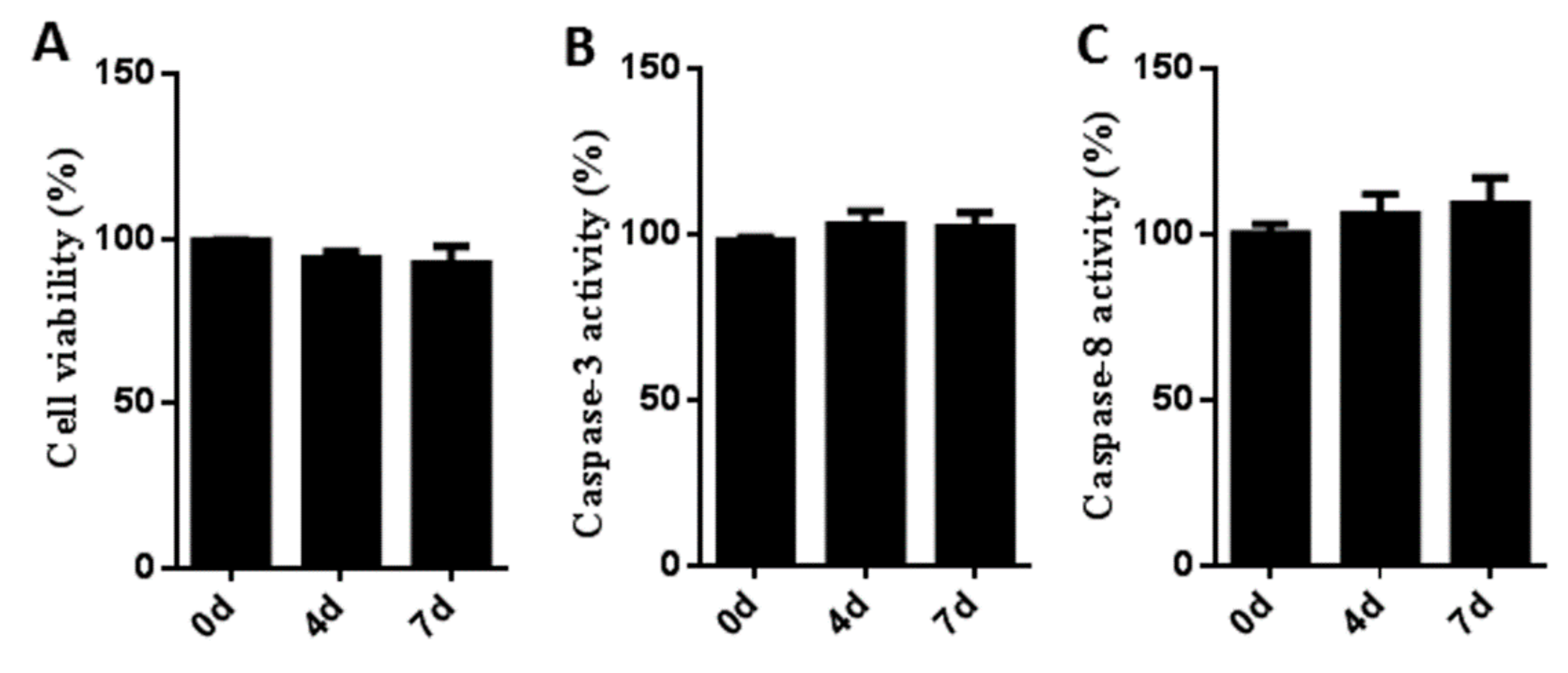
3.2. Cytotoxicity and Stress Effects of Sb216763 on Muscle Satellite Cells
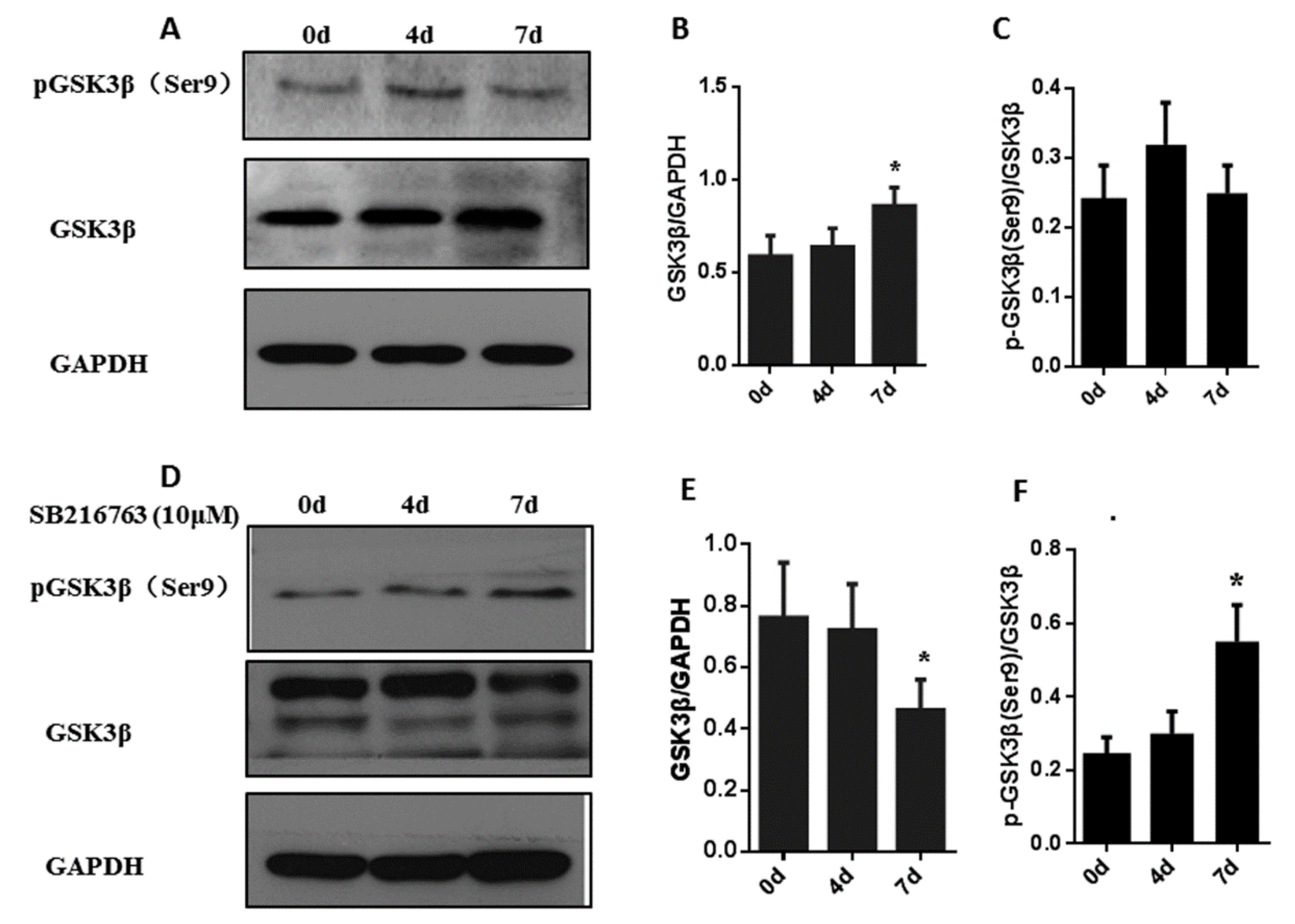
3.3. SB216763 Affects the Levels of Pgsk3β (Ser9) and Gsk3β In Muscle Satellite Cells
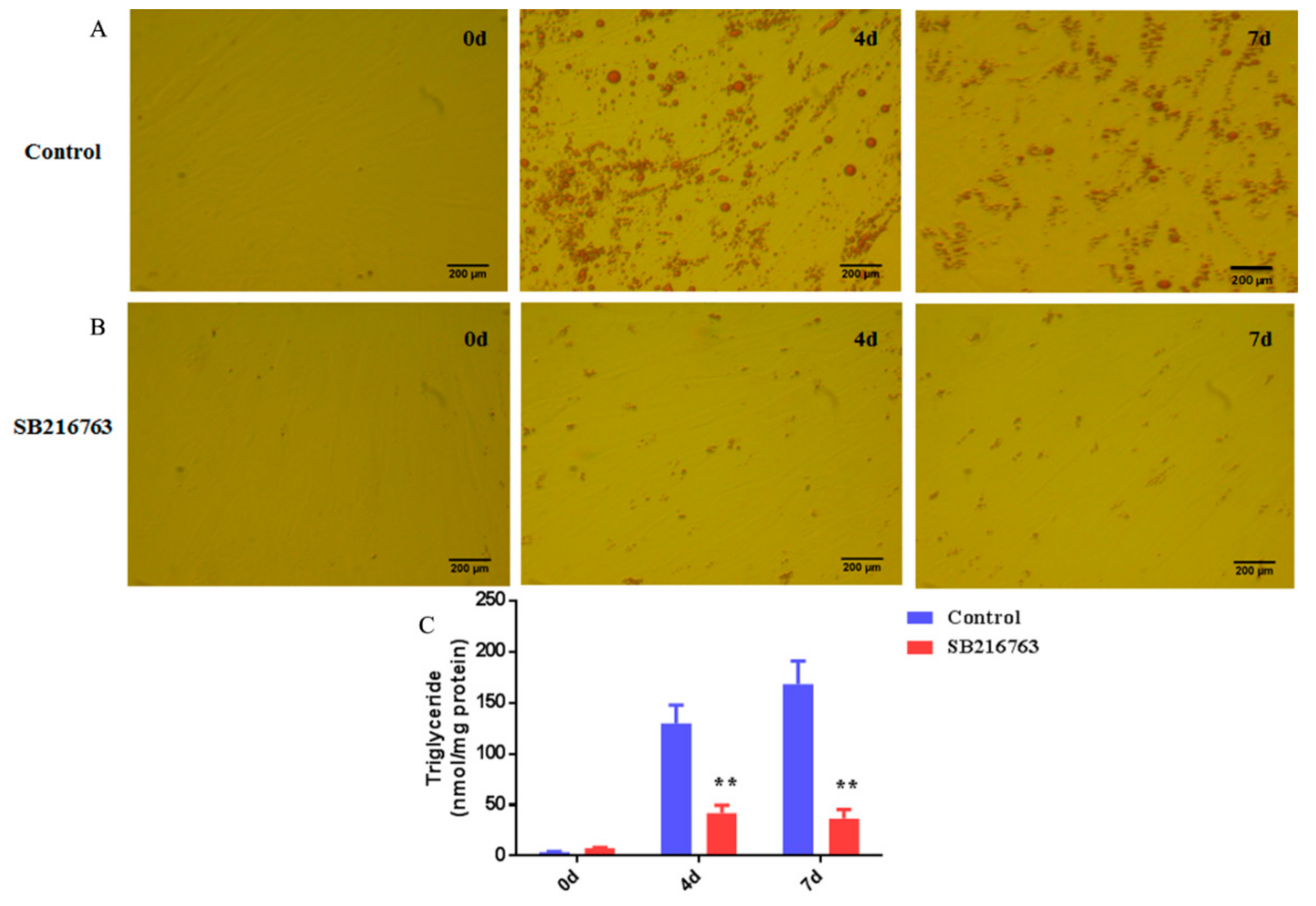
3.4. SB216763 Inhibits Lipid Accumulation in Muscle Satellite Cells
3.5. GSK3β Regulates the Expression of Lipogenesis-Related Genes in the Adipogenic Differentiation of Muscle Satellite Cells
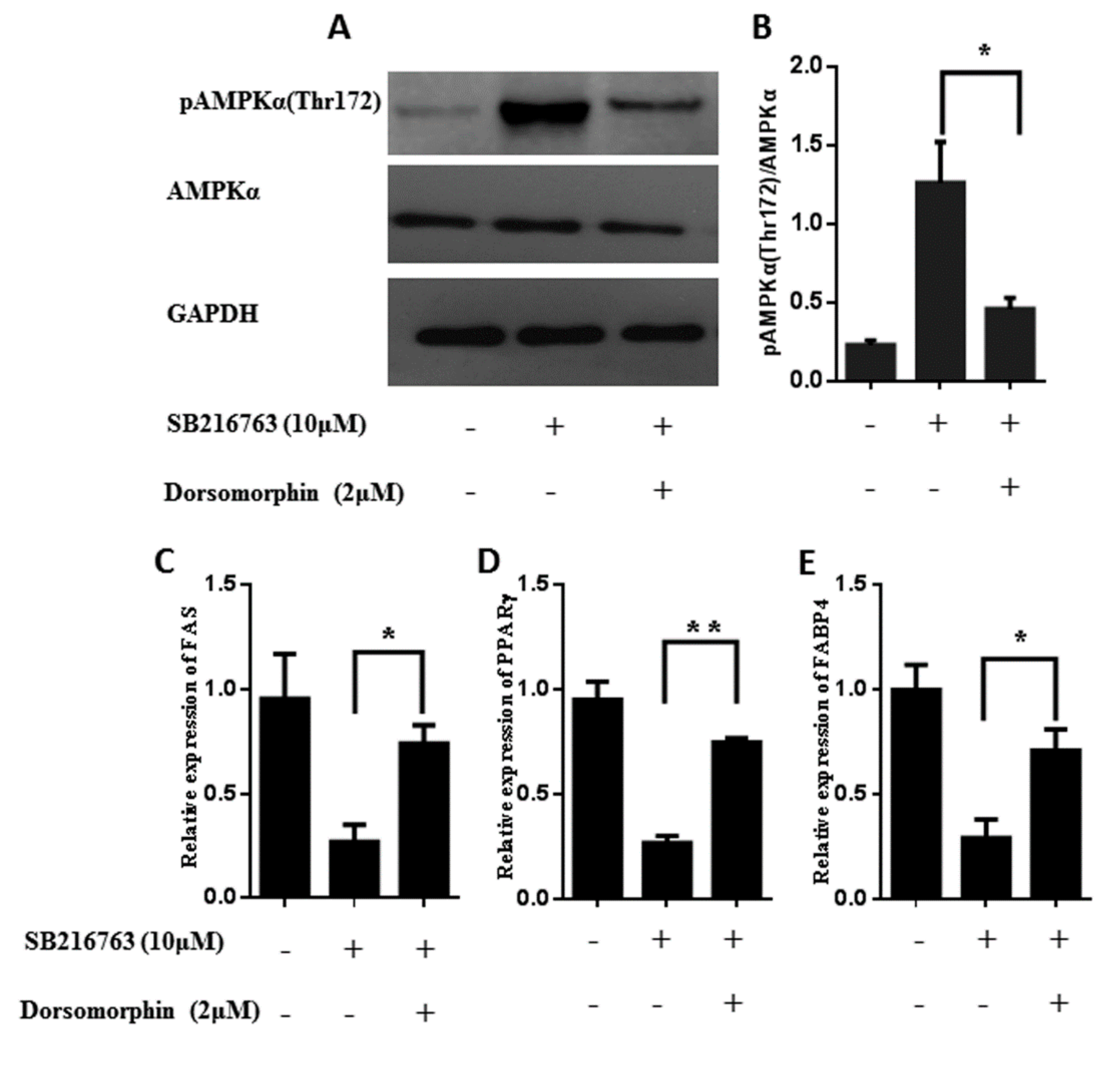
3.6. GSK3β Regulates the Lipid Accumulation in Muscle Satellite Cells through Activating the Ampk Pathway
3.7. Inhibition of GSK3β-Induced Autophagy in Muscle Satellite Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Boil. 2011, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.V.; Hausman, G.J.; Guan, L.; Du, M.; Rasmussen, T.P.; Poulos, S.P.; Mir, P.; Bergen, W.G.; Fernyhough, M.E.; McFarland, D.C.; et al. Skeletal muscle stem cells from animals i. Basic cell biology. Int. J. Biol. Sci. 2010, 6, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009, 460, 1154. [Google Scholar] [CrossRef]
- Yin, H.; Pasut, A.; Soleimani, V.D.; Bentzinger, C.F.; Antoun, G.; Thorn, S.; Seale, P.; Fernando, P.; Van Ijcken, W.; Grosveld, F.; et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013, 17, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Zhang, P.; Bi, P.; Kuang, S. Lkb1 deletion promotes ectopic lipid accumulation in muscle progenitor cells and mature muscles. J. Cell. Physiol. 2015, 230, 1033–1041. [Google Scholar] [CrossRef]
- Vertino, A.M.; Taylor-Jones, J.M.; Longo, K.A.; Bearden, E.D.; Lane, T.F.; McGehee, R.E.; MacDougald, O.A.; Peterson, C.A. Wnt10b Deficiency Promotes Coexpression of Myogenic and Adipogenic Programs in Myoblasts. Mol. Boil. Cell 2005, 16, 2039–2048. [Google Scholar] [CrossRef]
- Duda, P.; Wiśniewski, J.; Wójtowicz, T.; Wójcicka, O.; Jaśkiewicz, M.; Drulis-Fajdasz, D.; Rakus, D.; McCubrey, J.A.; Gizak, A. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets 2018, 22, 833–848. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3beta in cell survival and nf-kappab activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef]
- Patel, S.; Doble, B.W.; MacAulay, K.; Sinclair, E.M.; Drucker, D.J.; Woodgett, J.R. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol. Cell Biol. 2008, 28, 6314–6328. [Google Scholar] [CrossRef]
- Pansters, N.A.; Schols, A.M.; Verhees, K.J.; de Theije, C.C.; Snepvangers, F.J.; Kelders, M.C.; Ubags, N.D.; Haegens, A.; Langen, R.C. Muscle-specific gsk-3beta ablation accelerates regeneration of disuse-atrophied skeletal muscle. Biochim. Biophys. Acta 2015, 1852, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Pansters, N.A.; van der Velden, J.L.; Kelders, M.C.; Laeremans, H.; Schols, A.M.; Langen, R.C. Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of gsk-3beta. Cell Mol. Life Sci. 2011, 68, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, J.L.; Langen, R.C.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M.; Schols, A.M. Inhibition of glycogen synthase kinase-3beta activity is sufficient to stimulate myogenic differentiation. Am. J. Physiol. Cell Physiol. 2006, 290, C453–C462. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, M.P.; A Culbert, A.; Cross, D.A.; Corcoran, S.L.; Yates, J.W.; Pearce, N.J.; Rausch, O.L.; Murphy, G.J.; Carter, P.S.; Cox, L.R.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Boil. 2000, 7, 793–803. [Google Scholar] [CrossRef]
- Ter Haar, E.; Coll, J.T.; Austen, D.; Hsiao, H.M.; Swenson, L.; Jain, J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat. Genet. 2001, 8, 593–596. [Google Scholar]
- Wang, L.; Zhu, Y.; Liu, X.; Chao, Z.; Wang, Y.; Zhong, T.; Guo, J.; Zhan, S.; Li, L.; Zhang, H. Glycogen synthase kinase 3beta (gsk3beta) regulates the expression of myhc2a in goat skeletal muscle satellite cells (smscs). Anim. Sci. J. 2019, 90, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Sciorati, C.; Clementi, E.; Manfredi, A.A.; Rovere-Querini, P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: cellular and molecular players. Cell. Mol. Life Sci. 2015, 72, 2135–2156. [Google Scholar] [CrossRef]
- Tardif, N.; Salles, J.; Guillet, C.; Tordjman, J.; Reggio, S.; Landrier, J.F.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; Migne, C.; et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eif2alpha activation. Aging Cell 2014, 13, 1001–1011. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baeza, E.; Medale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Wang, L. In vitro characterization of goat skeletal muscle satellite cells. Anim. Biotechnol. 2019, 1–7. [Google Scholar] [CrossRef]
- Zaragosi, L.-E.; Wdziekonski, B.; Fontaine, C.; Villageois, P.; Peraldi, P.; Dani, C. Effects of GSK3 inhibitors on in vitro expansion and differentiation of human adipose-derived stem cells into adipocytes. BMC Cell Boil. 2008, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Ko, R.; Jung, H.J.; Lee, S.Y. Glycogen synthase kinase 3beta promotes osteogenic differentiation of murine adipose-derived stromal cells. PLoS ONE 2013, 8, e54551. [Google Scholar] [CrossRef] [PubMed]
- Orena, S.J. Inhibition of Glycogen-synthase Kinase 3 Stimulates Glycogen Synthase and Glucose Transport by Distinct Mechanisms in 3T3-L1 Adipocytes. J. Boil. Chem. 2000, 275, 15765–15772. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Meng, Y.; Zhang, C.; Di, L. GSK3-activated STAT5 regulates expression of SFRPs to modulate adipogenesis. FASEB J. 2018, 32, 4714–4726. [Google Scholar] [CrossRef]
- Kuri-Harcuch, W.; Velez-delValle, C.; Vazquez-Sandoval, A.; Hernandez-Mosqueira, C.; Fernandez-Sanchez, V. A cellular perspective of adipogenesis transcriptional regulation. J. Cell Physiol. 2019, 234, 1111–1129. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Siersbaek, R.; Nielsen, R.; Mandrup, S. Ppargamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010, 584, 3242–3249. [Google Scholar] [CrossRef]
- Farmer, S.R. Regulation of ppargamma activity during adipogenesis. Int. J. Obes. 2005, 1, S13–S16. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Ahn, J.-C. Melatonin attenuated adipogenesis through reduction of the CCAAT/enhancer binding protein beta by regulating the glycogen synthase 3 beta in human mesenchymal stem cells. J. Physiol. Biochem. 2016, 72, 145–155. [Google Scholar] [CrossRef]
- Liu, D.; Yu, H.; Gao, L.; Li, A.; Deng, H.; Zhang, Z.; Tao, S.; Liu, Z.; Yang, Q.; Pang, Q. The inhibition of gsk-3beta promotes the production of reactive oxygen species via beta-catenin/c/ebpalpha signaling in the spleen of zebrafish (danio rerio). Fish Shellfish Immunol. 2018, 76, 110–120. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Zhao, W.; Yang, Y.; Tian, H.; Shi, H.; Bionaz, M. Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J. Dairy Sci. 2016, 99, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Bridges, D.; Nakada, D.; Skiniotis, G.; Morrison, S.J.; Lin, J.D.; Saltiel, A.R.; Inoki, K. Inhibition of AMPK catabolic action by GSK3. Mol. Cell 2013, 50, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Ni, M.; Yue, S.; Xia, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Lu, L.; Wang, X.; Zhai, Y. Glycogen synthase kinase 3beta promotes liver innate immune activation by restraining amp-activated protein kinase activation. J. Hepatol. 2018, 69, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.-Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature 2011, 13, 1016. [Google Scholar] [CrossRef]
- Sun, A.J.; Li, C.L.; Chen, R.B.; Huang, Y.L.; Chen, Q.; Cui, X.J.; Liu, H.F.; Thrasher, J.B.; Li, B.Y. Gsk-3 beta controls autophagy by modulating lkb1-ampk pathway in prostate cancer cells. Prostate 2016, 76, 172–183. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, L.; Zhang, X.; Shi, H.; Wen, T.; Bai, L.; Zheng, S.; Chen, Y.; Chen, D.; Li, L.; et al. Inhibition of glycogen synthase kinase 3 beta promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor alpha. Cell Death Dis. 2016, 7, e2151. [Google Scholar]
- Guo, L.; Chen, D.K.; Yin, X.; Shu, Q.F. Gsk-3 beta promotes cell migration and inhibits autophagy by mediating the ampk pathway in breast cancer. Oncol. Res. 2019, 27, 487–494. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, X.; Zhan, S.; Guo, J.; Yang, S.; Zhong, T.; Li, L.; Zhang, H.; Wang, Y. Inhibition of GSK3β Reduces Ectopic Lipid Accumulation and Induces Autophagy by The AMPK Pathway in Goat Muscle Satellite Cells. Cells 2019, 8, 1378. https://doi.org/10.3390/cells8111378
Wang L, Liu X, Zhan S, Guo J, Yang S, Zhong T, Li L, Zhang H, Wang Y. Inhibition of GSK3β Reduces Ectopic Lipid Accumulation and Induces Autophagy by The AMPK Pathway in Goat Muscle Satellite Cells. Cells. 2019; 8(11):1378. https://doi.org/10.3390/cells8111378
Chicago/Turabian StyleWang, Linjie, Xin Liu, Siyuan Zhan, Jiazhong Guo, Shizhong Yang, Tao Zhong, Li Li, Hongping Zhang, and Yan Wang. 2019. "Inhibition of GSK3β Reduces Ectopic Lipid Accumulation and Induces Autophagy by The AMPK Pathway in Goat Muscle Satellite Cells" Cells 8, no. 11: 1378. https://doi.org/10.3390/cells8111378
APA StyleWang, L., Liu, X., Zhan, S., Guo, J., Yang, S., Zhong, T., Li, L., Zhang, H., & Wang, Y. (2019). Inhibition of GSK3β Reduces Ectopic Lipid Accumulation and Induces Autophagy by The AMPK Pathway in Goat Muscle Satellite Cells. Cells, 8(11), 1378. https://doi.org/10.3390/cells8111378




