Differentiation of Baboon (Papio anubis) Induced-Pluripotent Stem Cells into Enucleated Red Blood Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Baboon CD34+ Cell Reprogramming
2.4. Adaptation and Maintenance of Baboon iPSCs in Feeder-Free Culture Conditions
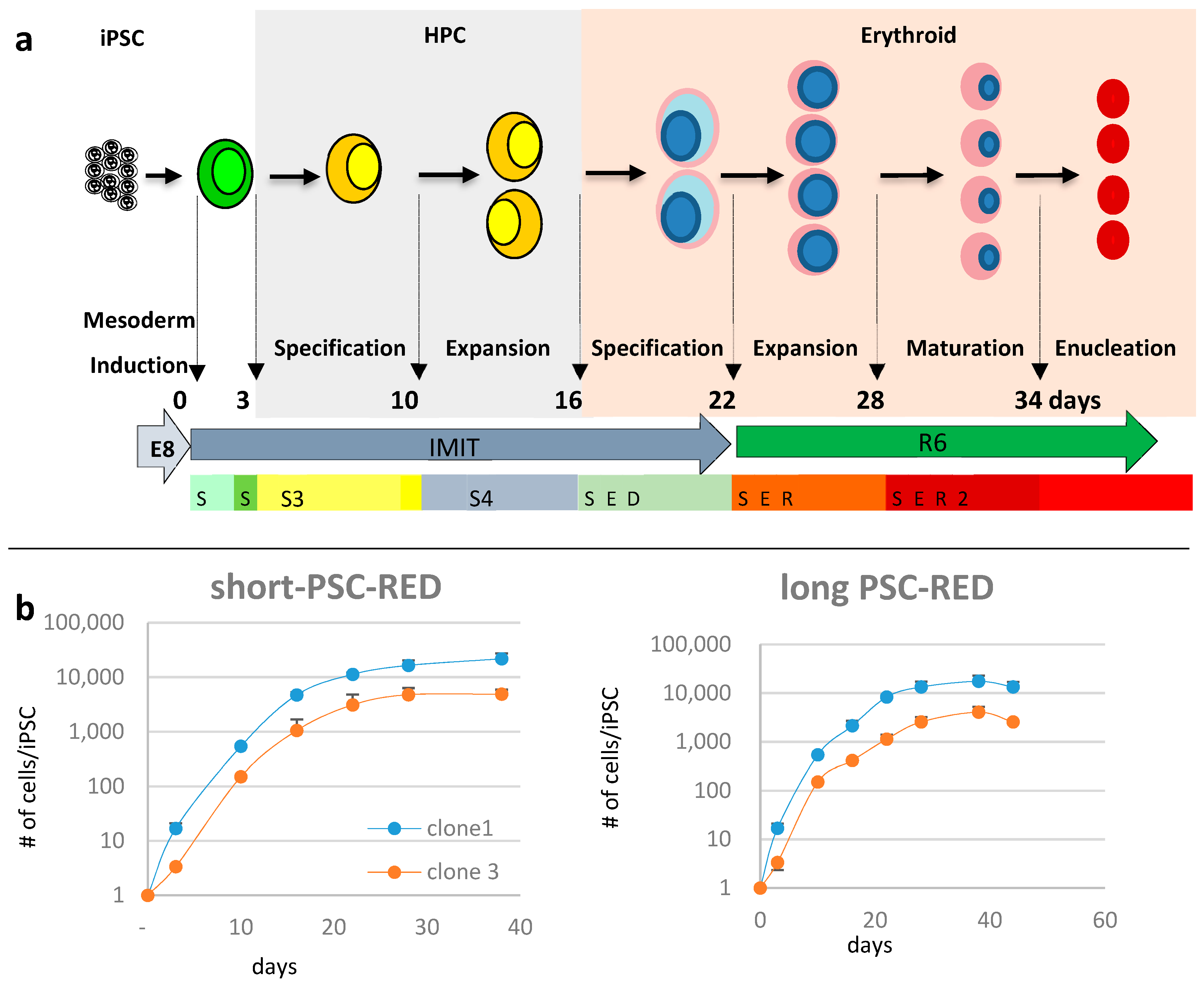
2.5. Short version of the differentiation protocol
2.6. Long version of the differentiation protocol
2.7. Analysis and Characterization
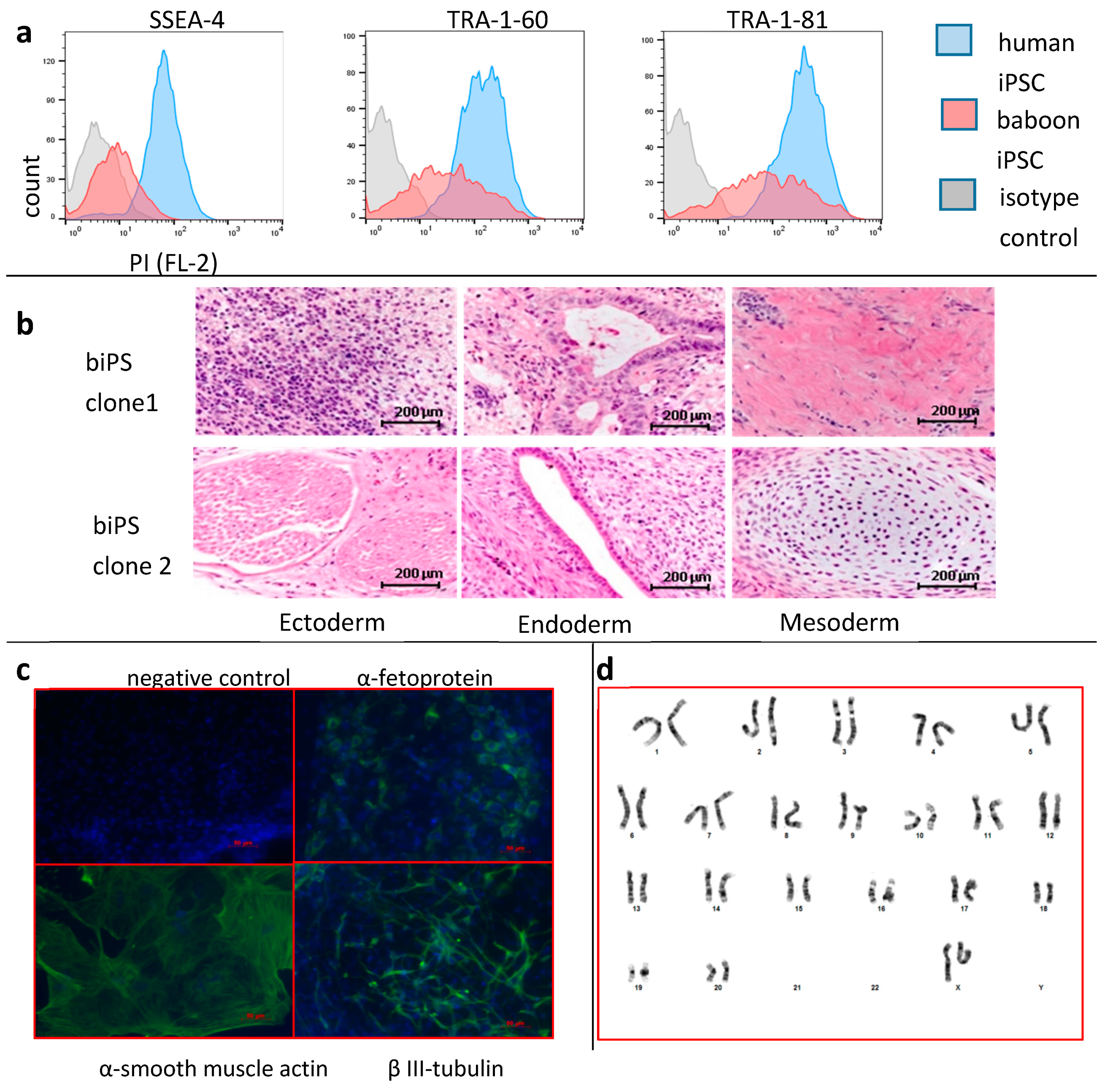
2.7.1. Teratoma Formation
2.7.2. Flow Cytometry
2.7.3. Embryoid Body Formation and Immunohistochemistry
2.7.4. Karyotype
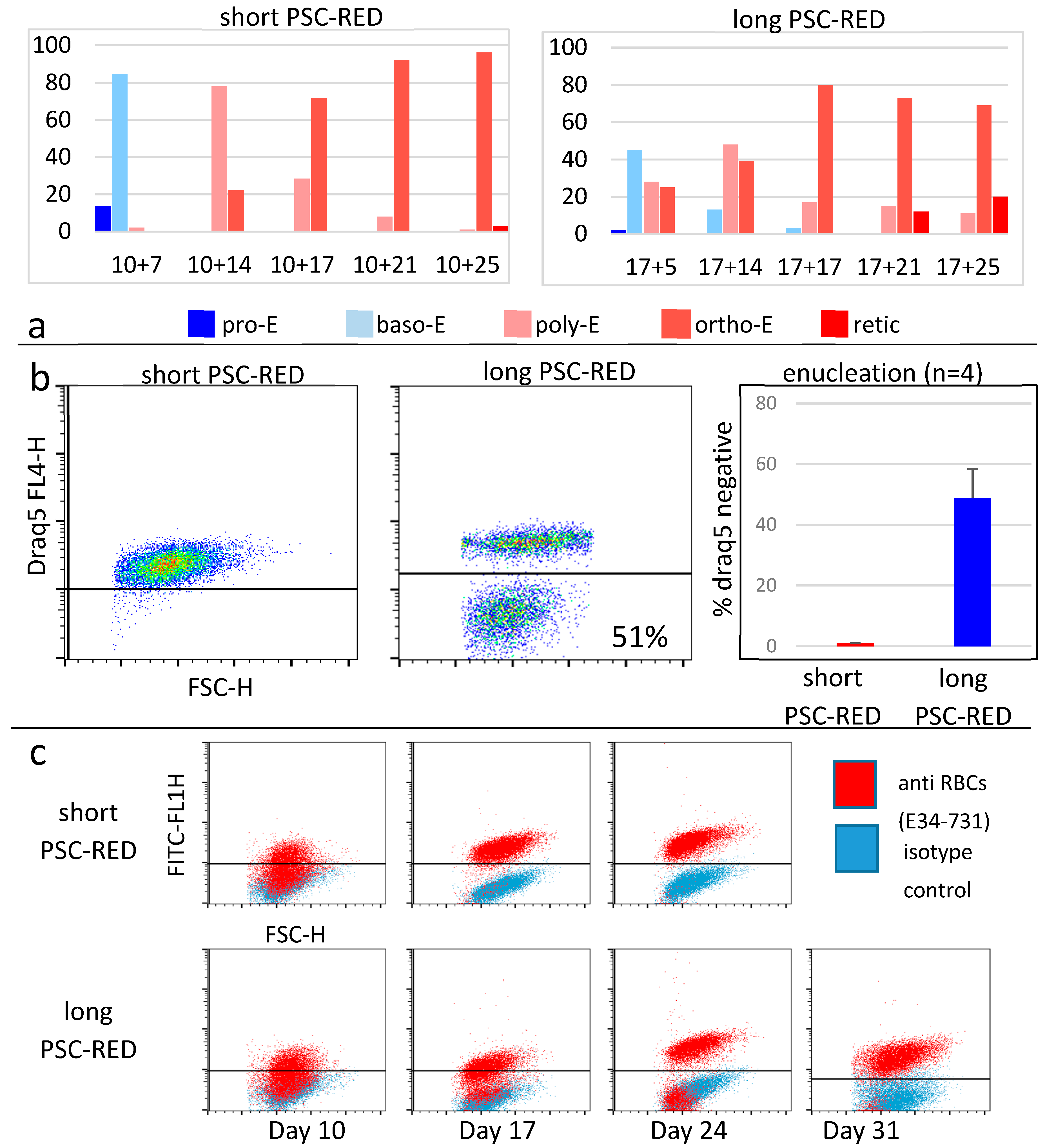
2.7.5. Enucleation
2.7.6. Cell Enumeration
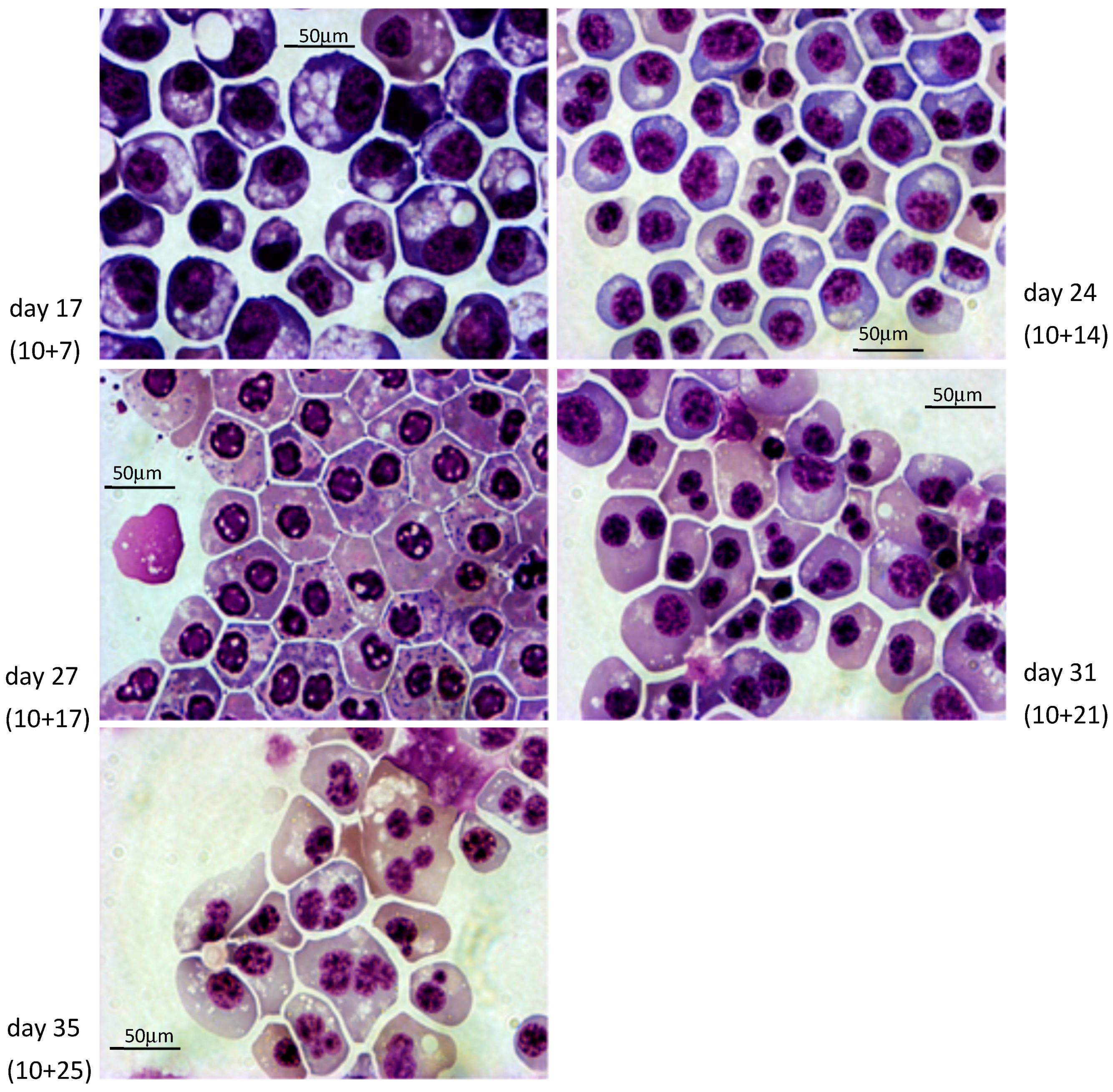
2.7.7. Cytological Staining
2.7.8. HPLC Analysis
2.7.9. Statistical Analysis
3. Results
3.1. Generation of Baboon iPSCs from Peripheral Blood Cells
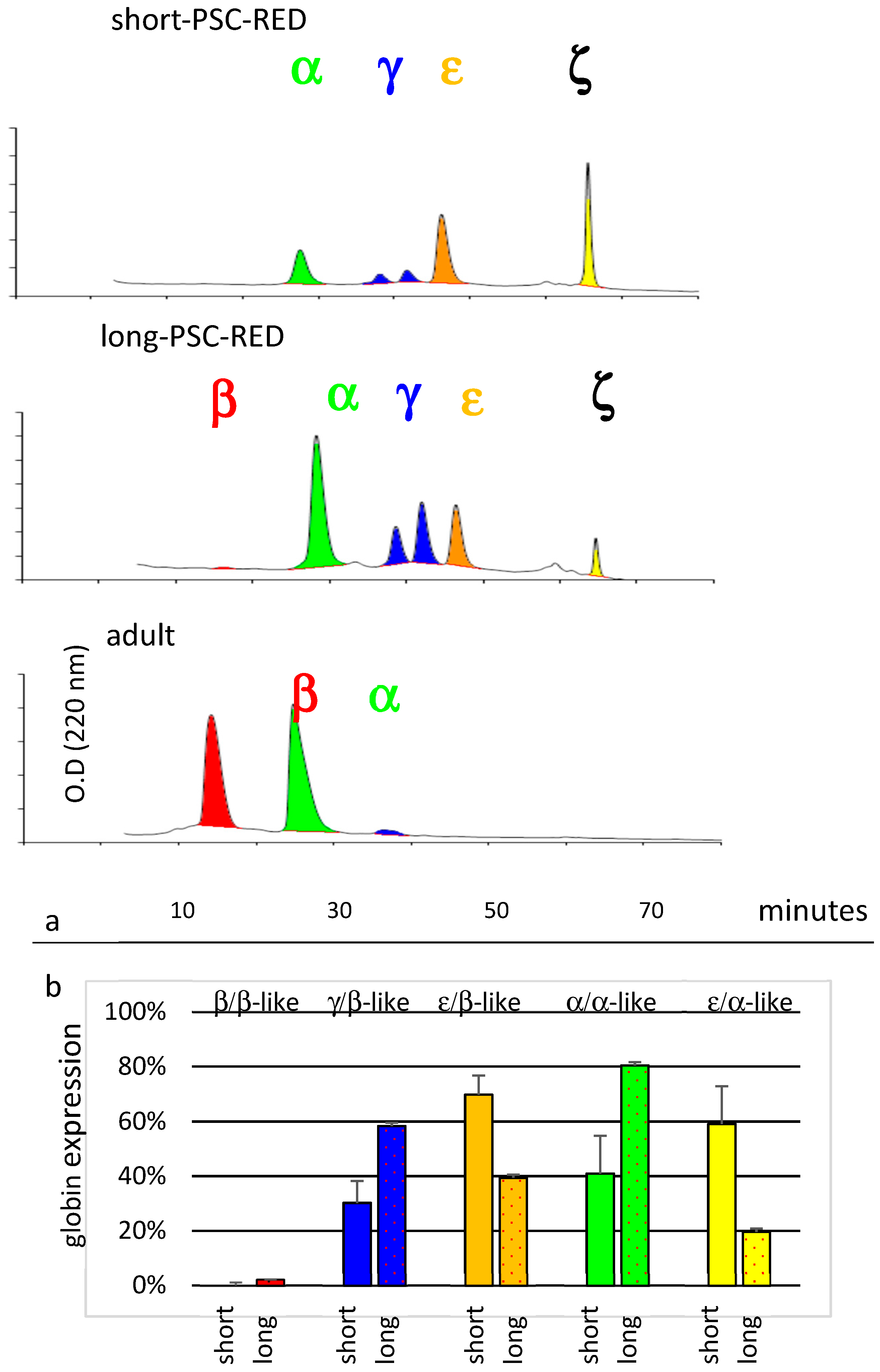
3.2. Differentiation into Erythroid Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Kravtzoff, R.; Ropars, C.; Desbois, I.; Chassaigne, M.; Lamagnere, J.P.; Colombat, P.; Muh, J.P.; Valat, C. Improved pharmacodynamics of l-asparaginase-loaded in human red blood cells. Eur. J. Clin. Pharmacol. 1996, 49, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.H.; Cines, D.B.; Siegel, D.L.; Muzykantov, V. Erythrocytes as Carriers for Drug Delivery in Blood Transfusion and Beyond. Transfus. Med. Rev. 2017, 31, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bagnis, C.; Chiaroni, J.; Bailly, P. Elimination of blood group antigens: Hope and reality. Br. J. Haematol. 2011, 152, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tsong, T.Y.; Kinosita, K. Use of voltage pulses for the pore opening and drug loading, and the subsequent resealing of red blood cells. Bibl. Haematol. 1985, 51, 108–114. [Google Scholar]
- Millán, C.G.; Marinero, M.L.S.; Castañeda, A.Z.; Lanao, J.M. Drug, enzyme and peptide delivery using erythrocytes as carriers. J. Control. Release 2004, 95, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Murciano, J.-C.; Medinilla, S.; Eslin, D.; Atochina, E.; Cines, D.B.; Muzykantov, V.R. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat. Biotechnol. 2003, 21, 891–896. [Google Scholar] [CrossRef]
- Zaitsev, S.; Kowalska, M.A.; Neyman, M.; Carnemolla, R.; Tliba, S.; Ding, B.-S.; Stonestrom, A.; Spitzer, D.; Atkinson, J.P.; Poncz, M.; et al. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood 2012, 119, 4779–4785. [Google Scholar] [CrossRef]
- Shi, J.; Kundrat, L.; Pishesha, N.; Bilate, A.; Theile, C.; Maruyama, T.; Dougan, S.K.; Ploegh, H.L.; Lodish, H.F. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl. Acad. Sci. USA 2014, 111, 10131–10136. [Google Scholar] [CrossRef]
- Huang, N.-J.; Pishesha, N.; Mukherjee, J.; Zhang, S.; Deshycka, R.; Sudaryo, V.; Dong, M.; Shoemaker, C.B.; Lodish, H.F. Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin. Nat. Commun. 2017, 8, 423. [Google Scholar] [CrossRef]
- Hunault-Berger, M.; Leguay, T.; Huguet, F.; Leprêtre, S.; Deconinck, E.; Ojeda-Uribe, M.; Bonmati, C.; Escoffre-Barbe, M.; Bories, P.; Himberlin, C.; et al. A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2-2008 study. Am. J. Hematol. 2015, 90, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Coker, S.A.; Szczepiorkowski, Z.M.; Siegel, A.H.; Ferrari, A.; Mambrini, G.; Anand, R.; Hartman, R.D.; Benatti, L.; Dumont, L.J. A Study of the Pharmacokinetic Properties and the In Vivo Kinetics of Erythrocytes Loaded With Dexamethasone Sodium Phosphate in Healthy Volunteers. Transfus. Med. Rev. 2018, 32, 102–110. [Google Scholar] [CrossRef]
- Kaufman, D.S.; Hanson, E.T.; Lewis, R.L.; Auerbach, R.; Thomson, J.A. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 10716–10721. [Google Scholar] [CrossRef] [PubMed]
- Zambidis, E.T.; Peault, B.; Park, T.S.; Bunz, F.; Civin, C.I. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood 2005, 106, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Hanson, E.; Olivier, E.; Inada, M.; Kaufman, D.S.; Gupta, S.; Bouhassira, E.E. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp. Hematol. 2005, 33. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.F.; Giarratana, M.-C.; Douay, L. Large-scale production of red blood cells from stem cells: What are the technical challenges ahead? Biotechnol. J. 2014, 9, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shah, S.; Wang, J.; Ye, Z.; Dowey, S.N.; Tsang, K.M.; Mendelsohn, L.G.; Kato, G.J.; Kickler, T.S.; Cheng, L. Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. Mol. Ther. 2014, 22, 451–463. [Google Scholar] [CrossRef]
- Olivier, E.; Qiu, C.; Bouhassira, E.E. Novel, high-yield red blood cell production methods from CD34-positive cells derived from human embryonic stem, yolk sac, fetal liver, cord blood, and peripheral blood. Stem Cells Transl. Med. 2012, 1, 604–614. [Google Scholar] [CrossRef]
- Qiu, C.; Olivier, E.N.; Velho, M.; Bouhassira, E.E. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood 2008, 111, 2400–2408. [Google Scholar] [CrossRef]
- Fujita, A.; Uchida, N.; Haro-Mora, J.J.; Winkler, T.; Tisdale, J. β-Globin-Expressing Definitive Erythroid Progenitor Cells Generated from Embryonic and Induced Pluripotent Stem Cell-Derived Sacs. Stem Cells 2016, 34, 1541–1552. [Google Scholar] [CrossRef]
- Olivier, E.N.; Zhang, S.; Yan, Z.; Suzuka, S.; Roberts, K.; Wang, K.; Bouhassira, E.E. PSC-RED and MNC-RED: Albumin-free and low-transferrin robust erythroid differentiation protocols to produce human enucleated red blood cells. Exp. Hematol. 2019, 75, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Fan, W.; Zou, J.; Zhang, S.; He, J.; Shu, C.; Zhao, G.; Sun, T.; Hu, Z.; Yang, Y.-G. Complement Depletion Improves Human Red Blood Cell Reconstitution in Immunodeficient Mice. Stem Cell Rep. 2017, 9, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Marshall, V.S. 4 Primate Embryonic Stem Cells. Curr. Top. Dev. Biol. 1997, 38, 133–165. [Google Scholar]
- Suemori, H.; Tada, T.; Torii, R.; Hosoi, Y.; Kobayashi, K.; Imahie, H.; Kondo, Y.; Iritani, A.; Nakatsuji, N. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev. Dyn. 2001, 222, 273–279. [Google Scholar] [CrossRef]
- Shimozawa, N.; Nakamura, S.; Takahashi, I.; Hatori, M.; Sankai, T. Characterization of a novel embryonic stem cell line from an ICSI-derived blastocyst in the African green monkey. Reproduction 2010, 139, 565–573. [Google Scholar] [CrossRef][Green Version]
- Simerly, C.R.; Navara, C.S.; Castro, C.A.; Turpin, J.C.; Redinger, C.J.; Mich-Basso, J.D.; Jacoby, E.S.; Grund, K.J.; McFarland, D.A.; Oliver, S.L.; et al. Establishment and characterization of baboon embryonic stem cell lines: An Old World Primate model for regeneration and transplantation research. Stem Cell Res. 2009, 2, 178–187. [Google Scholar] [CrossRef]
- Navara, C.S.; Hornecker, J.; Grow, D.; Chaudhari, S.; Hornsby, P.J.; Ichida, J.K.; Eggan, K.; McCarrey, J.R. Derivation of Induced Pluripotent Stem Cells from the Baboon: A Nonhuman Primate Model for Preclinical Testing of Stem Cell Therapies. Cell. Reprogram. 2013, 15, 495–502. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, F.; Yong, J.; Zhang, P.; Hou, P.; Li, H.; Jiang, W.; Cai, J.; Liu, M.; Cui, K.; et al. Generation of Induced Pluripotent Stem Cells from Adult Rhesus Monkey Fibroblasts. Cell Stem Cell 2008, 3, 587–590. [Google Scholar] [CrossRef]
- Vermilyea, S.C.; Guthrie, S.; Meyer, M.; Smuga-Otto, K.; Braun, K.; Howden, S.; Thomson, J.A.; Zhang, S.-C.; Emborg, M.E.; Golos, T.G. Induced Pluripotent Stem Cell-Derived Dopaminergic Neurons from Adult Common Marmoset Fibroblasts. Stem Cells Dev. 2017, 26, 1225–1235. [Google Scholar] [CrossRef]
- Zhong, B.; Trobridge, G.D.; Zhang, X.; Watts, K.L.; Ramakrishnan, A.; Wohlfahrt, M.; Adair, J.E.; Kiem, H.-P. Efficient Generation of Nonhuman Primate Induced Pluripotent Stem Cells. Stem Cells Dev. 2011, 20, 795–807. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef]
- Jörundsson, E.; Lumsden, J.H.; Jacobs, R.M. Rapid staining techniques in cytopathology: A review and comparison of modified protocols for hematoxylin and eosin, Papanicolaou and Romanowsky stains. Vet. Clin. Pathol. 1999, 28, 100–108. [Google Scholar] [CrossRef]
- Fabry, M.E.; Bouhassira, E.E.; Suzuka, S.M.; Nagel, R.L. Transgenic Mice and Hemoglobinopathies. In Hemoglobin Disorders; Humana Press: Totowa, NJ, USA, 2003; Volume 82, pp. 213–241. [Google Scholar]
- Navara, C.S.; Chaudhari, S.; McCarrey, J.R. Optimization of culture conditions for the derivation and propagation of baboon (Papio anubis) induced pluripotent stem cells. PLoS ONE 2018, 13, e0193195. [Google Scholar] [CrossRef]
- Johnson, R.M.; Gumucio, D.; Goodman, M. Globin gene switching in primates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 877–883. [Google Scholar] [CrossRef]
- De Simone, J.; Mueller, A.L. Fetal hemoglobin (HbF) synthesis in baboons, Papio cynocephalus. Analysis of fetal and adult hemoglobin synthesis during fetal development. Blood 1979, 53, 19–27. [Google Scholar] [CrossRef]
- Wang, K.; Guzman, A.K.; Yan, Z.; Zhang, S.; Hu, M.Y.; Hamaneh, M.B.; Yu, Y.K.; Tolu, S.; Zhang, J.; Kanavy, H.E.; et al. BE Ultra-High Frequency Reprogramming Of Individual Long-Term-Hematopoietic Stem Cells Yields Low Somatic Variant Induced-Pluripotent Stem Cells. Cell Rep. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S.; Tardif, S.D. (Eds.) The Baboon in Biomedical Research; Springer: New York, NY, USA, 2009; ISBN 9780387759906. [Google Scholar]
- Valeri, C.R.; Ragno, G. The survival and function of baboon red blood cells, platelets, and plasma proteins: A review of the experience from 1972 to 2002 at the Naval Blood Research Laboratory, Boston, Massachusetts. Transfusion 2006, 46, 1–42. [Google Scholar] [CrossRef]
- Mahmud, N.; Khanal, A.; Taioli, S.; Koca, E.; Gaitonde, S.; Petro, B.; Sweiss, K.; Halliday, L.; Wang, X.; Patel, P.; et al. Preclinical IV busulfan dose-finding study to induce reversible myeloablation in a non-human primate model. PLoS ONE 2018, 13, e0206980. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivier, E.N.; Wang, K.; Grossman, J.; Mahmud, N.; Bouhassira, E.E. Differentiation of Baboon (Papio anubis) Induced-Pluripotent Stem Cells into Enucleated Red Blood Cells. Cells 2019, 8, 1282. https://doi.org/10.3390/cells8101282
Olivier EN, Wang K, Grossman J, Mahmud N, Bouhassira EE. Differentiation of Baboon (Papio anubis) Induced-Pluripotent Stem Cells into Enucleated Red Blood Cells. Cells. 2019; 8(10):1282. https://doi.org/10.3390/cells8101282
Chicago/Turabian StyleOlivier, Emmanuel N., Kai Wang, Joshua Grossman, Nadim Mahmud, and Eric E. Bouhassira. 2019. "Differentiation of Baboon (Papio anubis) Induced-Pluripotent Stem Cells into Enucleated Red Blood Cells" Cells 8, no. 10: 1282. https://doi.org/10.3390/cells8101282
APA StyleOlivier, E. N., Wang, K., Grossman, J., Mahmud, N., & Bouhassira, E. E. (2019). Differentiation of Baboon (Papio anubis) Induced-Pluripotent Stem Cells into Enucleated Red Blood Cells. Cells, 8(10), 1282. https://doi.org/10.3390/cells8101282





