Abstract
One of the greatest challenges in neuro-oncology is diagnosis and therapy (theranostics) of leptomeningeal metastasis (LM), brain metastasis (BM) and brain tumors (BT), which are associated with poor prognosis in patients. Retrospective analyses suggest that cerebrospinal fluid (CSF) is one of the promising diagnostic targets because CSF passes through central nervous system, harvests tumor-related markers from brain tissue and, then, delivers them into peripheral parts of the human body where CSF can be sampled using minimally invasive and routine clinical procedure. However, limited sensitivity of the established clinical diagnostic cytology in vitro and MRI in vivo together with minimal therapeutic options do not provide patient care at early, potentially treatable, stages of LM, BM and BT. Novel technologies are in demand. This review outlines the advantages, limitations and clinical utility of emerging liquid biopsy in vitro and photoacoustic flow cytometry (PAFC) in vivo for assessment of CSF markers including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), microRNA (miRNA), proteins, exosomes and emboli. The integration of in vitro and in vivo methods, PAFC-guided theranostics of single CTCs and targeted drug delivery are discussed as future perspectives.
1. Introduction
Leptomeningeal and brain metastasis (LM and BM) as a result of metastatic dissemination of solid tumors (e.g., melanoma, breast cancer, lung cancer and colorectal cancer) and hematological neoplasms as well as primary brain tumors (BTs, e.g., glioma) are commonly fatal with minimum treatment options [1,2,3,4,5,6,7,8,9,10,11]. Relatively high number of underdiagnosed LM, BM and BT and often ineffective therapy are the major challenges. For example, autopsy data demonstrate that BM contribute to death in ~75% of melanoma patients but they are clinically diagnosed in only 37% cases [8].
Among other parts of central nervous system (CNS), cerebrospinal fluid (CSF) is the easiest accessible medium that can directly uptake tumor markers from different parts of CNS [12,13,14,15,16,17]. Normally, CSF is a colorless liquid (a total volume of 130–150 mL for human) that contains up to 5 cells/µL, mainly leukocytes (white blood cells [WBCs]) [18,19,20]. CSF is produced by the choroidal plexus of the ventricular system and ependymal brain cells from blood [18,20,21].
In tumor patients with CNS involvement, CSF contains various markers associated with disease progression and responses to therapy [2,3,4,13,14,15,16,17,22,23,24,25,26,27,28,29,30]. Among others, circulating tumor cells (CTCs) are direct seeds of metastasis and, therefore, their diagnostic significance encourages high attention of researchers and clinicians. Furthermore, multiple recent reports suggested that detection of tumor-derived markers such as exosomes, circulating tumor DNA (ctDNA), micro-RNA (miRNA) and proteins is relevant to LM, BM and BT. The diagnostic significance of these markers seems especially important for BT because some BTs are not metastatic and do not typically shed CTCs but may release tumor-derived markers in CSF. CTC aggregates (so-called clusters or emboli) in CSF may also have diagnostic value. This speculation is based on: (1) finding CTC emboli in CSF samples of patients with lung cancer and LM [30]; (2) detection of CTC clusters in blood of patients with BT (e.g., glioblastoma) assuming their leaving CNS through the compromised blood-brain barrier (BBB) [31]; and (3) experimental and clinical evidences that multicellular CTC aggregates in peripheral blood represent the aggressive cell subset responsible for initiating and promoting metastasis [31,32,33,34,35,36,37,38,39,40].
Based on the physiology of CNS and mechanisms of tumor development (e.g., compromising BBB to penetrate tumor cells [41]), CTCs, their aggregates and other tumor-derived markers may invade CSF through different mechanisms that include (1) crossing the compromised BBB by blood and lymphatic CTCs and/or (2) shedding tumor cells by existing BM and BT. The latter mechanism provides a solid basis for using CSF tumor markers to diagnose progression of BM and BT, and to estimate responses to therapy. The first mechanism likely works for LM and BM and suggests the origin of CSF tumor biomarkers from blood or/and lymph and their possible entry to CSF before colonization of brain tissue and meninges.
Thus, testing CSF might predict deadly LM, BM and BT; and advanced methods to assess CSF tumor markers in CSF are urgently needed to prolong life of patients suffering from CNS tumor lesions.
2. In Vitro Detection of CSF Tumor Markers
The gold standard for routine clinical examination of CSF is cytology after lumbar puncture [9,10,11,24,42,43]. The detection approach is based on cytomorphology of tumor cells after staining samples with Wright-Giemsa or Papanicolaou dyes. However, the sensitivity of CSF cytology is estimated as low as 50% [9]. Furthermore, cytology is a relatively subjective method since its results depend on the ability of a laboratory technician to correctly identify types of cells, for example, to distinguish tumor cells from normal leukocytes [24,25,26]. This may lead to delaying of therapeutic interventions until other diagnostic criteria (e.g., abnormal magnetic resonance imaging [MRI]) and/or strong clinical symptoms emerge. As a result, involvement of CNS in some patients is found at autopsy only.
The limitations of cytology and deadly nature of LM, BM and BT encouraged researchers and clinicians to develop more sensitive and accurate markers using modern technologies. During the past decade, substantial efforts have been made to assess CSF samples using new concept of liquid biopsy (Figure 1) [2,15,16,17,23,26,27,28,29,30,44,45,46,47,48].
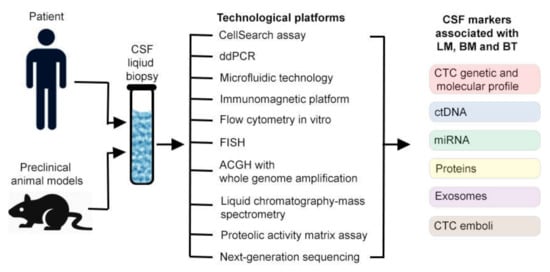
Figure 1.
Cerebrospinal fluid (CSF) liquid biopsy detection of tumor markers in vitro.
CSF Liquid Biopsy
Several years ago, Patel et al. showed that FDA-approved CellSearch method can be used to identify CTCs in 7.5 mL CSF samples of breast cancer patients [22]. Compared to traditional cytology, the CellSearch assay has been demonstrated significantly higher number of CTCs [22,28,30,49,50]. Despite promise, this technological platform is limited in detection of only a few tumor markers, typically EpCam, for patients with epithelial cancers (e.g., breast cancer) and CD 146 and HMW-MMA for patients with melanoma [21,49]. Thus, CellSearch obviously cannot identify a highly heterogeneous population of CTCs and not suitable for diagnosis of many tumors such as glioblastoma. These limitations somewhat reduced enthusiasm to recommend this method in routine clinical practice.
Using real-time polymerase chain reaction (RT-PCR) for examination of patients with BM and LM has demonstrated higher sensitivity than conventional cytology [51]. However, relatively high rate of false-negatives during RT-PCR analysis make it a suboptimal method for CSF testing [9]. To solve this problem, cancer researchers and clinical oncologists recently explored the use of high-sensitive droplet-digital PCR (ddPCR) [52,53,54,55,56]. It was shown that ddPCR provides accurate and reliable CSF analysis. It can work with poor DNA quality and measure multiple parameters including absolute allele quantification, rare mutation, copy number variations, DNA methylation and gene rearrangements [52]. In a few clinical studies, ddPCR of CSF was able to detect ctDNA in patients with melanoma and CNS metastasis; and the obtained results were strongly correlated with cytology results and detection of abnormalities in MRI [52,56]. It is interesting that some patients with high level of ctDNA showed negative cytological results [56]. The small volume of CSF fluid required for testing ctDNA is definitely an additional advantage but high level of false results is a challenge. Overall, to date, it is too early to make conclusions on diagnostic value of ctDNA.
Another promising emerging data of CSF liquid biopsy have been obtained using immunofluorescence in situ hybridization (FISH) technology [24,57,58,59,60]. The published results hold promise to provide more accurate diagnosis of CSF CTCs than cytology. The main advantage of FISH is phenotypic and karyotypic identification and characterization of the highly heterogeneous CTCs, which can be assessed by both chromosome ploidy and the expression of various tumor markers [57]. However, FISH is not currently standardized for liquid biopsy and requires future development and research to clarify whether or not this method is reliable for identification of CTCs.
Integration of array comparative genomic hybridization (ACGH) analysis and whole genome amplification provided achieving the genomic characterization of rare CSF CTCs [61,62]. The clonal similarity between CSF CTCs and primary tumor genomic profiles with more copy number alterations in CTCs was demonstrated using samples of CSF and primary tumor from breast cancer patients with LM [62].
Analysis of CSF samples with conventional flow cytometry in vitro has been reported to diagnose CTCs in CSF [63,64,65]. Flow cytometry immunophenotypic testing of bulk breast cancer receptors, cancer stem cell markers and various WBC subpopulations looks interesting and suggests interplay of CSF and lymph fluid during CTC migration [63]. However, well-known limitation of flow cytometry to detect rare events might reduce enthusiasm for its use of assessment of CTCs which is supposed to be rare (up to 1-5 CTCs per sample) at early stage of CNS involvement.
In the past few years, the clinical potential of some other technological platforms including microfluidic technology, immunomagnetic platform, high performance liquid chromatography-mass spectrometry, next generation sequencing (NGS) and proteolytic activity matrix assay (PrAMA) has also been demonstrated [25,50,55,66,67,68,69]. Despite interest and promises, the singularity of these reports does not allow yet making conclusions on suitability of these methods to improve prognosis in patients.Overall, despite CSF liquid biopsy is expected to yield clinically significant biomarkers and assays, the main drawback to all aforementioned approaches in vitro is that their sensitivity is substantially limited by the volume of the sample [70,71]. Typically, up to 10 mL of CSF is used for examination, which is estimated to be less than 6–7% of the total 130–150 mL volume of human CSF. It means that in vitro testing misses up to 93–94% of CTCs [71]. A simple recalculation of the results in vitro, which detected minimum 1–2 CTCs per CSF patient sample (5–10 mL) with the existing LM and BM [21,49], shows that the real number of CTCs at the time of diagnosis was more than 15–20 cells in the total CSF volume. Serial analysis of multiple samples from repeated punctures increases sensitivity [28]. However, repeated punctures are a challenge because it can be performed over several days and may lead to delaying of therapies. In addition, the existing methods in vitro are burdened with: (1) low throughput, which may require many hours (if not, days) to assess a typical CSF sample and (2) multiple time-consuming sample-processing steps including staining, immunomagnetic capture, isolation and washing, which result in loss of many CTCs [21,23,30,49,51]. As a result, CTCs in small quantities may escape detection, which also contributes to late diagnosis and poor outcomes.
Based on this, liquid biopsy in vitro can provide advanced molecular and genetic analysis of tumor associated markers in CSF but it cannot detect rare CTCs at early stage of LM and BM and possibly, before LM and BM initiation (Table 1). The rarity of CSF CTCs definitely demands a new strategy. An attractive solution to these problems is to monitor almost entire CSF volume in vivo (Table 1).

Table 1.
New and emerging technologies for detection of tumor biomarkers in CSF.
3. In Vivo Diagnosis of CSF
Despite significant progress in neuroimaging in vivo (e.g., MRI, computed tomography [CT], radiography) [9,11,50,64,72,73,74], existing diagnosis, even advanced multi-modal imaging is not sufficient to make judgments about early LM and BM. The low spatial and temporal resolution of CT and MRI allows identification of only macroscopic changes in the CNS (e.g., metastases ≥10 mm by CT). Therefore, the diagnosis is typically based on the indirect signs of LM including pathological meningeal contrast enhancement at the MRI examination, which are often equivocal. In addition, a recent study has found that immunotherapy might be a source of MRI false positivity (‘pseudomeningeosis’) [73]. New generations of MRI, such as phase-contrast MRI, enable quantitative measurements of CSF flow but not suitable for detection of relatively fast moving single CTCs and particles due to slow time response [75]. The same limitation applies to intravital fluorescence microscopy which has been used for imaging CSF plasma (so-called, cisternography) but not single cells in CSF [76]. Furthermore, the translation of fluorescent neuroimaging to humans in vivo is problematic due to (1) cytotoxicity of fluorophores, (2) undesirable immune responses to tags and (3) assessing only superficial fluid flows due to strong influence of autofluorescent and scattering background.
Photoacoustic Flow Cytometry In Vivo
The most promising method for detecting CTCs in CSF is photoacoustic (PA) flow cytometry (PAFC), which compared to other in vivo diagnostic techniques, demonstrated ultra-sensitive molecular detection and counting of single cells in different body fluids (e.g., blood, lymph and CSF) [40,70,77,78,79,80,81,82,83]. The principle of multicolor PAFC is based on noninvasive (i.e., through intact skin) irradiation of the selected fluid with short laser pulses at different wavelengths followed by the detection of laser-induced acoustic waves (referred to as PA signals) using an ultrasound transducer placed on the skin (Figure 2a). PA methods provide higher sensitivity and resolution in deeper tissues (up to 2–3 cm, with potential up to 5–7 cm [70,84]) than other optical modalities. These benefits make possible detection of CTCs in CSF through the atlanto-occipital membrane. In PAFC, this allows distinguishing signals from single fast-moving particles (e.g., CTCs, exosomes, and emboli) at laser energies within the safety standards for humans [70,77,81,83,85,86]. In regards of CSF detection, PAFC has advantages compared to other in vivo methods. Specifically, the colorlessness and optical transparency of CSF, commonly accepted as a diagnostic limitation, provides low absorbance and, therefore, extremely low PA background signal, which significantly improves distinguishing stronly light absorbing objects [71]. It means that CTCs, exosomes or emboli with strong absorbing molecules (e.g., natural melanin or nanoparticles) are predominated over the absorption of CSF by a few orders of magnitude, especially in the near-infrared window of transparency for biotissues (“first window”: 700–1100 nm). Based on this, some strong absorbing cells such as melanoma CTCs with natural intracellular high absorbing melanin as intrinsic non-toxic PA contrast agents, can be easily detected by PAFC in label-free mode. To detect low absorbing tumor-related CSF markers (e.g., breast cancer CTCs), they should be labeled by exogenous PA contrast agents conjugated with ligands (e.g., antibodies, peptides, or folic acid) against specific surface receptor(s). The key requirements for in vivo use of contrast agents include low toxicity and high PA contrast. Some of the best candidates are gold and magnetic nanoparticles [77,87,88].
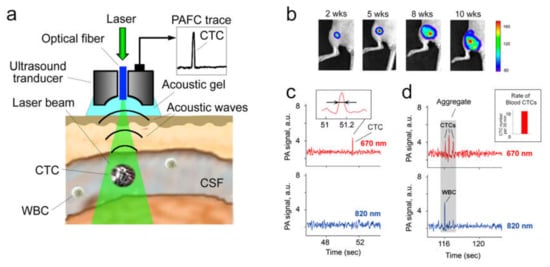
Figure 2.
Assessment of circulating tumor markers in CSF in vivo with multicolor PAFC. (a) Principle of diagnosis with PAFC. (b) Intravital luminescence imaging of metastatic breast cancer progression in orthotopic xenograft mouse model after inoculation of human MDA-MB-231-luc2-GFP cells. (c) Two-color PAFC of the spontaneous CSF CTCs in vivo; inset: the photoacoustic signal width (indicated by arrows), which is associated with a single circulating tumor cell (CTC). (d) PAFC of circulating CTC-containing embolus in tumor-bearing mice; gray rectangle: aggregate of CSF-CTCs and leukocyte (WBC); insert: the blood CTC rate at the time of CSF monitoring.
The first successful demonstration of PAFC’s capability to diagnose CSF tumor markers was reported using preclinical models of breast metastatic cancer (Figure 2b–d) [71]. It was shown that PAFC was able to detect CSF CTCs with 10–20 times higher sensitivity compared to in vitro methods. The most important finding is that some tumor-bearing mice without histologically detectable BM exhibited rare CSF CTCs (e.g., 1–3 signals every 40–60 min). The presence of blood CTCs in these mice suggests the possible origin of CSF CTCs to be from blood CTCs and indicates the potential of CSF CTCs as a predictive biomarker of BM. The obtained experimental evidence is in line with the aforementioned suggestion that blood and lymphatic CTCs might pass the compromised BBB and enter brain tissue, meninges and CSF to form BM and LM. This may serve as a scientific foundation for prognosis and prediction of LM and BM in patients.
Another interesting finding is the existence of CTC-containing emboli in CSF in vivo (Figure 2d). Identification of embolus is based on the width and shape of PA signal, assuming that embolus’ multicellular structure produces a relatively wider PA signal containing a set of narrower peaks.
Overall, the success of preclinical studies together with the simplicity and safety of PAFC give confidence to rapidly translate this method into clinical practice. PAFC diagnosis of CSF in human subarachnoid space and spinal canal at a depth of 1–3 cm seems possible and was supported by the reports on high sensitivity and resolution of PA methods in deeper tissues. Recently, the clinical relevance of PAFC was successfully demonstrated in clinical trials with melanoma patients by detecting blood CTCs in 1–2 mm hand vessels at depth of 1-3 mm with a detection limit of 1 CTC/1000 mL (i.e.,103 –fold increased sensitivity compared to existing CTC assays) [40].
4. Future Directions
To date, crucial steps in increasing the survival of patients with LM and BM are (1) early diagnosis; (2) initiating preventive therapy such as targeted therapy of single CSF CTCs and their emboli and (3) assessing therapeutic efficacy in order to optimize an individual course of therapy.
4.1. Advance Diagnosis
Although many promising technologies to detect various CSF tumor markers during liquid biopsy have been reported, there is no standardized and validated assay that is currently ready to introduce for daily clinical practice as an advanced alternative or supplement of conventional cytology.
Novel approaches integrating unprecedented high sensitivity of in vivo flow cytometry and comprehensive molecular and genetic characterization of tumor markers in CSF in vitro are highly desired for clinical needs.
In addition, one of the possible future alternatives is CSF diagnosis in vivo using updated GILUPI CellCollector. This method was introduced in 2016 for EpCam-based detection of CTCs in blood by introduction of EpCAM-coated wire into a vein of the patient [89]. However, the invasive nature of the method and possibility of missing CTCs, which transit outside the wire, somewhat reduce enthusiasm of using GILUPI device for CSF assessment.
The new looks are also suggesting continuous cell exchange between CSF, blood, lymph and brain tissue [90,91,92] that should be considered at the diagnosis. The prognostic value of CTCs, if they are simultaneously tested in blood, lymph and CSF, would provide a new, highly sensitive and accurate prognostic biomarker of metastasis progression and therapy efficacy.
4.2. Therapeutic Perspectives
Minimal treatment options in current management of LM and BM lead to poor prognosis for patients due to low efficacy, late therapy initiation, use of common (i.e., not-personalized) therapeutic schematics and high toxicity. From this, one of the top future priorities is development of novel targeted and immune therapies. The molecular-targeted nanotechnology platform is highly promising for targeted drug delivery. For this purpose, nanoparticles should have high sensitivity, specificity and selectivity as well as safety, multifunctionality, multimodality, ability to penetrate BBB and high efficiency of drug delivery to tumor. Among existing nanoparticle-based drug cargoes, the most promising candidates include low toxic individual nanoparticles, high-contrast spasers, liposomes, polymer micelles, lipid micelles packaged with semiconducting polymer dots as simultaneous MRI and PA imaging and photodynamic and photothermal dual-modal therapeutic agents, layer by layer based composite structures (core-shells) and microcapsules (shells) and biocompatible natural magnetic nanoparticles [87,88,93,94,95,96,97,98]. The targeting could be achieved by surface modification using targeted molecules specific to CTCs, exosomes and emboli [88,99]. For example, a single injection of core-shells in CSF has shown the effectiveness of their use for the long-term delivery of painkillers in the treatment of persistent pain [100]. Potentially, these drug delivery systems may be effective for treating CTCs in CSF.
There is a high therapeutic potential of modern technologies for creating synthetic truncated antibodies [101] and scaffolds [102]. The revolutionary progress in genetic and protein engineering methods make it possible to directionally modify the molecular size, affinity, specificity and immunogenicity of an antibody, their derivatives and analogues, oriented to the use in the diagnosis and targeted therapy of cancer. Today, rational design and molecular engineering allow modelling of the compounds with preprogrammed properties and to create biotechnological producers of therapeutic medicines [102,103,104,105,106]. A promising direction is conjugation of these unique theranostic agents with nanoparticles. The advantages of using nanoparticles in these conjugates include developed surface of nanoparticles, which can be decorated with biocompatible functional moieties for targeted delivery; and diagnosis that guides and monitors effects of the nanoparticle-assisted therapy [107,108,109,110]. Recently, the design of a hybrid nanocomplex based on an upconversion nanoparticle (UCNP) was reported [111]. Owing to their unique photophysical properties, UCNPs are high-potential platform for theranostics complexes. Conversion of near-infrared light, which can deeply penetrate in biological tissue, to the higher photon energy visible, ultra-violet and near-infrared light is among UCNP’s most useful properties. Two toxic agents––beta-emitting radionuclide yttrium-90 and a highly efficient targeted toxin DARPin-exotoxin A from Pseudomonas aeruginosa––were coupled to UCNP core to exert toxicity to cancer cells. As a result, on the one hand, the photophysical properties of hybrid nanocomplex enable background-free imaging of its distribution in cells and animals. On the other hand, specific delivery of UCNP complexes to cancer cells results in combined therapy by two toxic agents with markedly increased synergetic effect [111]. The design of the hybrid multifunctional nanoheterocomplex proves the principle “when the whole is greater than the sum of the parts.”
The novel targeted CSF therapy may also use the advanced design of heterostructures based on the barnase:barstar pair [112]. The ribonuclease barnase and its inhibitor, barstar, are small stable proteins. They form extremely tight complex with a Kd~10−14 M. The strategy is applicable to any proteins or nanoparticles that can be functionally attached to the barstar and barnase, especially for production of heterooligomeric constructs because the extremely specific barnase barstar interaction eliminates reliably the mispairing problems. This universal platform is a promising alternative to commonly used chemical conjugation techniques in nanobiotechnology, theranostics and clinical applications. It provides a straightforward technology to design wide range of multifunctional nanoheterostructures for the highly efficient delivery of active agents to tumor cells for theranostics [112,113,114,115,116,117].
A very exciting future direction is the possibility of integration of in vivo molecular diagnosis, targeted therapy and estimation of therapeutic efficacy in one technological platform of PAFC [40,79,118]. PAFC’s capability to identify a single high-absorbing CTC and immediately “kill” it through photothermal-indiced nanobubbles with photomechanical action on CTC membranes and vital intracellular structures was demonstrated for blood CTCs in experiments and, recently, in clinical research in blood circulation [40]. Furthermore, the following disappearance of the CTC-associated PA signals might serve as the criterion of effective therapy. These data bring hope that earliest rare CTCs might be identified and “killed” directly in CSF before colonization of brain tissue and formation of BM and LM.
It is expected that technological innovations and ongoing clinical trials would contribute to the finding of novel approaches to provide advances in BM and LM theranostics at the earliest possible stages before development of overt deadly lesions, to select patients with high risk of BM and LM for personalized therapy, to identify early disease progression and thereby improve survival rates of cancer patients.
Funding
This work was supported in part by grants CA131164, EB009230, EB022698 and R21CA230059 from the National Institute of Health (NIH), grants from the Arkansas Biosciences Institute and the Translational Research Institute at UAMS, the Arkansas EPSCoR-NSF Program and RF 14.Z50.31.0044.
Acknowledgments
We thank V.T., M.J., D.N. and many other colleagues who participated in this work and co-authored the cited joint papers.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Taillibert, S.; Chamberlain, M.C. Leptomeningeal metastasis. Handb. Clin. Neurol. 2018, 149, 169–204. [Google Scholar] [PubMed]
- Steeg, P.S.; Camphausen, K.A.; Smith, Q.R. Brain metastases as preventive and therapeutic targets. Nat. Rev. Cancer 2011, 11, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.R.; Fidler, I.J. The biology of brain metastasis. Clin. Chem. 2013, 59, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Figura, N.B.; Rizk, V.T.; Armaghani, A.J.; Arrington, J.A.; Etame, A.B.; Han, H.S.; Czerniecki, B.J.; Forsyth, P.A.; Ahmed, K.A. Breast leptomeningeal disease: A review of current practices and updates on management. Breast Cancer Res. Treat. 2019, 177, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Karlin, N.; Halfdanarson, T.R.; Coppola, K.; Grothey, A. Leptomeningeal Carcinomatosis in Colorectal Cancer: The Mayo Clinic Experience. Clin. Colorectal. Cancer 2018, 17, e183–e187. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, J.O.; Coons, S.J.; Corboy, J.R.; Kline Leidy, N.; Mendoza, T.R.; Wefel, J.S. Clinical outcome assessment in malignant glioma trials: Measuring signs, symptoms, and functional limitations. Neuro Oncol. 2016, 18 (Suppl. 2), ii13–ii20. [Google Scholar] [CrossRef]
- Sloan, A.E.; Nock, C.J.; Einstein, D.B. Diagnosis and treatment of melanoma brain metastasis: A literature review. Cancer Control 2009, 16, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Glantz, M.; Groves, M.D.; Wilson, W.H. Diagnostic tools for neoplastic meningitis: Detecting disease, identifying patient risk, and determining benefit of treatment. Semin. Oncol. 2009, 36, S35–S45. [Google Scholar] [CrossRef]
- Nayar, G.; Ejikeme, T.; Chongsathidkiet, P.; Elsamadicy, A.A.; Blackwell, K.L.; Clarke, J.M.; Lad, S.P.; Fecci, P.E. Leptomeningeal disease: Current diagnostic and therapeutic strategies. Oncotarget 2017, 8, 73312–73328. [Google Scholar] [CrossRef] [PubMed]
- Pellerino, A.; Bertero, L.; Rudà, R.; Soffietti, R. Neoplastic meningitis in solid tumors: From diagnosis to personalized treatments. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418759618. [Google Scholar] [CrossRef] [PubMed]
- Boon, J.M.; Abrahams, P.H.; Meiring, J.H.; Welch, T. Lumbar puncture: Anatomical review of a clinical skill. Clin. Anat. 2004, 17, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Verheul, C.; Kleijn, A.; Lamfers, M.L.M. Cerebrospinal fluid biomarkers of malignancies located in the central nervous system. Handb. Clin. Neurol. 2017, 146, 139–169. [Google Scholar] [PubMed]
- Hepnar, D.; Adam, P.; Žáková, H.; Krušina, M.; Kalvach, P.; Kasík, J.; Karpowicz, I.; Nasler, J.; Bechyně, K.; Fiala, T.; et al. Recommendations for cerebrospinal fluid analysis. Folia Microbiol. 2019, 64, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Nevel, K.S.; Wilcox, J.A.; Robell, L.J.; Umemura, Y. The Utility of Liquid Biopsy in Central Nervous System Malignancies. Curr. Oncol. Rep. 2018, 20, 60. [Google Scholar] [CrossRef]
- Fontanilles, M.; Duran-Peña, A.; Idbaih, A. Liquid Biopsy in Primary Brain Tumors: Looking for Stardust! Curr. Neurol. Neurosci. Rep. 2018, 18, 13. [Google Scholar] [CrossRef]
- Shankar, G.M.; Balaj, L.; Stott, S.L.; Nahed, B.; Carter, B.S. Liquid biopsy for brain tumors. Expert Rev. Mol. Diagn. 2017, 17, 943–947. [Google Scholar] [CrossRef]
- Johanson, C.E.; Duncan, J.A.; Klinge, P.M.; Brinker, T.; Stopa, E.G.; Silverberg, G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008, 5, 10. [Google Scholar] [CrossRef]
- Sweetman, B.; Linninger, A.A. Cerebrospinal fluid flow dynamics in the central nervous system. Ann. Biomed. Eng. 2011, 39, 484–496. [Google Scholar] [CrossRef]
- Huff, T.; Tadi, P.; Varacallo, M. Neuroanatomy, Cerebrospinal Fluid. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef]
- Patel, A.S.; Allen, J.E.; Dicker, D.T.; Peters, K.L.; Sheehan, J.M.; Glantz, M.J.; El-Deiry, W.S. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2011, 2, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.F.; Wolff, A.C. Detection of circulating tumor cells in the cerebrospinal fluid: A new frontier. Cell Cycle 2012, 11, 203–204. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Zhang, Y.; Ding, J.; Wang, M.; Li, N.; Yang, H.; Wang, K.; Wang, D.; Lin, P.P.; Li, M.; et al. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget 2017, 9, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fleisher, M.; Rosenblum, M.; Lin, O.; Boire, A.; Briggs, S.; Bensman, Y.; Hurtado, B.; Shagabayeva, L.; DeAngelis, L.M.; et al. Cerebrospinal fluid circulating tumor cells: A novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2017, 19, 1248–1254. [Google Scholar] [CrossRef]
- Zorofchian, S.; Iqbal, F.; Rao, M.; Aung, P.P.; Esquenazi, Y.; Ballester, L.Y. Circulating tumour DNA, microRNA and metabolites in cerebrospinal fluid as biomarkers for central nervous system malignancies. J. Clin. Pathol. 2019, 72, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Duran-Peña, A.; Alentorn, A.; Lacroix, L.; Massard, C.; Idbaih, A. Emerging circulating biomarkers in glioblastoma: Promises and challenges. Expert Rev. Mol. Diagn. 2015, 15, 1311–1323. [Google Scholar] [CrossRef]
- Lee, J.S.; Melisko, M.E.; Magbanua, M.J.; Kablanian, A.T.; Scott, J.H.; Rugo, H.S.; Park, J.W. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res. Treat. 2015, 154, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Chunhua, M.; Rong, J.; Yuan, L.; Jinduo, L.; Bin, W.; Liwei, S. Diagnostic value of circulating tumor cells in cerebrospinal fluid. Open Med. 2016, 11, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Wu, X.; Le Rhun, E.; Blonski, M.; Wittwer, B.; Taillandier, L.; De Carvalho Bittencourt, M.; Faure, G.C. CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer 2015, 90, 352–357. [Google Scholar] [CrossRef]
- Krol, I.; Castro-Giner, F.; Maurer, M.; Gkountela, S.; Szczerba, B.M.; Scherrer, R.; Coleman, N.; Carreira, S.; Bachmann, F.; Anderson, S.; et al. Detection of circulating tumour cell clusters in human glioblastoma. Br. J. Cancer 2018, 119, 487–491. [Google Scholar] [CrossRef]
- Young, A.; Chapman, O.; Connor, C.; Poole, C.; Rose, P.; Kakar, A.K. Thrombosis and cancer. Nat. Rev. Clin. Oncol. 2012, 9, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Storey, B.D.; Moore, J.C.; Tang, Q.; Chen, Y.L.; Javaid, S.; Sarioglu, A.F.; Sullivan, R.; Madden, M.W.; O’Keefe, R.; et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Wang, C.; Ye, Z.; Austin, L.; Civan, J.; Hyslop, T.; Palazzo, J.P.; Jaslow, R.; Li, B.; Myers, R.E.; et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res. Treat. 2015, 154, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.K.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci. Rep. 2017, 7, 2433. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Schmidt, H.; Perry, C.; Whitfield, B.; Kenny, L.; Nelson, C.; Warkiani, M.E.; Punyadeera, C. A Collective Route to Head and Neck Cancer Metastasis. Sci. Rep. 2018, 8, 746. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Kapeleris, J.; Cooper, C.; Warkiani, M.E.; O’Byrne, K.; Punyadeera, C. Phenotypic Characterization of Circulating Lung Cancer Cells for Clinically Actionable Targets. Cancers 2019, 11, 380. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Menyaev, Y.A.; Yadem, A.C.; Sarimollaoglu, M.; Juratli, M.A.; Nedosekin, D.A.; Foster, S.R.; Jamshidi-Parsian, A.; Siegel, E.R.; Makhoul, I.; et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci. Transl. Med. 2019, 11, eaat5857. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Toborek, M. Blood–brain Barrier Remodeling during Brain Metastasis Formation. Mol. Med. 2016, 22, 32–40. [Google Scholar] [CrossRef]
- Deeken, J.F.; Loscher, W. The Blood-Brain Barrier a Overview of cerebrospinal fluid cytology and Cancer: Transporters, Treatment, and Trojan Horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, J.; Woehrer, A. Overview of cerebrospinal fluid cytology. Handb. Clin. Neurol. 2017, 145, 563–571. [Google Scholar] [PubMed]
- Pantel, K.; Alix-Panabières, C. The potential of circulating tumor cells as a liquid biopsy to guide therapy in prostate cancer. Cancer Discov. 2012, 2, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy: Potential and challenges. Mol. Oncol. 2016, 10, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Connolly, I.D.; Li, Y.; Gephart, M.H.; Nagpal, S. The “Liquid Biopsy”: The Role of Circulating DNA and RNA in Central Nervous System Tumors. Curr. Neurol. Neurosci. Rep. 2016, 16, 25. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Zijl, S.; Reijneveld, J.C.; Wesseling, P.; Wurdinger, T. Liquid biopsies in patients with diffuse glioma. Acta Neuropathol. 2015, 129, 849–865. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Le Rhun, E.; Tu, Q.; De Carvalho Bittencourt, M.; Farre, I.; Mortier, L.; Cai, H.; Kohler, C.; Faure, G.C. Detection and quantification of CSF malignant cells by the CellSearch technology in patients with melanoma leptomeningeal metastasis. Med. Oncol. 2013, 30, 538. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Li, Y.S.; Guo, W.B.; Zhang, X.C.; Chen, Z.H.; Su, J.; Zhong, W.Z.; Yang, X.N.; Yang, J.J.; Shao, Y.; et al. Detection of Driver and Resistance Mutations in Leptomeningeal Metastases of NSCLC by Next-Generation Sequencing of Cerebrospinal Fluid Circulating Tumor Cells. Clin. Cancer Res. 2017, 23, 5480–5488. [Google Scholar] [CrossRef]
- Hoon, D.S.; Kuo, C.T.; Wascher, R.A.; Fournier, P.; Wang, H.J.; O’Day, S.J. Molecular detection of metastatic melanoma cells in cerebrospinal fluid in melanoma patients. J. Investig. Dermatol. 2001, 117, 375–378. [Google Scholar] [CrossRef][Green Version]
- Olmedillas-López, S.; García-Arranz, M.; García-Olmo, D. Current and Emerging Applications of Droplet Digital PCR in Oncology. Mol. Diagn. Ther. 2017, 21, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, P.; Pentsova, E.; Abdel-Wahab, O.; Diamond, E.; Hyman, D.; Merghoub, T.; You, D.; Gasmi, B.; Viale, A.; Chapman, P.B. Quantification of tumor-derived cell free DNA(cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget 2016, 7, 85430–85436. [Google Scholar] [CrossRef] [PubMed]
- Hiemcke-Jiwa, L.S.; Minnema, M.C.; Radersma-van Loon, J.H.; Jiwa, N.M.; de Boer, M.; Leguit, R.J.; de Weger, R.A.; Huibers, M.M.H. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol. Oncol. 2018, 36, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ye, X.; Xu, Y.; Chen, M.; Zhong, W.; Sun, Y.; Yang, Z.; Zhu, G.; Gu, Y.; Wang, M. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother. Pharmacol. 2016, 78, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Glitza Oliva, I.C.; Douse, D.Y.; Chen, M.M.; Lan, C.; Haydu, L.E.; Huse, J.T.; Roy-Chowdhuri, S.; Luthra, R.; Wistuba, I.I.; et al. Evaluating Circulating Tumor DNA From the Cerebrospinal Fluid of Patients with Melanoma and Leptomeningeal Disease. J. Neuropathol. Exp. Neurol. 2018, 77, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhang, H.; Wang, D.D.; Li, L.; Lin, P.P. Enhanced detection and comprehensive in situ phenotypic characterization of circulating and disseminated heteroploid epithelial and glioma tumor cells. Oncotarget 2015, 6, 27049–27064. [Google Scholar] [CrossRef]
- Jiao, X.D.; Ding, C.; Zang, Y.S.; Yu, G. Rapid symptomatic relief of HER2-positive gastric cancer leptomeningeal carcinomatosis with lapatinib, trastuzumab and capecitabine: A case report. BMC Cancer 2018, 18, 206. [Google Scholar] [CrossRef]
- Lv, Y.; Mu, N.; Ma, C.; Jiang, R.; Wu, Q.; Li, J.; Wang, B.; Sun, L. Detection value of tumor cells in cerebrospinal fluid in the diagnosis of meningeal metastasis from lung cancer by immuno-FISH technology. Oncol. Lett. 2016, 12, 5080–5084. [Google Scholar] [CrossRef][Green Version]
- Ma, C.; Lv, Y.; Jiang, R.; Li, J.; Wang, B.; Sun, L. Novel method for the detection and quantification of malignant cells in the CSF of patients with leptomeningeal metastasis of lung cancer. Oncol. Lett. 2016, 11, 619–623. [Google Scholar] [CrossRef]
- Magbanua, M.J.; Melisko, M.; Roy, R.; Sosa, E.V.; Hauranieh, L.; Kablanian, A.; Eisenbud, L.E.; Ryazantsev, A.; Au, A.; Scott, J.H.; et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 2013, 73, 7134–7143. [Google Scholar] [CrossRef]
- Magbanua, M.J.; Roy, R.; Sosa, E.V.; Hauranieh, L.; Kablanian, A.; Eisenbud, L.E.; Ryazantsev, A.; Au, A.; Scott, J.H.; Melisko, M.; et al. Genome-wide copy number analysis of cerebrospinal fluid tumor cells and their corresponding archival primary tumors. Genom. Data 2014, 2, 60–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cordone, I.; Masi, S.; Summa, V.; Carosi, M.; Vidiri, A.; Fabi, A.; Pasquale, A.; Conti, L.; Rosito, I.; Carapella, C.M.; et al. Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: A cerebrospinal fluid flow cytometry study. Breast Cancer Res. 2017, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Milojkovic Kerklaan, B.; Pluim, D.; Bol, M.; Hofland, I.; Westerga, J.; van Tinteren, H.; Beijnen, J.H.; Boogerd, W.; Schellens, J.H.; Brandsma, D. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2016, 18, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, D.; Wang, H.; Wang, Y.; Liu, B.; Wei, H.; Zhou, C.; Liu, K.; Wei, S.; Gong, B.; et al. Flow cytometric analysis of cerebrospinal fluid in adult patients with acute lymphoblastic leukemia during follow-up. Eur. J. Haematol. 2018, 100, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Dorzweiler, K.; Miller, M.A.; Lauffenburger, D.A.; Strik, H.; Bartsch, J.W. Profiling of metalloprotease activities in cerebrospinal fluids of patients with neoplastic meningitis. Fluids Barriers CNS 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.; Wu, L.; Tay, A.K.; Bhagat, A.A.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8. [Google Scholar] [CrossRef]
- Turetsky, A.; Lee, K.; Song, J.; Giedt, R.J.; Kim, E.; Kovach, A.E.; Hochberg, E.P.; Castro, C.M.; Lee, H.; Weissleder, R. On chip analysis of CNS lymphoma in cerebrospinal fluid. Theranostics 2015, 5, 796–804. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Shashkov, E.V.; Spring, P.; Suen, J.Y.; Zharov, V.P. In vivo noninvasive label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009, 69, 7926–7934. [Google Scholar] [CrossRef]
- Nedosekin, D.A.; Juratli, M.A.; Sarimollaoglu, M.; Moore, C.L.; Rusch, N.J.; Smeltzer, M.S.; Zharov, V.P.; Galanzha, E.I. Photoacoustic and photothermal detection of circulating tumor cells, bacteria and nanoparticles in cerebrospinal fluid in vivo and ex vivo. J. Biophotonics 2013, 6, 523–533. [Google Scholar] [CrossRef]
- Barajas, R.F., Jr.; Cha, S. Imaging diagnosis of brain metastasis. Prog. Neurol. Surg. 2012, 25, 55–73. [Google Scholar] [PubMed]
- Bier, G.; Klumpp, B.; Roder, C.; Garbe, C.; Preibsch, H.; Ernemann, U.; Hempel, J.M. Meningeal enhancement depicted by magnetic resonance imaging in tumor patients: Neoplastic meningitis or therapy-related enhancement? Neuroradiology 2019, 61, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Salehi Ravesh, M.; Huhndorf, M.; Moussavi, A. Non-contrast enhanced molecular characterization of C6 rat glioma tumor at 7 T. Magn. Reason. Imaging 2019, 61, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sakhare, A.R.; Barisano, G.; Pa, J. Assessing test-retest reliability of phase contrast MRI for measuring cerebrospinal fluid and cerebral blood flow dynamics. Magn. Reason. Med. 2019, 82, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Kruskal, J.B.; Palmer, M.R. Imaging of cerebrospinal fluid space and movement of hydrocephalus mice using near infrared fluorescence. Neurol. Sci. 2007, 28, 87–92. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Shashkov, E.V.; Kelly, T.; Kim, J.-W.; Yang, L.; Zharov, V.P. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009, 4, 855–860. [Google Scholar] [CrossRef]
- Zharov, V.P. Ultrasharp nonlinear photothermal and photoacoustic resonances and holes beyond the spectral limit. Nat. Photonics 2011, 5, 110–116. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Zharov, V.P. Circulating tumor cell detection and capture by photoacoustic flow cytometry in vivo and ex vivo. Cancers 2013, 5, 1691–1738. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Viegas, M.G.; Malinsky, T.I.; Melerzanov, A.V.; Juratli, M.A.; Sarimollaoglu, M.; Nedosekin, D.A.; Zharov, V.P. In vivo acoustic and photoacoustic focusing of circulating cells. Sci. Rep. 2016, 6, 21531. [Google Scholar] [CrossRef]
- Nolan, J.; Sarimollaoglu, M.; Nedosekin, D.A.; Jamshidi-Parsian, A.; Galanzha, E.I.; Kore, R.A.; Griffin, R.J.; Zharov, V.P. In Vivo Flow Cytometry of Circulating Tumor-Associated Exosomes. Anal. Cell. Pathol. 2016, 2016, 1628057. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Tarnok, A.; Zharov, V.P. In vivo flow cytometry: A horizon of opportunities. Cytom. Part A 2011, 79A, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Zharov, V.P. Photoacoustic flow cytometry. Methods 2012, 57, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Juratli, M.A.; Menyaev, Y.A.; Sarimollaoglu, M.; Siegel, E.R.; Nedosekin, D.A.; Suen, J.Y.; Melerzanov, A.V.; Juratli, T.A.; Galanzha, E.I.; Zharov, V.P. Real-Time Label-Free Embolus Detection Using In Vivo Photoacoustic Flow Cytometry. PLoS ONE 2016, 11, e0156269. [Google Scholar] [CrossRef] [PubMed]
- Juratli, M.A.; Sarimollaoglu, M.; Nedosekin, D.A.; Melerzanov, A.V.; Zharov, V.P.; Galanzha, E.I. Dynamic Fluctuation of Circulating Tumor Cells during Cancer Progression. Cancers 2014, 6, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Galanzha, E.I.; Shashkov, E.V.; Moon, H.-M.; Zharov, V.P. Golden carbon nanotubes as multimodal photoacoustic and photothermal molecular agents. Nat. Nanotechnol. 2009, 4, 688–694. [Google Scholar] [CrossRef]
- Kim, J.W.; Galanzha, E.I.; Zaharoff, D.A.; Griffin, R.J.; Zharov, V.P. Nanotheranostics of circulating tumor cells, infections and other pathological features in vivo. Mol. Pharm. 2013, 10, 813–830. [Google Scholar] [CrossRef]
- Gorges, T.M.; Penkalla, N.; Schalk, T.; Joosse, S.A.; Riethdorf, S.; Tucholski, J.; Lücke, K.; Wikman, H.; Jackson, S.; Brychta, N.; et al. Enumeration and Molecular Characterization of Tumor Cells in Lung Cancer Patients Using a Novel In Vivo Device for Capturing Circulating Tumor Cells. Clin. Cancer Res. 2016, 22, 2197–2206. [Google Scholar] [CrossRef]
- Garzia, L.; Kijima, N.; Morrissy, A.S.; De Antonellis, P.; Guerreiro-Stucklin, A.; Holgado, B.L.; Wu, X.; Wang, X.; Parsons, M.; Zayne, K.; et al. A Hematogenous Route for Medulloblastoma Leptomeningeal Metastases. Cell 2018, 172, 1050–1062. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Zharov, V.P.; Galanzha, E.I. Biophotonics for lymphatic theranostics in animals and humans. J. Biophotonics 2018, 11, e201811001. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Beziere, N.; Lozano, N.; Nunes, A.; Salichs, J.; Queiros, D.; Kostarelos, K.; Ntziachristos, V. Dynamic imaging of PEGylated indocyanine green (ICG) liposomes within the tumor microenvironment using multi-spectral optoacoustic tomography (MSOT). Biomaterials 2015, 37, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Weingold, R.; Nedosekin, D.A.; Sarimollaoglu, M.; Nolan, J.; Harrington, W.; Kuchyanov, A.S.; Parkhomenko, R.G.; Watanabe, F.; Nima, Z.; et al. Spaser as a biological probe. Nat. Commun. 2017, 8, 15528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, M.; Zeng, Y.; Liao, N.; Cai, Z.; Liu, G.; Liu, J. Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. J. Mater. Chem. B 2016, 4, 589–599. [Google Scholar] [CrossRef]
- Yashchenok, M.; Jose, J.; Trochet, P.; Sukhorukov, G.B.; Gorin, D.A. Multifunctional polyelectrolyte microcapsules as a contrast agent for photoacoustic imaging in blood. J. Biophotonics 2016, 9, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, M.V.; Bratashov, D.N.; Sarimollaoglu, M.; Nedosekin, D.A.; Harrington, W.; Watts, A.; Han, M.; Khlebtsov, B.N.; Galanzha, E.I.; Gorin, D.A.; et al. Photoacoustic and fluorescent effects in multilayer plasmon-dye interfaces. J. Biophotonics 2019, 12, e201800265. [Google Scholar] [CrossRef]
- Nima, Z.A.; Watanabe, F.; Jamshidi-Parsian, A.; Sarimollaoglu, M.; Nedosekin, D.A.; Han, M.; Watts, J.A.; Biris, A.S.; Zharov, V.P.; Galanzha, E.I. Bioinspired magnetic nanoparticles as multimodal photoacoustic, photothermal and photomechanical contrast agents. Sci. Rep. 2019, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.K.; Chen, Y.; Kang, J.; Lisok, A.; Minn, I.; Pomper, M.G.; Boctor, E.M. Prostate-specific membrane antigen-targeted photoacoustic imaging of prostate cancer in vivo. J. Biophotonics 2018, 11, e201800021. [Google Scholar] [CrossRef]
- Kopach, O.; Zheng, K.; Dong, L.; Sapelkin, A.; Voitenko, N.; Sukhorukov, G.B.; Rusakov, D.A. Nano-engineered microcapsules boost the treatment of persistent pain. Drug Deliv. 2018, 25, 435–447. [Google Scholar] [CrossRef]
- Mironova, K.E.; Proshkina, G.M.; Ryabova, A.V.; Stremovskiy, O.A.; Lukyanov, S.A.; Petrov, R.V.; Deyev, S.M. Genetically encoded immunophotosensitizer 4D5scFV-miniSOG is a highly selective agent for targeted photokilling of tumor cells in vitro. Theranostics 2013, 3, 831–840. [Google Scholar] [CrossRef]
- Deyev, S.M.; Lebedenko, E.N.; Petrovskaya, L.E.; Dolgikh, D.A.; Gabibov, A.G.; Kirpichnikov, M.P. Man-made antibodies and immunoconjugates with desired properties: Function optimization using structural engineering. Rus. Chem. Rev. 2015, 84, 1–26. [Google Scholar] [CrossRef]
- Martsev, S.P.; Chumanevich, A.A.; Vlasov, A.P.; Dubnovitsky, A.P.; Tsybovsky, Y.I.; Deyev, S.M.; Cozzi, A.; Arosio, P.; Kravchuk, Z.I. Antiferritin single-chain Fv fragment is a functional protein with properties of a partially structured state: Comparison with the completely folded V(L) domain. Biochemistry 2000, 39, 8047–8057. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.; Bragina, O.; Altai, M.; Mitran, B.; Orlova, A.; Shulga, A.; Proshkina, G.; Chernov, V.; Tolmachev, V.; Deyev, S. Comparative Evaluation of Radioiodine and Technetium-Labeled DARPin 9_29 for Radionuclide Molecular Imaging of HER2 Expression in Malignant Tumors. Contrast Media Mol. Imaging 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, G.M.; Shilova, O.N.; Ryabova, A.V.; Stremovskiy, O.A.; Deyev, S.M. A new anticancer toxin based on HER2/neu-specific DARPin and photoactive flavoprotein miniSOG. Biochimie 2015, 118, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Nikitin, M.P.; Nikitin, P.I.; Deyev, S.M. MPQ-cytometry: A magnetism-based method for quantification of nanoparticle-cell interactions. Nanoscale 2016, 8, 12764–12772. [Google Scholar] [CrossRef] [PubMed]
- Deyev, S.; Proshkina, G.; Ryabova, A.; Tavanti, F.; Menziani, M.C.; Eidelshtein, G.; Avishai, G.; Kotlyar, A. Synthesis, Characterization, and Selective Delivery of DARPin-Gold Nanoparticle Conjugates to Cancer Cells. Bioconjug. Chem. 2017, 28, 2569–2574. [Google Scholar] [CrossRef]
- Balalaeva, I.V.; Zdobnova, T.A.; Krutova, I.V.; Brilkina, A.A.; Lebedenko, E.N.; Deyev, S.M. Passive and active targeting of quantum dots for whole-body fluorescence imaging of breast cancer xenografts. J. Biophotonics 2012, 5, 860–867. [Google Scholar] [CrossRef]
- Zdobnova, T.A.; Stremovskiy, O.A.; Lebedenko, E.N.; Deyev, S.M. Self-Assembling Complexes of Quantum Dots and scFv Antibodies for Cancer Cell Targeting and Imaging. PLoS ONE 2012, 7, e48248. [Google Scholar] [CrossRef]
- Deyev, S.; Proshkina, G.; Baryshnikova, O.; Ryabova, A.; Avishai, G.; Katrivas, L.; Giannini, C.; Levi-Kalisman, Y.; Kotlyar, A. Selective staining and eradication of cancer cells by protein-carrying DARPin-functionalized liposomes. Eur. J. Pharm. Biopharm. 2018, 130, 296–305. [Google Scholar] [CrossRef]
- Guryev, E.L.; Volodina, N.O.; Shilyagina, N.Y.; Gudkov, S.V.; Balalaeva, N.Y.; Volovetskiy, A.B.; Lyubeshkin, A.V.; Sen', A.V.; Ermilov, S.A.; Vodeneev, V.A.; et al. Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. Proc. Natl. Acad. Sci. USA 2018, 11, 9690–9695. [Google Scholar]
- Nikitin, M.P.; Zdobnova, T.A.; Lukash, S.V.; Stremovskiy, O.A.; Deyev, S.M. Protein-assisted self-assembly of multifunctional nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 5827–5832. [Google Scholar] [CrossRef] [PubMed]
- Aghayeva, U.F.; Nikitin, M.P.; Lukash, S.V.; Deyev, S.M. Denaturation-Resistant Bifunctional Colloidal Superstructures Assembled via the Proteinaceous Barnase-Barstar Interface. ACS Nano 2013, 7, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Zelepukin, I.V.; Stremovskiy, O.A.; Nikitin, M.P.; Care, A.; Sunna, A.; Zvyagin, A.V.; Deyev, S.M. Versatile Platform for Nanoparticle Surface Bioengineering Based on SiO2-Binding Peptide and Proteinaceous Barnase*Barstar Interface. ACS Appl. Mater. Interfaces 2018, 10, 17437–17447. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Kotelnikova, P.A.; Aghayeva, U.F.; Stremovskiy, O.A.; Novikov, I.A.; Schulga, A.A.; Nikitin, M.P.; Deyev, S.M. Self-assembling nanoparticles biofunctionalized with magnetite-binding protein for the targeted delivery to HER2/neu overexpressing cancer cells. J. Magn. Magn. Mater. 2019, 469, 450–455. [Google Scholar] [CrossRef]
- Sreenivasan, V.K.A.; Kelf, T.A.; Grebenik, E.A.; Stremovskiy, O.A.; Say, J.M.; Rabeau, J.R.; Zvyagin, A.V.; Deyev, S.M. A modular design of low-background bioassays based on a high-affinity molecular pair barstar:barnase. Proteomics 2013, 13, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Grebenik, E.A.; Nadort, A.; Generalova, A.N.; Nechaev, A.V.; Sreenivasan, V.K.; Khaydukov, E.V.; Semchishen, V.A.; Popov, A.P.; Sokolov, V.I.; Akhmanov, A.S.; et al. Feasibility study of the optical imaging of a breast cancer lesion labeled with upconversion nanoparticle biocomplexes. J. Biomed. Opt. 2013, 18, 76004. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Shashkov, E.V.; Galanzha, E.I.; Kotagiri, N.; Zharov, V.P. Photothermal antimicrobial nanotherapy and nanodiagnostics with self-assembling carbon nanotube clusters. Lasers Surgery Med. 2007, 39, 622–634. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).