Characterization of the Roles of Vimentin in Regulating the Proliferation and Migration of HSCs during Hepatic Fibrogenesis
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Animals
2.3. Analysis of Transcripts of α-SMA, Vimentin, and Procollagen Genes
2.4. Western Blot Analysis
2.5. Histology and Immunohistochemistry
2.6. Clinical Cases
2.7. Cell Culture
2.8. Gene Silencing by Small Interfering RNA
2.9. Wound-Migration Assay
2.10. Immunofluorescence
2.11. Statistical Analysis
3. Results
3.1. Liver Pathological Changes and Vimentin Expression Induced by DMN Administration
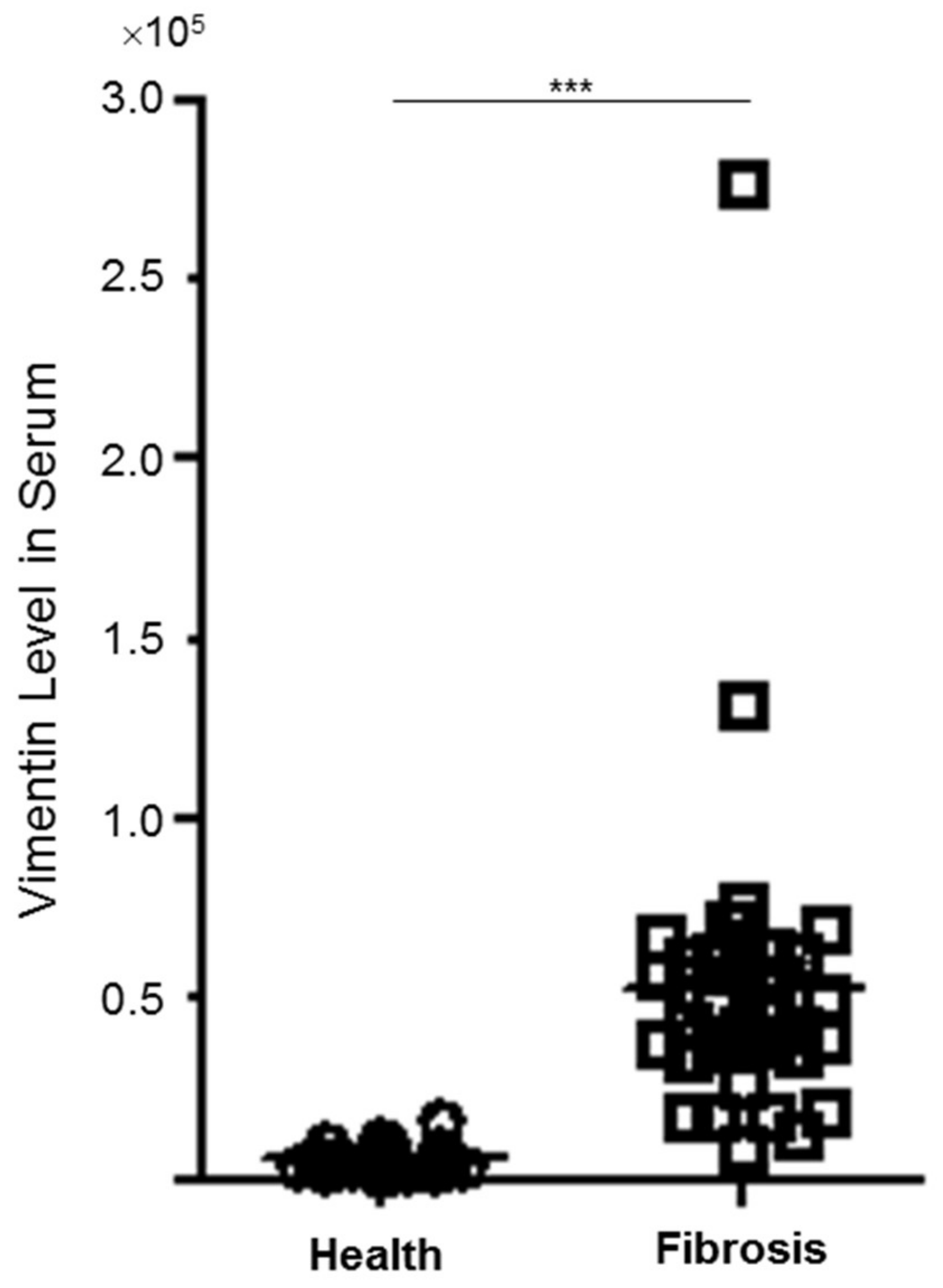
3.2. Plasma Levels of Vimentin between Control and Patients with Hepatic Fibrosis/Cirrhosis
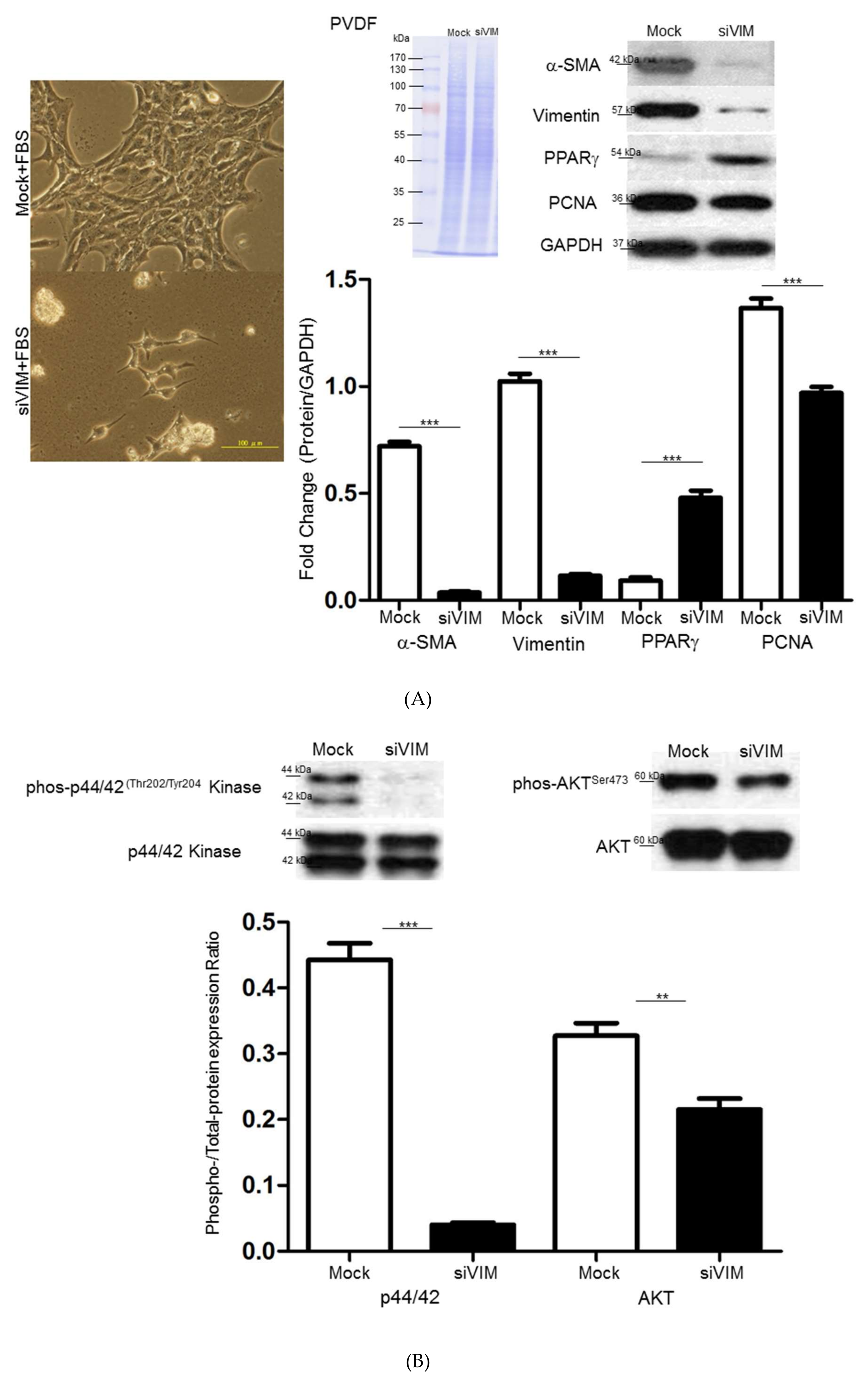
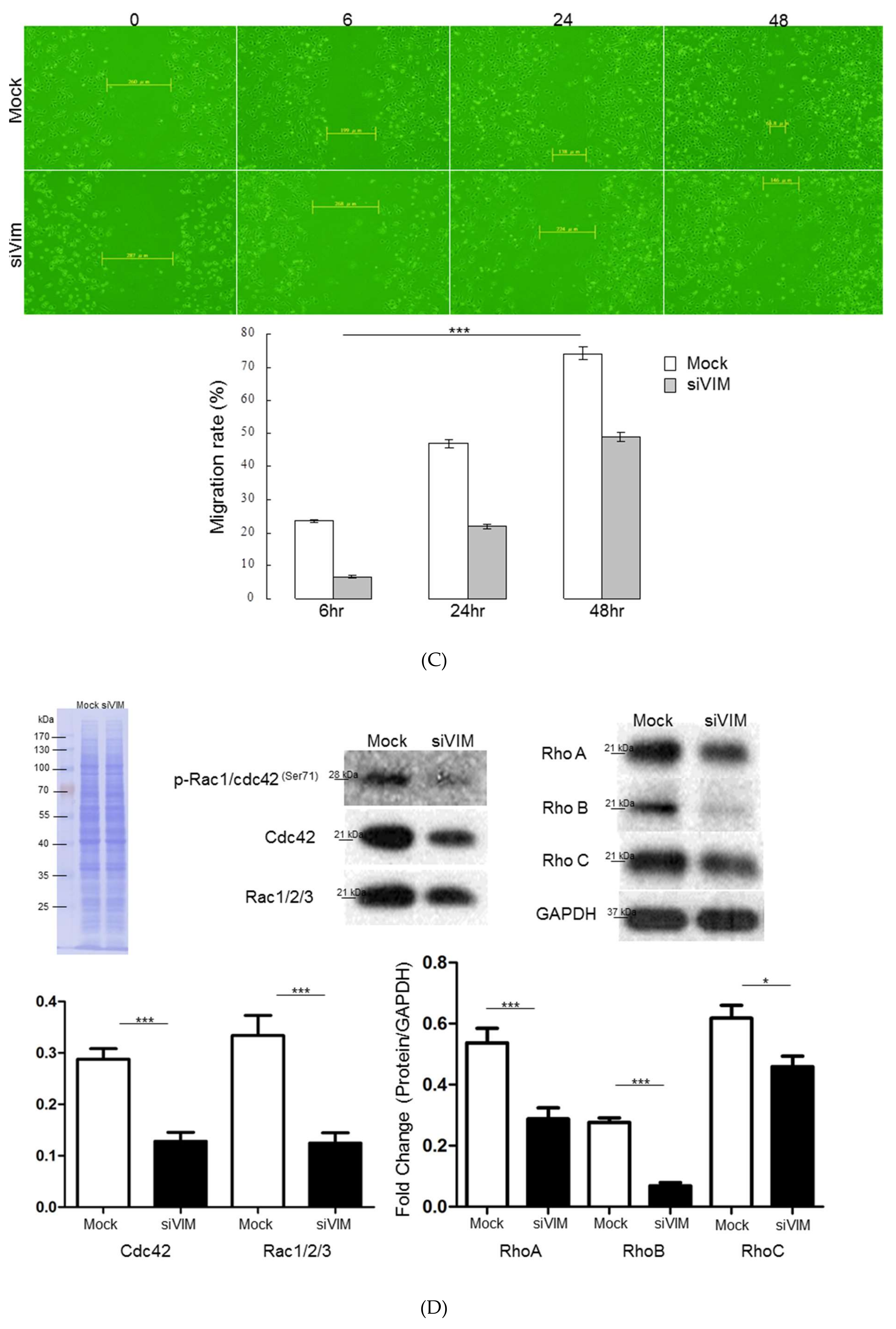
3.3. Functional Roles of Vimentin in Regulating HSC Activation
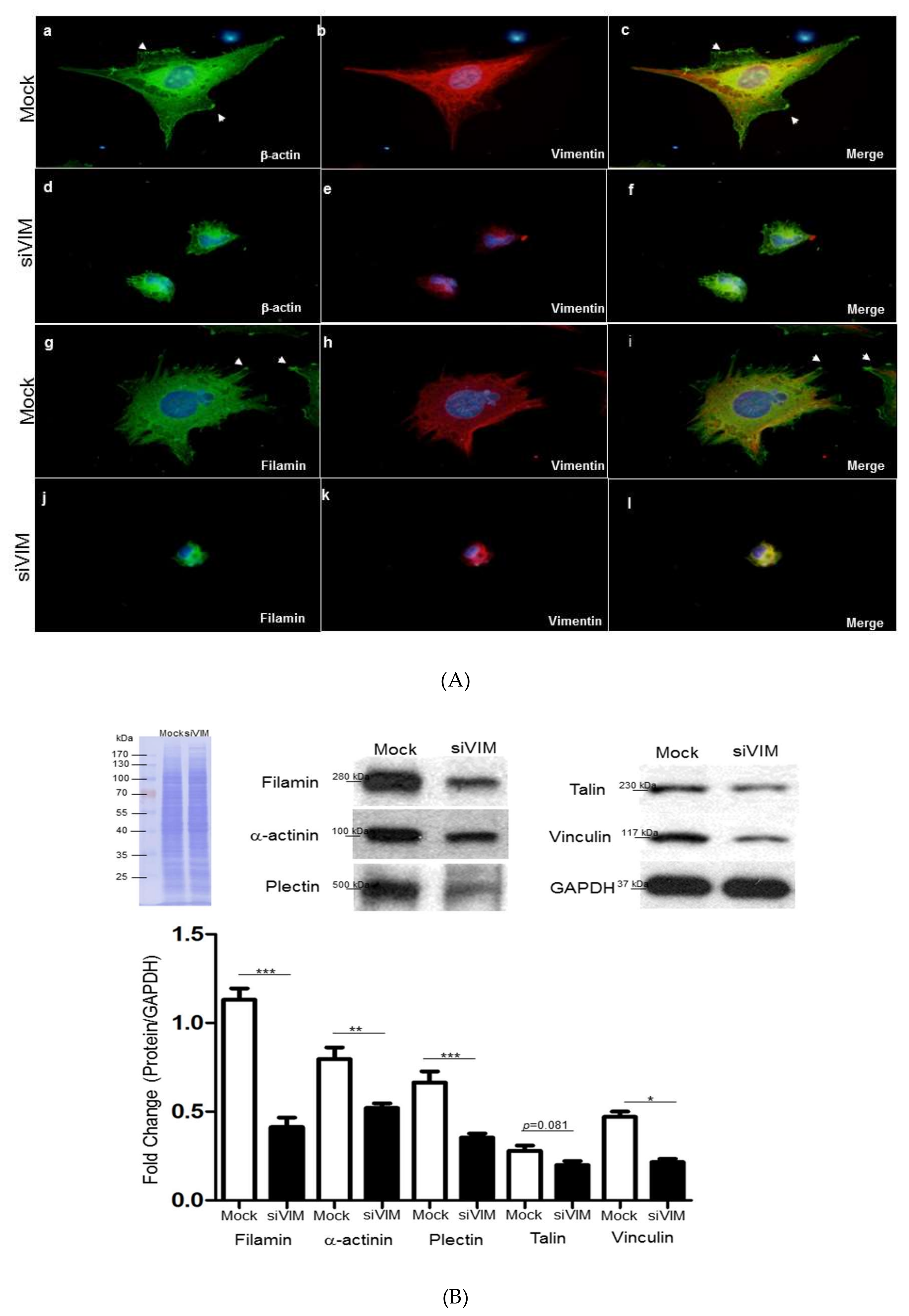
3.4. Verifiction of Vimentin-Dependent Regulation of Cytoskeletal Proteins
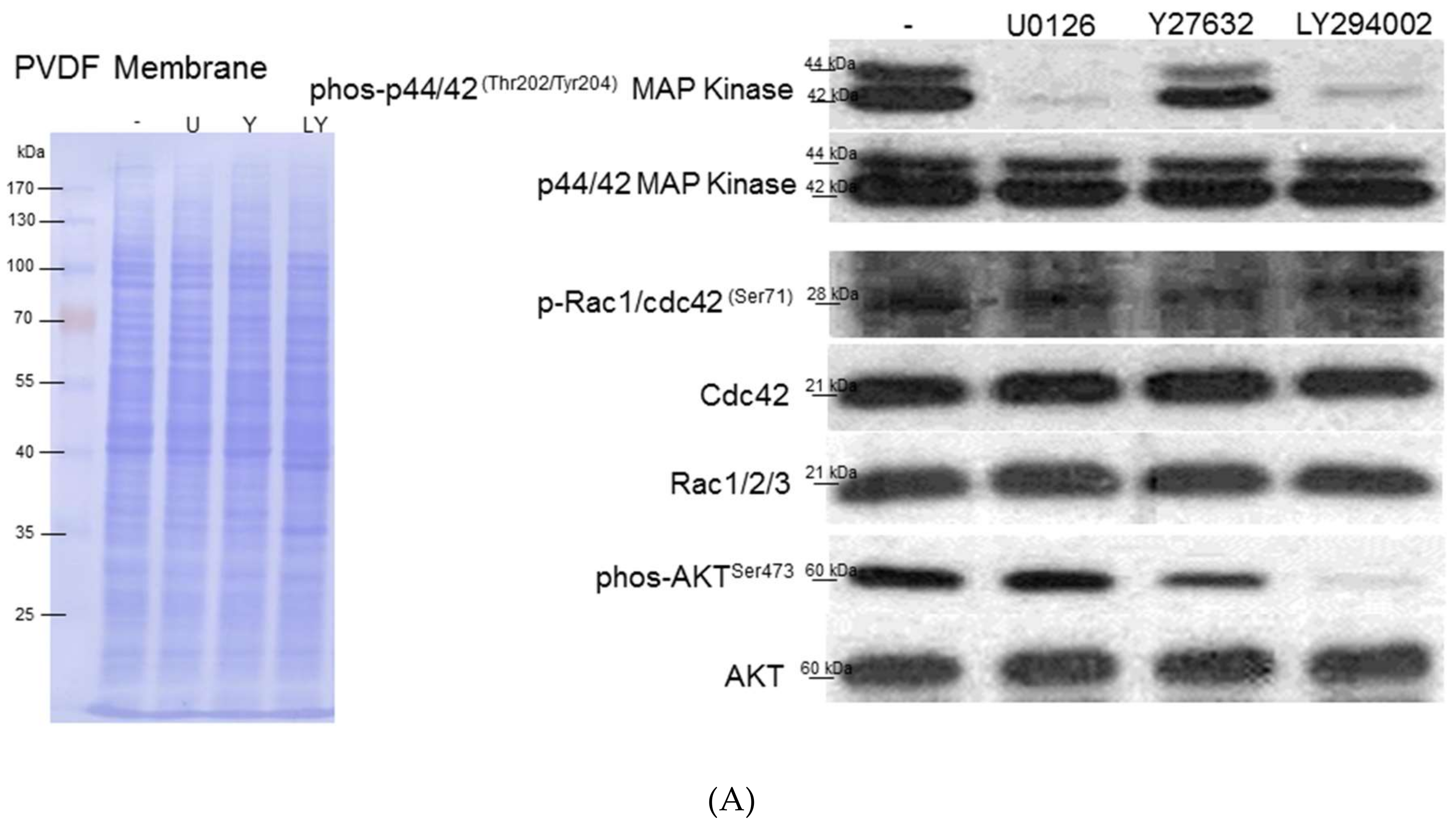
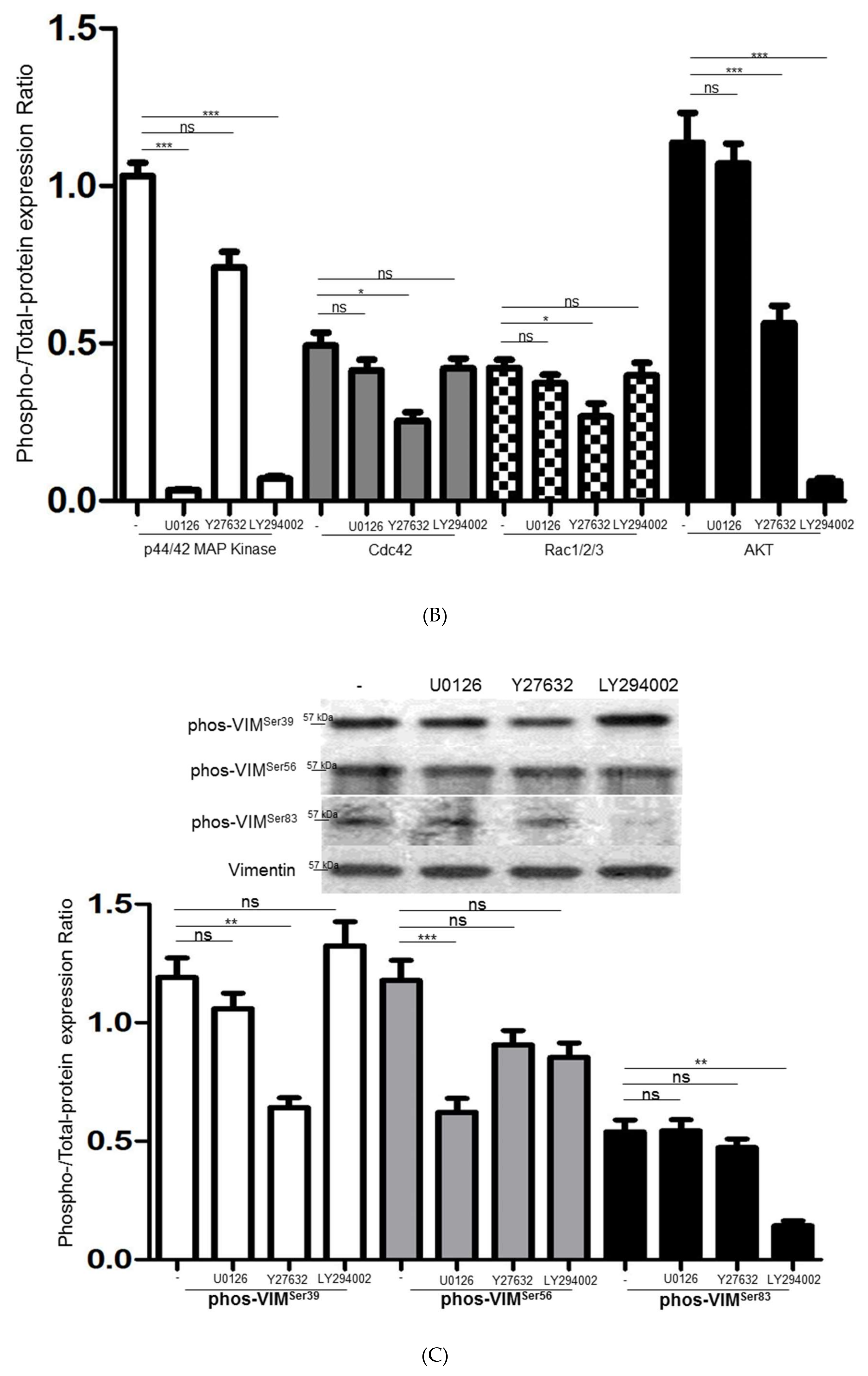
3.5. Intracellular Signaling of ERK, AKT and Rho Affecting HSC Proliferation and Motility
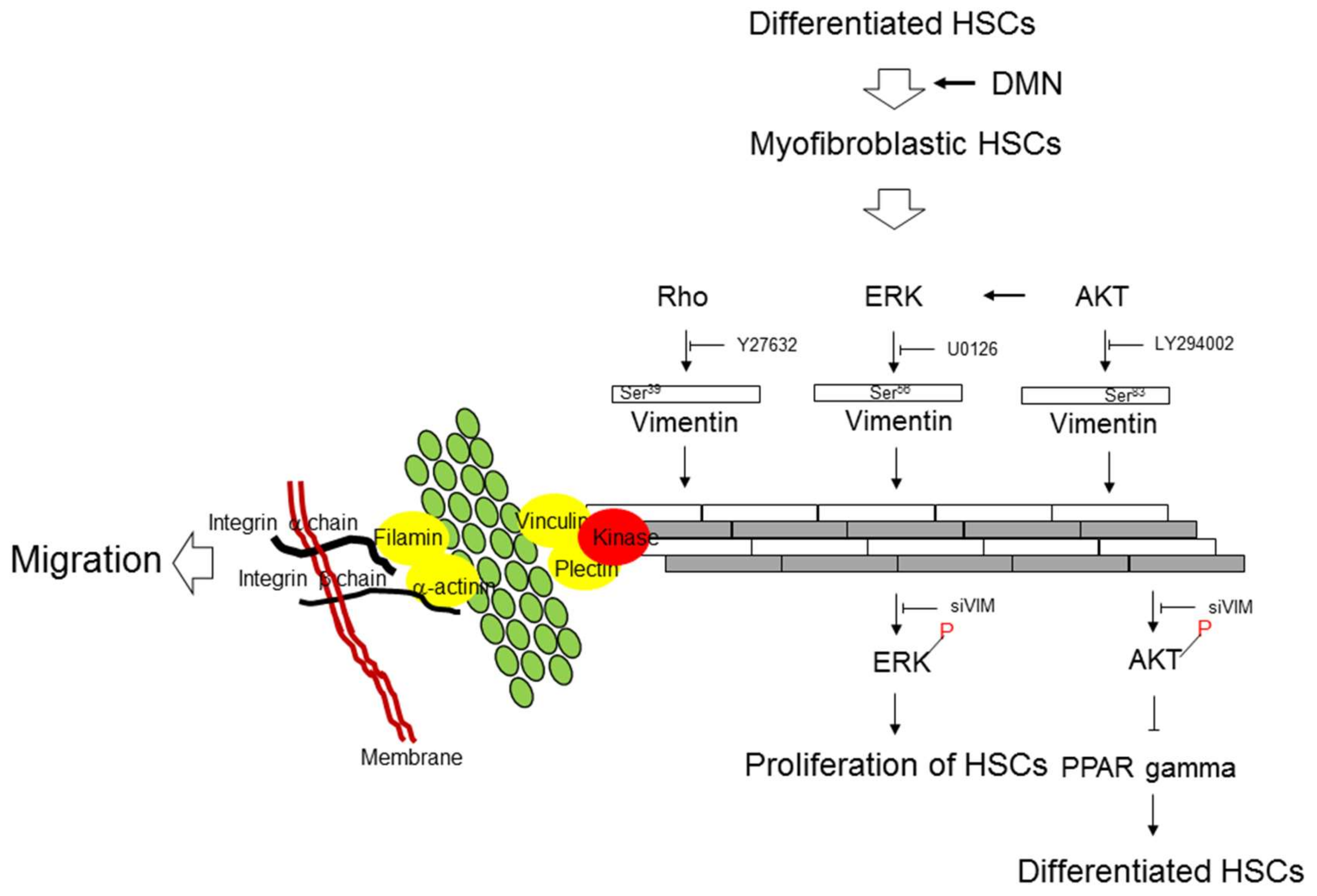
3.6. Interplays among ERK, AKT, and Rho Signaling Pathways and Different Vimentin Phosphorylated Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Invest. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Kim, W.R. The global impact of hepatic fibrosis and end-stage liver disease. Clin. Liver Dis. 2008, 12, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef] [PubMed]
- Carloni, V.; Luong, T.V.; Rombouts, K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014, 34, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L. Prediction of fibrosis progression in chronic viral hepatitis. Clin. Mol. Hepatol. 2014, 20, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Non-alcoholic fatty liver disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Tacke, F.; Trautwein, C. Mechanisms of liver fibrosis resolution. J. Hepatol. 2015, 63, 1038–1039. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Wang, X.M.; Yu, D.M.; McCaughan, G.W.; Gorrell, M.D. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology 2005, 42, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Thymosin Beta 4 Is a Potential Regulator of Hepatic Stellate Cells. Vitam. Horm. 2016, 102, 121–149. [Google Scholar] [PubMed]
- Lowery, J.; Kuczmarski, E.R.; Herrmann, H.; Goldman, R.D. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J. Biol. Chem. 2015, 290, 17145–17153. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Dai, F.; Liu, Y.; Yu, X.; Huang, C.; Wang, Y.; Yao, W. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol. Res. 2018, 133, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.M.; Bayless, K.J. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation 2014, 21, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Pallari, H.M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Greuter, T.; Shah, V.H. Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights. J. Gastroenterol. 2016, 51, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Bokoch, G.M.; Waterman-Storer, C.M. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 2003, 161, 845–851. [Google Scholar] [CrossRef]
- Byrne, K.M.; Monsefi, N.; Dawson, J.C.; Degasperi, A.; Bukowski-Wills, J.C.; Volinsky, N.; Dobrzyński, M.; Birtwistle, M.R.; Tsyganov, M.A.; Kiyatkin, A.; et al. Bistability in the Rac1, PAK, and RhoA Signaling Network Drives Actin Cytoskeleton Dynamics and Cell Motility Switches. Cell Syst. 2016, 2, 38–48. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Leu, Y.L.; Wu, T.H.; Wu, T.S. Inhibitory effects of Scutellaria baicalensis extract on hepatic stellate cells through inducing G2/M cell cycle arrest and activating ERK-dependent apoptosis via Bax and caspase pathway. J. Ethnopharmacol. 2012, 139, 829–837. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chiu, Y.T.; Lee, C.Y.; Lin, Y.L.; Huang, Y.T. Increases in fibrosis-related gene transcripts in livers of dimethylnitrosamine-intoxicated rats. J. Biomed. Sci. 2004, 11, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Wu, T.H.; Huang, C.H.; Wang, P.W.; Chen, C.C.; Wu, Y.C.; Pan, T.L. Proteomics reveals plasma profiles for monitoring the toxicity caused by chromium compounds. Clin. Chim. Acta. 2013, 423, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Wang, P.W.; Huang, C.H.; Leu, Y.L.; Wu, T.H.; Wu, Y.R.; You, J.S. Herbal formula, Scutellariae radix and Rhei rhizoma attenuate dimethylnitrosamine-induced liver fibrosis in a rat model. Sci. Rep. 2015, 5, 11734. [Google Scholar] [CrossRef]
- Hsu, C.W.; Liang, K.H.; Huang, S.F.; Tsao, K.C.; Yeh, C.T. Development of a non-invasive fibrosis test for chronic hepatitis B patients and comparison with other unpatented scores. BMC Res. Notes 2013, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Wang, P.W.; Huang, C.C.; Yeh, C.T.; Hu, T.H.; Yu, J.S. Network analysis and proteomic identification of vimentin as a key regulator associated with invasion and metastasis in human hepatocellular carcinoma cells. J. Proteomics. 2012, 75, 4676–4692. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Wang, P.W. Explore the Molecular Mechanism of Apoptosis Induced by Tanshinone IIA on Activated Rat Hepatic Stellate Cells. Evid. Based Complement. Alternat. Med. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Van Lonkhuyzen, D.R.; Hollier, B.G.; Shooter, G.K.; Leavesley, D.I.; Upton, Z. Chimeric vitronectin:insulin-like growth factor proteins enhance cell growth and migration through co-activation of receptors. Growth Factors. 2007, 25, 295–308. [Google Scholar] [CrossRef]
- George, J.; Tsuchishima, M.; Tsutsumi, M. Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis. Cell Death Dis. 2019, 10, 18. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Zhu, N.L.; Wang, J.; Asahina, K.; Machida, K. Morphogens and hepatic stellate cell fate regulation in chronic liver disease. J. Gastroenterol. Hepatol. 2012, 27, 94–98. [Google Scholar] [CrossRef]
- Kisseleva, T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 2017, 65, 1039–1043. [Google Scholar] [CrossRef]
- Novo, E.; Cannito, S.; Morello, E.; Paternostro, C.; Bocca, C.; Miglietta, A.; Parola, M. Hepatic myofibroblasts and fibrogenic progression of chronic liver diseases. Histol. Histopathol. 2015, 30, 1011–1132. [Google Scholar] [PubMed]
- Bernal, S.D.; Stahel, R.A. Cytoskeleton-associated proteins: their role as cellular integrators in the neoplastic process. Crit. Rev. Oncol. Hematol. 1985, 3, 191–204. [Google Scholar] [CrossRef]
- Hohmann, T.; Dehghani, F. The Cytoskeleton-A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Yanguas, S.C.; Cogliati, B.; Willebrords, J.; Maes, M.; Colle, I.; van den Bossche, B.; de Oliveira, C.P.M.S.; Andraus, W.; Alves, V.A.F.; Leclercq, I.; et al. Experimental models of liver fibrosis. Arch. Toxicol 2016, 90, 1025–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nakamura, F.; Lee, W.; Shifrin, Y.; Arora, P.; McCulloch, C.A. Filamin A is required for vimentin-mediated cell adhesion and spreading. Am. J. Physiol. Cell Physiol. 2010, 298, C221–C236. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Sugimura, K.; Ohoka, A.; Burden, J.; Suganuma, H.; Ikegawa, M.; Shimada, T.; Kitamura, T.; Shindoh, M.; Ishikawa, S.; et al. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun. 2014, 5, 4428. [Google Scholar] [CrossRef]
- Jiu, Y.; Peränen, J.; Schaible, N.; Cheng, F.; Eriksson, J.E.; Krishnan, R.; Lappalainen, P. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J. Cell Sci. 2017, 130, 892–902. [Google Scholar] [CrossRef]
- Ehrenreiter, K.; Piazzolla, D.; Velamoor, V.; Sobczak, I.; Small, J.V.; Takeda, J.; Leung, T.; Baccarini, M. Raf-1 regulates Rho signaling and cell migration. J. Cell Biol. 2005, 168, 955–964. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017, 18, 54. [Google Scholar] [CrossRef]
- Sihag, R.K.; Inagaki, M.; Yamaguchi, T.; Shea, T.B.; Pant, H.C. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 2007, 313, 2098–2109. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H. Fat paradox in liver disease. Keio. J. Med. 2005, 54, 190–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koo, J.B.; Nam, M.O.; Jung, Y.; Yoo, J.; Kim, D.H.; Kim, G.; Shin, S.J.; Lee, K.M.; Hahm, K.B.; Kim, J.W.; et al. Anti-fibrogenic effect of PPAR-γ agonists in human intestinal myofibroblasts. BMC Gastroenterol. 2017, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Menko, A.S.; Bleaken, B.M.; Libowitz, A.A.; Zhang, L.; Stepp, M.A.; Walker, J.L. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol. Biol. Cell. 2014, 25, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Schoumacher, M.; Goldman, R.D.; Louvard, D.; Vignjevic, D.M. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 2010, 189, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.Y.; Yang, C.Q.; Jiang, W. Effect of RhoA on transforming growth factor β1-induced rat hepatic stellate cell migration. Liver Int. 2012, 32, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Van Beuge, M.M.; Prakash, J.; Lacombe, M.; Gosens, R.; Post, E.; Reker-Smit, C.; Beljaars, L.; Poelstra, K. Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J. Pharmacol. Exp. Ther. 2011, 337, 628–635. [Google Scholar] [CrossRef]
- Iwamoto, H.; Nakamuta, M.; Tada, S.; Sugimoto, R.; Enjoji, M.; Nawata, H. A p160ROCK-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth. J. Hepatol. 2000, 32, 762–770. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-W.; Wu, T.-H.; Lin, T.-Y.; Chen, M.-H.; Yeh, C.-T.; Pan, T.-L. Characterization of the Roles of Vimentin in Regulating the Proliferation and Migration of HSCs during Hepatic Fibrogenesis. Cells 2019, 8, 1184. https://doi.org/10.3390/cells8101184
Wang P-W, Wu T-H, Lin T-Y, Chen M-H, Yeh C-T, Pan T-L. Characterization of the Roles of Vimentin in Regulating the Proliferation and Migration of HSCs during Hepatic Fibrogenesis. Cells. 2019; 8(10):1184. https://doi.org/10.3390/cells8101184
Chicago/Turabian StyleWang, Pei-Wen, Tung-Ho Wu, Tung-Yi Lin, Mu-Hong Chen, Chau-Ting Yeh, and Tai-Long Pan. 2019. "Characterization of the Roles of Vimentin in Regulating the Proliferation and Migration of HSCs during Hepatic Fibrogenesis" Cells 8, no. 10: 1184. https://doi.org/10.3390/cells8101184
APA StyleWang, P.-W., Wu, T.-H., Lin, T.-Y., Chen, M.-H., Yeh, C.-T., & Pan, T.-L. (2019). Characterization of the Roles of Vimentin in Regulating the Proliferation and Migration of HSCs during Hepatic Fibrogenesis. Cells, 8(10), 1184. https://doi.org/10.3390/cells8101184






