Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Production of Tissue-Engineered Skin Substitutes
2.3. Histologic and Morphometric Analyses
2.4. Percutaneous Absorption
2.5. Gas Chromatography
2.6. Statistical Analysis
3. Results
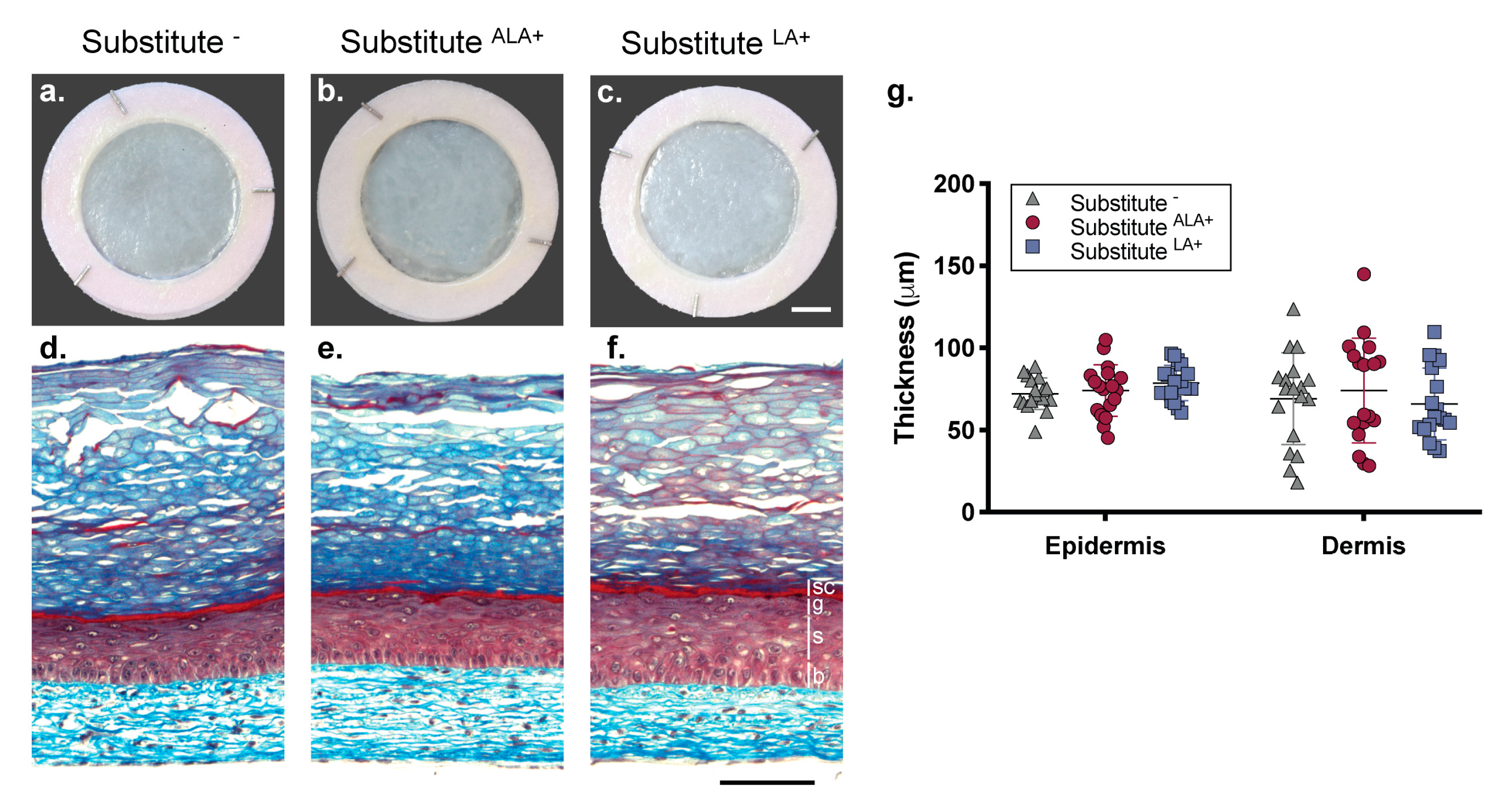
3.1. Morphology of the Skin Substitutes
3.2. Effects of Essential Fatty Acids on the Percutaneous Absorption of Testosterone and Benzoic Acid Through Skin Substitutes
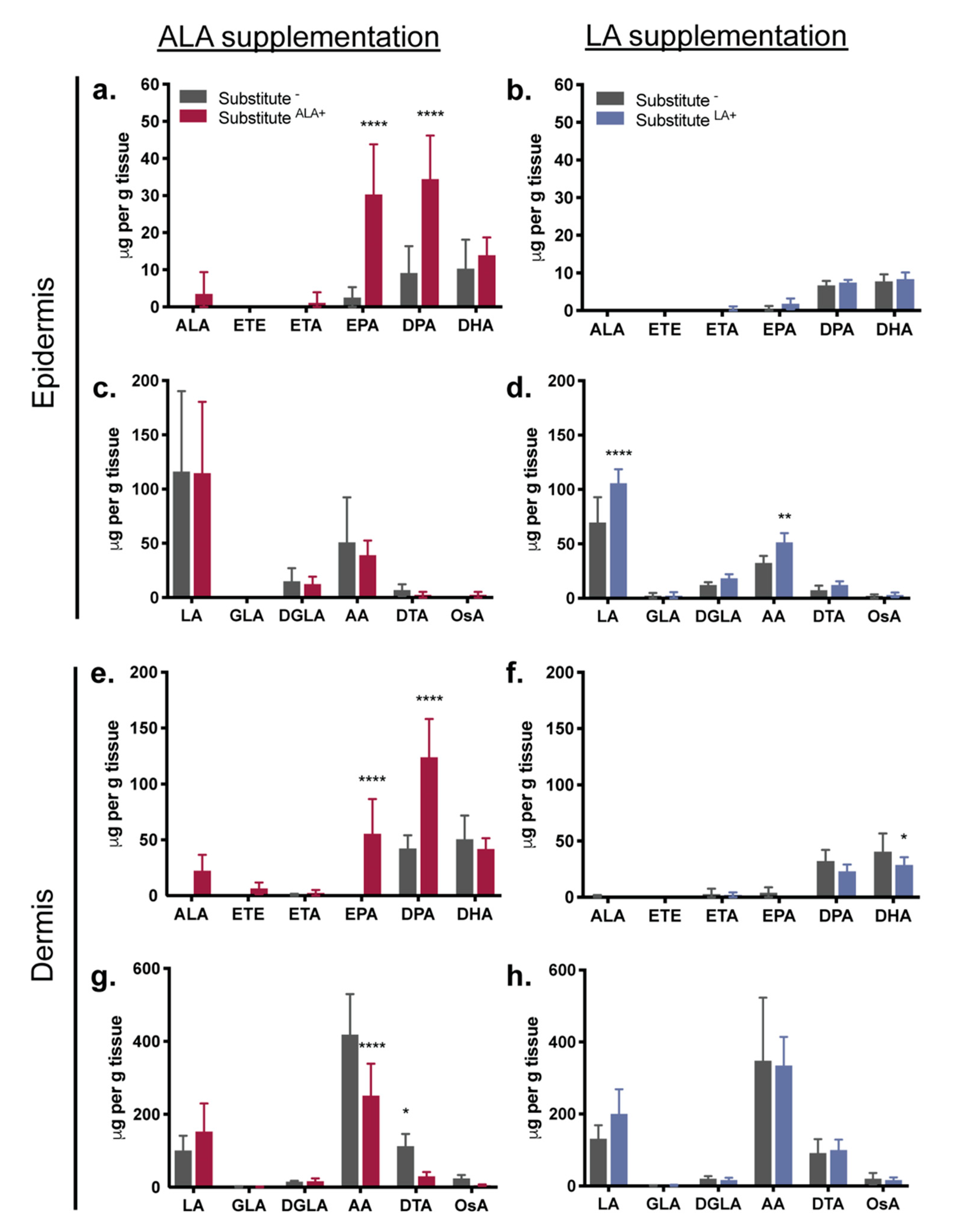
3.3. Fatty Acids Added to the Culture Media: Incorporation into the Epidermal and Dermal Phospholipids
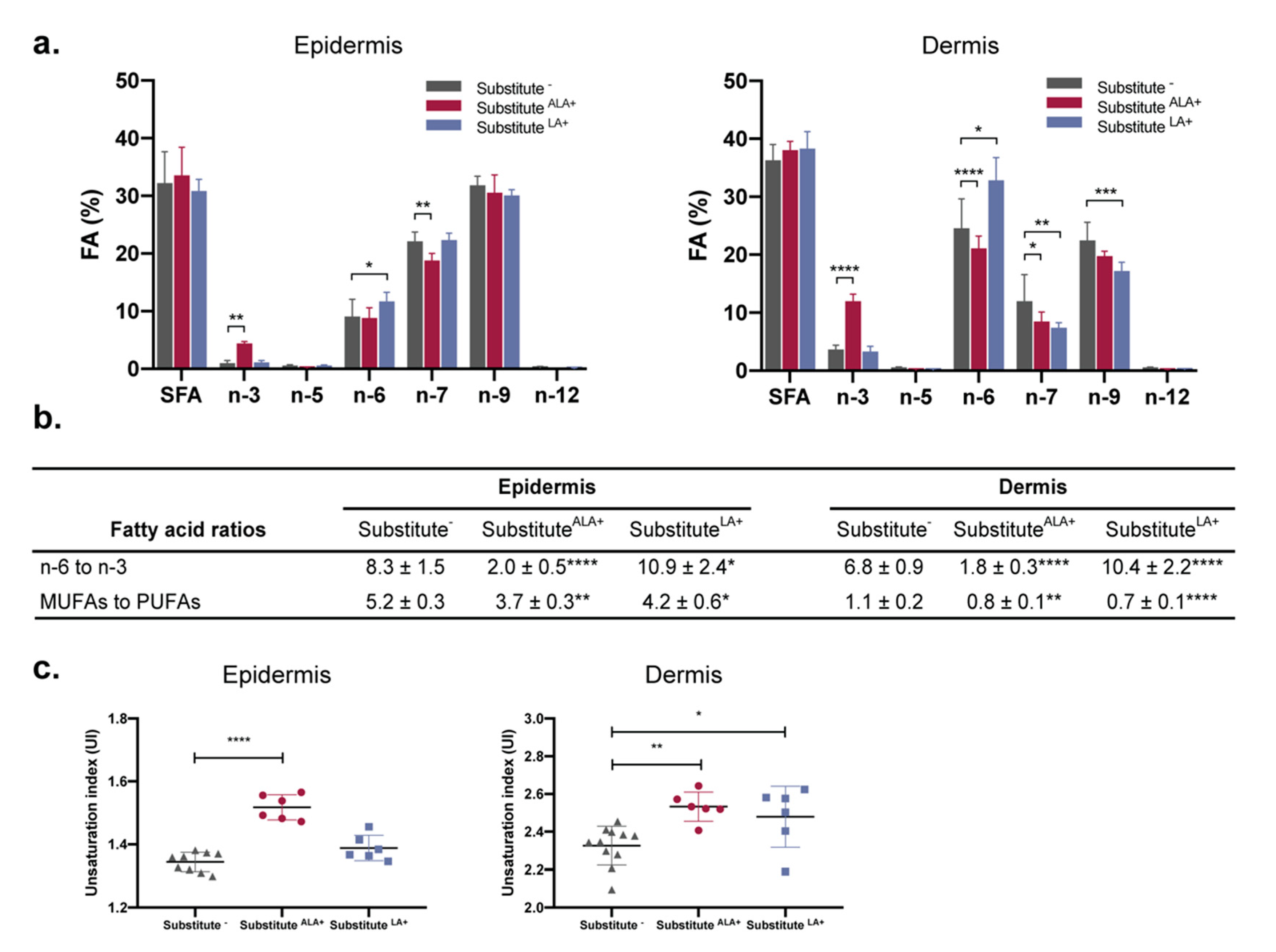
3.4. The Proportion of n-3 and n-6 Fatty Acids Increases in Response to ALA and LA Supplementation, Causing Changes in n-3 to n-6 Ratios and Unsaturation Indexes
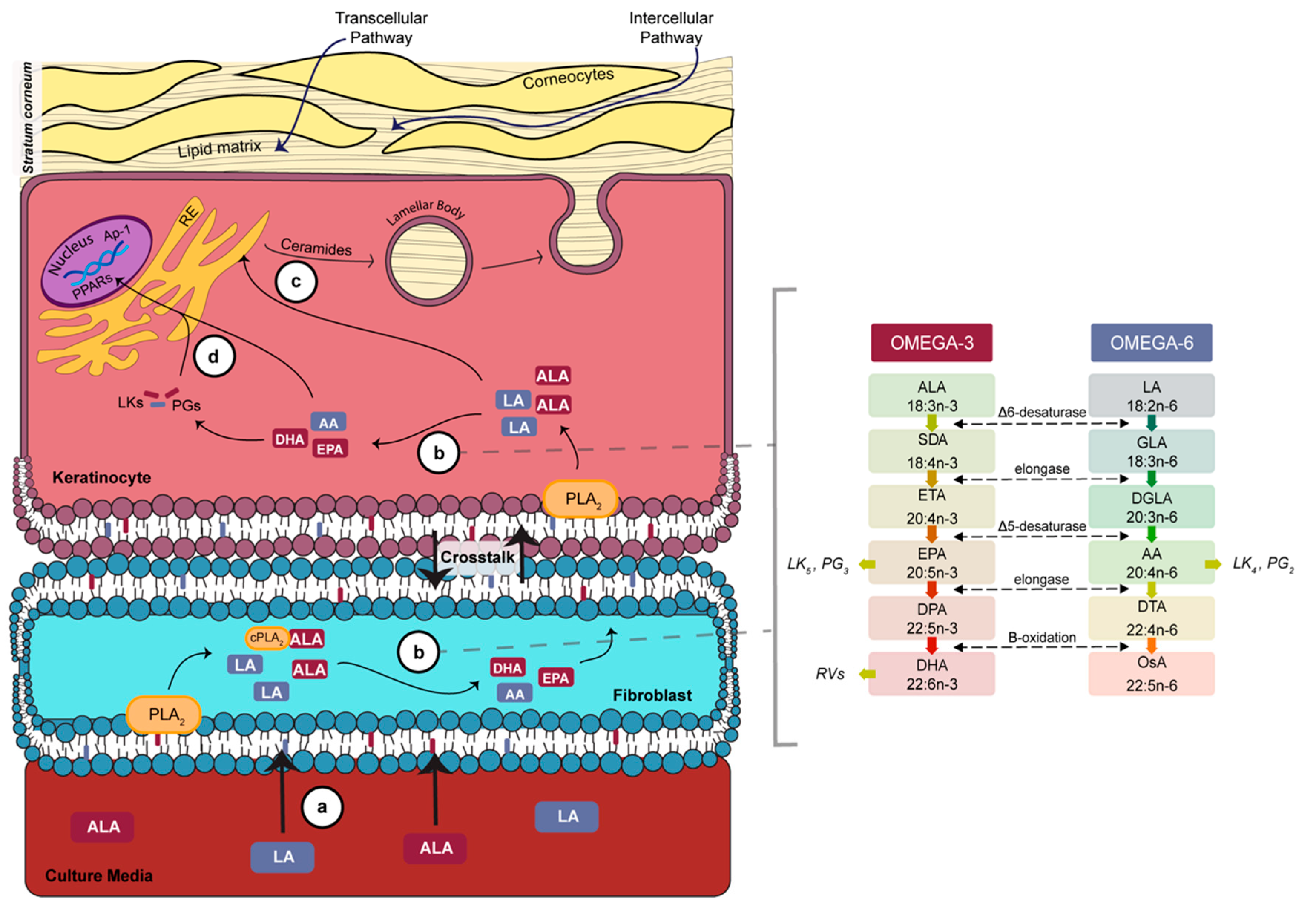
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madison, K.C. Barrier function of the skin: “la raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta 2014, 1841, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Kruse, V.; Neess, D.; Faergeman, N.J. The Significance of Epidermal Lipid Metabolism in Whole-Body Physiology. Trends Endocrinol. Metab. 2017, 28, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Freinkel, R.K.; Traczyk, T.N. Flux of fatty acids during epidermal differentiation. J. Investig. Dermatol. 1977, 69, 413–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yardley, H.J.; Summerly, R. Lipid composition and metabolism in normal and diseased epidermis. Pharmacol. Ther. 1981, 13, 357–383. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model. Biochim. Biophys. Acta 2017, 1859, 1679–1689. [Google Scholar] [CrossRef]
- Burr, G.O.; Burr, M.M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929, 82, 345–367. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharm. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Gooris, G.S.; Dubbelaar, F.E.; Weerheim, A.M.; Ijzerman, A.P.; Ponec, M. Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J. Lipid Res. 1998, 39, 186–196. [Google Scholar] [PubMed]
- Melton, J.L.; Wertz, P.W.; Swartzendruber, D.C.; Downing, D.T. Effects of essential fatty acid deficiency on epidermal O-acylsphingolipids and transepidermal water loss in young pigs. Biochim. Biophys. Acta 1987, 921, 191–197. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Gooris, G.S.; Groen, D.; Barlow, D.J.; Lawrence, M.J.; Deme, B.; Bouwstra, J.A. Stratum corneum lipid matrix: Location of acyl ceramide and cholesterol in the unit cell of the long periodicity phase. Biochim. Biophys. Acta 2016, 1858, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Behne, M.; Uchida, Y.; Seki, T.; de Montellano, P.O.; Elias, P.M.; Holleran, W.M. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J. Investig. Dermatol. 2000, 114, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Watson, A.L.; Solgadi, A.; Butowski, C.; Allaway, D.; Portoukalian, J. Linoleate-enriched diet increases both linoleic acid esterified to omega hydroxy very long chain fatty acids and free ceramides of canine stratum corneum without effect on protein-bound ceramides and skin barrier function. Arch. Dermatol. Res. 2018, 310, 579–589. [Google Scholar] [CrossRef]
- Nicolaou, A. Eicosanoids in skin inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Pilkington, S.M.; Massey, K.A.; Sassano, G.; Rhodes, L.E.; Nicolaou, A. Distribution of bioactive lipid mediators in human skin. J. Investig. Dermatol. 2015, 135, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Mayser, P.; Grimm, H.; Grimminger, F. n-3 fatty acids in psoriasis. Br. J. Nutr. 2002, 87 (Suppl. 1), S77–S82. [Google Scholar] [CrossRef]
- Clark, C.C.T.; Taghizadeh, M.; Nahavandi, M.; Jafarnejad, S. Efficacy of omega-3 supplementation in patients with psoriasis: A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2019, 38, 977–988. [Google Scholar] [CrossRef]
- Netzlaff, F.; Lehr, C.M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin, SkinEthic and EpiDerm: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Sarmento, B.; Rodrigues, F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int. J. Pharm. 2017, 519, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, C.L.; Duell, E.A.; Rhodes, L.M.; Dunham, W.R. In vitro model of essential fatty acid deficiency. J. Investig. Dermatol. 1992, 99, 703–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Smeden, J.; Boiten, W.A.; Hankemeier, T.; Rissmann, R.; Bouwstra, J.A.; Vreeken, R.J. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim. Biophys. Acta 2014, 1841, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Duque-Fernandez, A.; Gauthier, L.; Simard, M.; Jean, J.; Gendreau, I.; Morin, A.; Soucy, J.; Auger, M.; Pouliot, R. A 3D-psoriatic skin model for dermatological testing: The impact of culture conditions. Biochem. Biophys. Rep. 2016, 8, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Netzlaff, F.; Kaca, M.; Bock, U.; Haltner-Ukomadu, E.; Meiers, P.; Lehr, C.M.; Schaefer, U.F. Permeability of the reconstructed human epidermis model Episkin in comparison to various human skin preparations. Eur. J. Pharm. Biopharm. 2007, 66, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Celli, A.; Crumrine, D.; Hupe, M.; Adame, L.; Pennypacker, S.; Park, K.; Uchida, Y.; Feingold, K.R.; Elias, P.M.; et al. Functional Epidermal Permeability Barrier in Human Epidermal Equivalents. Tissue Eng. 2014. [Google Scholar] [CrossRef]
- Marcelo, C.L.; Rhodes, L.M.; Dunham, W.R. Normalization of essential-fatty-acid-deficient keratinocytes requires palmitic acid. J. Investig. Dermatol. 1994, 103, 564–568. [Google Scholar] [CrossRef][Green Version]
- Garner, W.L.; Oyatsu, Y.; Zuccaro, C.; Rodriquez, J.L.; Smith, D.J.; Marcelo, C.L. The effect of essential fatty acid supplementation on keratinocyte replication. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 349–355. [Google Scholar] [CrossRef]
- Breiden, B.; Gallala, H.; Doering, T.; Sandhoff, K. Optimization of submerged keratinocyte cultures for the synthesis of barrier ceramides. Eur. J. Cell Biol. 2007, 86, 657–673. [Google Scholar] [CrossRef]
- Vicanova, J.; Weerheim, A.M.; Kempenaar, J.A.; Ponec, M. Incorporation of linoleic acid by cultured human keratinocytes. Arch. Dermatol. Res. 1999, 291, 405–412. [Google Scholar] [PubMed]
- Boyce, S.T.; Williams, M.L. Lipid supplemented medium induces lamellar bodies and precursors of barrier lipids in cultured analogues of human skin. J. Investig. Dermatol. 1993, 101, 180–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thakoersing, V.S.; Smeden, J.; Boiten, W.A.; Gooris, G.S.; Mulder, A.A.; Vreeken, R.J.; El Ghalbzouri, A.; Bouwstra, J.A. Modulation of stratum corneum lipid composition and organization of human skin equivalents by specific medium supplements. Exp. Dermatol. 2015, 24, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Ohyanagi, C.; Kawaguchi, N.; Matsuda, H.; Miyamoto, Y.; Ohya, S.; Nabe, T. Eicosapentaenoic acid ethyl ester ameliorates atopic dermatitis-like symptoms in special diet-fed hairless mice, partly by restoring covalently bound ceramides in the stratum corneum. Exp. Dermatol. 2018, 27, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Rouabhia, M.; Guignard, R.; Carrier, L.; Bouvard, V.; Auger, F.A. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993, 19, 99–104. [Google Scholar] [CrossRef]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef]
- Pouliot, R.; Larouche, D.; Auger, F.A.; Juhasz, J.; Xu, W.; Li, H.; Germain, L. Reconstructed human skin produced in vitro and grafted on athymic mice. Transplantation 2002, 73, 1751–1757. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Auger, F.A.; Germain, L. Anchored skin equivalent cultured in vitro: A new tool for percutaneous absorption studies. In Vitro Cell. Dev. Biol. Anim. 1993, 29A, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Berthiaume, L.; Hadj-Tahar, A.; Rajput, A.H.; Bedard, P.J.; Di Paolo, T.; Julien, P.; Calon, F. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem. Int. 2006, 48, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Gevariya, N.; Besancon, M.; Robitaille, K.; Picard, V.; Diabate, L.; Alesawi, A.; Julien, P.; Fradet, Y.; Bergeron, A.; Fradet, V. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate 2019, 79, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yago, M.D.; Diaz, R.J.; Ramirez, R.; Martinez, M.A.; Manas, M.; Martinez-Victoria, E. Dietary-induced changes in the fatty acid profile of rat pancreatic membranes are associated with modifications in acinar cell function and signalling. Br. J. Nutr. 2004, 91, 227–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elias, P.M.; Brown, B.E.; Ziboh, V.A. The permeability barrier in essential fatty acid deficiency: Evidence for a direct role for linoleic acid in barrier function. J. Investig. Dermatol. 1980, 74, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Cho, E.S.; Downing, D.T. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rat. Biochim. Biophys. Acta 1983, 753, 350–355. [Google Scholar] [CrossRef]
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochim. Biophys. Acta 1985, 834, 357–363. [Google Scholar] [CrossRef]
- Fujii, M.; Nakashima, H.; Tomozawa, J.; Shimazaki, Y.; Ohyanagi, C.; Kawaguchi, N.; Ohya, S.; Kohno, S.; Nabe, T. Deficiency of n-6 polyunsaturated fatty acids is mainly responsible for atopic dermatitis-like pruritic skin inflammation in special diet-fed hairless mice. Exp. Dermatol. 2013, 22, 272–277. [Google Scholar] [CrossRef]
- Van de Sandt, J.J.; van Burgsteden, J.A.; Cage, S.; Carmichael, P.L.; Dick, I.; Kenyon, S.; Korinth, G.; Larese, F.; Limasset, J.C.; Maas, W.J.; et al. In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: A multi-centre comparison study. Regul. Toxicol. Pharmacol. 2004, 39, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Mahmoud, A.; Vuia, A.; Rubbelke, M.K.; Schmidt, E.; Schaller, M.; Kandarova, H.; Haberland, A.; Schafer, U.F.; Bock, U.; et al. Reconstructed epidermis versus human and animal skin in skin absorption studies. Toxicol. In Vitro 2005, 19, 813–822. [Google Scholar] [CrossRef]
- Michel, M.; Germain, L.; Belanger, P.M.; Auger, F.A. Functional evaluation of anchored skin equivalent cultured in vitro: Percutaneous absorption studies and lipid analysis. Pharm. Res. 1995, 12, 455–458. [Google Scholar] [CrossRef]
- Barry, B.W. Lipid-Protein-Partitioning theory of skin penetration enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Wilkinson, S.C.; Maas, W.J.; Nielsen, J.B.; Greaves, L.C.; van de Sandt, J.J.; Williams, F.M. Interactions of skin thickness and physicochemical properties of test compounds in percutaneous penetration studies. Int. Arch. Occup. Environ. Health 2006, 79, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Holub, B.J. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. CMAJ 2002, 166, 608–615. [Google Scholar] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Hibbeln, J.R.; Lin, Y.; Goodson, S.; Riggs, P.; Sebring, N.; Brown, G.L.; Salem, N., Jr. Effects of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. Am. J. Clin. Nutr. 2003, 77, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef] [PubMed]
- Drouin, G.; Rioux, V.; Legrand, P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie 2019, 159, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Okada, N.; Nakatani, S.; Yoshikawa, K. Eicosapentaenoic acid-induced changes in membrane fluidity and cell adhesion molecules in cultured human keratinocytes. Br. J. Dermatol. 1995, 133, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Structure and function of the stratum corneum extracellular matrix. J. Investig. Dermatol. 2012, 132, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol 2005, 6, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Kew, S.; Banerjee, T.; Minihane, A.M.; Finnegan, Y.E.; Williams, C.M.; Calder, P.C. Relation between the fatty acid composition of peripheral blood mononuclear cells and measures of immune cell function in healthy, free-living subjects aged 25–72 y. Am. J. Clin. Nutr. 2003, 77, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.; Jiang, Y.; He, S.S.; Friedman, M.; Elias, P.M.; Bikle, D.D.; Williams, M.L.; Feingold, K.R. Keratinocyte differentiation is stimulated by activators of the nuclear hormone receptor PPARalpha. J. Investig. Dermatol. 1998, 110, 368–375. [Google Scholar] [CrossRef]
- Chon, S.H.; Tannahill, R.; Yao, X.; Southall, M.D.; Pappas, A. Keratinocyte differentiation and upregulation of ceramide synthesis induced by an oat lipid extract via the activation of PPAR pathways. Exp. Dermatol. 2015, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Batheja, P.; Song, Y.; Wertz, P.; Michniak-Kohn, B. Effects of growth conditions on the barrier properties of a human skin equivalent. Pharm. Res. 2009, 26, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Moosbrugger-Martinz, V.; Blunder, S.; Dubrac, S. Role of PPAR, LXR, and PXR in epidermal homeostasis and inflammation. Biochim. Biophys. Acta 2014, 1841, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, D.S.; Brentnall, M.; Mietla, J.A.; Hoeferlin, L.A.; Diegelmann, R.F.; Boise, L.H.; Chalfant, C.E. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J. Lipid Res. 2014, 55, 1298–1309. [Google Scholar] [CrossRef] [PubMed]

| Molecules | Donors | Substitute− | SubstituteALA+ | p-Value | Substitute− | SubstituteLA+ | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flux (μg/cm2/h) | n | Flux (μg/cm2/h) | n | Flux (μg/cm2/h) | n | Flux (μg/cm2/h) | n | ||||
| Testosterone | |||||||||||
| 1 | 62.8 ± 7.6 | 7 | 37.6 ± 12.6 | 7 | NS | 35.1 ± 2.9 | 8 | 32.6 ± 3.7 | 8 | NS | |
| 2 | 94.9 ± 7.2 | 6 | 49.4 ± 7.3 | 6 | 0.0013 | 94.9 ± 7.2 | 6 | 101.5 ± 13.7 | 6 | NS | |
| 3 | 79.7 ± 29.4 | 6 | 54.9 ± 4.0 | 6 | NS | 37.1 ± 3.7 | 7 | 37.3 ± 5.5 | 7 | NS | |
| Mean | 78.3 ± 9.9 | 19 | 46.8 ± 5.3 | 19 | 0.008 | 52.9 ± 6.4 | 21 | 48.8 ± 9.7 | 21 | NS | |
| Benzoic Acid | |||||||||||
| 1 | 456.2 ± 25.7 | 7 | 369.8 ± 11.2 | 7 | 0.0095 | 456.2 ± 25.7 | 7 | 396.3 ± 11.0 | 7 | NS | |
| 2 | 417.7 ± 3.3 | 6 | 389.2 ± 9.1 | 4 | 0.0087 | 417.7 ± 3.3 | 6 | 399.3 ± 4.3 | 5 | 0.0072 | |
| 3 | 437.9 ± 7.7 | 5 | 444.3 ± 11.9 | 5 | NS | 380.2 ± 20.8 | 7 | 410.7 ± 16.5 | 7 | NS | |
| Mean | 438.3 ± 10.6 | 18 | 397.9 ± 10.4 | 16 | 0.0105 | 418.0 ± 13.2 | 20 | 402.4 ± 7.2 | 19 | NS | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simard, M.; Julien, P.; Fradette, J.; Pouliot, R. Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells 2019, 8, 1142. https://doi.org/10.3390/cells8101142
Simard M, Julien P, Fradette J, Pouliot R. Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells. 2019; 8(10):1142. https://doi.org/10.3390/cells8101142
Chicago/Turabian StyleSimard, Mélissa, Pierre Julien, Julie Fradette, and Roxane Pouliot. 2019. "Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability" Cells 8, no. 10: 1142. https://doi.org/10.3390/cells8101142
APA StyleSimard, M., Julien, P., Fradette, J., & Pouliot, R. (2019). Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells, 8(10), 1142. https://doi.org/10.3390/cells8101142







