Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise
Abstract
1. Introduction
2. Cardiac Cellular Changes in Exercise
2.1. Cellular Regeneration and Physiological and Pathological Hypertrophy
2.2. Animal Models of Exercise
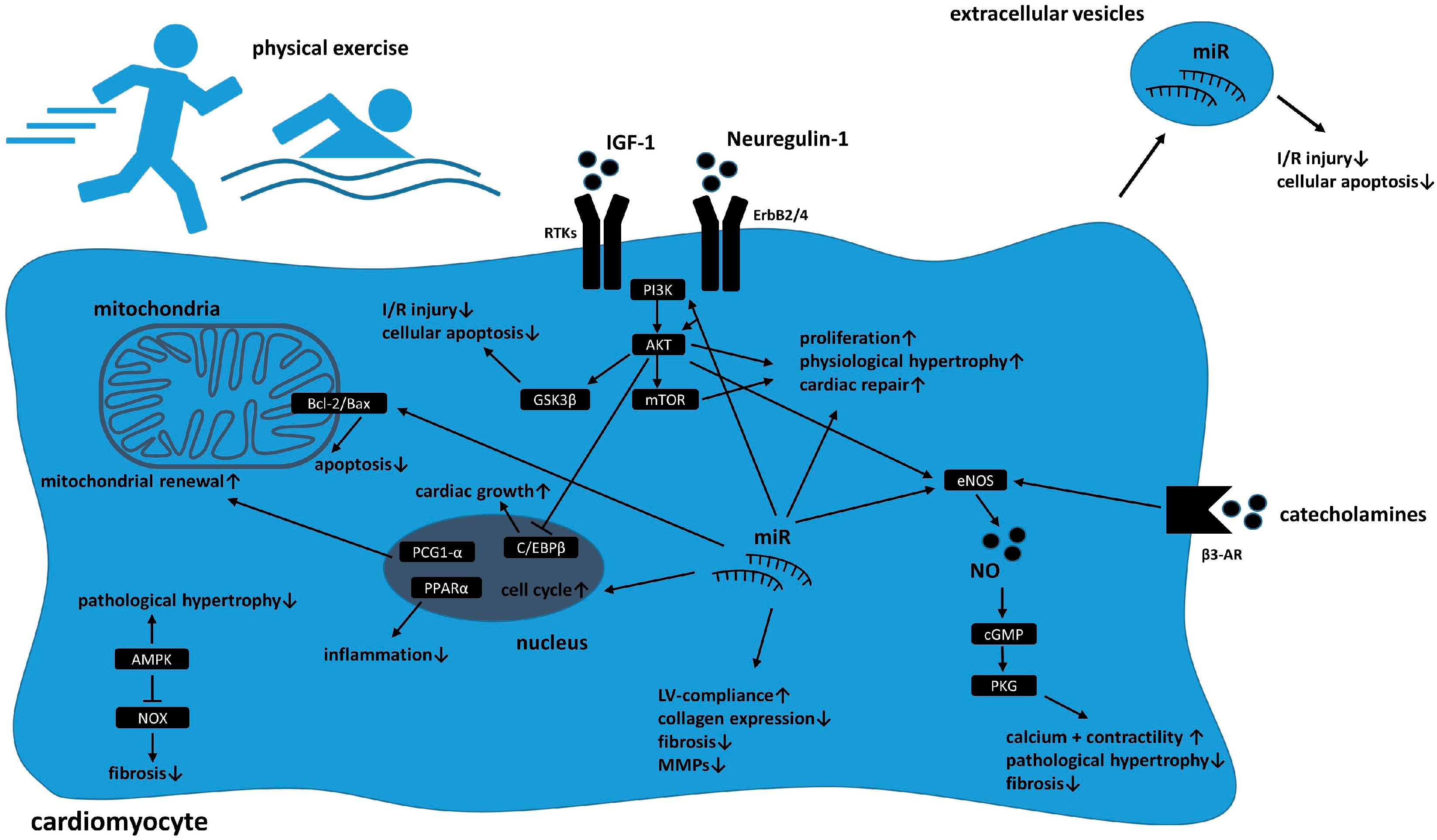
2.3. Major Signaling Pathways in Exercise-Induced Cardiac Remodeling
2.3.1. Akt-Signaling
2.3.2. Neuregulin-1/ErbB-Signaling
2.3.3. Nitric Oxide (NO) Signaling
2.3.4. Other Pathways and Extracellular Vesicles
3. MicroRNAs
4. Metabolic and Mitochondrial Cardiac Changes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Golbidi, S.; Laher, I. Exercise and the cardiovascular system. Cardiol. Res. Pract. 2012, 2012, 210852. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Katan, M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am. J. Med. 2002, 113 (Suppl. 9B), 13S–24S. [Google Scholar] [CrossRef]
- Wilson, K.; Gibson, N.; Willan, A.; Cook, D. Effect of smoking cessation on mortality after myocardial infarction: Meta-analysis of cohort studies. Arch. Intern. Med. 2000, 160, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P. Physical Activity Guidelines for Americans from the US Department of Health and Human Services. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005263. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.; Montgomery, H.E.; Mullen, M.J.; Donald, A.E.; Powe, A.J.; Bull, T.; Jubb, M.; World, M.; Deanfield, J.E. Exercise training enhances endothelial function in young men. J. Am. Coll. Cardiol. 1999, 33, 1379–1385. [Google Scholar] [CrossRef]
- Vona, M.; Codeluppi, G.M.; Iannino, T.; Ferrari, E.; Bogousslavsky, J.; von Segesser, L.K. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation 2009, 119, 1601–1608. [Google Scholar] [CrossRef]
- Pearson, M.J.; Smart, N.A. Aerobic Training Intensity for Improved Endothelial Function in Heart Failure Patients: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2017, 2017, 2450202. [Google Scholar] [CrossRef]
- Calvert, J.W.; Condit, M.E.; Aragon, J.P.; Nicholson, C.K.; Moody, B.F.; Hood, R.L.; Sindler, A.L.; Gundewar, S.; Seals, D.R.; Barouch, L.A.; et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circ. Res. 2011, 108, 1448–1458. [Google Scholar] [CrossRef]
- Taylor, R.S.; Long, L.; Mordi, I.R.; Madsen, M.T.; Davies, E.J.; Dalal, H.; Rees, K.; Singh, S.J.; Gluud, C.; Zwisler, A.D. Exercise-Based Rehabilitation for Heart Failure: Cochrane Systematic Review, Meta-Analysis, and Trial Sequential Analysis. JACC Heart Fail. 2019, 7, 691–705. [Google Scholar] [CrossRef]
- Long, L.; Anderson, L.; He, J.; Gandhi, M.; Dewhirst, A.; Bridges, C.; Taylor, R. Exercise-based cardiac rehabilitation for stable angina: Systematic review and meta-analysis. Open Heart 2019, 6, e000989. [Google Scholar] [CrossRef]
- Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.; Dalal, H.; Rees, K.; Singh, S.J.; Taylor, R.S. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 2019, 1, CD003331. [Google Scholar] [CrossRef]
- Fagard, R. Athlete’s heart. Heart 2003, 89, 1455–1461. [Google Scholar] [CrossRef]
- Maillet, M.; van Berlo, J.H.; Molkentin, J.D. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, B. Cardiomyocyte proliferation: Remove brakes and push accelerators. Cell Res. 2017, 27, 959–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Ding, Y.; He, L.; Kim, T.; Vasudevarao, M.D.; Long, Q.; Yang, K.; Wickramasinghe, N.; Renikunta, H.V.; Dubois, N.; et al. Live cell screening platform identifies PPARdelta as a regulator of cardiomyocyte proliferation and cardiac repair. Cell Res. 2017, 27, 1002–1019. [Google Scholar] [CrossRef]
- Bassat, E.; Mutlak, Y.E.; Genzelinakh, A.; Shadrin, I.Y.; Baruch Umansky, K.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Riabov Bassat, D.; et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017, 547, 179–184. [Google Scholar] [CrossRef]
- Morikawa, Y.; Heallen, T.; Leach, J.; Xiao, Y.; Martin, J.F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 2017, 547, 227–231. [Google Scholar] [CrossRef]
- Hunter, J.J.; Chien, K.R. Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 1999, 341, 1276–1283. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef]
- Bostrom, P.; Mann, N.; Wu, J.; Quintero, P.A.; Plovie, E.R.; Panakova, D.; Gupta, R.K.; Xiao, C.; MacRae, C.A.; Rosenzweig, A.; et al. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 2010, 143, 1072–1083. [Google Scholar] [CrossRef]
- Bezzerides, V.J.; Platt, C.; Lerchenmuller, C.; Paruchuri, K.; Oh, N.L.; Xiao, C.; Cao, Y.; Mann, N.; Spiegelman, B.M.; Rosenzweig, A. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight 2016, 1, e85904. [Google Scholar] [CrossRef]
- Bei, Y.; Fu, S.; Chen, X.; Chen, M.; Zhou, Q.; Yu, P.; Yao, J.; Wang, H.; Che, L.; Xu, J.; et al. Cardiac cell proliferation is not necessary for exercise-induced cardiac growth but required for its protection against ischaemia/reperfusion injury. J. Cell. Mol. Med. 2017, 21, 1648–1655. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, W.H.; Su, C.; Wu, F.; Zhang, Y.Y.; Xu, S.Y.; Liu, X.; Zhang, X.Y.; Ou, Z.J.; Lai, G.H.; et al. Regular exercise-induced increased number and activity of circulating endothelial progenitor cells attenuates age-related decline in arterial elasticity in healthy men. Int. J. Cardiol. 2013, 165, 247–254. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, L.; Su, C.; Xia, W.H.; Wang, Y.; Wang, J.M.; Chen, F.; Zhang, Y.Y.; Wu, F.; Xu, S.Y.; et al. Impaired endothelial progenitor cell activity is associated with reduced arterial elasticity in patients with essential hypertension. Clin. Exp. Hypertens. 2010, 32, 444–452. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef]
- Buja, L.M. Cardiac repair and the putative role of stem cells. J. Mol. Cell. Cardiol. 2019, 128, 96–104. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Ingwall, J.S. Energy metabolism in heart failure and remodelling. Cardiovasc. Res. 2009, 81, 412–419. [Google Scholar] [CrossRef]
- Dirkx, E.; da Costa Martins, P.A.; De Windt, L.J. Regulation of fetal gene expression in heart failure. Biochim. Biophys. Acta 2013, 1832, 2414–2424. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; De Luca, R.; Di Paolo, F.M.; Spataro, A.; Culasso, F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef]
- Maron, B.J.; Pelliccia, A. The heart of trained athletes: Cardiac remodeling and the risks of sports, including sudden death. Circulation 2006, 114, 1633–1644. [Google Scholar] [CrossRef]
- Asif, I.M.; Harmon, K.G. Incidence and Etiology of Sudden Cardiac Death: New Updates for Athletic Departments. Sports Health 2017, 9, 268–279. [Google Scholar] [CrossRef]
- Thu, V.T.; Kim, H.K.; Han, J. Acute and Chronic Exercise in Animal Models. Adv. Exp. Med. Biol. 2017, 999, 55–71. [Google Scholar] [CrossRef]
- Rovira, M.; Borras, D.M.; Marques, I.J.; Puig, C.; Planas, J.V. Physiological Responses to Swimming-Induced Exercise in the Adult Zebrafish Regenerating Heart. Front. Physiol. 2018, 9, 1362. [Google Scholar] [CrossRef]
- Gonzalez-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef]
- Gemberling, M.; Karra, R.; Dickson, A.L.; Poss, K.D. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. eLife 2015, 4, e05871. [Google Scholar] [CrossRef]
- Karra, R.; Knecht, A.K.; Kikuchi, K.; Poss, K.D. Myocardial NF-kappaB activation is essential for zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 13255–13260. [Google Scholar] [CrossRef]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L.A. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell Metab. 2017, 25, 1012–1026. [Google Scholar] [CrossRef]
- Perrino, C.; Gargiulo, G.; Pironti, G.; Franzone, A.; Scudiero, L.; De Laurentis, M.; Magliulo, F.; Ilardi, F.; Carotenuto, G.; Schiattarella, G.G.; et al. Cardiovascular effects of treadmill exercise in physiological and pathological preclinical settings. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1983–H1989. [Google Scholar] [CrossRef]
- de Carvalho Cunha, V.N.; Dos Santos Rosa, T.; Sales, M.M.; Sousa, C.V.; da Silva Aguiar, S.; Deus, L.A.; Simoes, H.G.; de Andrade, R.V. Training Performed Above Lactate Threshold Decreases p53 and Shelterin Expression in Mice. Int. J. Sports Med. 2018, 39, 704–711. [Google Scholar] [CrossRef]
- Merino, D.; Gil, A.; Gomez, J.; Ruiz, L.; Llano, M.; Garcia, R.; Hurle, M.A.; Nistal, J.F. Experimental modelling of cardiac pressure overload hypertrophy: Modified technique for precise, reproducible, safe and easy aortic arch banding-debanding in mice. Sci. Rep. 2018, 8, 3167. [Google Scholar] [CrossRef]
- Kemi, O.J.; Ceci, M.; Wisloff, U.; Grimaldi, S.; Gallo, P.; Smith, G.L.; Condorelli, G.; Ellingsen, O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J. Cell. Physiol. 2008, 214, 316–321. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006, 20, 3347–3365. [Google Scholar] [CrossRef]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef]
- Kemi, O.J.; Loennechen, J.P.; Wisloff, U.; Ellingsen, O. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J. Appl. Physiol. 2002, 93, 1301–1309. [Google Scholar] [CrossRef]
- Rahimi, M.; Shekarforoush, S.; Asgari, A.R.; Khoshbaten, A.; Rajabi, H.; Bazgir, B.; Mohammadi, M.T.; Sobhani, V.; Shakibaee, A. The effect of high intensity interval training on cardioprotection against ischemia-reperfusion injury in wistar rats. EXCLI J. 2015, 14, 237–246. [Google Scholar] [CrossRef]
- Zhang, K.R.; Liu, H.T.; Zhang, H.F.; Zhang, Q.J.; Li, Q.X.; Yu, Q.J.; Guo, W.Y.; Wang, H.C.; Gao, F. Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism. Apoptosis 2007, 12, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Clauss, S.; Bleyer, C.; Schuttler, D.; Tomsits, P.; Renner, S.; Klymiuk, N.; Wakili, R.; Massberg, S.; Wolf, E.; Kaab, S. Animal models of arrhythmia: Classic electrophysiology to genetically modified large animals. Nat. Rev. Cardiol. 2019, 16, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.M.; White, F.C.; Nichols, M.L.; Dobbs, S.L.; Longhurst, J.C.; Bloor, C.M. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation 1990, 82, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Affuso, F.; Conza, P.D.; Fazio, S. The GH/IGF-1 Axis and Heart Failure. Curr. Cardiol. Rev. 2009, 5, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Duerr, R.L.; Huang, S.; Miraliakbar, H.R.; Clark, R.; Chien, K.R.; Ross, J., Jr. Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. J. Clin. Investig. 1995, 95, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Chen, J.W.; Ting, C.T.; Ishiwata, T.; Lin, S.J.; Korc, M.; Wang, P.H. Insulin-like growth factor I improves cardiovascular function and suppresses apoptosis of cardiomyocytes in dilated cardiomyopathy. Endocrinology 1999, 140, 4831–4840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nindl, B.C.; Pierce, J.R. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med. Sci. Sports Exerc. 2010, 42, 39–49. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.R.; Shioi, T.; Zhang, L.; Tarnavski, O.; Sherwood, M.C.; Kang, P.M.; Izumo, S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2003, 100, 12355–12360. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.R.; Amirahmadi, F.; Woodcock, E.A.; Schinke-Braun, M.; Bouwman, R.D.; Hewitt, K.A.; Mollica, J.P.; Zhang, L.; Zhang, Y.; Shioi, T.; et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA 2007, 104, 612–617. [Google Scholar] [CrossRef]
- Weeks, K.L.; Gao, X.; Du, X.J.; Boey, E.J.; Matsumoto, A.; Bernardo, B.C.; Kiriazis, H.; Cemerlang, N.; Tan, J.W.; Tham, Y.K.; et al. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ. Heart Fail. 2012, 5, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights into the Role of mTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Investig. 2005, 115, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Qi, J.; Meng, S.; Wen, B.; Zhang, J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur. J. Appl. Physiol. 2013, 113, 2473–2486. [Google Scholar] [CrossRef] [PubMed]
- Schuettler, D.; Piontek, G.; Wirth, M.; Haller, B.; Reiter, R.; Brockhoff, G.; Pickhard, A. Selective inhibition of EGFR downstream signaling reverses the irradiation-enhanced migration of HNSCC cells. Am. J. Cancer Res. 2015, 5, 2660–2672. [Google Scholar] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Sugden, P.H.; Fuller, S.J.; Weiss, S.C.; Clerk, A. Glycogen synthase kinase 3 (GSK3) in the heart: A point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S137–S153. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, I.; Jang, Y.; Song, W.; Cosio-Lima, L.M.; Roltsch, M.H. Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J. Physiol. Sci. 2017, 67, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Ahmad, F.; Woodgett, J.; Force, T. The GSK-3 family as therapeutic target for myocardial diseases. Circ. Res. 2015, 116, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, R.; Miller, T.A.; Kuramochi, Y.; Frantz, S.; Kim, Y.D.; Marchionni, M.A.; Kelly, R.A.; Sawyer, D.B. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J. Mol. Cell. Cardiol. 2003, 35, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.X.; Shi, X.C.; Chen, T.; Tan, Z.N.; Lin, Q.Q.; Du, S.J.; Tian, Z.J. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 2016, 149, 1–9. [Google Scholar] [CrossRef]
- D’Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Galindo, C.L.; Ryzhov, S.; Sawyer, D.B. Neuregulin as a heart failure therapy and mediator of reverse remodeling. Curr. Heart Fail. Rep. 2014, 11, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Ryzhov, S.; Gupte, M.; Sengsayadeth, S.; Gumina, R.J.; Sawyer, D.B.; Galindo, C.L. Neuregulin-1beta induces proliferation, survival and paracrine signaling in normal human cardiac ventricular fibroblasts. J. Mol. Cell. Cardiol. 2017, 105, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Waring, C.D.; Vicinanza, C.; Papalamprou, A.; Smith, A.J.; Purushothaman, S.; Goldspink, D.F.; Nadal-Ginard, B.; Torella, D.; Ellison, G.M. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur. Heart J. 2014, 35, 2722–2731. [Google Scholar] [CrossRef] [PubMed]
- Barbier, J.; Rannou-Bekono, F.; Marchais, J.; Tanguy, S.; Carre, F. Alterations of beta3-adrenoceptors expression and their myocardial functional effects in physiological model of chronic exercise-induced cardiac hypertrophy. Mol. Cell. Biochem. 2007, 300, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Talukder, M.A.; Varadharaj, S.; Velayutham, M.; Zweier, J.L. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc. Res. 2013, 97, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Otani, H. The role of nitric oxide in myocardial repair and remodeling. Antioxid. Redox Signal. 2009, 11, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Alogna, A.; Post, H.; Hamdani, N. Is enhancing cGMP-PKG signalling a promising therapeutic target for heart failure with preserved ejection fraction? Neth. Heart J. 2016, 24, 268–274. [Google Scholar] [CrossRef]
- Blanton, R.M.; Takimoto, E.; Lane, A.M.; Aronovitz, M.; Piotrowski, R.; Karas, R.H.; Kass, D.A.; Mendelsohn, M.E. Protein kinase g ialpha inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J. Am. Heart Assoc. 2012, 1, e003731. [Google Scholar] [CrossRef]
- Iemitsu, M.; Miyauchi, T.; Maeda, S.; Tanabe, T.; Takanashi, M.; Matsuda, M.; Yamaguchi, I. Exercise training improves cardiac function-related gene levels through thyroid hormone receptor signaling in aged rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1696–H1705. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Xu, T.; Lv, D.; Yu, P.; Xu, J.; Che, L.; Das, A.; Tigges, J.; Toxavidis, V.; Ghiran, I.; et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res. Cardiol. 2017, 112, 38. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: A review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Verdoorn, K.S.; Matsuura, C.; Borges, J.P. Exercise for cardiac health and regeneration: Killing two birds with one stone. Ann. Transl. Med. 2017, 5, S13. [Google Scholar] [CrossRef] [PubMed]
- Ultimo, S.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Capitani, S.; Neri, L.M. Cardiovascular disease-related miRNAs expression: Potential role as biomarkers and effects of training exercise. Oncotarget 2018, 9, 17238–17254. [Google Scholar] [CrossRef]
- Clauss, S.; Sinner, M.F.; Kaab, S.; Wakili, R. The Role of MicroRNAs in Antiarrhythmic Therapy for Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2015, 4, 146–155. [Google Scholar] [CrossRef]
- Clauss, S.; Wakili, R.; Hildebrand, B.; Kaab, S.; Hoster, E.; Klier, I.; Martens, E.; Hanley, A.; Hanssen, H.; Halle, M.; et al. MicroRNAs as Biomarkers for Acute Atrial Remodeling in Marathon Runners (The miRathon Study—A Sub-Study of the Munich Marathon Study). PLoS ONE 2016, 11, e0148599. [Google Scholar] [CrossRef]
- Dawson, K.; Wakili, R.; Ordog, B.; Clauss, S.; Chen, Y.; Iwasaki, Y.; Voigt, N.; Qi, X.Y.; Sinner, M.F.; Dobrev, D.; et al. MicroRNA29: A mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 2013, 127, 1466–1475. [Google Scholar] [CrossRef]
- Chen, Y.; Wakili, R.; Xiao, J.; Wu, C.T.; Luo, X.; Clauss, S.; Dawson, K.; Qi, X.; Naud, P.; Shi, Y.F.; et al. Detailed characterization of microRNA changes in a canine heart failure model: Relationship to arrhythmogenic structural remodeling. J. Mol. Cell. Cardiol. 2014, 77, 113–124. [Google Scholar] [CrossRef]
- Cahill, T.J.; Choudhury, R.P.; Riley, P.R. Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Nat. Rev. Drug Discov. 2017, 16, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Dimmeler, S. Exercise controls non-coding RNAs. Cell Metab. 2015, 21, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Heallen, T.R.; Kadow, Z.A.; Kim, J.H.; Wang, J.; Martin, J.F. Stimulating Cardiogenesis as a Treatment for Heart Failure. Circ. Res. 2019, 124, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Bei, Y.; Kong, X.; Liu, X.; Lei, Z.; Xu, T.; Wang, H.; Xuan, Q.; Chen, P.; Xu, J.; et al. miR-17-3p Contributes to Exercise-Induced Cardiac Growth and Protects against Myocardial Ischemia-Reperfusion Injury. Theranostics 2017, 7, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, J.; Zhu, H.; Wei, X.; Platt, C.; Damilano, F.; Xiao, C.; Bezzerides, V.; Bostrom, P.; Che, L.; et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. [Google Scholar] [CrossRef]
- Palabiyik, O.; Tastekin, E.; Doganlar, Z.B.; Tayfur, P.; Dogan, A.; Vardar, S.A. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed. Rep. 2019, 10, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.D., Jr.; Fernandes, T.; Soci, U.P.; Monteiro, A.W.; Phillips, M.I.; de Oliveira, E.M. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med. Sci. Sports Exerc. 2012, 44, 1453–1462. [Google Scholar] [CrossRef]
- Dong, D.L.; Chen, C.; Huo, R.; Wang, N.; Li, Z.; Tu, Y.J.; Hu, J.T.; Chu, X.; Huang, W.; Yang, B.F. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: A novel mechanism for progressive cardiac hypertrophy. Hypertension 2010, 55, 946–952. [Google Scholar] [CrossRef]
- Soci, U.P.; Fernandes, T.; Hashimoto, N.Y.; Mota, G.F.; Amadeu, M.A.; Rosa, K.T.; Irigoyen, M.C.; Phillips, M.I.; Oliveira, E.M. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol. Genom. 2011, 43, 665–673. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Hou, N.; Song, Y.; An, X.; Zhang, Y.; Yang, X.; Wang, J. miR-199-sponge transgenic mice develop physiological cardiac hypertrophy. Cardiovasc. Res. 2016, 110, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, Z. Swimming training affects apoptosis-related microRNAs and reduces cardiac apoptosis in mice. Gen. Physiol. Biophys. 2016, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Mano, M.; Dal Ferro, M.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Tang, L.; Zhuang, Y.; Zhao, P. miR-17-3P regulates the proliferation and survival of colon cancer cells by targeting Par4. Mol. Med. Rep. 2018, 17, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Song, K.; Zhang, G. MicroRNA-17-3p promotes keratinocyte cells growth and metastasis via targeting MYOT and regulating Notch1/NF-kappaB pathways. Die Pharm. 2017, 72, 543–549. [Google Scholar] [CrossRef]
- Hammoud, L.; Burger, D.E.; Lu, X.; Feng, Q. Tissue inhibitor of metalloproteinase-3 inhibits neonatal mouse cardiomyocyte proliferation via EGFR/JNK/SP-1 signaling. Am. J. Physiol. Cell Physiol. 2009, 296, C735–C745. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Dixon, J.E. Pten. Annu. Rev. Biochem. 2014, 83, 641–669. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, C.K.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE 2006, 1, e116. [Google Scholar] [CrossRef]
- Iemitsu, M.; Maeda, S.; Jesmin, S.; Otsuki, T.; Miyauchi, T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1290–H1298. [Google Scholar] [CrossRef]
- Uhlemann, M.; Mobius-Winkler, S.; Fikenzer, S.; Adam, J.; Redlich, M.; Mohlenkamp, S.; Hilberg, T.; Schuler, G.C.; Adams, V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur. J. Prev. Cardiol. 2014, 21, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Viereck, J.; Kruger, K.; Thum, T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H557–H563. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Smuder, A.J.; Willis, W.T.; Powers, S.K. Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med. Sci. Sports Exerc. 2012, 44, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C., Jr. An “Exercise” in Cardiac Metabolism. Front. Cardiovasc. Med. 2018, 5, 66. [Google Scholar] [CrossRef]
- Strom, C.C.; Aplin, M.; Ploug, T.; Christoffersen, T.E.; Langfort, J.; Viese, M.; Galbo, H.; Haunso, S.; Sheikh, S.P. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 2005, 272, 2684–2695. [Google Scholar] [CrossRef]
- Aitman, T.J.; Glazier, A.M.; Wallace, C.A.; Cooper, L.D.; Norsworthy, P.J.; Wahid, F.N.; Al-Majali, K.M.; Trembling, P.M.; Mann, C.J.; Shoulders, C.C.; et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat. Genet. 1999, 21, 76–83. [Google Scholar] [CrossRef]
- Glatz, J.F.; Angin, Y.; Steinbusch, L.K.; Schwenk, R.W.; Luiken, J.J. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 71–77. [Google Scholar] [CrossRef]
- Kuang, M.; Febbraio, M.; Wagg, C.; Lopaschuk, G.D.; Dyck, J.R. Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation 2004, 109, 1550–1557. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. PGC-1 proteins and heart failure. Trends Cardiovasc. Med. 2012, 22, 98–105. [Google Scholar] [CrossRef]
- Wang, H.; Bei, Y.; Lu, Y.; Sun, W.; Liu, Q.; Wang, Y.; Cao, Y.; Chen, P.; Xiao, J.; Kong, X. Exercise Prevents Cardiac Injury and Improves Mitochondrial Biogenesis in Advanced Diabetic Cardiomyopathy with PGC-1alpha and Akt Activation. Cell. Physiol. Biochem. 2015, 35, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase--development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Yin, R.; Chen, D.; Liu, D.; Wang, D.; Yang, Q.; Dong, Y.G. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J. Cell. Biochem. 2007, 100, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, X.; Xu, X.; Fassett, J.; Zhu, G.; Viollet, B.; Xu, W.; Wiczer, B.; Bernlohr, D.A.; Bache, R.J.; et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension 2008, 52, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fu, Y.; Xiao, H.; Song, Y.; Chen, R.; Shen, J.; An, X.; Shen, Q.; Li, Z.; Zhang, Y. Cardiac Fibrosis Alleviated by Exercise Training Is AMPK-Dependent. PLoS ONE 2015, 10, e0129971. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Li, H.; Shen, Q.; Shen, J.; An, X.; Wu, J.; Zhang, J.; Wu, Y.; Xiao, H.; et al. Exacerbated cardiac fibrosis induced by beta-adrenergic activation in old mice due to decreased AMPK activity. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1029–1037. [Google Scholar] [CrossRef]
- Li, L.; Muhlfeld, C.; Niemann, B.; Pan, R.; Li, R.; Hilfiker-Kleiner, D.; Chen, Y.; Rohrbach, S. Mitochondrial biogenesis and PGC-1alpha deacetylation by chronic treadmill exercise: Differential response in cardiac and skeletal muscle. Basic Res. Cardiol. 2011, 106, 1221–1234. [Google Scholar] [CrossRef]
- Chen, L.; Knowlton, A.A. Mitochondria and heart failure: New insights into an energetic problem. Minerva Cardioangiol. 2010, 58, 213–229. [Google Scholar]
- Kavazis, A.N.; McClung, J.M.; Hood, D.A.; Powers, S.K. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H928–H935. [Google Scholar] [CrossRef]
- Kwak, H.B. Effects of aging and exercise training on apoptosis in the heart. J. Exerc. Rehabil. 2013, 9, 212–219. [Google Scholar] [CrossRef]
- Kwak, H.B.; Song, W.; Lawler, J.M. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006, 20, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Grieve, D.J. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacol. Ther. 2009, 122, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.H.; Higuchi Mde, L.; Tucci, P.J.; Garavelo, S.M.; Reis, M.M.; Antonio, E.L.; Serra, A.J.; Maranhao, R.C. Previous exercise training increases levels of PPAR-alpha in long-term post-myocardial infarction in rats, which is correlated with better inflammatory response. Clinics 2016, 71, 163–168. [Google Scholar] [CrossRef]
- Ibarra-Lara Mde, L.; Sanchez-Aguilar, M.; Soria, E.; Torres-Narvaez, J.C.; Del Valle-Mondragon, L.; Cervantes-Perez, L.G.; Perez-Severiano, F.; Ramirez-Ortega Mdel, C.; Pastelin-Hernandez, G.; Oidor-Chan, V.H.; et al. Peroxisome proliferator-activated receptors (PPAR) downregulate the expression of pro-inflammatory molecules in an experimental model of myocardial infarction. Can. J. Physiol. Pharmacol. 2016, 94, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Iemitsu, M.; Miyauchi, T.; Maeda, S.; Tanabe, T.; Takanashi, M.; Irukayama-Tomobe, Y.; Sakai, S.; Ohmori, H.; Matsuda, M.; Yamaguchi, I. Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1750–H1760. [Google Scholar] [CrossRef] [PubMed]
| MicroRNA | Cellular Target | Cardiac Function | Animal Model and Exercise Modality | References |
|---|---|---|---|---|
| miR-17-3p | TIMP3, PTEN | Cardiac hypertrophy Myocyte proliferation Cardiac apoptosis | Mice, swimming and wheel exercise | [94] |
| miR-222 | P27, Hipk1, Hmbox1 | Cell cycle Cardiac apoptosis Cardiac hypertrophy Myocyte proliferation | Mice, swimming and wheel exercise | [95] |
| miR-124 | PI3K | Cardiac hypertrophy | Rats, swimming exercise | [63] |
| miR-21 | PTEN | Cardiac hypertrophy | Rats, swimming exercise | [63,96] |
| miR-144 | PTEN | Cardiac hypertrophy | Rats, swimming exercise | [63] |
| miR-145 | TSC | Cardiac hypertrophy | Rats, swimming exercise | [63] |
| miR-126 | Spred-1 Raf-1/ERK 1/2 signaling | Cardiac angiogenesis | Rats, swimming exercise | [97] |
| miR-133 | Calcineurin PI3K/Akt signaling | Cardiac hypertrophy | Rats, swimming exercise | [96,98] |
| miR-29c | Collagen I und III TGFβ pathway | Left ventricular compliance | Rats, swimming exercise | [99] |
| miR-29b | MMP9 | Fibrosis, matrix degradation | Mice, treadmill running | [100] |
| miR-455 | MMP9 | Fibrosis, matrix degradation | Mice, treadmill running | [100] |
| miR-199a | PGC1α | Cardiac hypertrophy | Mice, treadmill running | [101] |
| mi-R1 | Bcl-2 | Cardiac apoptosis | Mice, swimming exercise | [102] |
| miR-30 | P53, Drp-1 | Cardiac apoptosis | Mice, swimming exercise | [102] |
| miR-21 | PDCD4 | Cardiac apoptosis | Mice, swimming exercise | [102] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schüttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. https://doi.org/10.3390/cells8101128
Schüttler D, Clauss S, Weckbach LT, Brunner S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells. 2019; 8(10):1128. https://doi.org/10.3390/cells8101128
Chicago/Turabian StyleSchüttler, Dominik, Sebastian Clauss, Ludwig T. Weckbach, and Stefan Brunner. 2019. "Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise" Cells 8, no. 10: 1128. https://doi.org/10.3390/cells8101128
APA StyleSchüttler, D., Clauss, S., Weckbach, L. T., & Brunner, S. (2019). Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells, 8(10), 1128. https://doi.org/10.3390/cells8101128






