Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss
Abstract
1. Introduction
2. Materials and Methods
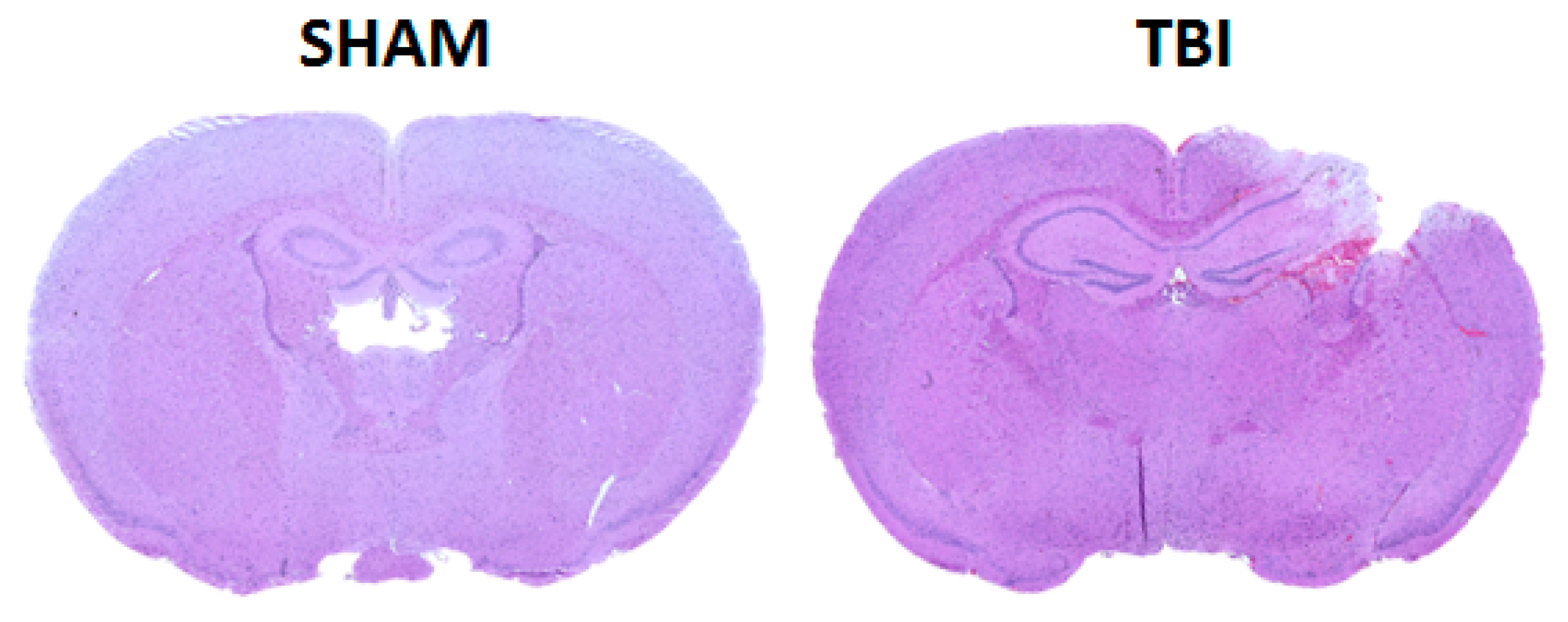
2.1. Controlled Cortical Impact
2.2. Micro-Computed Tomography Analyses (µCT)
2.3. Isolation of Bone Marrow Cells for Colony Forming and Osteoclast Differentiation Assay
2.4. Tartrate-Resistant Acid Phosphatase Staining
2.5. Isolation of RNA, Synthesis of cDNA, and Real-Time PCR
2.6. Extracellular Vesicles Isolation from Bone Marrow
2.7. Extracellular Vesicles Treatment
2.8. miRNA Isolation and Real Time PCR on Extracellular Vesicles
2.9. Statistics Analysis
3. Results
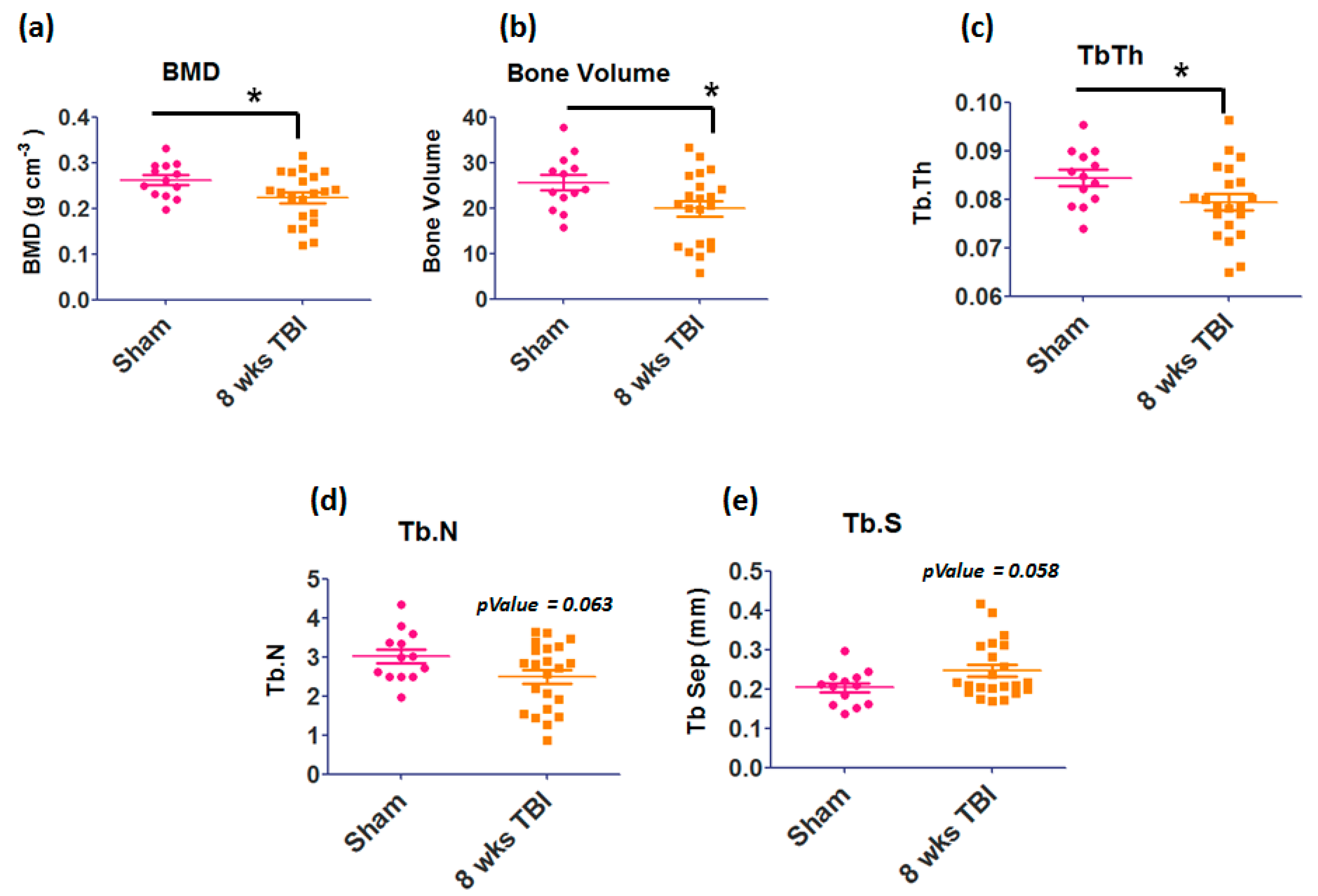
3.1. Micro-Computed Tomography Analysis of Femur Bone
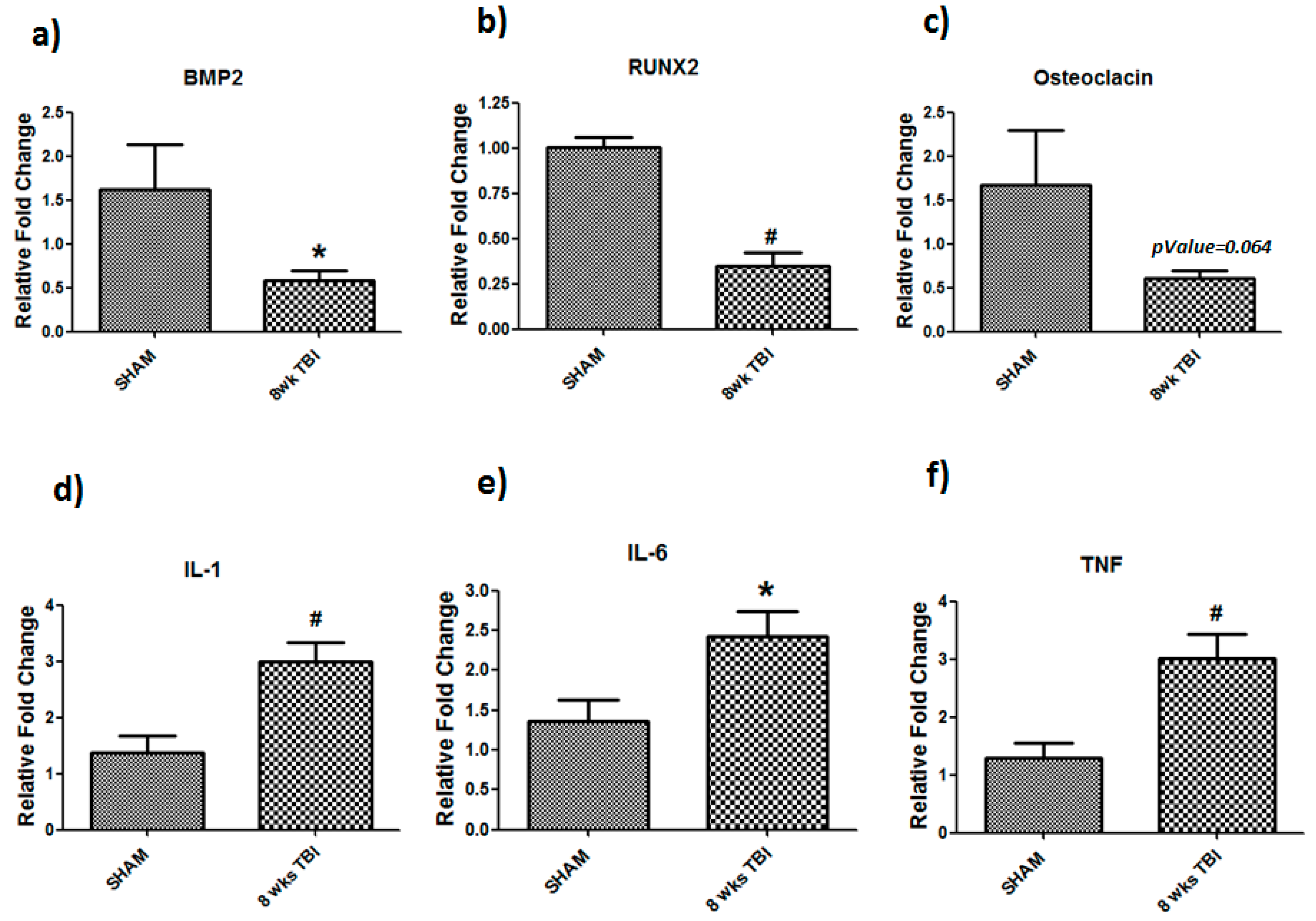
3.2. TBI Decreased Bone Formation Markers and Increased Cytokines Expression in Bone
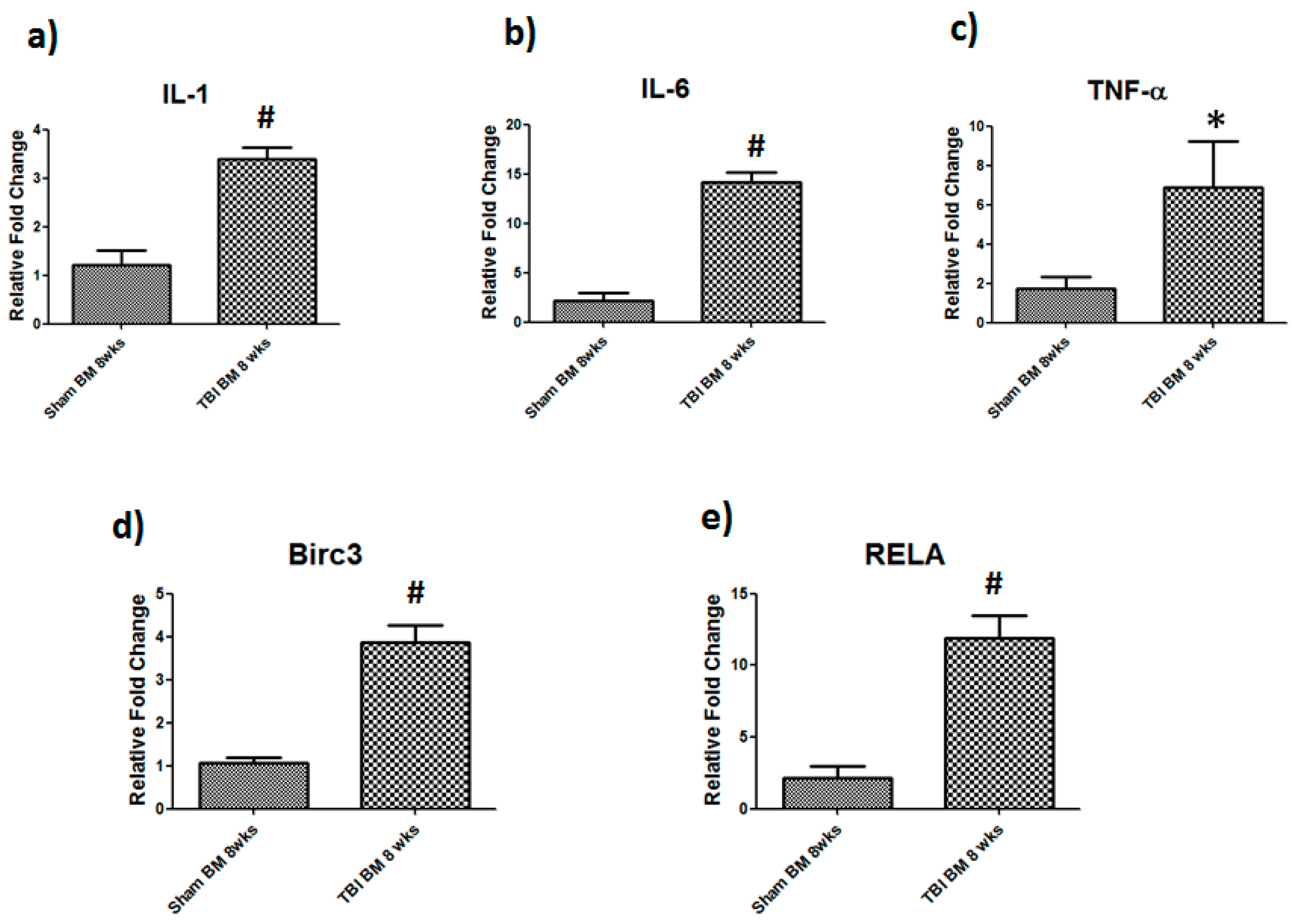
3.3. Elevated Chronic Inflammation and NF-κB Signaling Genes in Bone Marrow after TBI
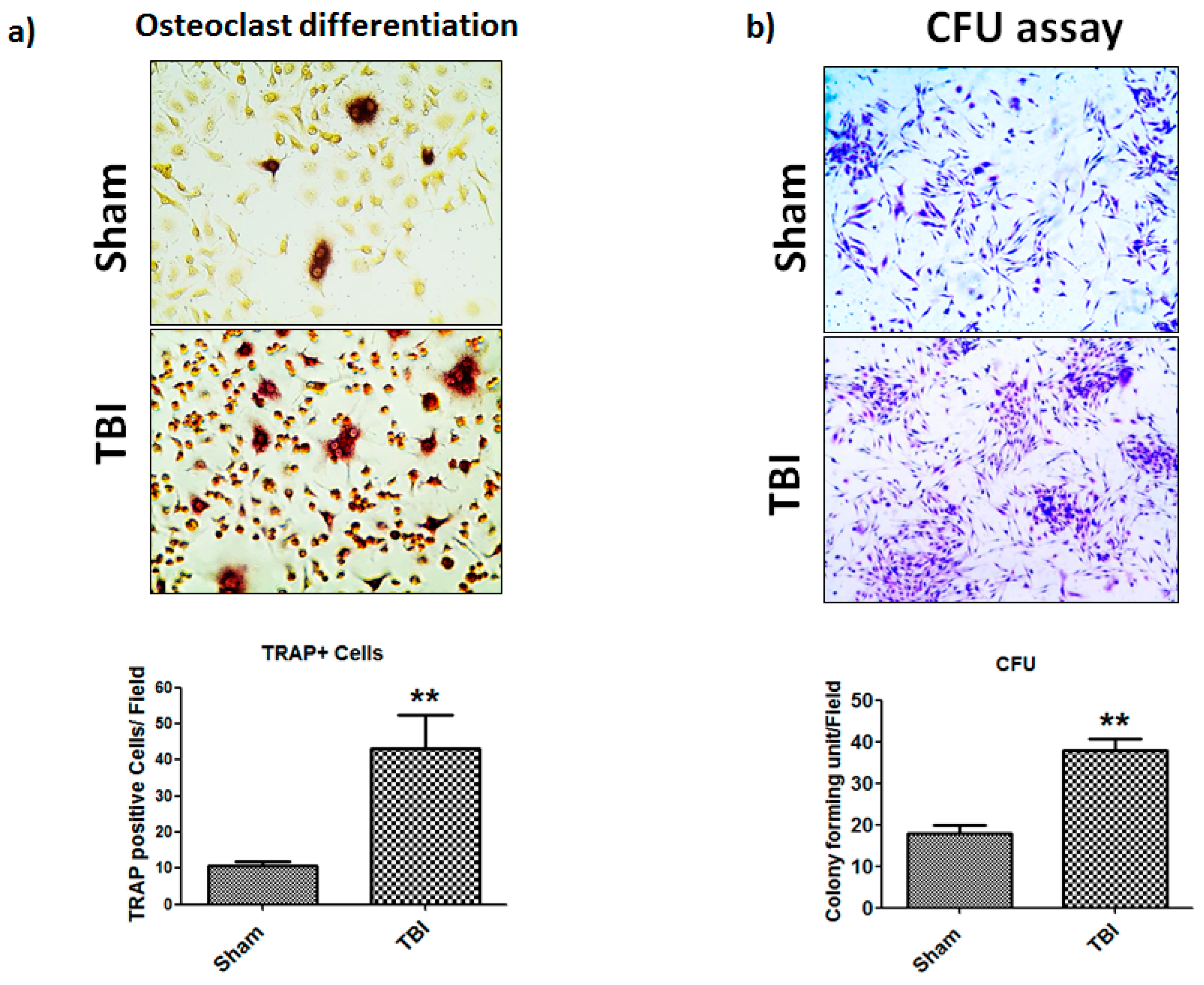
3.4. TBI Affects Colony Forming Unit (CFU) Efficiency and Osteoclast Differentiation of Bone Marrow Cells
3.5. Extracellular Vesicle Isolation and Characterization
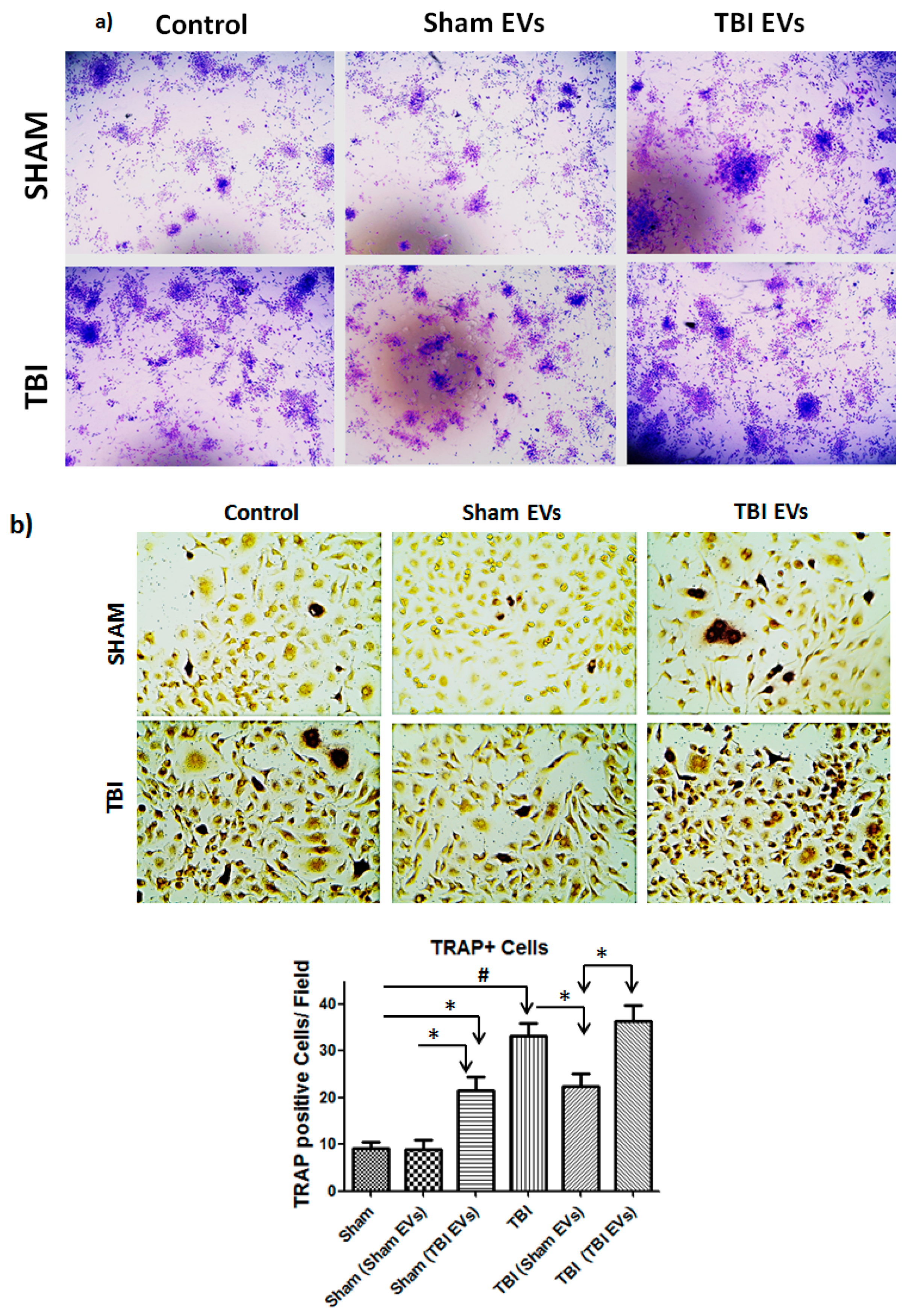
3.6. EVs Derived from TBI Bone Marrow Enhance Osteoclast Differentiation of Bone Marrow Cells
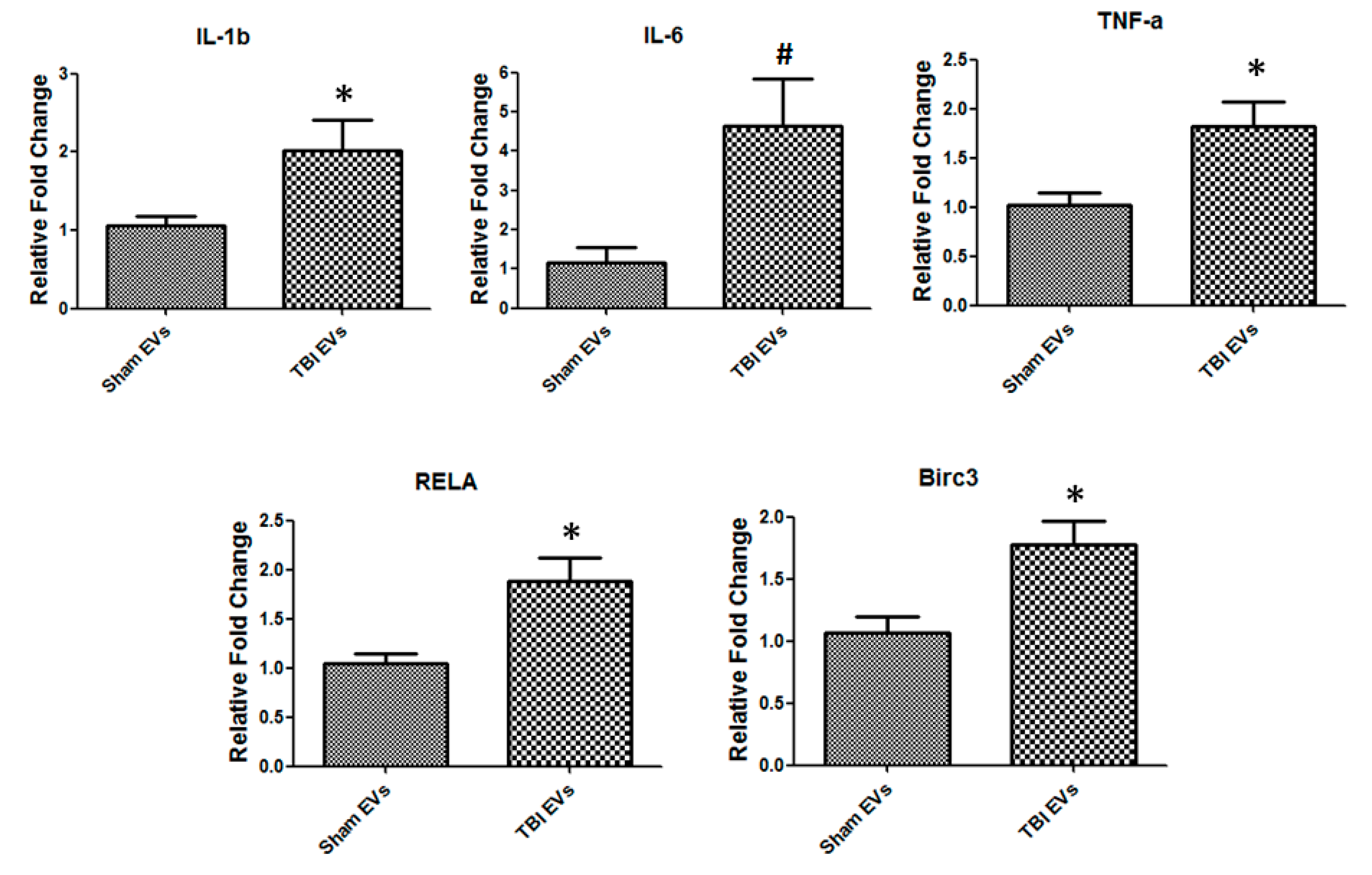
3.7. TBI-Derived EVs Isolated from Bone Marrow Regulate Inflammatory and NF-κB Signaling
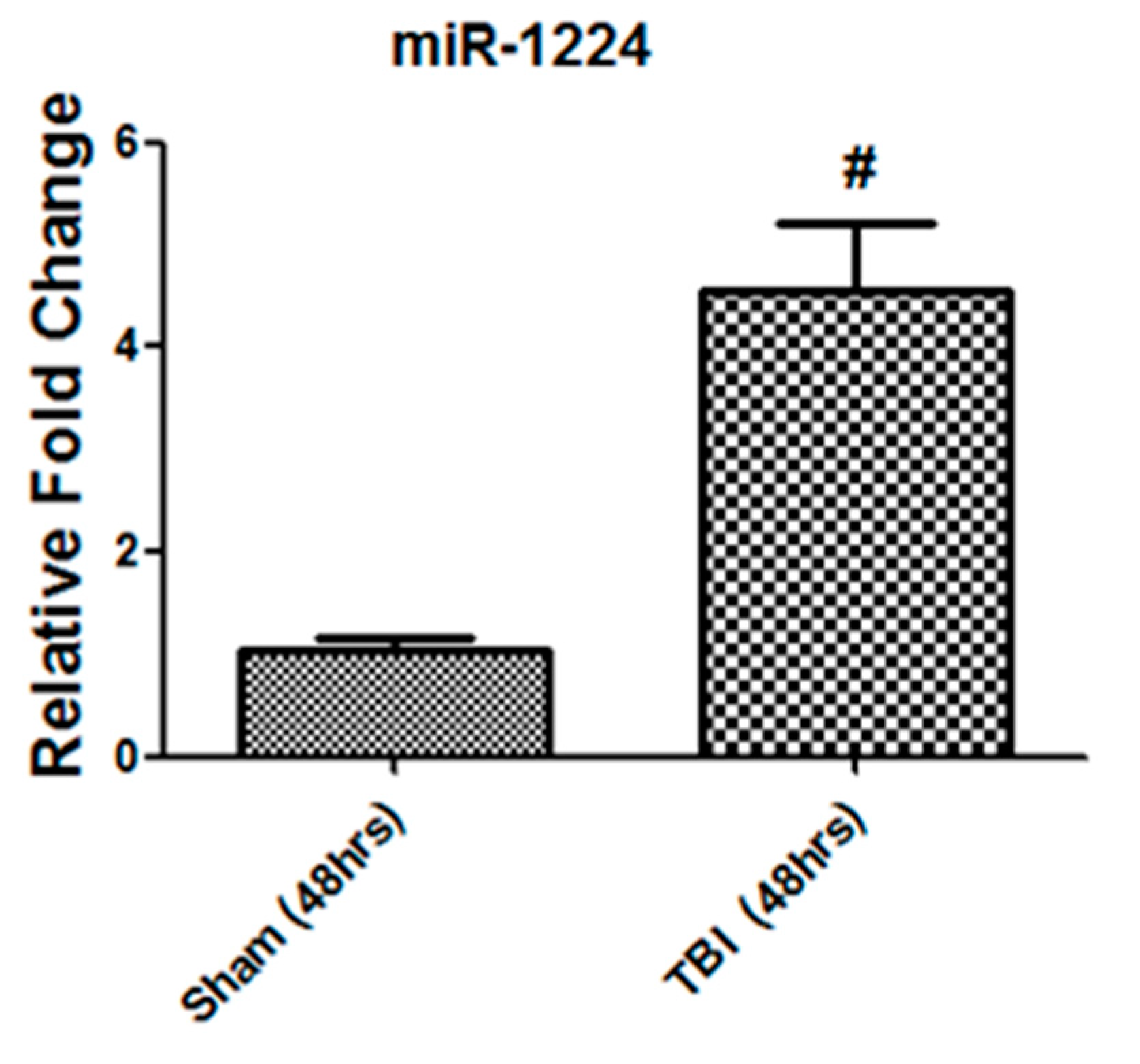
3.8. The miRNA-1224 Cargo Changed in TBI-Derived EVs
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| EVs | Extracellular vesicles |
| M-CSF | Macrophage colony-stimulating factor |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| BMP2 | Bone morphogenetic protein 2 |
| RUNX2 | Runt-related transcription factor 2 |
| TRAP | Tartrate-resistant acid phosphatase |
References
- Rutland-Brown, W.; Langlois, J.A.; Thomas, K.E.; Xi, Y.L. Incidence of Traumatic Brain Injury in the United States, 2003. J. Head Trauma Rehabil. 2006, 21, 544–548. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. Fdg-pet imaging in mild traumatic brain injury: A critical review. Front. Neuroenerget. 2014, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Losoi, H.; Silverberg, N.D.; Wäljas, M.; Turunen, S.; Rosti-Otajärvi, E.; Helminen, M.; Luoto, T.M.; Julkunen, J.; Öhman, J.; Iverson, G.L. Recovery from mild traumatic brain injury in previously healthy adults. J. Neurotrauma 2016, 33, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Andelic, N.; Ye, J.; Tornas, S.; Roe, C.; Lu, J.; Bautz-Holter, E.; Moger, T.; Sigurdardottir, S.; Schanke, A.-K.; Aas, E. Cost-effectiveness analysis of an early-initiated, continuous chain of rehabilitation after severe traumatic brain injury. J. Neurotrauma 2014, 31, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, R.L.; Hanley-Peterson, P.; Englander, J. Group psychotherapy for persons with traumatic brain injury: Management of frustration and substance abuse. J. Head Trauma Rehabil. 1998, 13, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Julien, J.; Joubert, S.; Ferland, M.C.; Frenette, L.C.; Boudreau-Duhaime, M.M.; Malo-Véronneau, L.; de Guise, E. Association of traumatic brain injury and Alzheimer disease onset: A systematic review. Ann. Phys. Rehabil. Med. 2017, 60, 347–356. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Long-term consequences of traumatic brain injury: Current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef]
- Utagawa, A.; Truettner, J.S.; Dietrich, W.D.; Bramlett, H.M. Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp. Neurol. 2008, 211, 283–291. [Google Scholar] [CrossRef]
- Hilz, M.J.; Wang, R.; Markus, J.; Ammon, F.; Hösl, K.M.; Flanagan, S.R.; Winder, K.; Koehn, J. Severity of traumatic brain injury correlates with long-term cardiovascular autonomic dysfunction. J. Neurol. 2017, 264, 1956–1967. [Google Scholar] [CrossRef]
- Catania, A.; Lonati, C.; Sordi, A.; Gatti, S. Detrimental consequences of brain injury on peripheral cells. Brainbehav. Immun. 2009, 23, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, P.; Guo, F.; Zhang, Z.-Y.; Zhang, Z. Correction: Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Banham-Hall, N.; Kothwal, K.; Pipkin, J.; Bentley, J.; Dickens, G.L. Prevalence of low bone mineral density in inpatients with traumatic brain injury receiving neurobehavioural rehabilitation: A postoperative, observational study. Physiotherapy 2013, 99, 328–334. [Google Scholar] [CrossRef]
- Smith, É.; Comiskey, C.; Carroll, Á. Prevalence of and risk factors for osteoporosis in adults with acquired brain injury. Irish J. Med. Sci. 2016, 185, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.D.; Grills, B.L.; Romano, T.; Wark, J.D.; O’Brien, T.J.; Shultz, S.R.; McDonald, S.J. Sodium selenate treatment mitigates reduction of bone volume following traumatic brain injury in rats. J. Musculoskelet. Neuronal. Interact. 2016, 16, 369–376. [Google Scholar] [PubMed]
- Brady, R.D.; Shultz, S.R.; Sun, M.; Romano, T.; van der Poel, C.; Wright, D.K.; Wark, J.D.; O’Brien, T.J.; Grills, B.L.; McDonald, S.J. Experimental traumatic brain injury induces bone loss in rats. J. Neurotrauma 2016, 33, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Kim, J.H.; Kim, H.W.; Choi, E.S.; Lim, S.H.; Ko, Y.J.; Han, Y.M. Changes in bone metabolism in a rat model of traumatic brain injury. Brain Inj. 2005, 19, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.-S.; Kuo, P.-J.; Wu, S.-C.; Chen, Y.-C.; Hsieh, H.-Y.; Hsieh, C.-H. Association between the osteoporosis self-assessment tool for asians score and mortality in patients with isolated moderate and severe traumatic brain injury: A propensity score-matched analysis. Int. J. Environ. Res. Public Health 2016, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Su, Y.F.; Chan, H.M.; Huang, S.L.; Lin, C.L.; Kwan, A.L.; Lou, Y.T.; Chen, C.W. Osteoporosis self-assessment tool for asianscan predict neurologic prognosis in patients with isolated moderate traumatic brain injury. PLoS ONE 2015, 10, e0132685. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Watt, H.; Mohan, S. The negative impact of traumatic brain injury (tbi) on bone in a mouse model. Brain Inj. 2013, 28, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Odén, A.; McCloskey, E.V.; Leslie, W.D. Overview of Fracture Prediction Tools. J. Clin. Densitom. 2017, 20, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Divittorio, G.; Jackson, K.L.; Chindalore, V.L.; Welker, W.; Walker, J.B. Examining the relationship between bone mineral density and fracture risk reduction during pharmacologic treatment of osteoporosis. Pharmacotherapy 2006, 26, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, C.A. Is bone mineral density predictive of fracture risk reduction? Curr. Med. Res. Opin. 2004, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Smith, É.; Carroll, Á. bone mineral density in adults disabled through acquired neurological conditions: A review. J. Clin. Densitom. 2011, 14, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Vaibhav, K.; Saad, N.; Fatima, S.; Brann, D.W.; Vender, J.R.; Wang, L.P.; Hoda, M.N.; Baban, B.; Dhandapani, K.M. Activation of Myeloid TLR4 Mediates T Lymphocyte Polarization after Traumatic Brain Injury. J. Immunol. 2017, 198, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Sangani, R.; Naime, M.; Zakhary, I.; Ahmad, S.; Chutkan, N.; Zhu, A.; Ha, Y.; Hamrick, M.; Isales, C.; Elsalanty, M.; et al. Regulation of vitamin c transporter in the type 1 diabetic mouse bone and bone marrow. Exp. Mol. Pathol. 2013, 95, 298–306. [Google Scholar] [CrossRef]
- Kolhe, R.; Hunter, M.; Liu, S.; Jadeja, R.N.; Pundkar, C.; Mondal, A.K.; Mendhe, B.; Drewry, M.; Rojiani, M.V.; Liu, Y.; et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Davis, C.; Dukes, A.; Drewry, M.; Helwa, I.; Johnson, M.H.; Isales, C.M.; Hill, W.D.; Liu, Y.; Shi, X.; Fulzele, S.; et al. MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng. Part A 2017, 23, 1231–1240. [Google Scholar] [CrossRef]
- Consortium, E.-T.; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, O.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Niu, Y.; Mo, D.; Qin, L.; Wang, C.; Li, A.; Zhao, X.; Wang, X.; Xiao, S.; Wang, Q.; Xie, Y.; et al. Lipopolysaccharide-induced miR-1224 negatively regulates tumour necrosis factor-α gene expression by modulating Sp1. Immunology 2011, 133, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Nakamura, S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J. Period. Res. 2013, 48, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Taira, M. Expression of MicroRNAs in the Extracellular Microvesicles of Murine Osteoclasts. J. Oral Tissue Eng. 2013, 10, 142–150. [Google Scholar]
- Villapol, S. Consequences of hepatic damage after traumatic brain injury: Current outlook and potential therapeutic targets. Neural. Regen. Res. 2016, 11, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Kisser, J.E.; Allen, A.J.; Katzel, L.I.; Wendell, C.R.; Siegel, E.L.; Lefkowitz, D.; Waldstein, S.R. Relations of blood pressure and head injury to regional cerebral blood flow. J. Neurol. Sci. 2016, 365, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Gaio, A.R.; Monteiro, E.; Barbosa, S.; Cerejo, A.; Donnelly, J.; Felgueiras, Ó.; Smielewski, P.; Paiva, J.-A.; Czosnyka, M. Kidney-brain link in traumatic brain injury patients? A preliminary report. Neurocrit. Care 2014, 22, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Hayakata, T.; Shiozaki, T.; Tasaki, O.; Ikegawa, H.; Inoue, Y.; Toshiyuki, F.; Hosotubo, H.; Kieko, F.; Yamashita, T.; Tanaka, H.; et al. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock 2004, 22, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Benedetto, M.C.D.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Hong, J.Y.; Kim, S.C.; Joo, J.K.; Na, Y.J.; Lee, K.S. The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet. Gynecol. Sci. 2015, 58, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vaira, S.; Alhawagri, M.; Anwisye, I.; Kitaura, H.; Faccio, R.; Novack, D.V. Rela/p65 promotes osteoclast differentiation by blocking a rankl-induced apoptotic jnk pathway in mice. J. Clin. Investig. 2008, 118, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Novack, D.V. Role of NF-κB in the skeleton. Cell Res. 2011, 21, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, M.W.; Dhandapani, K.M.; Brann, D.W. Regulatory role of NADPH oxidase 2 in the polarization dynamics and neurotoxicity of microglia/macrophages after traumatic brain injury. Free Radic. Biol. Med. 2017, 113, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2017, 68, 224–237. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Brann, D.W. NADPH Oxidase 2 Regulates NLRP3 Inflammasome Activation in the Brain after Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2017, 6057609. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.D.; Shields, J.S.; Sukumari-Ramesh, S.; Kimbler, D.E.; Fessler, R.D.; Shakir, B.; Youssef, P.; Yanasak, N.; Vender, J.R.; Dhandapani, K.M. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 2013, 62, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ekström, K.; Omar, O.; Granéli, C.; Wang, X.; Vazirisani, F.; Thomsen, P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS ONE 2013, 8, e75227. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-F.; Yang, G.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; et al. Altered microrna expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef] [PubMed]
- Marton, N.; Kovács, O.T.; Baricza, E.; Kittel, Á.; Győri, D.; Mócsai, A.; Meier, F.M.P.; Goodyear, C.S.; McInnes, I.B.; Buzás, E.I.; et al. Extracellular vesicles regulate the human osteoclastogenesis: Divergent roles in discrete inflammatory arthropathies. Cell. Mol. Life Sci. 2017, 74, 3599–3611. [Google Scholar] [CrossRef] [PubMed]
- Bretz, N.P.; Ridinger, J.; Rupp, A.-K.; Rimbach, K.; Keller, S.; Rupp, C.; Marme, F.; Umansky, L.; Umansky, V.; Eigenbrod, T.; et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via toll-like receptor signaling. J. Biol. Chem. 2013, 288, 36691–36702. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Reference/Accession Number |
|---|---|---|
| GAPDH | CAT GGC CTC CAA GGA GTA AGA GAG GGA GAT GCT CAG TGT TGG | M32599 |
| BMP-2 | TGT TTG GCC TGA AGC AGA GA TGA GTG CCT GCG GTA CAG AT | NM_007553.2 |
| RUNX-2 | GGA AAG GCA CTG ACT GAC CTA ACA AAT TCT AAG CTT GGG AGG A | NM_009820 |
| Osteocalcin | ATT TAG GAC CTG TGC TGC CCT A GGA GCT GCT GTG ACA TCC ATA C | U11542.1 |
| IL-6 | TAG TCC TTC CTA CCC CAA TTT CC TTG GTC CTT AGC CAC TCC TTC | NM_031168.1| |
| IL-1 | GCA CCT TAC ACC TAC CAG AGT AAA CTT CTG CCT GAC GAG CTT | NM_031168.1| |
| TNF | CCC TCA CAC TCA GAT CAT CTT CT GTC ACG ACG TGG GCT ACA G | NM_013693.2| |
| RELA | GGA GGA TGC CTC CTG CAA AC TGT AGT GGA AGC CCT GTC CT | AF199371 |
| Birc3 | ACG CAG CAA TCG TGC ATT TTG CCT ATA ACG AGG TCA CTG ACG G | AJ401388 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singleton, Q.; Vaibhav, K.; Braun, M.; Patel, C.; Khayrullin, A.; Mendhe, B.; Lee, B.R.; Kolhe, R.; Kaiser, H.; Awad, M.E.; et al. Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss. Cells 2019, 8, 63. https://doi.org/10.3390/cells8010063
Singleton Q, Vaibhav K, Braun M, Patel C, Khayrullin A, Mendhe B, Lee BR, Kolhe R, Kaiser H, Awad ME, et al. Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss. Cells. 2019; 8(1):63. https://doi.org/10.3390/cells8010063
Chicago/Turabian StyleSingleton, Quante, Kumar Vaibhav, Molly Braun, Chandani Patel, Andrew Khayrullin, Bharati Mendhe, Byung R. Lee, Ravindra Kolhe, Helen Kaiser, Mohamed E. Awad, and et al. 2019. "Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss" Cells 8, no. 1: 63. https://doi.org/10.3390/cells8010063
APA StyleSingleton, Q., Vaibhav, K., Braun, M., Patel, C., Khayrullin, A., Mendhe, B., Lee, B. R., Kolhe, R., Kaiser, H., Awad, M. E., Fariyike, T., Elsayed, R., Elsalanty, M., Isales, C. M., Liu, Y., Hamrick, M. W., Dhandapani, K. M., & Fulzele, S. (2019). Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss. Cells, 8(1), 63. https://doi.org/10.3390/cells8010063







