Abstract
Parkinson’s disease (PD) is a neurodegenerative disease characterized by a progressive loss of dopaminergic neurons from the nigrostriatal pathway, formation of Lewy bodies, and microgliosis. During the past decades multiple cellular pathways have been associated with PD pathology (i.e., oxidative stress, endosomal-lysosomal dysfunction, endoplasmic reticulum stress, and immune response), yet disease-modifying treatments are not available. We have recently used genetic data from familial and sporadic cases in an unbiased approach to build a molecular landscape for PD, revealing lipids as central players in this disease. Here we extensively review the current knowledge concerning the involvement of various subclasses of fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterols, and lipoproteins in PD pathogenesis. Our review corroborates a central role for most lipid classes, but the available information is fragmented, not always reproducible, and sometimes differs by sex, age or PD etiology of the patients. This hinders drawing firm conclusions about causal or associative effects of dietary lipids or defects in specific steps of lipid metabolism in PD. Future technological advances in lipidomics and additional systematic studies on lipid species from PD patient material may improve this situation and lead to a better appreciation of the significance of lipids for this devastating disease.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease affecting 1% of the population above 60 years and up to 4% of individuals in the highest age groups [1]. Parkinson’s disease is characterized by motor symptoms, such us tremor, rigidity, bradykinesia (slowed movement) and impaired balance [2], and non-motor manifestations, including sleep disorders, and autonomic, gastrointestinal, sensory, and neuropsychiatric symptoms [3]. These symptoms are associated with a progressive loss of dopaminergic (DA) neurons from the nigrostriatal pathway, formation of Lewy bodies (LB), and microgliosis [4]. In familial PD, which explains 5–10% of all cases, these abnormalities may be caused by a mutation in one of the thus far known 19 familial genes, including SNCA, LRRK2, PRKN, PINK1 and DJ-1, among others [5]. The remaining 90–95% of PD cases are of sporadic nature with unknown etiology.
Despite a large number of studies on familial forms of PD or toxin-induced cell and animal PD models (e.g., use of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone or 6-hydroxydopamine (6-OHDA)) [6,7,8], no disease-modifying treatment for PD has been developed yet. Thus, additional approaches are necessary to advance the field of PD. Previously, we used data from genome-wide association studies and other genetic studies of PD patients to build a molecular landscape [9]. This enabled us to identify, in an unbiased way, various processes and pathways that might be involved in PD. Interestingly, we found that lipids play a key role in most of the processes that have been (classically) associated with PD (i.e., oxidative stress, endosomal-lysosomal function, endoplasmic reticulum stress, and immune response), and thus in PD etiology. In agreement with this observation, not only mutations in the gene encoding the lipid-producing enzyme glucocerebroside (GBA) are associated with familial PD [10,11,12], but also multiple single-nucleotide polymorphisms (SNPs) located in other genes involved in lipid metabolism, e.g., SREBF1 [13], DGKQ [14], ASAH1 [15] or SMPD1 [16], have been linked to sporadic PD.
Lipids are biomolecules soluble in nonpolar organic solvents, usually insoluble in water, and primarily known for their metabolic role in energy storage [17,18]. Furthermore, they are the main constituents of cellular membranes, part of membrane rafts and protein anchors, and signaling and transport molecules [19,20,21,22,23]. There are eight different classes of lipids, classified as fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterols, prenols, saccharolipids, and polyketides [24]. Here we will review the current knowledge of the role of the first five lipid classes and of lipoproteins in PD (Figure 1). Certain aspects of the relationship between PD and lipids are beyond the scope of this review, including the complex interaction between (membrane) glycerophospholipids and α-synuclein, the interaction between lipid classes, and the role of cholesterol derivatives, such as bile acids, tocopherols, and tocotrienols (vitamin E), vitamin A and carotenoids, vitamin D, steroidal hormones (e.g., estrogen) and coenzyme Q10.
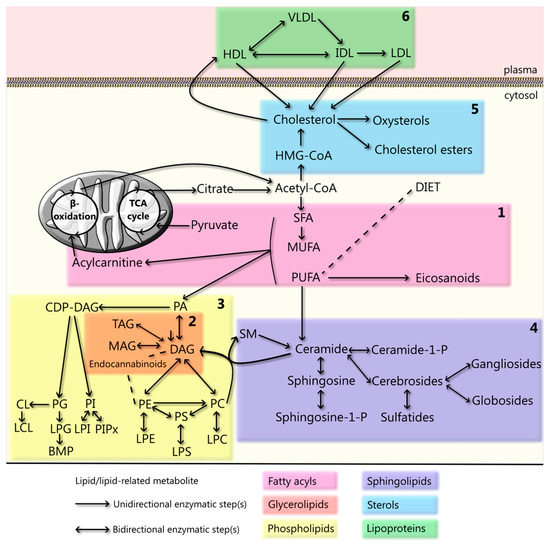
Figure 1.
Cellular lipid metabolism and lipoprotein cycle. Schematic representation of lipid metabolism, whereby each colored box represents one lipid class: (1) fatty acyls, which include saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids, their mitochondrial-transporter, acylcarnitine, and the PUFA-derivatives eicosanoids; (2) glycyerolipids, including monoacylglycerol (MAG), diacylglycerol (DAG), and triacylglycerol (TAG), together with endocannabinoids (even though only some of them belong to this lipid class); (3) phospholipids, which include phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylglycerol (PG), cardiolipin (CL), and their lyso derivatives (lysoPC (LPC), lysoPS (LPS), lysoPE (LPE), lysoPI (LPI), lysoPG (LPG) and lysoCL (LCL)), and Bis(monoacylglycero)phosphate (BMP); (4) sphingolipids, including ceramide(-1-phosphate), sphingosine(-1-phosphate), sphingomyelin (SM), cerebrosides, sulfatides, gangliosides, and globosides; (5) sterols, which include the metabolites of cholesterol synthesis, such as β-hydroxy β-methylglutaryl-CoA (HMG-CoA), cholesterol, and its derivatives cholesterol esters and oxysterols; and (6) lipoproteins, including high-density lipoproteins (HDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and very low-density lipoproteins (VLDL). A depiction of the various lipid structures and of all the metabolic steps involved in their generation and interconversion(s) is given in Figures 2a,b–6a,b, respectively.
2. Fatty Acyls
Fatty acyls are carboxylic acids formed by a hydrocarbon chain and a terminal carboxyl group (Figure 2) [25]. They are synthesized by chain elongation of acetyl-CoA with malonyl-CoA groups by enzymes named elongases. While humans can synthesize most fatty acyls, linoleic acid (LA) and alpha-linoleic acid (ALA) need to be obtained through the diet [26]. Fatty acyls are not only energy sources, but also the building blocks of complex lipids and as such form a key category of metabolites. Additionally, they are membrane constituents and regulate intracellular signaling, transcription factors, gene expression, bioactive lipid production, and inflammation [27,28]. Below, we will discuss the current knowledge of the roles of fatty acyls, more specifically of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturatedfatty acids (PUFA), eicosanoids and (acyl)carnitine, in PD, and an overview can be found in Supplementary Materials Table S1.
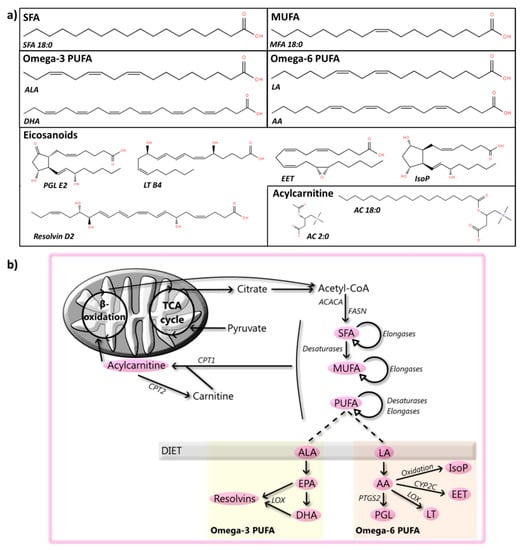
Figure 2.
Fatty acyls: structures and metabolic steps involved. (a) Schematic representation of the chemical structures of fatty acyls, including saturated fatty acids (SFA 18:0), monounsaturated fatty acids (MUFA 18:1), omega-3 polyunsaturated fatty acids (PUFA, alpha-linoleic acid (ALA, top) and docosahexaenoic acid (DHA, bottom)), omega-6 PUFA (linoleic acid (LA, top) and arachidonic acid (AA, bottom)), eicosanoids (from left to right, prostaglandin E2 (PGL E2), leukotriene B4 (LT), 14,15-Epoxyeicosatrienoic acid (EET), 15-F2t-Isoprostane (IsoP), and resolvin D2 (bottom)), and acetylcarnitine (AC 2:0) and acylcarnitine (AC 18:0). Chemical structures are adapted from the LIPID MAPS structure database [25]. (b) Schematic overview of steps involved in the metabolism of fatty acyls, where fatty acids (FAs) can be obtained through the diet or by a multi-enzymatic reaction starting from acetyl-CoA and performed by enzymes such as acetyl-CoA carboxylase 1 (ACACA) and fatty acid synthase (FASN). Multiple steps of elongation, performed by elongases, and desaturation, carried out by desaturases, produce MUFA and PUFA. PUFA include, among others, omega-3 PUFA, such as ALA, which can be converted by a multistep reaction into eicosapentaenoic acid (EPA) and DHA, and omega-6 PUFA, including LA, which can be transformed by a multistep reaction to AA. PUFA can be further metabolized by enzymes such as lipoxygenase (LOX), prostaglandin-endoperoxide synthase 2 (PTGS2, also known as COX2), cytochrome p450 2C to various eicosanoids, including resolvins, PGL, LT, EET, or oxidized to isoP. Furthermore, transport of FA into mitochondria for their metabolism is preceded by their association with carnitine, which is catalyzed by the enzyme carnitine O-palmitoyltransferase 1 (CPT1) and reversed by carnitine O-palmitoyltransferase 2 (CPT2).
2.1. SFA
The simplest fatty acids are the straight-chain SFA. Their intake does not seem to be linked to PD risk in humans [29,30,31] per se, but SFA intake in individuals exposed to rotenone increases PD risk, when compared to pesticide exposure alone [32]. Thus, SFA could exacerbate PD-linked pathology. Interestingly, higher levels of SFA (mainly 16:0 and 18:0) have been observed in lipid rafts from the frontal cortex of PD patients compared to controls [33], but not in their temporal cortex [34]. These area-dependent changes combined with a lack of differences in SFA intake between PD patients and controls point to defects in their absorption or metabolism and region-specific and/or cell-compartment differences. Dietary supplementation with SFA 18:0, which seems to be a less potent pro-inflammatory lipid than other SFA species [35], regulates mitochondrial function and rescues the PD-like phenotype of PINK and PRKN mutant flies [36,37,38]. Similarly, both acute and repeated intra-gastric gavage of SFA 8:0 reduces the impairment of DA neurotransmission in MPTP-treated mice [39]. These findings, together with the observed higher SFA levels in the frontal cortical lipid rafts, may point towards a compensatory mechanism in PD patients. In contrast, exposure of SH-SY5Y cells, primary neurons, and astrocytes to SFA 16:0 leads to apoptosis, reduces peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A, PGC-1alpha) and estrogen receptor alpha (ER-alpha) expression, promotes inflammation, and activates cyclooxygenase-2 (COX-2) [35,40,41,42], features that have also been observed in the brains of PD patients [43,44,45]. Since α-synuclein modulates the uptake of SFA 16:0 into the brain [46], accumulation of this protein in PD brains might lead to increased levels of SFA 16:0, which in turn can trigger some of its neuropathological activities.
2.2. MUFA
Variants of SFA containing one double bond are known as MUFA. Higher MUFA intake has been variably associated with decreased PD risk [31], reduced risk only in women [29] or unchanged risk [30]. These discrepancies in findings could be due to variation in the ethnicity of the subjects, differences in the type of study (cohort or case-control), the number of participants, questionnaires employed to asses MUFA intake, the corrections used, or even PD etiology, since different MUFA levels in cerebrospinal fluid (CSF) have been described in PD patients carrying a GBA mutation and those that do not [47]. Of note, no abnormalities in MUFA level have been observed in the temporal cortex of PD patients [34]. Some MUFA, such as oleic acid and cis-vaccenic acid, trigger the production of dopamine in MN9D cells [48], and the amide of oleic acid and dopamine (N-oleoyl-dopamine) modulates the firing of nigrostriatal DA neurons [49]. Interestingly, α-synuclein has a motif homologous to a region in fatty acid-binding proteins, allowing it to bind to oleic acid [50], which facilitates the interaction of α-synuclein with lipid rafts [51]. Based on these suggestive but still tentative findings, the effect of MUFA intake on PD risk, and specifically, its effect on dopamine production and the intracellular location and function of α-synuclein, should be further examined.
2.3. PUFA
Fatty acids containing two or more double bonds are known as PUFA and are usually classified according to the position of the first double bond counted from the tail (omega). The omega-3 family, for which ALA is the essential parent fatty acid, forms metabolic products that include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-6 PUFA, for which LA is the parent fatty acid, include arachidonic acid (AA), an intensely studied precursor of signaling lipids [52]. In general, PUFA are known to play a role in inflammation [53], epigenetics [54], and brain development [55] and function [56]. As such, they have been widely examined in PD patients, and animal and cellular PD models.
2.3.1. Human Studies on PUFA
Higher intake of omega-3 PUFA and ALA, but not other PUFA, such as omega-6 PUFA or LA, has been associated with reduced risk of PD [31,32], while other studies have reported a weak positive association between omega-6 PUFA and LA intake and PD risk [57], or have provided evidence against an association between PUFA intake and PD risk [30]. A link between AA intake and PD risk is also controversial: one study described a positive association [30], while another reported an inverse association [58]. Serum of PD patients has decreased concentrations of long-chain PUFA, including ALA, LA, and AA, compared to controls [59], while the CSF of PD patients has increased levels of 4-hydroxynonenal, a toxic product generated by AA peroxidation [60]. However, as described for MUFA, PUFA levels in CSF may depend on PD etiology [47]. The levels of PUFA in the anterior cingulate cortex and the occipital cortex of PD patients are increased or not changed, respectively [61]. Additionally, the levels of DHA and AA are decreased in frontal cortex lipid rafts from PD patients [33], while reduced LA, increased DHA and docosatetraenoic acid (an omega-6 PUFA), and no changes in AA have been reported for the cytosolic fraction of PD frontal cortex [62]. Moreover, no changes of PUFA were observed in the temporal cortex of PD patients [34]. Therefore, there is no agreement on the impact of PUFA intake on PD risk and little information on PUFA levels in the blood, CSF and brain of PD patients is available. The only consistent finding is the altered intracellular distribution of PUFA in neurons from the frontal cortex of PD patients, i.e., reduced levels of DHA in the lipid rafts and increased DHA in the cytosolic fraction.
2.3.2. Animal and Cellular Studies on PUFA
Omega-3 PUFA exert neuroprotective actions in MPTP-treated mice [63] by increasing the expression of brain-derived neurotrophic factor [64] and have also neuroprotective activity in 6-OHDA-treated rats [65]. A decrease in the level of this class of PUFA has been observed in the brains of an MPTP-induced goldfish PD model [66]. Furthermore, omega-3 PUFA deficiency leads to a reduced ability of the nigrostriatal system to maintain homeostasis under oxidative conditions, increasing the risk for PD [67]. Maternal omega-3 PUFA seem to partially protect a lipopolysaccharide (LPS)-model for PD [68]. Likewise, the omega-3 PUFA DHA protects DA neurons against MPTP–[69,70,71], paraquat—[72] or rotenone-induced toxicity [73] in rodent models and against effects of 6-OHDA-treatment in Caenorhabditis elegans, mice and rats [74,75,76], also when administered as TAG-DHA [77]. Moreover, DHA plays a crucial role in the differentiation of induced pluripotent stem cells (iPSCs) into functional DA neurons [78] and DHA supplementation protects DA neurons from the SN in MPTP-treated mice [79]. EPA and ethyl-EPA attenuate 1-methyl-4-phenylpyridinium (MPP+)-induced cell death in SH-SY5Y cells, primary mesencephalic neurons, and brain slices [80,81], and in vivo reduce MPTP/probecenid-induced dyskinesia and memory deficits (without preventing nigrostriatal DA loss) [82]. Thus, omega-3 PUFA appear to have a neuroprotective role in animal models for PD.
Additionally, pretreatment of rats with fish oil (which is rich in omega-3 PUFA) for 25 days before 6-OHDA treatment mitigates the loss of substantia nigra (SN) DA neurons [83]. In contrast, a chronic supplementation of fish oil in rats does not protect DA neurons but increases dopamine turnover [84]. These differential effects could be explained by the finding that the ethyl ester of DHA, a PUFA present in fish oil, enhances 6-OHDA-induced neuronal damage by triggering lipid peroxidation in mouse striatum [85]. Lipid peroxidation, which occurs frequently in PUFA, may lead to mitochondrial dysfunction [86] and α-synuclein oligomerization [87]. It is therefore not surprising that a number of studies have demonstrated beneficial effects when using deuterium-reinforced (deuterated) PUFA (which protects the PUFA sites susceptible for oxidation) [88,89,90], or PUFA in combination with antioxidants [91]. Therefore, omega-3 PUFA, probably in combination with the prevention of lipid peroxidation, should be further studied as a complementary therapy for PD.
Increased levels of omega-6 PUFA (LA and AA) have been reported in mice brain slices upon MPP+ treatment [81]. Similarly, upregulated AA signaling has been observed in the caudate-putamen and frontal cortex of 6-OHDA-treated rats [92], and the striatum and midbrain of MPTP-treated mice [93]. Both LA and AA are able to inhibit MPP+-induced toxicity in PC12 cells [94], while excess AA aggravates α-synuclein oligomerization in PC12 cells [95]. Interestingly, a mouse model with impaired incorporation of AA in the brain is resistant to MPTP treatment [96]. Hence, pharmacologically induced PD is linked to an increase in AA, the consequences of which are at present unclear and could be dose dependent.
2.3.3. Alpha-Synuclein and PUFA
Under physiological conditions, α-synuclein and PUFA are involved in endocytic mechanisms linked to synaptic vesicle recycling upon neuronal stimulation [97]. Moreover, α-synuclein and PUFA regulate each other, since α-synuclein increases endogenous levels of AA and DHA [62], and its oligomers control the ability of AA to stimulate SNARE-complex formation and endocytosis [98]. Reciprocally, PUFA strongly interact with the N-terminal region of α-synuclein [99], enhancing its oligomerization both in vivo and in vitro [100,101,102]. This might precede the formation of protective (LB-like) inclusions in DA cells [103].
Studies on specific PUFA species have shown that DHA induces α-synuclein oligomerization [104] by activating retinoic X receptor and PPAR-gamma 2 [105], effects that were prevented by co-administering aspirin [106]. The oligomers formed in the presence of DHA seem to be cytotoxic [107] and affect membrane integrity [108] and the physical properties of DHA itself (triggering formation of lipid droplets) [109]. Alpha-synuclein aggregation is also induced by AA [110], but the oligomers that are formed seem to be less toxic (more prone to disaggregation and enzymatic digestion) [111] and their formation is prevented or enhanced by low or high doses of dopamine, respectively [112]. The enhanced toxicity of dopamine might be related to its ability to form adducts with AA, which are able to trigger apoptosis [113].
Interestingly, a diet poor in omega-3 PUFA (with or without DHA supplementation) did not affect α-synuclein expression [114]. Accordingly, a DHA-rich diet had no effect on the DA system, motor impairments or α-synuclein levels in α-synuclein-overexpressing mice but increased the longevity of the mice [115]. This latter phenomenon might be related to the role of monomeric α-synuclein in sequestering early DHA peroxidation products and thus reducing oxidative stress [116]. The interaction between α-synuclein and (peroxidated) PUFA has recently been reviewed in more detail elsewhere [87,117].
2.4. Eicosanoids and Docosanoids
Eicosanoids and docosanoids constitute a family of bioactive fatty acyls mainly generated by AA, EPA, and DHA oxidation. They play a local role in infection and inflammation [118]. The family includes PGL, LT, EET, isoprostanes, HETE, isofurans, and resolvins, among others. Interestingly, one of the enzymes responsible for the formation of eicosanoids, COX-2, has been linked to PD pathology. Its role in the disease has been reviewed elsewhere [119,120].
2.4.1. PGL
No changes in or increased PGL E2 levels have been observed in the CSF and SN of PD patients, respectively [121,122]. In animal and cellular PD models, PGL E2 secretion is induced by LPS [123,124,125], 6-OHDA [126,127,128], rotenone [129,130], MPTP [131,132], and α-synuclein aggregation [133,134]. Nevertheless, PGL E2 levels in the striatum, hippocampus, and cortex of 6-OHDA-treated mice are decreased following a four-week exposure [135]. The eicosanoid PGL E2 mainly mediates its effects by binding to PGL E2 receptors (EP1-4), which trigger various intracellular pathways [136]. The EP1 receptor knock-out (KO) has neuroprotective effects on 6-OHDA-treated mice [137] and an EP1 antagonist protects embryonic rat mesencephalic primary cultures from 6-OHDA toxicity [138]. An agonist of EP2 protects primary neuronal cultures from 6-OHDA-induced toxicity [139] and an agonist of EP4 prevents DA loss in the SN of MPTP-treated mice [140]. Therefore, the effect of PGL E2 is also dependent on which receptor it binds. Interestingly, astrocytes KO for the familial PD gene DJ-1 secrete less PGL E2 than WT astrocytes [141]. This could impair DA neuron survival mediated by EP2 [142]. Thus, the sparse data that are available regarding the effects of PGL suggest that increased PGL E2 levels may play a role in the pathology of animal and cellular PD models, but that this occurs in a time-, location-, phenotype- and receptor-dependent manner.
Both PGL A1 and lipocalin-type PGL D synthase (the enzyme that isomerizes PGL H2 to PGL D2) inhibit rotenone- and paraquat-induced apoptosis in SH-SY5Y cells, respectively [143,144]. Furthermore, enhanced prostacyclin synthesis seems to reduce glial activation and ameliorate motor dysfunction in 6-OHDA-treated rats [145]. Conversely, PGL J2 treatment of SK-N-SH cells leads to the formation of aggregates containing ubiquitinated α-synuclein [146], and infusion into the SN of mice induces a pathology that mimics the slow-onset cellular and behavioral pathology of PD, including loss of DA neurons in the SN, α-synuclein aggregation, posture impairment, and microgliosis [115,147,148]. Hence, PGL other than PGL E2 seem to play a role in PD pathology as well, with effects being protective or detrimental. To resolve this complexity and obtain deeper insight into the contributions of these oxidized PUFAs to PD pathology further research is needed.
2.4.2. LT
Increased plasma LT B3 has been suggested as a biomarker for PD [149]. However, the role of LT has only been tested in animal and cellular PD models, in which MPTP treatment upregulates arachidonate 5-lipoxygenase (5-LOX, the enzyme that synthesizes LT from AA). This work has demonstrated that 5-LOX inhibition has neuroprotective effects [150], a finding which would be in agreement with the observation that LT B4 enhances MPP+-induced neurotoxicity in midbrain cultures [150]. Moreover, inhibition of cysteinyl LT receptor 1 has neuroprotective effects in a rotenone-induced rat PD model [151,152]. Interestingly, 5-LOX KO in mice reduces striatal dopamine levels under normal conditions [153]. Thus, 5-LOX seems to be necessary for maintaining the DA tone but can become deleterious upon toxicant challenge.
2.4.3. EET
In PD patients, the SNP rs10889162 located in CYP2J2 (the enzyme that metabolizes AA into EET) is associated with age of diagnosis [154]. Interestingly, EETs are known to have cytoprotective effects in other diseases and may therefore also play a role in PD neuroinflammation [155]. In PD models, 14,15-EET, which is released from astrocytes, enhances cell viability against oxidative stress [156] and protects DA neuronal loss in MPTP-treated mice [157]. Inhibition or KO of the soluble epoxide hydrolase (sEH, inhibition of which elevates endogenous EET) protects MPTP-treated mice [157,158], and a double sEH and COX-2 inhibitor has protective effects on a rotenone-induced Drosophila melanogaster PD model [159]. Combined, these findings suggest that EET has widespread neuroprotective effects, not necessarily relevant for PD only.
2.4.4. Isoprostanes
The role of isoprostanes in PD is controversial. Both higher levels and no change in F2-isoprostane have been found in urine and plasma [160,161,162] of (early) PD patients. Moreover, no changes have been observed in CSF [163] or SN [164] of PD patients, but higher levels of F2-isoprostane have been described in anterior cingulate cortex of PD patients [61]. Higher F2-isoprostane levels have also been observed in rotenone-, but not manganese-treated DA neurons derived from healthy iPSC [165]. Thus, more research and proper stratification of findings need to be performed to understand the role of isoprostanes in PD.
2.4.5. Other Eicosanoids and Docosanoids
The classic and non-classic AA-derived eicosanoids, HETE and isofurans, are increased in plasma [161,162] and SN [164] of PD patients, respectively. The docosanoid resolvin D1 attenuates MPP+-induced PD by inhibiting inflammation in PC12 cells [166], and resolvin D2 seems to restore LPS-induced neural injury in a rat model, also by suppression of inflammation [167]. Thus, resolvins seem to have a protective role in PD.
2.5. Carnitine and Acylcarnitine
Carnitine is a trimethyllysine derivative that can associate with various fatty acids, forming acylcarnitine. This association facilitates their transport from the cytosol to the mitochondrial matrix, where fatty acids undergo β-oxidation. Both carnitine and acylcarnitines are involved in processes such as neurotransmission and apoptosis [168]. Decreased levels of carnitine and (long-chain) acylcarnitines have been detected in plasma from PD patients [149,169,170], while no changes in acylcarnitine levels have been found in either CSF or plasma from PD patients when compared to controls [171]. Acetylcarnitine (acylcarnitine 2:0) protects SK-N-MC cells from rotenone-induced toxicity [172], and carnitine reduces the effects of MPP+ on rat forebrain primary cultures [173] and LPS in SIM-A9 microglial cells [174]. Moreover, the neuroprotective properties of acetylcarnitine have been found in rats treated with 6-OHDA [175,176,177] and rotenone [178,179], and non-human primates treated with MPTP [180]. Additionally, increased levels of carnitine and acylcarnitine 16:0 and 18:0 have been detected in the striatum of 6-OHDA-treated rats and the mesencephalon of MPTP-treated mice, respectively [181,182], suggesting a compensatory mechanism against PD-associated toxicity. Thus, while it remains unclear whether the levels of acylcarnitine change in PD patients, mounting evidence points towards decreased plasma levels and a protective role of acylcarnitine in animal and cellular PD models.
3. Glycerolipids
The esterification of one, two or three fatty acyls with glycerol gives rise to the glycerolipids mono-, di-, and tri-substituted glycerol, known as monoacylglycerol (MAG), diacylglycerol (DAG), and triacylglycerol (TAG), respectively (Figure 3). There is little information available on the function of MAG, while DAG is a neutral lipid involved in the formation of membranes [55] and in the synaptic vesicle cycle [183]. Additionally, DAG fulfills a role as secondary lipid messenger [184]. The neutral lipid TAG is the main energy storage molecule [185]. Below, we will discuss the current knowledge of the roles of all glycerolipids with potential significance for PD, including MAG, endocannabinoids, DAG, and TAG, and an overview is given in Supplementary Materials Table S1.
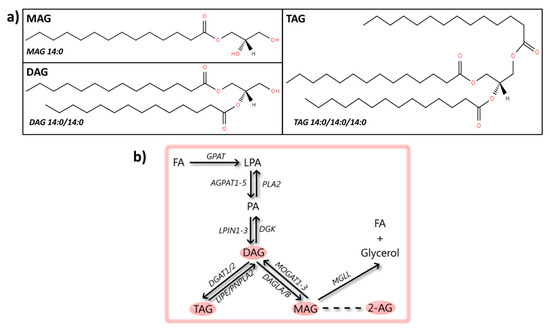
Figure 3.
Glycerolipids: structures and metabolic steps involved. (a) Schematic representation of the chemical structures of glycerolipids, including monoacylglycerol (MAG 14:0), diacylglycerol (DAG 14:0/14:0), and triacylglycerol (TAG 14:0/14:0/14:0). Chemical structures are adapted from the LIPID MAPS structure database [25]. (b) Schematic overview of metabolic steps involved in the synthesis and conversion of glycerolipids: synthesis starts from fatty acids (FAs) by sequential conversion into LPA and PA, which are phospholipids (process described in Figure 4b). The enzyme phosphatide phosphatase (LPIN1-3) converts PA into DAG, a step that can be reversed by diacylglycerol kinase (DGK). From DAG, one FA can be added to the glycerol backbone by diacylglycerol O-acyltransferase 1/2 (DGAT1/2), creating TAG, a step that can be reversed by the hormone-sensitive lipase (LIPE) or patatin-like phospholipase domain-containing protein 2 (PNPLA2). Additionally, one FA can be removed from DAG by the enzyme sn1-specific diacylglycerol lipase alpha/beta (DAGLA/B), giving rise to MAG, which can be transformed back to DAG by 2-acylglycerol O-acyltransferase 1-3 (MOGAT1-3). One of the mostly studied MAG species is the endocannabinoid 2-arachidonoylglycerol (2-AG). MAG can be degraded to glycerol and a FA by monoglyceride lipase (MGLL).
3.1. MAG
Decreased and increased expression of MAG lipase, the enzyme that degrades MAG to glycerol and free fatty acids, has been observed in the SN and the putamen of PD patients, respectively [186]. These differential effects could be associated with the different mechanisms leading to the degeneration of these brain areas; in PD patients, the SN presents with cell loss [187], while the putamen has DA depletion [188]. In PD models, pharmacological inhibition of MAG lipase has neuroprotective effects in both SH-SY5Y cells treated with MPP+ [189] and chronic MPTP/probenecid mouse models [190,191]. Although there is no information on the levels of MAG in PD patients, the model studies suggest that MAG lipase inhibition, and thus higher levels of MAG, may be protective for PD.
Endocannabinoids
MAG include 2-arachidonoylglycerol (2-AG), which is classified as an endocannabinoid. Endocannabinoids are a heterogeneous and thus difficult to classify group of lipids, including not only 2-AG, but also fatty acyl amides, such as anandamide (AEA). They have been previously linked to PD [192].
Two studies have reported high AEA levels in the CSF of untreated PD patients, which were restored upon DA treatment [193,194]. Moreover, higher cannabinoid 1 receptor (CB1R) levels in the putamen, and higher and lower cannabinoid 2 receptor (CB2R) levels have been reported in the SN and putamen of PD patients, respectively [186]. Treatment of PD patients displaying no psychiatric comorbidities with cannabidiol, a naturally occurring cannabinoid constituent of cannabis which appears to lack psychoactive effects, improves quality of life measures, but does not improve Unified Parkinson’s Disease Rating Scale scores [195]. The toxin 6-OHDA has been found to increase CB1R mRNA expression [196], downregulate CB1R protein density in multiple brain regions [197,198] or not produce any changes [199]. These differential effects may be explained by the fact that 6-OHDA seems to change the expression of CB1R protein in a region- and time-specific manner [200]. Decreased CB1R mRNA expression has been described in the striatum of reserpine-treated rats [201] and increased CB1R protein density has been observed in PRKN KO female mice [202]. Moreover, CB1R agonists fail to modulate spontaneous excitatory postsynaptic currents in cortical synapses of PINK1 KO mice [203], which points towards a CB1R dysfunction in these synapses. Hence, there is no agreement on the modulation of CB1R in different PD models and its correlation with the pathogenesis of PD in humans, making further studies necessary.
Drug-induced animal PD models (using rotenone, 6-OHDA or LPS) show increased CB2R mRNA expression [204,205], while a genetic model for PD (LRRK2 KO) does not display changes in CB2R mRNA levels [206]. However, CB2R agonists appear to improve PD-linked impairments in both drug- and genetically-induced rodent PD models [206,207,208]. Thus, CB2R upregulation in animal models may reflect a compensatory mechanism, since the administration of CB2R agonists has positive effects on PD-linked pathology.
Reduced levels of the AEA precursor synthesizing enzyme, N-acyl-transferase [209], and reduced activities of the AEA membrane transporter and hydrolase [210,211] have been observed in the striatum of 6-OHDA-treated rats. Moreover, 6-OHDA treatment has been found to both decrease [212] and increase [210,211] striatal levels of AEA, while MPTP-lesions in monkeys increase striatal AEA levels [213]. Interestingly, an increase of AEA levels, by inhibition of the fatty acid amide hydrolase or administration of AM404 (an endogenous cannabinoid reuptake inhibitor), has neuroprotective effects [209,210,214,215]. Hence, AEA seems to have neuroprotective effects, which have been suggested to be mediated by activation of PI3K and inhibition of JNK signaling [216].
All MPTP, rotenone, and reserpine treatments lead to increased 2-AG levels in a time- and region-specific manner in various animal PD models [213,217,218,219], and 2-AG administration provides protection against MPTP-induced cell death [217]. The endocannabinoid N-arahidonoyl-dopamine has anti-inflammatory effects on both macrophages and activated BV-2 cells [96] and modulates the activity of SN neurons [220], together with the endocannabinoid-like N-oleoyl-dopamine [49]. This effect could be linked to the fact that, together with an inhibitor of endocannabinoid degradation, administration of a D2 receptor agonist improves motor performance in both a 6-OHDA-and a reserpine-model for PD [221]. Similar to AEA, different members of the endocannabinoid group of lipids may thus be neuroprotective.
3.2. DAG
Parkinson’s disease patients have decreased plasma levels of DAG [222,223], and increased DAG levels in frontal cortex [224] and primary visual cortex [225]. Interestingly, SNPs from the chromosomal region that includes the gene encoding diacylglycerol kinase theta (DGKQ), which mediates the production of phosphatidic acid (PA) from DAG, are associated with PD susceptibility [14,226,227], and DGKQ is linked to increased PA 36:2 production and consequent α-synuclein aggregation [228]. The dysregulation of integral DAG metabolism in PD patients could be related to the observed genetic association between DGKQ and PD.
3.3. TAG
TAG levels are decreased in serum and plasma of (male) PD patients [222,223,229,230,231,232,233], even before diagnosis [234], and higher serum TAG is associated with reduced risk of idiopathic PD [235]. However, other studies have found no differences in blood TAG levels of PD patients and controls [236,237]. In the primary visual cortex of PD patients, the levels of TAG are decreased [225]. Thus, reduced levels of TAG seem to be linked to PD, although high heterogeneity has been described for TAG in PD patients [238]. Gender, ethnicity, or the technique used to measure TAG could bias the obtained results and contribute to the observed heterogeneity. Nevertheless, the trend from the majority of the results is in line with findings in animal models for PD, in which α-synuclein A53T overexpression leads to deceased serum TAG levels [239], and 6-OHDA treatment decreases TAG levels in retroperitoneal white adipose tissue [240]. Both rotenone and α-synuclein overexpression have been linked to intracellular deposition of TAG [241,242], which forms lipid droplets to which α-synuclein binds, and as such, the turnover of stored TAG is reduced and α-synuclein aggregation is enhanced [243]. In agreement, α-synuclein A53T overexpression in N27 cells leads to increased intracellular levels of TAG [244]. Thus, intracellular deposition and reduced turnover of TAG may explain the reduced levels of this acylglycerol in PD serum. Interestingly, Saccharomyces cereviciae that are unable to synthesize TAG are more tolerant to α-synuclein overexpression [242].
4. Glycerophospholipids
Glycerophospholipids, or phospholipids, have a glycerol backbone and a polar head group, which allows their classification into distinct subgroups, known as PA, phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidyllycerol (PG) and cardiolipin (CL) (Figure 4). The hydrolysis of one acyl derivative gives rise to the lipid species known as lysophospholipids. Glycerophospholipids are key components of the lipid bilayers of cells, and as such play a role in organelle function [245] and processes like endocytosis [246] or mitophagy [247]. Moreover, they also act as signaling molecules [248,249,250] and regulate lipid metabolism-related gene expression [251]. Below, we will discuss the current research on glycerophospholipids in PD, more specifically PA, PE, PS, PC, PI, PG and CL, and an overview is given in Supplementary Materials Table S1.
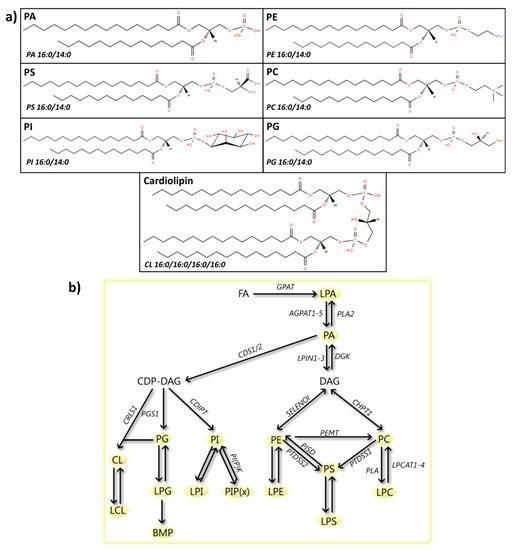
Figure 4.
Phospholipids: structures and metabolic steps involved. (a) Schematic representation of the chemical structures of phospholipids, including phosphatidic acid (PA 16:0/14:0), phosphatidylethanolamine (PE 16:0/14:0), phosphatidylserine (PS 16:0/14:0), phosphatidylcholine (PC 16:0/14:0), phosphatidylinositol (PI 16:0/14:0), phosphatidylglycerol (PG 16:0/14:0), and cardiolipin (CL 16:0/16:0/16:0/16:0). Chemical structures are adapted from the LIPID MAPS structure database [25]. (b) Schematic overview of phospholipid metabolism. Synthesis starts with the conversion of fatty acids into lysophosphatidic acid (LPA) by glycerol-3-phosphate acyltransferase (GPAT). LPA is then metabolized to PA by 1-acyl-sn-glycerol-3-phosphate acyltransferase 1-5 (AGPAT1-5), a reaction that can be reversed by phospholipase A2 (PLA2). PA can then be metabolized to diacylglycerol (DAG) (process described in Figure 3b), which can be subsequently transformed to PE by ethanolaminephosphotransferase 1 (SELENOI) or PC by cholinephosphotransferase 1 (CHPT1). PE can also be converted into PC by the enzyme phosphatidylethanolamine N-methyltransferase (PEMT). Both compounds can be precursors for the synthesis of PS by the enzymes phosphatidylserine synthase1/2 (PTDSS1/2). The conversion of PE to PS can be reversed by phosphatidylserine decarboxylase proenzyme (PISD). Additionally, PA can be metabolized by phosphatidate cytidylyltransferase 1/2 (CDS1/2) to cytidine diphosphate DAG (CDP-DAG), which can then be transformed to either PI, PG or CL, by CDP-diacylglycerol--inositol 3-phosphatidyltransferase (CDIPT), CDP-diacylglycerol--glycerol-3-phosphate 3-phosphatidyltransferase (PGS1) and cardiolipin synthase (CRLS1), respectively. PI can be phosphorylated by PI (phosphate) kinases (PI(P)K), to produce PI phosphate (PIP(x)). Moreover, all phospholipids can be metabolized to their lyso-forms (LPC, LPS, LPE, LPI, LPG, and LCL) by PLA2, a reaction reversed by lysophospholipid acyltransferases (LPCATs). LPG can be further metabolized to Bis(monoacylglycero)phosphate (BMP).
4.1. PA
One of the best-known glycerophospholipid messengers is PA, which has a broad spectrum of functions, including intracellular vesicular trafficking, cell survival, cytoskeletal organization, neuronal development, and mitochondrial function [252,253,254]. Increased plasma PA (18:2/15:0) levels have been suggested as a biomarker for PD [149]. Additionally, PA is known to interact with residues 1–102 of α-synuclein [255,256], thus enhancing the formation of multimeric and protease-resistant α-synuclein aggregates [257,258]. ATP13A2, a lysosomal ATPase, which, when mutated, causes familial PD, constitutes another link between PA and PD. This ATPase requires the interaction with PA, and also PI(3,5)P2, to protect cells against rotenone-induced mitochondrial stress or other PD-related stress conditions, such as exposure to Fe(3+) [259,260]. Furthermore, overexpression of phospholipase D2 appears to induce DA neuronal cell loss via a mechanism involving PA signaling [261]. Given PA’s role in the subcellular distribution and aggregation of α-synuclein, and in ATP13A2-mediated neuroprotection, it would be of interest to study PA levels and its partitioning in the brains of PD patients.
LPA
Similar to PA, LPA is a lipid mediator in a wide range of biological actions, including cell proliferation, (nervous system) development, and cytokine secretion [262,263,264]. Furthermore, LPA is involved in neuronal (DA) differentiation [265]. The expression of LPA receptor 1 is reduced in the SN of a 6-OHDA rat PD model [265], and an LPA receptor ligand attenuates the MPTP mouse PD model [266]. Unfortunately, nothing is known about LPA in PD patients.
4.2. PE
The glycerophospholipid PE has a structural role in biological membranes, and it is a regulator of cell division, membrane fusion/fission, and hepatic secretion of very low-density lipoproteins (VLDL) [267]. Patients with PD show decreased plasma levels of PE 34:2 [222], and those carrying a GBA mutation have decreased serum levels of PE compared to non-GBA mutation carriers [268]. Decreased total PE levels have also been observed in the SN of PD patients before treatment [269], in males only after treatment [270], and in the primary visual cortex [225]. In contrast, increased PE has been found in frontal cortex lipid rafts from PD patients [33]. Of note, one of the enzymes linked to PE synthesis, phosphoethanolamine cytidylyltransferase, is elevated in the SN of PD patients [271]. All findings combined, most evidence points towards decreased levels of PE in PD patients, but the biological implications of the reduced levels need to be examined further.
In vitro, PE is necessary for the interaction between α-synuclein and biological membranes [272] and for the formation of stable, highly conductive channels by α-synuclein [273]. Both processes might have a role in the normal function of α-synuclein. Accordingly, in yeast and worm models, PE deficiency disrupts α-synuclein homeostasis and induces its aggregation [274,275]. This deficiency, also seen in PD patients, could be due to increased formation of LPC from PE, which occurs in MPP+ models [276]. Moreover, the inhibition of this metabolic step offers significant protection against cytotoxicity [277].
4.3. PS
The glycerophospholipid PE is involved in the triggering of both intracellular and extracellular cascades, such as the activation of kinases or the clearance of apoptotic cells [250,278]. It plays a role in neuronal survival and differentiation, and neurotransmitter release [279]. Plasma levels of PS 40:4 are decreased in PD patients [222], but higher levels of PS 36:1, PS 36:1, 36:2, and 38:3, or overall PS, have been found in parkin-mutant fibroblasts [280], frontal cortex [224], and primary visual cortex [225] of PD patients, respectively. This is in agreement with the increased PS synthase activity that has been observed in the SN of PD patients [271]. However, some groups reported contrasting findings and claimed that total PS levels in PD SN and frontal cortex lipid rafts are not significantly altered [33,270]. Yet another interesting finding is that parkin-mutant iPSC-derived neurons have a different subcellular distribution of PS [281], with increased and decreased PS in the mitochondrial and ER fractions, respectively.
The exposure of PS on the cellular surface, which acts as an “eat-me” signal for phagocytosis, is triggered by 6-OHDA [282], rotenone [283], paraquat [284], MPP+ [285], and WT, A53T and A30P α-synuclein [286]. Blockade by an antibody against PS is protective in a rotenone-induced neuronal/glial PD model [287], pointing towards a role of microglial-mediated phagocytosis in PD. This glyceroplipid is known to be associated with the N-terminal- and mid-region of α-synuclein [256,288], with some preference for acetylated α-synuclein [289,290]. This association correlates with membrane penetration [255], alpha-helix formation [256] and aggregation [291], and vesicle [256,292] and liposome [51] binding. Taken together, these findings suggest that PS is a modulator of apoptosis and α-synuclein-mediated pathology.
4.4. PC
The most-abundant glycerophospholipid in eukaryotic membranes, including mitochondrial membranes [293], where it plays a structural role, is PC. It is involved in anti-inflammation [294], cholesterol metabolism [295], and neuronal differentiation [296]. Decreased levels of PC 34:2 and 46:2, PC 34:5, 36:5, and 38:5, and total PC, have been observed in plasma and frontal cortex from PD patients [222,224], and in SN from only male PD patients [270], respectively. One of the enzymes involved in PC synthesis, PC cytidylyltransferase, is elevated in the SN of PD patients [271]. Interestingly, components of the pathway “PC biosynthesis”, together with “PPAR signaling” components, allowed accurate classification of PD and control samples [297], highlighting altered PC metabolism as a consistent feature of PD.
Decreased PC levels have been found in the SN of a mouse model of early PD [298] and in brain tissue from MPTP-treated goldfish [66]. Interestingly, α-synuclein does not bind to but rather remodels pure PC membranes through weak interactions with this phospholipid [299,300], and α-synuclein E46K mutants form functionally distinct ion channels in PC membranes [301]. However, others observed binding of the physiologically relevant N-terminally acetylated α-synuclein to pure PC membranes, with preference for highly curved and ordered membranes [302]. Based on these multiple links, the significance of PC metabolism for PD pathology is an interesting and important topic for further study.
LPC
The most-abundant lysophospholipid in the blood is LPC. Its levels are critically related to major alterations in mitochondrial function (e.g., oxidation rate) and to minor defects in mitochondrial permeability [303,304]. Of note, saturated acyl LPCs have inflammatory properties, such as leukocyte extravasation and formation of pro-inflammatory mediators, which can be compensated by polyunsaturated acyl LPC, such as LPC 20:4 and LPC 22:6 [305]. Higher levels of LPC 16:0 and 18:1 have been found in the lipid profile of parkin-mutant fibroblasts compared to healthy controls [280]. Moreover, increased plasma LPC 18:2 has been suggested as a biomarker for PD [149].
Treatment with MPTP induces LPC formation, which leads to cytotoxic changes, dopamine release and inhibition of its uptake, a decreased mitochondrial potential, and increased reactive oxygen species (ROS) formation in PC12 cells [277]. The lysophospholipid LPC inhibits D1 and D2 receptor binding activities in the striatum of rats, inhibits the dopamine transporter, and decreases striatal dopamine turnover rate [306], leading to hypokinesia [307]. Interestingly, 6-OHDA treatment of rats gives rise to an overall decrease in LPC species, with the exception of LPC 16:0 and 18:1, which are increased in the SN [298]. Thus, LPC has negative effects on the DA system, but LPC levels in PD models seem to depend on the type of pharmacological treatment used and the LPC species involved.
4.5. PI
The glycerophospholipids PI and PI phosphates are part of intracellular signal transduction systems [308], but relatively little is known about their role in PD. In humans, higher levels of PI 34:1 and no changes of PI 36:1, 36:2, 38:4, 38:5, 40:5, and 40:6, or no changes in total PI have been observed in parkin-mutant skin fibroblasts [280], and in the lipid rafts of frontal cortex from PD patients [33], respectively. Decreased levels of overall PI have been observed in the SN of male PD patients [270]. In rodents, MPTP decreases the expression level of striatal PI-transfer protein [309], which is involved in the transfer of PI across membranes [310].
PI Phosphate (PIPx)
The role of PIPx species in PD is also poorly defined. However, PI and PIP2 effectively influence self-oligomerization of α-synuclein [311], while α-synuclein seems to prefer binding membranes containing PI(4,5)P2 [312]. Moreover, PIP3 is decreased in the nuclear fraction and whole-tissue homogenate, while PIP2 is increased in whole-tissue homogenate of SN from PD patients [313]. As mentioned above, ATP13A2 requires the interaction with PI(3,5)P2 to protect cells against PD-related stress conditions [259,260], an interaction that is able to reduce proteasomal inhibitor-induced accumulation of ubiquitin proteins [314]. Future systematic studies on the roles of the various PIPx species are required to understand their function in PD pathogenesis.
4.6. PG
Less than 1% of total glycerophospholipids in intracellular membranes is composed of PG and it is mainly localized to mitochondrial membranes, where it can be synthesized locally [315]. The levels of total PG are not changed in lipid rafts from PD frontal cortex [33], while increased PG 32:0 has been described in total extracts of the same brain area [224]. Alpha-synuclein is able to bind PG with various degrees of affinity depending on the variability in its structure (WT ≈ truncated > A53T > A30P) [316], and PG-containing membranes can promote α-synuclein aggregation [317,318,319]. Additionally, α-synuclein oligomers are able to induce PG clustering [319], connect PG-containing vesicles [320] and disrupt PG vesicles [321] through large membrane bilayer defects, rather than through a pore-like mechanism [322], leading to vesicle docking and fusion problems. Furthermore, low concentrations of α-synuclein inhibit and high concentrations stimulate lipid peroxidation of PG [323]. Unfortunately, information on the levels of PG in animal PD models is lacking.
4.7. CL
The glycerophospholipid specific for mitochondrial membranes is CL. Here, it plays both a structural and functional role [324,325,326]. No changes in total CL levels have been detected in the SN of PD patients [270]. However, PINK1 KO mouse embryonic fibroblasts display decreased CL levels and supplementation with CL rescues mitochondrial dysfunction [327]. Moreover, rotenone induces oxidation of highly unsaturated CL in human peripheral blood lymphocytes [328], and increases levels of plasma PUFA CLs, but decreases oxidizable PUFA-containing CL levels and increases mono-oxygenated CL species in the SN of rats [329]. A proper CL content in the inner mitochondrial membrane and the presence of acyl side chains are crucial for α-synuclein localization [330,331], while CL content in the outer mitochondrial membrane buffers synucleinopathy [332]. Moreover, α-synuclein is able to disrupt artificial membranes containing CL [333], and its overexpression reduces CL content in MN9D cells [334] and in mouse brain [335]. Additionally, the formation of complexes between CL and α-synuclein, together with cytochrome c, may be a source of oxidative stress [336]. The role that CL plays in the interaction of α-synuclein with membranes and in mitophagy has been previously reviewed [337,338].
5. Sphingolipids
Sphingolipids constitute a family of lipids characterized by the presence of a sphingoid-base backbone. This complex family of compounds includes the sphingoid bases (e.g., sphingosine and sphingosine-1-phosphate), ceramides, phosphosphingolipids (e.g., sphingomyelin (SM)) and glycosphingolipids (e.g., cerebrosides, ganglisodes, and sulfatides) (Figure 5). Sphingolipids are not only structural components of cell membranes, but they also play a role in apoptosis, autophagy, and immune response [339]. Here, we will specifically focus on the involvement of sphingosine(-1-phosphate), ceramide, SM, cerebrosides, gangliosides, and sulfatides, and an overview is given in Supplementary Materials Table S1.
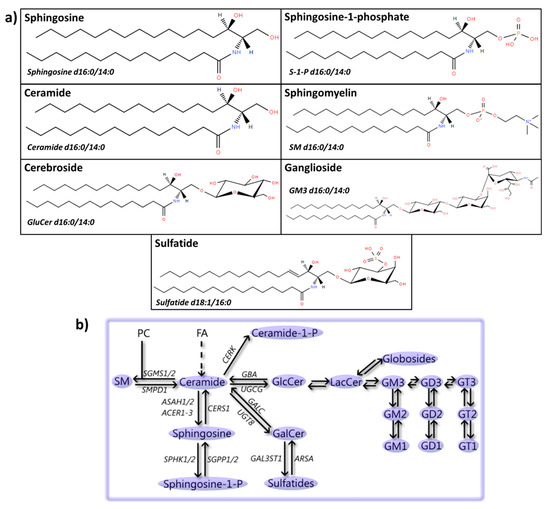
Figure 5.
Sphingolipids: structures and metabolic steps involved. (a) Schematic representation of the chemical structures of sphingolipids, including sphingosine (d16:0/14:0), sphingosine-1-phosphate (S-1-P d16:0/14:0), ceramide (d16:0/14:0), sphingomyelin (SM d16:0/14:0), cerebroside (glucosylceramide, GluCer, d16:0/14:0), ganglioside (GM3 d16:0/14:0), and sulfatide (d18:1/16:0). Chemical structures are adapted from the LIPID MAPS structure database [25]. (b) Schematic overview of steps involved in the formation and metabolic conversion of sphingolipids. Synthesis of sphingolipids starts by a multistep process to convert fatty acids (FAs) into ceramide. Phosphatidylcholine can be fused to ceramide by phosphatidylcholine:ceramide cholinephosphotransferase 1/2 (SGMS1/2) to produce SM, which can be converted back into ceramide by sphingomyelin phosphodiesterase (SMPD1). Ceramide can also be phosphorylated by ceramide kinase (CERK) to ceramide-1-P and converted into sphingosine by acid or alkaline ceramidases (ASAH1/2 or ACER1-3), and phosphorylated by sphingosine kinase 1/2 (SPHK1/2) to sphingosine-1-phosphate. This process can be reversed by the sequential action of sphingosine-1-phosphate phosphatase 1/2 (SGPP1/2) and ceramide synthase 1 (CERS1). Furthermore, ceramide can be glycosylated via the addition of a galactose molecule by 2-hydroxyacylsphingosine 1-beta-galactosyltransferase (UGT8) to produce galactosylceramide (GalCer). Further addition of a sulfate group by galactosylceramide sulfotransferase (GAL3ST1) results in the formation of sulfatides. This process can be reversed by the sequential actions of arylsulfatase A (ARSA) and galactocerebrosidase (GALC). Finally, ceramide can also by glycosylated via the addition of a glucose molecule by ceramide glucosyltransferase (UGCG) to produce GlcCer, which can be further glycosylated to produce both globosides and gangliosides, such as GM3, GD2 and GT1, by multiple enzymes [340], or it can be converted back into ceramide by glucosylceramidase (GBA).
5.1. Sphingosine(-1-Phosphate)
Sphingosine is a bioactive lipid known to induce apoptosis and regulate endocytosis, while its phosphorylated form, sphingosine-1-phosphate (S1P), promotes cell survival and triggers diverse intracellular signaling pathways through G-protein-coupled receptors [339,341,342]. Sphingosine induces the formation of oligomeric α-synuclein species, which serve as template for the formation of endogenous α-synuclein aggregates in human and mammalian neurons [343]. Similarly, S1P accumulation, e.g., due to GBA deficiency, promotes α-synuclein aggregation [343]. Alpha-synuclein itself inhibits the expression and activity of sphingosine kinase 1, the enzyme that catalyzes the phosphorylation of sphingosine to S1P [344] and modulates S1P receptor-mediated signaling [345,346]. Sphingosine-1-phosphate supplementation of MPP+-treated cells is neuroprotective [347,348,349], and a selective S1P receptor agonist is protective in mouse and cellular models treated with 6-OHDA and rotenone [350]. Therefore, while S1P is protective in animal and cellular PD models, presumably through its pro-survival effects, it is clear that both sphingosine and S1P are linked to α-synuclein aggregation. Unfortunately, the lack of studies on human samples does not allow drawing a conclusion regarding the relevance of these lipids for PD pathogenesis.
5.2. Ceramide
Ceramide is involved in apoptosis, lipid raft formation, and regulation of the mitochondrial respiratory chain [340,351,352,353]. Both higher [354] and lower [222,355] plasma levels of ceramide have been reported in PD patients, while lower ceramide 18:0 and no differences in total ceramide levels are observed in their frontal cortex [224] and SN [270], respectively. Reduced levels of ceramide may be associated with α-synuclein accumulation [356,357]. This is in line with the finding that reduced and increased levels of ceramide have been observed in the anterior cingulate cortex and primary visual cortex of PD patients [225,356,358], which display and lack α-synuclein aggregation, respectively [359]. Thus, variation in the levels of ceramide in different tissues may be linked to α-synuclein accumulation.
Mimicking PD with PLA2G6 KO, LRRK2 KO, PINK1 KO or rotenone treatment increases ceramide levels in fly brain, mouse brain, mouse olfactory bulb, and human erythrocytes, respectively [283,360,361,362]. C2-ceramide initiates a series of events leading to neuronal death, including an early inactivation of PI3K/AKT and ERK pathways, followed by activation of JNK, GSK3β activation and neuronal death [363]. Additionally, C2-ceramide induces cytotoxicity and ROS production in neuronal(-like) cells [364,365,366], which can be prevented by WT α-synuclein [367], PINK1 [368,369] and DJ-1 [370]. However, both in vivo and in vitro C2-ceramide seems to suppress microglial activation [371], protect neurons against α-synuclein-induced cell injury [372], and reverse rotenone-induced phosphorylation and aggregation of α-synuclein [373]. An increase in ceramide levels is thus commonly found in animal and cellular models for PD, but its effects are unclear and may be both beneficial and detrimental for different PD-related traits.
5.3. SM
The most abundant sphingolipid in eukaryotic cells and plasma is SM. It is one of the building blocks of the cellular membrane and a source of bioactive lipids, such as ceramide, ceramide-1-phosphate and S1P, which are involved in inflammation [374,375], cell death [376,377] and autophagy [378]. In the nervous system, SM is a major constituent of myelin. Mutations in sphingomyelinase-1, which lead to SM accumulation, are a risk factor for PD [16,379,380]. This feature may be linked to the increase in α-synuclein expression observed upon SM treatment [381] and the presence of SM in LB inclusions [382]. Parkinson’s disease patients carrying GBA mutations have elevated levels of total plasma SM compared to PD patients not carrying the mutation [268]. Moreover, SM 18:1 and SM 26:1 are increased and decreased in the anterior cingulate cortex [358], respectively, while increased SM levels have been described in the primary visual cortex [225], and, in males only, in the SN of PD patients [270]. However, no changes have been found in the putamen or cerebellum of sporadic PD patients [383]. The role that SM accumulation appears to play in PD pathogenesis may thus be multifold, being linked to inflammation, autophagy dysfunction, and/or α-synuclein expression and aggregation.
5.4. Cerebrosides
Cerebrosides are lipids glycosylated via the addition of either glucose or galactose and known to be involved in intracellular membrane transport and cell survival [384]. In PD patients, cerebrosides are increased in plasma (of GBA mutation carriers) and, in males only, in the SN, whereas they are decreased in lipid rafts from the frontal cortex [33,268,270,385]. More specifically, PD patients have increased levels of glucosylceramide [223,354] in plasma but no changes in cerebroside levels in the temporal cortex [386], putamen or cerebellum [383], and decreased levels of galactosylceramide 24:1 and lactosylceramide 18:1 in the frontal cortex [224]. Thus, whereas a consistent coupling between PD and increased cerebrosides in plasma has been found, cerebroside changes in the brain are region dependent and their significance for PD needs to be determined.
Interestingly, mutations in the enzymes responsible for the degradation of cerebrosoides, namely GBA and galactocerebosides (GALC), which cause Gaucher’s disease and Krabbe’s disease, respectively, have been associated with α-synuclein aggregation and PD [387,388]. Glucosylceramide, a product that accumulates upon GBA deficiency, destabilizes α-synuclein tetramers and related multimers and frees α-synuclein monomers and leads to cellular toxicity [389]. These effects are caused by colocalization of glucosylceramide with α-synuclein and induction of a pathogenic conformational change of the protein [390]. This promotes aggregation of WT (but not mutated) α-synuclein into a β-sheeted conformation [343,391], and conversion of α-synuclein into a proteinase-resistant form [392]. Conversely, α-synuclein inhibits normal activity of GBA [393], which increases glucosylceramide, creating a feedback loop. Inhibition of glucosylceramide synthase, which decreases glucosylceramide levels, slows α-synuclein accumulation [394] and partially protects mice against MPTP-induced toxicity [395]. Thus, it is well established that glucosylceramide accumulation leads to α-synuclein aggregation and toxicity. Interestingly, aging of WT mice leads to brain accumulation of both glucosylceramide and lactosylceramide [396], suggesting that age-associated changes in its metabolism might be related to PD onset.
5.5. Gangliosides
Gangliosides are synthesized by the addition of carbohydrate moieties to lactosylceramide. One of the simplest and most widely distributed ganglioside is monosialodihexosylganglioside (GM3) that consists of lactosylceramide and sialic acid [397]. Gangliosides were initially discovered in the brain where they are involved in neurotransmission, receptor regulation, and stabilization of neural circuits, including the nigro-striatal DA pathway [398,399]. Parkinson’s disease patients have higher plasma levels of gangliosides [385], GM3 gangliosides [223], and N-acetylneuraminic acid-3 (NANA-3) gangliosides [222] than controls. Likewise, higher GM2 and GM3 levels have been detected in parkin-mutant iPSCs compared to controls [280]. However, no accumulation of GM1, GM2 or GM3 has been observed in the putamen or cerebellum of sporadic (or heterozygous GBA-mutation) PD patients [383], nor in the SN of PD patients [270]. Even a GM1 deficiency, together with decreased expression of ganglioside biosynthetic enzymes (B3GALT4 and ST3GAL2), has been found in the SN from PD patients [400,401]. Hence, most publications point towards increased gangliosides in plasma of PD patients, but concomitant changes in ganglioside levels have not been observed in their brains.
Interestingly, GM1 supplementation seems to have a positive disease-modifying effect in PD patients [402,403,404,405,406,407]. Also, increased GM1 levels are neuroprotective in MPTP-treated animals [408]. For example, GM1 can partially protect against 6-OHDA treatment [409] and aging-related DA deficits [410] as well. However, studies on MPTP-treated non-human primates have shown that a short treatment with GM1 does not lead to any improvement [411], while a chronic treatment does have a positive effect [412], which might be restricted to the surviving DA neurons in the midbrain, rather than due to the prevention of cell death [413]. Mechanistically, GM1 treatment increases DA innervation, dopamine synthesis, and TH expression following an MPTP lesion [414,415,416,417,418,419,420,421,422,423]. Moreover, GM1 inhibits the inflammatory response triggered by 6-OHDA [424], protects against the toxic intracellular GPR37 aggregates observed in parkinsonism [425] and is involved in the internalization of α-synuclein into microglia [426]. Nonetheless, evidence for an α-synuclein-linked role of GM1 is controversial: in one study it was claimed that GM1 may accelerate α-synuclein aggregation [427] and the formation of proteinase-resistant α-synuclein [392], but other work demonstrated that it induces alpha-helical structure and inhibits or eliminates α-synuclein fibril formation (depending on the amount of GM1 present) [289,428]. It is also unclear whether membranes containing GM1 interact with α-synuclein [428,429]. Hence, GM1 is a promising candidate for PD treatment, but further clarification of its specific effects on α-synuclein is urgently needed.
Only a limited number of studies have analyzed the role of gangliosides other than GM1 in animal and cellular models. For instance, mice lacking GM2/GD2 synthase develop parkinsonism, which can be partially rescued by administration of GM1 [400,430]. However, GM2 accumulation, as seen in Tay Sachs and Sandhoff’s diseases, leads to α-synuclein aggregation [431]. Thus, both deficiency and excess of GM2 may lead to PD-like pathology. Likewise, GM3 accelerates α-synuclein aggregation [427] and regulates α-synuclein-induced channel formation in PC-containing membranes [301]. Furthermore, deletion of GD3 synthase, which decreases production of the pro-apoptotic GD3 ganglioside, protects against MPTP treatment in mice [432]. In contrast, ganglioside GT1b is neurotoxic in nigral DA neurons by triggering nitric oxide release from activated microglia [433]. The gangliosides GD3 and GT1b are unchanged and decreased in the SN of (male) PD patients, respectively [270]. Together, these results indicate that GM3, GD3, and GT1b play aggravating roles in PD pathology. Finally, 1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP, an inhibitor of glycosylceramide synthase that decreases ganglioside content) enhances α-synuclein toxicity, which can be rescued by ganglioside addition [434].
5.6. Sulfatides
Sulfatides, which are sulfated galactocerebrosides, form a group of lipids involved in protein trafficking, immune responses and neural plasticity, among others [435]. Higher levels of sulfatides have been detected in the plasma [385] and visual cortex [225] of PD patients, and in the SN of male PD patients [270]. Arylsulfatase A, an enzyme that breaks down sulfatides, has been linked to PD recurrence [436,437]. However, no changes or reductions in sulfatide levels have been described in lipid rafts from the frontal cortex of PD patients [33] and in brain samples from PD patients [438], respectively. Thus, most evidence points towards increased sulfatide levels in PD, although a number of studies have not confirmed this finding, suggesting patient, technique and/or tissue-type differences among the various investigations.
6. Sterols
Sterols are amphipathic lipids synthesized from acetyl-CoA via the β-hydroxy β-methylglutaryl-CoA reductase pathway and containing a fused four-ring core structure (Figure 6). Sterols are known to play a role in immune cell function [439], influence membrane fluidity and permeability, and serve as signaling molecules and hormones [440], among others. Here we will review the current findings on sterols in PD, more specifically cholesterol, its precursors, CE, and oxysterols (Supplementary Materials Table S1).
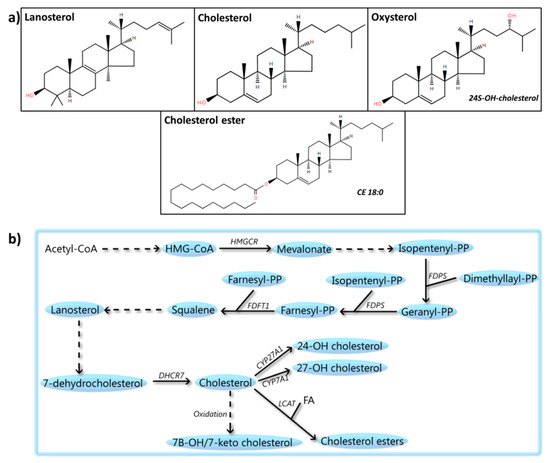
Figure 6.
Sterols: structures and metabolic steps involved. (a) Schematic representation of the chemical structures of sterols, including lanosterol, cholesterol, oxysterols (24S-hydroxy-cholesterol), and cholesterol esters (CE 18:0). Chemical structures are adapted from the LIPID MAPS structure database [25]. (b) Schematic overview of steps involved in sterol metabolism. Acetyl-CoA is used to synthesize β-hydroxy β-methylglutaryl-CoA (HMG-CoA), which is converted into mevalonate by 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR). Mevalonate is metabolized to isopentenyl-PP by a multistep process, followed by its conversion to geranyl-PP and farnesyl-PP by the enzyme farnesyl pyrophosphate synthase (FDPS). Two molecules of farnesyl-PP are condensed by squalene synthase (FDFT1) to create squalene, which is further metabolized to lanosterol and 7-dehydrocholesterol. Subsequently, cholesterol is synthesized from 7-hydrocholesterol by 7-dehydrocholesterol reductase (DHCR7). Finally, cholesterol can be oxidized to compounds such as 7-beta-hydroxycholesterol or 7-ketocholesterol. It can also be esterified to a fatty acid (FA) by phosphatidylcholine-sterol acyltransferase (LCAT) to create cholesterol esters or metabolized by the cytochrome p450 to produce compounds such as 24/27-hydroxycholesterol.
6.1. Cholesterol
6.1.1. Human Studies on Cholesterol
Cholesterol intake has been found to be negatively [29,441], positively [30,442], or not [31,443] correlated with PD risk. A meta-analysis indicates a lack of association between cholesterol intake and PD [444]. Lower plasma cholesterol has been associated with PD [229,232,445,446,447], and confirmed by a meta-analysis [238], and higher plasma cholesterol levels have been linked to reduced PD risk [235,448,449,450,451,452] and slower clinical progression of PD [453]. However, others, including a meta-analysis [454], have found no association between plasma cholesterol levels and PD [230,455] or PD risk [233,456]. Even higher plasma cholesterol levels in PD patients compared to controls [231,457] have been reported. The differential outcome of these studies could be attributed to factors such as age and gender, among others, since lower plasma cholesterol levels have been reported in PD male patients of more than 55 years compared to controls [458], a high total cholesterol baseline has been associated with increased risk of PD in subjects of 25–54 years (but not in those above 55) [459], and female PD patients seem to have higher cholesterol levels compared to male PD patients [460]. Thus, proper patient stratification is necessary to determine whether plasma cholesterol is associated with PD, which would point towards defects in cholesterol metabolism.
In PD patients, no significant changes in cholesterol levels have been observed in the putamen [383], SN [270] or frontal cortex lipid rafts [33], while elevated levels of cholesterol have been found in the visual cortex [225]. Finally, decreased cholesterol biosynthesis has been described in fibroblasts from PD patients [461]. The differences in these observations could be related to tissue or brain-region specificities, technique sensitivity, and/or choice of patients. Thus, validation studies and larger cohorts are needed to determine the relevance of cholesterol changes in PD patients and their pathology. Additionally, some studies [13,462,463] have found an association between PD and a SNP near the gene SREBF1, which encodes a transcription factor that regulates cholesterol biosynthesis, although other studies could not confirm the findings [464].
6.1.2. Animal and Cellular Studies on Cholesterol
In animal and cell model studies, the link between PD and cholesterol has been demonstrated multiple times. For example, the cholesterol biosynthetic pathway controls PRKN expression [465], which in turn regulates fat (and cholesterol) uptake in PRKN mutant mice and human cells [466]. Additionally, DJ-1 KO mouse embryonic fibroblasts and astrocytes display lower cellular (but not plasma [467]) cholesterol levels and impaired endocytosis [468], which can be rescued by increased membrane cholesterol [469]. In contrast, GBA KO and PRKN KO cells have increased cholesterol levels [470,471], and the N370S GBA mutation leads to cholesterol accumulation in lysosomes [472], while LRRK2 KO rats have higher serum cholesterol levels [473]. Thus, cholesterol biosynthesis seems to be impaired in PD, but the direction of the change differs among PD etiologies, which could explain part of the variation observed in different studies with PD patients.
Increased cholesterol reduces cell death [474] and modulates presynaptic DA phenotype by increasing TH and VMAT2 expression in SH-SY5Y cells [475] and enhancing ligand binding of DAT and VMAT2 in the brains from rats and monkeys [476]. However, hypercholesterolemia seems to cause DA neuronal loss and oxidative stress in the SN and the striatum, leading to motor impairment [477,478,479]. Together with the observation that cholesterol treatment of (MPP+-treated) SH-SY5Y cells reduces their viability [480], this finding suggests that the effect of cholesterol levels on PD is dose dependent.
6.1.3. Alpha-Synuclein and Cholesterol
Alpha-synuclein interacts with cholesterol [481] and cholesterol-containing vesicles [482], but it is unclear whether cholesterol facilitates the binding of α-synuclein to charge-neutral membranes [483,484]. Alpha-synuclein-cholesterol interaction seems to be associated with α-synuclein accumulation [474,485] and aggregation [486] and is a determining factor in α-synuclein’s ability to form pores [487,488]. Accordingly, reducing cholesterol levels leads to decreased α-synuclein accumulation and damage in the synapse [489,490,491]. Hence, high levels of cholesterol aggravate α-synuclein-associated pathology. Furthermore, α-synuclein potentiates cholesterol efflux [492], antagonizes cholesterol in lipid rafts [493], and enhances production of oxidative cholesterol metabolites [494]. Finally, A53T-α-synuclein-overexpressing mice have increased levels of serum cholesterol [239], while WT-α-synuclein-overexpressing mice have upregulation of genes involved in cholesterol biosynthesis in DA neurons from the SN [495], indicating a tight reciprocal relationship between α-synuclein and cholesterol metabolism.
6.1.4. Statins
Statins are cholesterol-lowering drugs that have been described to decrease [496,497,498,499,500,501] or not affect [502,503,504] PD risk. Interestingly, lipophilic, but not hydrophilic, statins increase PD risk [505]. In the current discussion on the contradictory findings regarding the effects of statins not enough attention is paid to confounding factors such as statin indication, statin-type effects or immortal time bias (span of cohort follow-up during which the outcome under study cannot occur), and healthy user effects [506]. In animal and cellular models, atorvastatin pretreatment seems to prevent early effects of MPTP administration in rats [507], and lovastatin has neuroprotective effects against MPP+ and 6-OHDA [474,508] and ameliorates α-synuclein accumulation [509,510]. Similarly, simvastatin is neuroprotective against 6-OHDA and MPTP treatments [511,512,513,514] and increases dopamine content in the striatum [515]. However, negative effects of simvastatin and atorvastatin on MPP+-mediated toxicity have also been reported [516], which could be explained by the fact that statin lactones, one of the statin metabolites, are able to inhibit mitochondrial complex III [517], potentiating MPP+ toxicity.
6.2. Cholesterol Precursors
In PD patients, the cholesterol-synthesizing enzymes isopentenyl diphosphate isomerases 1 and 2 have been observed in LB from the SN of PD patients [518]. The natural cholesterol intermediate squalene seems to prevent toxicity in the striatum of 6-OHDA-treated mice [519], whereas α-synuclein accumulation enhances squalene production [242], which could be a cellular response to oxidative damage. A derivative of squalene, squalane, exacerbates 6-OHDA toxicity [519]. The naturally occurring cholesterol precursor lanosterol induces mitochondrial uncoupling and protects DA neurons from cell death in the nigrostriatal region of MPTP-treated mice [520]. Thus, cholesterol precursors seem to have a protective role in PD. Interestingly, inhibitors of both geranylgeranyl transferase (GGTI) and farnesyl transferase (FTI), enzymes that transfer the prenyl group geranylgeranyl or farnesyl to proteins, protect nigrostriatal neurons in MPTP-intoxicated mice [521].
6.3. CEs
The esters between cholesterol and fatty acids, CEs, are synthesized from excess cholesterol in the cytosol by the enzyme acetyl-coA acetyltransferase 1, a process that can be reversed by the enzyme cholesteryl ester hydrolase. In PD patients, reduced cholesterol esterifying activity has been detected in fibroblasts [461] and CE 20:5 is reduced in their visual cortex [222]. Interestingly, in C. elegans the ortholog of neutral cholesteryl ester hydrolase 1 attenuates α-synuclein neurotoxicity when sufficient CE is present, while knockdown leads to neurodegeneration [522]. However, GBA KO cells have increased levels of CE 15:1, 22:6, and 24:1 [470], which could reflect either a protective or a pathological mechanism.
6.4. Oxysterols
The products of cholesterol oxidation, 7beta- and 27-hydroxycholesterol, and 7-ketocholesterol, are elevated in plasma from PD patients [162]. Additionally, 27-hydroxycholesterol CSF levels are increased in a subgroup of PD patients [523] Moreover, increased cholesterol lipid hydroperoxides have been observed in the SN of PD patients [524]. The CSF levels of 24-hydroxycholesterol appear to be correlated with PD duration [523], but higher levels have also been observed in early stage PD [525]. Conversely, 24-hydroxycholesterol esters are reduced in plasma from PD patients [526]. Of note, TH levels are increased by 24-hydroxycholesterol [527], while 27-hydroxycholesterol seems to reduce TH expression and increases α-synuclein levels [527,528,529,530]. An unexpected finding was that both 24- and 27-hydroxycholesterol seem to protect against staurosporine-induced cell death [531]. Interestingly, oxysterols, and more specifically 24(S),25-epoxycholesterol, increase DA neuronal differentiation via liver X receptors in both mouse and human embryonic stem cells [532,533].
7. Lipoproteins
Lipoproteins transport triglycerides and cholesteryl esters. Together, these lipids form the core of the lipoprotein, which is further surrounded by glycerophospholipids and free cholesterol [534]. Lipoproteins are classified according to their density, and thus their composition, as high-density lipoproteins (HDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL) or VLDL. Here, we will specifically review the current findings concerning HDL, LDL and VLDL (Supplementary Table S1).
7.1. HDL
The assembly complex HDL is composed of proteins (around 40%, mainly apolipoprotein A1 (ApoA1), but also apolipoprotein C (ApoC), apolipoprotein E (ApoE), and apolipoprotein J (ApoJ)) and lipids (including around 30% of glycerophospholipids, 25% of cholesterol/CE, and 5% of TAG). The main biological role of HDL is in cargo transport, in particular of lipids and proteins, but it is now also known to bring miRNAs to recipient cells [535]. Lower plasma HDL and ApoA1 levels have been associated with earlier PD onset [536] and higher PD risk [237,537,538,539], and HDL levels are positively correlated with disease duration [540]. Plasma levels of HDL-cholesterol are lower [229,385,541] or not different [230,233,446,447,457] in PD patients compared to controls. This controversial relationship is complex, as both sex [460] and APOE polymorphisms [231] seem to affect HDL-cholesterol levels in PD patients.
7.2. LDL
About 20% of LDL consists of proteins (mainly apolipoprotein B (ApoB)) and the remainder consists of lipids (including about 22% of glycerophospholipids, 50% of cholesterol/CE, and 8% of TAG). High LDL-cholesterol levels in plasma are protective for PD and associated with preserved executive and fine motor functions in PD [452,455,457,542], while lower LDL-cholesterol levels are associated with higher PD risk [229,445,446,447,500,543,544]. One study reported that plasma LDL levels are not different between PD patients and controls [237]. A number of other studies have reported no difference in baseline LDL-cholesterol [230,497], and two meta-analyses have found no association [238,496]. Furthermore, in contrast to HDL, LDL-cholesterol levels do not differ between male and female PD patients [460,540]. However, one study reported increased LDL-cholesterol levels in PD patients compared to controls [231]. Interestingly, compared to controls PD patients seem to have higher levels of oxidized LDL [545], which is able to enter neuronal cells and elicit neurotoxicity [546]. Finally, male DJ-1 KO mice have higher LDL-cholesterol levels in serum, which could be due to the fact that the LDLR is a transcriptional target of DJ-1 [467].
7.3. VLDL
Very low-density lipoproteins are mainly composed of lipids (including around 15% of glycerophospholipids, 20% of cholesterol/CE, and 60% of TAG) and only minor amounts of protein (around 5%, mainly ApoB and ApoC). Parkinson’s disease patients appear to have lower levels of both VLDL [230] and VLDL-cholesterol [231] than controls, but the role of VLDL in PD remains unclear.
8. The Cellular Lipidome
Above we have given an overview of the changes in lipid composition that have been observed in multiple studies involving PD patients, and animal and cellular PD models. The question arises what the significance of such changes is from a biological point of view. In mammalian cells, about 5% of the genes are involved in the generation and transport of an estimate of 10,000 individual lipid species [547,548], which have structural [549], signaling [549,550], and energy storage [17,18] roles. More specifically, above-mentioned molecules such as glycerophospholipids, sphingolipids, and sterols represent the main components of the cell’s plasma and mitochondrial membranes, endoplasmic reticulum, the Golgi complex, and endosomes. In a dynamic manner, lipid composition defines organelle identity [547], controls the recruitment of proteins, and lipid bilayer properties, such as thickness, elastic compression, and intrinsic curvature, can be an allosteric regulator of membrane protein function [551]. Alterations in membranes thus dynamically control important processes such as (synaptic) vesicle trafficking, endocytosis-exocytosis [552] or α-synuclein aggregation [553], processes that have already been associated with PD [554,555,556].
Lipids also play an important role in intracellular and intercellular signaling in the brain by direct interaction with receptors and other signal-transducing proteins [549,550] that regulate integral physiological processes linked to PD. For example, PUFA are involved in inflammation, neurogenesis, and neuroprotection [56]. Endocannabinoids are lipid-based retrograde neurotransmitters that modulate synaptic plasticity [557], and LPA modulates processes like proliferation, survival and migration [249]. Additionally, although most energy consumed by brain cells comes from glucose, lipids have been suggested to provide up to 20% of the total energy consumption of the adult brain [549,558,559]. Therefore, changes in lipid composition or content, such as the ones that have been described here for PD, can have vast consequences for key processes in the maintenance of normal neuronal and brain function. However, unlike what holds for genes and proteins, most lipid species cannot be associated with specific functions: their role is dictated by the concentration and location of individual lipid species, and, most importantly, by their interaction with other lipid species. Since most of the available information is a description of changes in lipid concentration, firm conclusions regarding the effects of these changes are hard to draw.
This lack of precise knowledge regarding the (patho)biological significance of lipidome abnormalities is predominantly caused by the fact that lipids form a vast and enormously complex group of biomolecules. This creates two major challenges. First, it is currently very difficult—if not impossible—to characterize all lipids present in the lipidome of a sample, due to limitations in the separation methods. This precludes simultaneous analysis of all lipid classes, which is especially hindered by the presence of isomeric (i.e., same mass) lipids. Second, no methodology is currently available to accurately determine the concentrations of the various lipid species [560]. This lack of information hampers the interpretation of lipidomic studies and the creation of reliable databases that, on its turn, impedes the identification of pathways in which a combination of lipid species plays a role [561].
As mentioned, not only their composition in the lipidome but also the tissue distribution and intracellular localization of individual lipids are crucial for their function, which makes it of great importance to develop techniques to identify and quantify lipids at the single-cell level and with the spatial organization of the cell still intact. These developments will help to elucidate the interplay of different lipid species in a time- and location-dependent manner both in health and disease. Indeed, it would allow us to obtain more information about (i) the lipidomes of various cell types, which are now identified in growing numbers within different tissues and organs by RNAseq [562], and (ii) the dynamic changes in lipidome composition that are associated with disease progression. Unfortunately, proper sample preparation, even more than the detection limits for lipids in mass-spectrometry, currently forms the biggest barrier to develop effective single-cell lipidomics [563]. Moreover, the interplay between lipids and other biomolecules necessitates the integration of lipidomics with other omics strategies [564].
It is also important to note that the lipidome composition is not only defined by the activity of genes involved in lipid metabolism, but also strongly depends on exogenous factors. These include (1) the direct dietary intake of lipids and lipid precursors from food, (2) life-style factors, i.e., exercise, sleep patterns, and intrinsic and extrinsic motivation factors that determine the choice of food composition, and (3) the effects of drugs that affect metabolism or cell behavior. For example, accumulating evidence suggests that tight bidirectional interactions exist between dietary lipids and composition and structure of the gastrointestinal tract microbiota [565,566,567]. This could be especially relevant for PD, since dysbiosis (i.e., the change in microbiota structure relative to that found in healthy individuals [568]), has been repeatedly observed in PD patients [569,570,571,572,573,574] from early stages of the disease onwards [575].
Filling in the current gaps in lipidomics technology and knowledge is crucial to exploit its potential to help us further understand the molecular mechanisms underlying PD, better define its stages and classification, and identify biomarkers, create dietary interventions, or perform compound screening, preclinical testing and monitoring of drug responses [576,577].
9. Conclusions
From this review, it is clear that a strong correlation exists between PD and abnormalities in lipid metabolism. More specifically, there is an association between PD and the levels of fatty acyls (SFA, MUFA, PUFA, a number of eicosanoids, and acylcarnitine), glycerolipids (MAG, DAG, and TAG), glycerophospholipids (PA, LPA, PE, PS, PC, LPC, PI, PIPx, PG, and CL), sphingolipids (sphingosine(-1P), ceramide, SM, cerebrosides, gangliosides, and sulfatides), sterols (cholesterol precursors, cholesterol, CE, and oxysterols) and lipoproteins (HDL, LDL, and VLDL). Furthermore, there is a conspicuous relationship between the folding, aggregation, and distribution of α-synuclein and the lipids that drive some of the neuropathological features of PD. Yet, it is presently unclear whether links exist between PD and some eicosanoids (eoxins, thromboxanes, oxoeicoanoids, hepoxilins, lipoxins, and epoxyeiconsatetraenoic acid), glycerophospholipids (lysoPE, lysoPS, lysoPI, lysoPG, lysoCL, and Bis(monoacylglycero)phosphate), sphingolipids (globosides), and lipoproteins (IDL).
One of the main concerns regarding the findings summarized in this review is that most lipid classes have not been consistently found to be associated with PD. Variables such as sex, age, PD etiology, specific DNA polymorphisms or the microbiome may have influenced the findings. Thus, proper stratification of PD patients is necessary to understand the biological implications of the lipid changes observed. Additionally, more accurate description of the lipid profiles of plasma, CSF and/or fibroblasts from PD patients will help to classify the patients more accurately.
A further concern is that most studies have focused on plasma levels of lipids, but these may not correlate with their brain levels, e.g., levels of ganglioside species are increased in plasma but not in brains of PD patients [270,385]. Thus, CSF (and brain) lipidomes of PD patients have to be determined to get insight into the actual pathological lipid composition and processes. Moreover, it is often unclear whether the changes in the levels of lipid species reflect a pathological or rather a compensatory mechanism. Finally, studies on cellular and animal PD models do not always show the same directionality of lipid level changes as found in studies on PD patients.
In conclusion, ample evidence for a central role of lipids in PD is available, but current data yield a picture that is still too fragmented. This hinders the unraveling of the specific pathological mechanisms in which lipids are involved. Technological advances to better characterize the lipidome and explore the functions of specific lipid species, together with additional studies on CSF and/or brain tissue from PD patients are now urgently needed to further our understanding of the pathobiology of the relationship between PD and lipids and will help us to identify biomarkers and druggable targets for the development of disease-modifying therapies for this devastating neurodegenerative disease.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4409/8/1/27/s1, Table S1: Lipid and lipoprotein levels in human PD body fluids and tissues, and their effects in animal/cellular models.
Funding
H.X. was funded by a junior-investigator grant from Radboudumc.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Mao, Z.H. Progression of motor symptoms in Parkinson’s disease. Neurosci. Bull. 2012, 28, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of Parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef]
- Cicchetti, F.; Drouin-Ouellet, J.; Gross, R.E. Environmental toxins and Parkinson’s disease: What have we learned from pesticide-induced animal models? Trends Pharmacol. Sci. 2009, 30, 475–483. [Google Scholar] [CrossRef]
- Klemann, C.J.H.M.; Martens, G.J.M.; Sharma, M.; Martens, M.B.; Isacson, O.; Gasser, T.; Visser, J.E.; Poelmans, G. Integrated molecular landscape of Parkinson’s disease. NPJ Park. Dis. 2017, 3, 14. [Google Scholar] [CrossRef]
- Houlden, H.; Singleton, A.B. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012, 124, 325–338. [Google Scholar] [CrossRef]
- Nichols, W.C.; Pankratz, N.; Marek, D.K.; Pauciulo, M.W.; Elsaesser, V.E.; Halter, C.A.; Rudolph, A.; Wojcieszek, J.; Pfeiffer, R.F.; Foround, T.; et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology 2009, 72, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sidransky, E.; Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012, 11, 986–998. [Google Scholar] [CrossRef]
- Do, C.B.; Tung, J.Y.; Dorfman, E.; Kiefer, A.K.; Drabant, E.M.; Francke, U.; Mountain, J.L.; Goldman, S.M.; Tanner, C.M.; Landston, J.W.; et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011, 7, e1002141. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, N.; Wilk, J.B.; Latourelle, J.C.; DeStefano, A.L.; Halter, C.; Pugh, E.W.; Doheny, K.F.; Gausella, J.F.; Nichols, W.C.; Foroud, T.; et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum. Genet. 2009, 124, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Robak, L.A.; Jansen, I.E.; van Rooij, J.; Uitterlinden, A.G.; Kraaij, R.; Jankovic, J.; Heutink, P.; Shulman, J.M.; Nalls, M.A.; Plagnol, V.; et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 2017, 140, 3191–3203. [Google Scholar] [CrossRef] [PubMed]
- Gan-Or, Z.; Ozelius, L.J.; Bar-Shira, A.; Saunders-Pullman, R.; Mirelman, A.; Kornreich, R.; Gana-Weisz, M.; Raymond, D.; Rozenkrantz, L.; Deik, A.; et al. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology 2013, 80, 1606–1610. [Google Scholar] [CrossRef]
- Horton, T.J.; Drougas, H.; Brachey, A.; Reed, G.W.; Peters, J.C.; Hill, J.O. Fat and carbohydrate overfeeding in humans: Different effects on energy storage. Am. J. Clin. Nutr. 1995, 62, 19–29. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- Fernandis, A.Z.; Wenk, M.R. Membrane lipids as signaling molecules. Curr. Opin. Lipidol. 2007, 18, 121–128. [Google Scholar] [CrossRef]
- Bieberich, E. It’s a lipid’s world: Bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochem. Res. 2012, 37, 1208–1229. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta 2017, 1862, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A. Expanding roles for lipid droplets. Curr. Biol. 2015, 25, R470–R481. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, D.; Wakelam, M.J.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential Fatty acids—A review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter Enteral Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Tan, L.C.; Methawasin, K.; Tan, E.K.; Tan, J.H.; Au, W.L.; Yuan, J.M.; Koh, W.P. Dietary cholesterol, fats and risk of Parkinson’s disease in the Singapore Chinese Health Study. J. Neurol. Neurosurg. Psychiatry 2016, 87, 86–92. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary fat intake and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2010, 288, 117–122. [Google Scholar] [CrossRef]
- De Lau, L.M.L.; Bornebroek, M.; Witteman, J.C.M.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Dietary fatty acids and the risk of Parkinson disease: The Rotterdam study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- Kamel, F.; Goldman, S.M.; Umbach, D.M.; Chen, H.; Richardson, G.; Barber, M.R.; Meng, C.; Marras, C.; Koerll, M.; Kasten, M.; et al. Dietary fat intake, pesticide use, and Parkinson’s disease. Park. Relat. Disord. 2014, 20, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Berthiaume, L.; Hadj-Tahar, A.; Rajput, A.H.; Bédard, P.J.; Di Paolo, T.; Julien, P.; Calon, F. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem. Int. 2006, 48, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [PubMed]
- Senyilmaz, D.; Virtue, S.; Xu, X.; Tan, C.Y.; Griffin, J.L.; Miller, A.K.; Vidal-Puig, A.; Teleman, A.A. Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature 2015, 525, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Bustamante, S.; Ballard, J.W.O. Stearic acid supplementation in high protein to carbohydrate (P:C) ratio diet improves physiological and mitochondrial functions of Drosophila melanogaster parkin null mutants. J. Gerontol. A Biol. Sci. Med. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Ballard, J.W.O. Dietary management and physical exercise can improve climbing defects and mitochondrial activity in Drosophila melanogaster parkin null mutants. Fly 2018, 12, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Joniec-Maciejak, I.; Wawer, A.; Turzyńska, D.; Sobolewska, A.; Maciejak, P.; Szyndler, J.; Mirowska-Guzel, D.; Płaźnik, A. Octanoic acid prevents reduction of striatal dopamine in the MPTP mouse model of Parkinson’s disease. Pharmacol. Rep. 2018, 70, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.W.; Say, Y.H. Palmitic acid induces neurotoxicity and gliatoxicity in SH-SY5Y human neuroblastoma and T98G human glioblastoma cells. PeerJ 2018, 6, e4696. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Fuente-Martin, E.; Finan, B.; Kim, M.; Frank, A.; Garcia-Caceres, C.; Navas, C.; Gordillo, R.; Neinast, M.; Kalainayakan, S.P.; et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014, 9, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chu, Y.; Kordower, J.H.; Li, B.; Cao, H.; Huang, L.; Nishida, M.; Song, L.; Wang, D.; Federoff, H.J. PGC-1α Promoter Methylation in Parkinson’s Disease. PLoS ONE 2015, 10, e0134087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L.; Leenders, K.L. Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr. Neuropharmacol. 2010, 8, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Golovko, M.Y.; Faergeman, N.J.; Cole, N.B.; Castagnet, P.I.; Nussbaum, R.L.; Murphy, E.J. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry 2005, 44, 8251–8259. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.P.; Schleicher, E.D.; Cegan, A.; Deuschle, C.; Baur, S.; Hauser, A.K.; Synofzik, M.; Srulijes, K.; Brockmann, K.; Berg, D.; et al. Cerebrospinal fluid fatty acids in glucocerebrosidase-associated Parkinson’s disease. Mov. Disord. 2012, 27, 288–292. [Google Scholar] [CrossRef]
- Heller, A.; Won, L.; Bubula, N.; Hessefort, S.; Kurutz, J.W.; Reddy, G.A.; Gross, M. Long-chain fatty acids increase cellular dopamine in an immortalized cell line (MN9D) derived from mouse mesencephalon. Neurosci. Lett. 2005, 376, 35–39. [Google Scholar] [CrossRef]
- Sergeeva, O.A.; De Luca, R.; Mazur, K.; Chepkova, A.N.; Haas, H.L.; Bauer, A. N-oleoyldopamine modulates activity of midbrain dopaminergic neurons through multiple mechanisms. Neuropharmacology 2017, 119, 111–122. [Google Scholar] [CrossRef]
- Sharon, R.; Goldberg, M.S.; Bar-Josef, I.; Betensky, R.A.; Shen, J.; Selkoe, D.J. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 9110–9115. [Google Scholar] [CrossRef]
- Kubo, S.; Nemani, V.M.; Chalkley, R.J.; Anthony, M.D.; Hattori, N.; Mizuno, Y.; Edwards, R.H.; Fortin, D.L. A combinatorial code for the interaction of alpha-synuclein with membranes. J. Biol. Chem. 2005, 280, 31664–31672. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Lillycrop, K.A. Fatty acids and epigenetics. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.F.F.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Beard, J.D.; Umbach, D.M.; Park, Y.; Huang, X.; Blair, A.; Kamel, F.; Chen, H. Dietary fat intake and risk for Parkinson’s disease. Mov. Disord. 2014, 29, 1623–1630. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.M.; Hernán, M.A.; Willett, W.C.; Ascherio, A. Dietary intakes of fat and risk of Parkinson’s disease. Am. J. Epidemiol. 2003, 157, 1007–1014. [Google Scholar] [CrossRef]
- Schulte, E.C.; Altmaier, E.; Berger, H.S.; Do, K.T.; Kastenmüller, G.; Wahl, S.; Adamski, J.; Peters, A.; Krumsiek, J.; Suhre, K.; et al. Alterations in Lipid and Inositol Metabolisms in Two Dopaminergic Disorders. PLoS ONE 2016, 11, e0147129. [Google Scholar] [CrossRef]
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174. [Google Scholar] [CrossRef]
- Abbott, S.K.; Jenner, A.M.; Spiro, A.S.; Batterham, M.; Halliday, G.M.; Garner, B. Fatty acid composition of the anterior cingulate cortex indicates a high susceptibility to lipid peroxidation in Parkinson’s disease. J. Park. Dis. 2015, 5, 175–185. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Mirick, G.E.; Serhan, C.N.; Selkoe, D.J. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J. Biol. Chem. 2003, 278, 49874–49881. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N.; Cicchetti, F.; Calon, F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008, 22, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Gibrat, C.; Saint-Pierre, M.; Julien, C.; Calon, F.; Cicchetti, F. Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkinsonian animal model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.S.; Crispim, R.Y.G.; Cavalcanti, J.U.; Souza, R.B.; Lemos, J.C.; Cristino Filho, G.; Bezerra, M.M.; Pinheiro, T.F.M.; de Vasconcelos, S.M.M.; Macêdo, D.S.; et al. Impact of the Chronic Omega-3 Fatty Acids Supplementation in Hemiparkinsonism Model Induced by 6-Hydroxydopamine in Rats. Basic Clin. Pharmacol. Toxicol. 2017, 120, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Li, M.; Liu, Q.; Wei, D.; Yang, M.; Kong, L. (1)H NMR-based metabolomics study on a goldfish model of Parkinson’s disease induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP). Chem. Biol. Interact. 2014, 223, 18–26. [Google Scholar] [CrossRef]
- Cardoso, H.D.; dos Santos Junior, E.F.; de Santana, D.F.; Gonçalves-Pimentel, C.; Angelim, M.K.; Isaac, A.R.; Lagranha, C.J.; Guedes, R.C.; Beltrão, E.I.; Morya, E.; et al. Omega-3 deficiency and neurodegeneration in the substantia nigra: Involvement of increased nitric oxide production and reduced BDNF expression. Biochim. Biophys. Acta 2014, 1840, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Delattre, A.M.; Carabelli, B.; Mori, M.A.; Kempe, P.G.; Rizzo de Souza, L.E.; Zanata, S.M.; Machado, R.B.; Suchecki, D.; Andrade da Costa, B.L.S.; Lima, M.M.S.; et al. Maternal Omega-3 Supplement Improves Dopaminergic System in Pre- and Postnatal Inflammation-Induced Neurotoxicity in Parkinson’s Disease Model. Mol. Neurobiol. 2017, 54, 2090–2106. [Google Scholar] [CrossRef]
- Tanriover, G.; Seval-Celik, Y.; Ozsoy, O.; Akkoyunlu, G.; Savcioglu, F.; Hacioglu, G.; Demir, N.; Agar, A. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochem. Cytobiol. 2010, 48, 434–441. [Google Scholar] [CrossRef]
- Hacioglu, G.; Seval-Celik, Y.; Tanriover, G.; Ozsoy, O.; Saka-Topcuoglu, E.; Balkan, S.; Agar, A. Docosahexaenoic acid provides protective mechanism in bilaterally MPTP-lesioned rat model of Parkinson’s disease. Folia Histochem. Cytobiol. 2012, 50, 228–238. [Google Scholar] [CrossRef]
- Ozkan, A.; Parlak, H.; Tanriover, G.; Dilmac, S.; Ulker, S.N.; Birsen, I.; Agar, A. The protective mechanism of docosahexaenoic acid in mouse model of Parkinson: The role of hemeoxygenase. Neurochem. Int. 2016, 101, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Han, J.; Jang, Y.; Kim, S.J.; Park, J.H.; Seo, K.S.; Jeong, S.; Shin, S.; Lim, K.; Heo, J.Y.; et al. Docosahexaenoic acid prevents paraquat-induced reactive oxygen species production in dopaminergic neurons via enhancement of glutathione homeostasis. Biochem. Biophys. Res. Commun. 2015, 457, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Serrano-García, N.; Fernández-Valverde, F.; Luis-Garcia, E.R.; Granados-Rojas, L.; Juárez-Zepeda, T.E.; Orozco-Suárez, S.A.; Pedraza-Chaverri, J.; Orozco-Ibarra, M.; Jiménez-Anguiano, A. Docosahexaenoic acid protection in a rotenone induced Parkinson’s model: Prevention of tubulin and synaptophysin loss, but no association with mitochondrial function. Neurochem. Int. 2018, 121, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Shashikumar, S.; Pradeep, H.; Chinnu, S.; Rajini, P.S.; Rajanikant, G.K. Alpha-linolenic acid suppresses dopaminergic neurodegeneration induced by 6-OHDA in C. elegans. Physiol. Behav. 2015, 151, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, K.; Saint-Pierre, M.; Cisbani, G.; St-Amour, I.; Gibrat, C.; Giguère-Rancourt, A.; Calon, F.; Cicchetti, F. Partial neurorescue effects of DHA following a 6-OHDA lesion of the mouse dopaminergic system. J. Nutr. Biochem. 2016, 30, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hernando, S.; Requejo, C.; Herran, E.; Ruiz-Ortega, J.A.; Morera-Herreras, T.; Lafuente, J.V.; Ugedo, L.; Gainza, E.; Pedraz, J.L.; Igartua, M.; et al. Beneficial effects of n-3 polyunsaturated fatty acids administration in a partial lesion model of Parkinson’s disease: The role of glia and NRf2 regulation. Neurobiol. Dis. 2019, 121, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Soler, M.; Cordobilla, B.; Morató, X.; Fernández-Dueñas, V.; Domingo, J.C.; Ciruela, F. Triglyceride Form of Docosahexaenoic Acid Mediates Neuroprotection in Experimental Parkinsonism. Front. Neurosci. 2018, 12, 604. [Google Scholar] [CrossRef]
- Chang, Y.L.; Chen, S.J.; Kao, C.L.; Hung, S.C.; Ding, D.C.; Yu, C.C.; Chen, Y.J.; Ku, H.H.; Lin, C.P.; Lee, K.H.; et al. Docosahexaenoic acid promotes dopaminergic differentiation in induced pluripotent stem cells and inhibits teratoma formation in rats with Parkinson-like pathology. Cell Transplant. 2012, 21, 313–332. [Google Scholar] [CrossRef]
- Parlak, H.; Ozkan, A.; Dilmac, S.; Tanriover, G.; Ozsoy, O.; Agar, A. Neuronal nitric oxide synthase phosphorylation induced by docosahexaenoic acid protects dopaminergic neurons in an experimental model of Parkinson’s disease. Folia Histochem. Cytobiol. 2015, 56, 27–37. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Meng, Q.; Wang, X.; Shao, D.; Song, C. ω-3 fatty acid eicosapentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J. Neurochem. 2013, 124, 855–868. [Google Scholar] [CrossRef]
- Meng, Q.; Luchtman, D.W.; El Bahh, B.; Zidichouski, J.A.; Yang, J.; Song, C. Ethyl-eicosapentaenoate modulates changes in neurochemistry and brain lipids induced by parkinsonian neurotoxin 1-methyl-4-phenylpyridinium in mouse brain slices. Eur. J. Pharmacol. 2010, 649, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Meng, Q.; Song, C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 226, 386–396. [Google Scholar] [CrossRef]
- Mori, M.A.; Delattre, A.M.; Carabelli, B.; Pudell, C.; Bortolanza, M.; Staziaki, P.V.; Visentainer, J.V.; Montanher, P.F.; Del Bel, E.A.; Ferraz, A.C. Neuroprotective effect of omega-3 polyunsaturated fatty acids in the 6-OHDA model of Parkinson’s disease is mediated by a reduction of inducible nitric oxide synthase. Nutr. Neurosci. 2018, 21, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Delattre, A.M.; Kiss, A.; Szawka, R.E.; Anselmo-Franci, J.A.; Bagatini, P.B.; Xavier, L.L.; Rigon, P.; Achaval, M.; Iagher, F.; de David, C.; et al. Evaluation of chronic omega-3 fatty acids supplementation on behavioral and neurochemical alterations in 6-hydroxydopamine-lesion model of Parkinson’s disease. Neurosci. Res. 2010, 66, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kabuto, H.; Amakawa, M.; Mankura, M.; Yamanushi, T.T.; Mori, A. Docosahexaenoic acid ethyl ester enhances 6-hydroxydopamine-induced neuronal damage by induction of lipid peroxidation in mouse striatum. Neurochem. Res. 2009, 34, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Katunga, L.A.; Willis, M.S. Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin. Exp. Pharmacol. Physiol. 2012, 39, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Shamoto-Nagai, M.; Hisaka, S.; Naoi, M.; Maruyama, W. Modification of α-synuclein by lipid peroxidation products derived from polyunsaturated fatty acids promotes toxic oligomerization: Its relevance to Parkinson disease. J. Clin. Biochem. Nutr. 2018, 62, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Horrocks, M.H.; Klenerman, D.; Gandhi, S.; Abramov, A.Y.; Shchepinov, M.S. Lipid peroxidation is essential for α-synuclein-induced cell death. J. Neurochem. 2015, 133, 582–589. [Google Scholar] [CrossRef]
- Shchepinov, M.S.; Chou, V.P.; Pollock, E.; Langston, J.W.; Cantor, C.R.; Molinari, R.J.; Manning-Boğ, A.B. Isotopic reinforcement of essential polyunsaturated fatty acids diminishes nigrostriatal degeneration in a mouse model of Parkinson’s disease. Toxicol. Lett. 2011, 207, 97–103. [Google Scholar] [CrossRef]
- Kinghorn, K.J.; Castillo-Quan, J.I.; Bartolome, F.; Angelova, P.R.; Li, L.; Pope, S.; Cochemé, H.M.; Khan, S.; Asghari, S.; Bhatia, K.P.; et al. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain 2015, 138 Pt 7, 1801–1816. [Google Scholar] [CrossRef]
- Denny Joseph, K.M.; Muralidhara. Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: Relevance to Parkinson’s disease. Neurochem. Res. 2015, 40, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Bazinet, R.P.; Rapoport, S.I.; Bhattacharjee, A.K. Brain arachidonic acid cascade enzymes are upregulated in a rat model of unilateral Parkinson disease. Neurochem. Res. 2010, 35, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Chalimoniuk, M.; Stolecka, A.; Ziemińska, E.; Stepień, A.; Langfort, J.; Strosznajder, J.B. Involvement of multiple protein kinases in cPLA2 phosphorylation, arachidonic acid release, and cell death in in vivo and in vitro models of 1-methyl-4-phenylpyridinium-induced parkinsonism—The possible key role of PKG. J. Neurochem. 2009, 110, 307–317. [Google Scholar] [CrossRef]
- Tang, K.S. Protective effect of arachidonic acid and linoleic acid on 1-methyl-4-phenylpyridinium-induced toxicity in PC12 cells. Lipids Health Dis. 2014, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Yabuki, Y.; Kobayashi, Y.; Onozato, M.; Owada, Y.; Fukunaga, K. FABP3 protein promotes α-synuclein oligomerization associated with 1-methyl-1, 2, 3, 6-tetrahydropiridine-induced neurotoxicity. J. Biol. Chem. 2014, 289, 18957–18965. [Google Scholar] [CrossRef]
- Wang, Y.; Plastina, P.; Vincken, J.P.; Jansen, R.; Balvers, M.; Ten Klooster, J.P.; Gruppen, H.; Witkamp, R.; Meijerink, J. N-Docosahexaenoyl Dopamine, an Endocannabinoid-like Conjugate of Dopamine and the n-3 Fatty Acid Docosahexaenoic Acid, Attenuates Lipopolysaccharide-Induced Activation of Microglia and Macrophages via COX-2. ACS Chem. Neurosci. 2017, 8, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Ben Gedalya, T.; Loeb, V.; Israeli, E.; Altschuler, Y.; Selkoe, D.J.; Sharon, R. Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic 2009, 10, 218–234. [Google Scholar] [CrossRef]
- Darios, F.; Ruipérez, V.; López, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Karube, H.; Sakamoto, M.; Arawaka, S.; Hara, S.; Sato, H.; Ren, C.H.; Goto, S.; Koyama, S.; Wada, M.; Kawanami, T.; et al. N-terminal region of alpha-synuclein is essential for the fatty acid-induced oligomerization of the molecules. FEBS Lett. 2008, 582, 3693–3700. [Google Scholar] [CrossRef]
- Israeli, E.; Sharon, R. Beta-synuclein occurs in vivo in lipid-associated oligomers and forms hetero-oligomers with alpha-synuclein. J. Neurochem. 2009, 108, 465–474. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Frosch, M.P.; Walsh, D.M.; Hamilton, J.A.; Selkoe, D.J. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 2003, 37, 583–595. [Google Scholar] [CrossRef]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 2001, 276, 41958–41962. [Google Scholar] [CrossRef] [PubMed]
- Assayag, K.; Yakunin, E.; Loeb, V.; Selkoe, D.J.; Sharon, R. Polyunsaturated fatty acids induce alpha-synuclein-related pathogenic changes in neuronal cells. Am. J. Pathol. 2007, 171, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Broersen, K.; van den Brink, D.; Fraser, G.; Goedert, M.; Davletov, B. Alpha-synuclein adopts an alpha-helical conformation in the presence of polyunsaturated fatty acids to hinder micelle formation. Biochemistry 2006, 45, 15610–15616. [Google Scholar] [CrossRef] [PubMed]
- Yakunin, E.; Loeb, V.; Kisos, H.; Biala, Y.; Yehuda, S.; Yaari, Y.; Selkoe, D.J.; Sharon, R. A-synuclein neuropathology is controlled by nuclear hormone receptors and enhanced by docosahexaenoic acid in a mouse model for Parkinson’s disease. Brain Pathol. 2012, 22, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhen, J.; Lu, Z. Synergetic Neuroprotective Effect of Docosahexaenoic Acid and Aspirin in SH-Y5Y by Inhibiting miR-21 and Activating RXRα and PPARα. DNA Cell Biol. 2017, 36, 482–489. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, G.; Frare, E.; Pivato, M.; Relini, A.; Penco, A.; Greggio, E.; Bubacco, L.; Fontana, A.; de Laureto, P.P. Structural and morphological characterization of aggregated species of α-synuclein induced by docosahexaenoic acid. J. Biol. Chem. 2011, 286, 22262–22274. [Google Scholar] [CrossRef]
- Fecchio, C.; De Franceschi, G.; Relini, A.; Greggio, E.; Dalla Serra, M.; Bubacco, L.; de Laureto, P.P. α-Synuclein oligomers induced by docosahexaenoic acid affect membrane integrity. PLoS ONE 2013, 8, e82732. [Google Scholar] [CrossRef]
- De Franceschi, G.; Frare, E.; Bubacco, L.; Mammi, S.; Fontana, A.; de Laureto, P.P. Molecular insights into the interaction between alpha-synuclein and docosahexaenoic acid. J. Mol. Biol. 2009, 394, 94–107. [Google Scholar] [CrossRef]
- Broersen, K.; Ruiperez, V.; Davletov, B. Structural and Aggregation Properties of Alpha-Synuclein Linked to Phospholipase A2 Action. Protein Pept. Lett. 2018, 25, 368–378. [Google Scholar] [CrossRef]
- Iljina, M.; Tosatto, L.; Choi, M.L.; Sang, J.C.; Ye, Y.; Hughes, C.D.; Bryant, C.E.; Gandhi, S.; Klenerman, D. Arachidonic acid mediates the formation of abundant alpha-helical multimers of alpha-synuclein. Sci. Rep. 2016, 6, 33928. [Google Scholar] [CrossRef]
- Jiang, P.; Gan, M.; Yen, S.H.C. Dopamine prevents lipid peroxidation-induced accumulation of toxic α-synuclein oligomers by preserving autophagy-lysosomal function. Front. Cell. Neurosci. 2013, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yamada, N.; Maruyama, W.; Osawa, T. Formation of dopamine adducts derived from brain polyunsaturated fatty acids: Mechanism for Parkinson disease. J. Biol. Chem. 2008, 283, 34887–34895. [Google Scholar] [CrossRef] [PubMed]
- Muntané, G.; Janué, A.; Fernandez, N.; Odena, M.A.; Oliveira, E.; Boluda, S.; Porero-Otin, M.; Naudí, A.; Boada, J.; Pamplona, R.; et al. Modification of brain lipids but not phenotype in alpha-synucleinopathy transgenic mice by long-term dietary n-3 fatty acids. Neurochem. Int. 2010, 56, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, K.; Kerdiles, O.; Tremblay, C.; Emond, V.; Lebel, M.; Boulianne, A.S.; Plourde, M.; Cicchetti, F.; Calon, F. Impact of DHA intake in a mouse model of synucleinopathy. Exp. Neurol. 2018, 301, 39–49. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, G.; Fecchio, C.; Sharon, R.; Schapira, A.H.V.; Proukakis, C.; Bellotti, V.; de Laureto, P.P. α-Synuclein structural features inhibit harmful polyunsaturated fatty acid oxidation, suggesting roles in neuroprotection. J. Biol. Chem. 2017, 292, 6927–6937. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, C.; Palazzi, L.; de Laureto, P.P. α-Synuclein and Polyunsaturated Fatty Acids: Molecular Basis of the Interaction and Implication in Neurodegeneration. Molecules 2018, 23, 1531. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Swanepoel, A.C.; Buys, A.V.; Vermeulen, N.; Duim, W.; Kell, D.B. Eryptosis as a marker of Parkinson’s disease. Aging 2014, 6, 788–819. [Google Scholar] [CrossRef]
- Teismann, P.; Tieu, K.; Choi, D.K.; Wu, D.C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zuo, L.J.; Wang, F.; Chen, Z.J.; Hu, Y.; Wang, Y.J.; Wang, X.M.; Zhang, W. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: A cross-sectional study. BMC Neurol. 2014, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Mattammal, M.B.; Strong, R.; Lakshmi, V.M.; Chung, H.D.; Stephenson, A.H. Prostaglandin H synthetase-mediated metabolism of dopamine: Implication for Parkinson’s disease. J. Neurochem. 1995, 64, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Fang, M.; Wang, J.; Yu, H.; Hu, Z.; Yew, D.T.; Chen, W. Triptolide down-regulates COX-2 expression and PGE2 release by suppressing the activity of NF-κB and MAP kinases in lipopolysaccharide-treated PC12 cells. Phytother. Res. 2012, 26, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Zhang, T.; Fu, H.; Liu, G.X.; Wang, X.M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur. J. Pharmacol. 2012, 692, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, X.; Li, X.; Liu, N.; Lou, F.; Ma, H.; Luo, X.; Ren, Y. Somatostatin prevents lipopolysaccharide-induced neurodegeneration in the rat substantia nigra by inhibiting the activation of microglia. Mol. Med. Rep. 2015, 12, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Song, R.; Yang, Z.; Shan, Q.; Chen, W. 6-Hydroxydopamine induces brain vascular endothelial inflammation. IUBMB Life 2017, 69, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Zhang, T.; Li, Q.; Huang, J.K.; Chen, R.F.; Sun, X.J. Inhibition of glycogen synthase kinase-3β by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem. Int. 2013, 63, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, F.B.; Ozsoy, O.; Tanriover, G.; Kaya, Y.; Ogut, E.; Gemici, B.; Dilmac, S.; Ozkan, A.; Agar, A.; Aslan, M. Mechanism of the beneficial effect of melatonin in experimental Parkinson’s disease. Neurochem. Int. 2014, 79, 1–11. [Google Scholar] [CrossRef]
- Zhou, F.; Yao, H.H.; Wu, J.Y.; Ding, J.H.; Sun, T.; Hu, G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J. Cell. Mol. Med. 2008, 12, 1559–1570. [Google Scholar] [CrossRef]
- Hu, J.H.; Zhu, X.Z. Rotenone-induced neurotoxicity of THP-1 cells requires production of reactive oxygen species and activation of phosphatidylinositol 3-kinase. Brain Res. 2007, 1153, 12–19. [Google Scholar] [CrossRef]
- Wang, T.; Pei, Z.; Zhang, W.; Liu, B.; Langenbach, R.; Lee, C.; Wilson, B.; Reece, J.M.; Miller, D.S.; Hong, J.S. MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005, 19, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, O.; Tanriover, G.; Derin, N.; Uysal, N.; Demir, N.; Gemici, B.; Kencebay, C.; Yargicoglu, P.; Agar, A.; Aslan, M. The effect of docosahexaenoic Acid on visual evoked potentials in a mouse model of Parkinson’s disease: The role of cyclooxygenase-2 and nuclear factor kappa-B. Neurotox. Res. 2011, 20, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Wang, Y.; Fong, H.; Murray, T.M.; Zhang, J. Identification of proteins involved in microglial endocytosis of alpha-synuclein. J. Proteome Res. 2007, 6, 3614–3627. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Armida, M.; Carnevale, D.; Ajmone-Cat, M.A.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Cassano, T.; Minghetti, L.; et al. Striatal 6-OHDA lesion in mice: Investigating early neurochemical changes underlying Parkinson’s disease. Behav. Brain Res. 2010, 208, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dey, I.; Lejeune, M.; Chadee, K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 2006, 149, 611–623. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Maruyama, T.; Narumiya, S.; Doré, S. PGE2 EP1 receptor deletion attenuates 6-OHDA-induced Parkinsonism in mice: Old switch, new target. Neurotox. Res. 2013, 23, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Casper, D.; Werner, P. PGE(2) receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE(2) neurotoxicity. J. Neurosci. Res. 2007, 85, 3109–3117. [Google Scholar] [CrossRef]
- Carrasco, E.; Werner, P.; Casper, D. Prostaglandin receptor EP2 protects dopaminergic neurons against 6-OHDA-mediated low oxidative stress. Neurosci. Lett. 2008, 441, 44–49. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Salinas, K.; Garduno, A.C.; Johansson, J.U.; Wang, Q.; Manning-Bog, A.; Andreasson, K.I. Anti-Inflammatory and Neuroprotective Effects of PGE2 EP4 Signaling in Models of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2017, 12, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.K.; Hinds, A.I.; Hanneman, W.H.; Tjalkens, R.B.; Legare, M.E. DJ-1 mutation decreases astroglial release of inflammatory mediators. Neurotoxicology 2016, 52, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Parga, J.A.; García-Garrote, M.; Martínez, S.; Raya, Á.; Labandeira-García, J.L.; Rodríguez-Pallares, J. Prostaglandin EP2 Receptors Mediate Mesenchymal Stromal Cell-Neuroprotective Effects on Dopaminergic Neurons. Mol. Neurobiol. 2018, 55, 4763–4776. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Z.H.; Leng, Y.; Wang, Y.; Jin, X.; Chase, T.N.; Bennett, M.C. Prostaglandin A1 inhibits rotenone-induced apoptosis in SH-SY5Y cells. J. Neurochem. 2002, 83, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Fukuhara, A.; Inui, T.; Allhorn, M. Prevention of paraquat-induced apoptosis in human neuronal SH-SY5Y cells by lipocalin-type prostaglandin D synthase. J. Neurochem. 2012, 120, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Weng, C.F.; Yu, N.C.; Liou, D.Y.; Kuo, F.S.; Huang, M.C.; Tam, K.; Shyue, S.K.; Cheng, H. Enhanced prostacyclin synthesis by adenoviral gene transfer reduced glial activation and ameliorated dopaminergic dysfunction in hemiparkinsonian rats. Oxid. Med. Cell. Longev. 2013, 2013, 649809. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, K.D.; Figueiredo-Pereira, M.E. Cytoskeleton/endoplasmic reticulum collapse induced by prostaglandin J2 parallels centrosomal deposition of ubiquitinated protein aggregates. J. Biol. Chem. 2006, 281, 23274–23284. [Google Scholar] [CrossRef] [PubMed]
- Shivers, K.Y.; Nikolopoulou, A.; Machlovi, S.I.; Vallabhajosula, S.; Figueiredo-Pereira, M.E. PACAP27 prevents Parkinson-like neuronal loss and motor deficits but not microglia activation induced by prostaglandin J2. Biochim. Biophys. Acta 2014, 1842, 1707–1719. [Google Scholar] [CrossRef]
- Pierre, S.R.; Lemmens, M.A.M.; Figueiredo-Pereira, M.E. Subchronic infusion of the product of inflammation prostaglandin J2 models sporadic Parkinson’s disease in mice. J. Neuroinflamm. 2009, 6, 18. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Zhao, N.; Li, W.; Yang, Z.; Liu, X.; Le, W.; Zhang, X. Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 101–108. [Google Scholar] [CrossRef]
- Kang, K.H.; Liou, H.H.; Hour, M.J.; Liou, H.C.; Fu, W.M. Protection of dopaminergic neurons by 5-lipoxygenase inhibitor. Neuropharmacology 2013, 73, 380–387. [Google Scholar] [CrossRef]
- Nagarajan, V.B.; Marathe, P.A. Effect of montelukast in experimental model of Parkinson’s disease. Neurosci. Lett. 2018, 682, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.M.; Ahmed, M.A.E.; El-Sahar, A.E.; El Sayed, N.S. Montelukast attenuates rotenone-induced microglial activation/p38 MAPK expression in rats: Possible role of its antioxidant, anti-inflammatory and antiapoptotic effects. Toxicol. Appl. Pharmacol. 2018, 358, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chou, V.P.; Ko, N.; Holman, T.R.; Manning-Boğ, A.B. Gene-environment interaction models to unmask susceptibility mechanisms in Parkinson’s disease. J. Vis. Exp. 2014, e50960. [Google Scholar] [CrossRef] [PubMed]
- Searles Nielsen, S.; Bammler, T.K.; Gallagher, L.G.; Farin, F.M.; Longstreth, W.; Franklin, G.M.; Swanson, P.D.; Checkoway, H. Genotype and age at Parkinson disease diagnosis. Int. J. Mol. Epidemiol. Genet. 2013, 4, 61–69. [Google Scholar] [PubMed]
- Lakkappa, N.; Krishnamurthy, P.T.; Hammock, B.D.; Velmurugan, D.; Bharath, M.M.S. Possible role of Epoxyeicosatrienoic acid in prevention of oxidative stress mediated neuroinflammation in Parkinson disorders. Med. Hypotheses 2016, 93, 161–165. [Google Scholar] [CrossRef]
- Terashvili, M.; Sarkar, P.; Nostrand, M.V.; Falck, J.R.; Harder, D.R. The protective effect of astrocyte-derived 14, 15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-dopaminergic neuronal cell line co-culture. Neuroscience 2012, 223, 68–76. [Google Scholar] [CrossRef]
- Qin, X.; Wu, Q.; Lin, L.; Sun, A.; Liu, S.; Li, X.; Cao, X.; Gao, T.; Luo, P.; et al. Soluble Epoxide Hydrolase Deficiency or Inhibition Attenuates MPTP-Induced Parkinsonism. Mol. Neurobiol. 2015, 52, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ma, M.; Yang, J.; Nonaka, R.; Yamaguchi, A.; Ishikawa, K.I.; Kobayashi, K.; Murayama, S.; Hwang, S.H.; Saiki, S.; et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E5815–E5823. [Google Scholar] [CrossRef] [PubMed]
- Lakkappa, N.; Krishnamurthy, P.T.; Yamjala, K.; Hwang, S.H.; Hammock, B.D.; Babu, B. Evaluation of antiparkinson activity of PTUPB by measuring dopamine and its metabolites in Drosophila melanogaster: LC-MS/MS method development. J. Pharm. Biomed. Anal. 2018, 149, 457–464. [Google Scholar] [CrossRef]
- Connolly, J.; Siderowf, A.; Clark, C.M.; Mu, D.; Pratico, D. F2 isoprostane levels in plasma and urine do not support increased lipid peroxidation in cognitively impaired Parkinson disease patients. Cogn. Behav. Neurol. 2008, 21, 83–86. [Google Scholar] [CrossRef]
- Lee, C.Y.J.; Seet, R.C.S.; Huang, S.H.; Long, L.H.; Halliwell, B. Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson’s disease patients: Cautions in the use of biomarkers of oxidative stress. Antioxid. Redox Signal. 2009, 11, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Lee, C.Y.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Wang, H.; Chang, Y.H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.C.; Yao, Y.; Hyman, B.T.; Growdon, J.H.; Praticò, D. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegener. Dis. 2007, 4, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J.P.; Hulette, C.; Powell, S.; Roberts, L.J.; Zhang, J. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. J. Neurochem. 2003, 85, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.D.; Davison, C.A.; Aschner, M.; Bowman, A.B. From the Cover: Manganese and Rotenone-Induced Oxidative Stress Signatures Differ in iPSC-Derived Human Dopamine Neurons. Toxicol. Sci. 2017, 159, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, X.; Yang, C.; Chen, L.; Chen, Z. Resolvin D1 Attenuates Mpp+-Induced Parkinson Disease via Inhibiting Inflammation in PC12 Cells. Med. Sci. Monit. 2017, 23, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Zhang, R.; Qiao, S.; Fan, J. Resolvin D2 recovers neural injury by suppressing inflammatory mediators expression in lipopolysaccharide-induced Parkinson’s disease rat model. Biochem. Biophys. Res. Commun. 2015, 460, 799–805. [Google Scholar] [CrossRef]
- Traina, G. The neurobiology of acetyl-l-carnitine. Front. Biosci. 2016, 21, 1314–1329. [Google Scholar] [CrossRef]
- Saiki, S.; Hatano, T.; Fujimaki, M.; Ishikawa, K.I.; Mori, A.; Oji, Y.; Okuzumi, A.; Fukuhara, T.; Koinuma, T.; Imamichi, Y.; et al. Decreased long-chain acylcarnitines from insufficient β-oxidation as potential early diagnostic markers for Parkinson’s disease. Sci. Rep. 2017, 7, 7328. [Google Scholar] [CrossRef]
- Crooks, S.A.; Bech, S.; Halling, J.; Christiansen, D.H.; Ritz, B.; Petersen, M.S. Carnitine levels and mutations in the SLC22A5 gene in Faroes patients with Parkinson’s disease. Neurosci. Lett. 2018, 675, 116–119. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Rubio, J.C.; Molina, J.A.; Martín, M.A.; Campos, Y.; Benito-León, J.; Ortí-Pareja, M.; Gassalla, T.; Arenas, J. Cerebrospinal fluid carnitine levels in patients with Parkinson’s disease. J. Neurol. Sci. 1997, 145, 183–185. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, H.; Liu, J.; Ao, N.; Yan, B.; Shen, W.; Wang, X.; Li, X.; Luo, C.; Liu, J. Combined R-alpha-lipoic acid and acetyl-l-carnitine exerts efficient preventative effects in a cellular model of Parkinson’s disease. J. Cell. Mol. Med. 2010, 14, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sadovova, N.; Ali, H.K.; Duhart, H.M.; Fu, X.; Zou, X.; Patterson, T.A.; Ninienda, Z.K.; Virmani, A.; Paule, M.G.; et al. L-carnitine protects neurons from 1-methyl-4-phenylpyridinium-induced neuronal apoptosis in rat forebrain culture. Neuroscience 2007, 144, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.L.; Raman, S.; Yost, R.A.; Garrett, T.J.; Vedam-Mai, V. l-Carnitine Inhibits Lipopolysaccharide-Induced Nitric Oxide Production of SIM-A9 Microglia Cells. ACS Chem. Neurosci. 2018, 9, 901–905. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Mishra, S.K.; Shukla, S. ALCAR promote adult hippocampal neurogenesis by regulating cell-survival and cell death-related signals in rat model of Parkinson’s disease like-phenotypes. Neurochem. Int. 2017, 108, 388–396. [Google Scholar] [CrossRef]
- Afshin-Majd, S.; Bashiri, K.; Kiasalari, Z.; Baluchnejadmojarad, T.; Sedaghat, R.; Roghani, M. Acetyl-l-carnitine protects dopaminergic nigrostriatal pathway in 6-hydroxydopamine-induced model of Parkinson’s disease in the rat. Biomed. Pharmacother. 2017, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, A.; Srivastava, N.; Shukla, R.; Shukla, S. Acetyl-l-Carnitine via Upegulating Dopamine D1 Receptor and Attenuating Microglial Activation Prevents Neuronal Loss and Improves Memory Functions in Parkinsonian Rats. Mol. Neurobiol. 2018, 55, 583–602. [Google Scholar] [CrossRef]
- Sarkar, S.; Gough, B.; Raymick, J.; Beaudoin, M.A.; Ali, S.F.; Virmani, A.; Binienda, Z.K. Histopathological and electrophysiological indices of rotenone-evoked dopaminergic toxicity: Neuroprotective effects of acetyl-l-carnitine. Neurosci. Lett. 2015, 606, 53–59. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Abo-Elmatty, D.M.; Shaalan, A.A. Acetyl-l-carnitine and α-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol. Biochem. Behav. 2012, 100, 347–360. [Google Scholar] [CrossRef]
- Bodis-Wollner, I.; Chung, E.; Ghilardi, M.F.; Glover, A.; Onofrj, M.; Pasik, P.; Samson, Y. Acetyl-levo-carnitine protects against MPTP-induced parkinsonism in primates. J. Neural Transm. Park Dis. Dement. Sect. 1991, 3, 63–72. [Google Scholar] [CrossRef]
- Vetel, S.; Sérrière, S.; Vercouillie, J.; Vergote, J.; Chicheri, G.; Deloye, J.B.; Dollé, F.; Bodard, S.; Tronel, C.; Nadal-Desbarats, L.; et al. Extensive exploration of a novel rat model of Parkinson’s disease using partial 6-hydroxydopamine lesion of dopaminergic neurons suggests new therapeutic approaches. Synapse 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Lu, F.; Liu, C.; Wang, Y.; Bai, Y.; Wang, N.; Liu, S.M. Cerebral metabonomics study on Parkinson’s disease mice treated with extract of Acanthopanax senticosus harms. Phytomedicine 2013, 20, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Tu-Sekine, B.; Goldschmidt, H.; Raben, D.M. Diacylglycerol, phosphatidic acid, and their metabolic enzymes in synaptic vesicle recycling. Adv. Biol. Regul. 2015, 57, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Almena, M.; Mérida, I. Shaping up the membrane: Diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 2011, 36, 593–603. [Google Scholar] [CrossRef]
- Ahmadian, M.; Duncan, R.E.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007, 2, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; García-Gutiérrez, M.S.; Aracil-Fernández, A.; Lanciego, J.L.; Manzanares, J. Cannabinoid CB1 and CB2 Receptors, and Monoacylglycerol Lipase Gene Expression Alterations in the Basal Ganglia of Patients with Parkinson’s Disease. Neurotherapeutics 2018, 15, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Aymerich, M.S.; Rojo-Bustamante, E.; Molina, C.; Celorrio, M.; Sánchez-Arias, J.A.; Franco, R. Neuroprotective Effect of JZL184 in MPP(+)-Treated SH-SY5Y Cells Through CB2 Receptors. Mol. Neurobiol. 2016, 53, 2312–2319. [Google Scholar] [CrossRef]
- Pasquarelli, N.; Porazik, C.; Bayer, H.; Buck, E.; Schildknecht, S.; Weydt, P.; Witting, A.; Ferger, B. Contrasting effects of selective MAGL and FAAH inhibition on dopamine depletion and GDNF expression in a chronic MPTP mouse model of Parkinson’s disease. Neurochem. Int. 2017, 110, 14–24. [Google Scholar] [CrossRef]
- Fernández-Suárez, D.; Celorrio, M.; Riezu-Boj, J.I.; Ugarte, A.; Pacheco, R.; González, H.; Oyarzabal, J.; Hillard, C.J.; Franco, R.; Aymerich, M.S. Monoacylglycerol lipase inhibitor JZL184 is neuroprotective and alters glial cell phenotype in the chronic MPTP mouse model. Neurobiol. Aging 2014, 35, 2603–2616. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in Parkinson’s Disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agrò, A.; Bernardi, G.; Bursa, L.; Pierantozzi, M.; Stanzione, P.; et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stacione, P.; Pisani, A.; Maccarrone, M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010, 25, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallack, J.E.; Crippa, J.A. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Kaneko, S.; Morise, S.; Takenouchi, N.; Hashizume, T.; Tsuge, A.; Nakamura, M.; Wate, R.; Kausaka, H. Zonisamide ameliorates levodopa-induced dyskinesia and reduces expression of striatal genes in Parkinson model rats. Neurosci. Res. 2017, 122, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mackovski, N.; Liao, J.; Weng, R.; Wei, X.; Wang, R.; Chen, Z.; Liu, X.; Yu, Y.; Meyer, B.J.; Xia, Y.; et al. Reversal effect of simvastatin on the decrease in cannabinoid receptor 1 density in 6-hydroxydopamine lesioned rat brains. Life Sci. 2016, 155, 123–132. [Google Scholar] [CrossRef]
- Casteels, C.; Lauwers, E.; Baitar, A.; Bormans, G.; Baekelandt, V.; Van Laere, K. In vivo type 1 cannabinoid receptor mapping in the 6-hydroxydopamine lesion rat model of Parkinson’s disease. Brain Res. 2010, 1316, 153–162. [Google Scholar] [CrossRef]
- Walsh, S.; Mnich, K.; Mackie, K.; Gorman, A.M.; Finn, D.P.; Dowd, E. Loss of cannabinoid CB1 receptor expression in the 6-hydroxydopamine-induced nigrostriatal terminal lesion model of Parkinson’s disease in the rat. Brain Res. Bull. 2010, 81, 543–548. [Google Scholar] [CrossRef]
- Chaves-Kirsten, G.P.; Mazucanti, C.H.Y.; Real, C.C.; Souza, B.M.; Britto, L.R.G.; Torrão, A.S. Temporal changes of CB1 cannabinoid receptor in the basal ganglia as a possible structure-specific plasticity process in 6-OHDA lesioned rats. PLoS ONE 2013, 8, e76874. [Google Scholar] [CrossRef]
- Silverdale, M.A.; McGuire, S.; McInnes, A.; Crossman, A.R.; Brotchie, J.M. Striatal cannabinoid CB1 receptor mRNA expression is decreased in the reserpine-treated rat model of Parkinson’s disease. Exp. Neurol. 2001, 169, 400–406. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Mena, M.A.; Lastres-Becker, I.; Serrano, A.; de Yébenes, J.G.; Ramos, J.A.; Fernández-Ruiz, J. Cannabinoid CB(1) receptors in the basal ganglia and motor response to activation or blockade of these receptors in parkin-null mice. Brain Res. 2005, 1046, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Madeo, G.; Schirinzi, T.; Maltese, M.; Martella, G.; Rapino, C.; Fezza, F.; Mastrengelo, N.; Bonsi, P.; Maccarrone, M.; Pisani, A. Dopamine-dependent CB1 receptor dysfunction at corticostriatal synapses in homozygous PINK1 knockout mice. Neuropharmacology 2016, 101, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2016, 283, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Differential upregulation of the cannabinoid CB2 receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2015, 269, 133–141. [Google Scholar] [CrossRef]
- Palomo-Garo, C.; Gómez-Gálvez, Y.; García, C.; Fernández-Ruiz, J. Targeting the cannabinoid CB2 receptor to attenuate the progression of motor deficits in LRRK2-transgenic mice. Pharmacol. Res. 2016, 110, 181–192. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef]
- García-Arencibia, M.; González, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef]
- Fernandez-Espejo, E.; Caraballo, I.; Rodriguez de Fonseca, F.; Ferrer, B.; El Banoua, F.; Flores, J.A.; Galan-Rodriguez, B. Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: A modulator of endocannabinoid function. Neuropsychopharmacology 2004, 29, 1134–1142. [Google Scholar] [CrossRef]
- Maccarrone, M.; Gubellini, P.; Bari, M.; Picconi, B.; Battista, N.; Centonze, D.; Bernardi, G.; Finazzi-Agrò, A.; Calabresi, P. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 2003, 85, 1018–1025. [Google Scholar] [CrossRef]
- Gubellini, P.; Picconi, B.; Bari, M.; Battista, N.; Calabresi, P.; Centonze, D.; Bernardi, G.; Finazzi-Agrò, A.; Maccarrone, M. Experimental parkinsonism alters endocannabinoid degradation: Implications for striatal glutamatergic transmission. J. Neurosci. 2002, 22, 6900–6907. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, B.; Asbrock, N.; Kathuria, S.; Piomelli, D.; Giuffrida, A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: Implications for the treatment of levodopa-induced dyskinesias. Eur. J. Neurosci. 2003, 18, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Van der Stelt, M.; Fox, S.H.; Hill, M.; Crossman, A.R.; Petrosino, S.; Di Marzo, V.; Brotchie, J.M. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005, 19, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Paredes, J.M.; Gonzalez-Castañeda, R.E.; Escalante-Castañeda, A.; Tejeda-Martínez, A.R.; Castañeda-Achutiguí, F.; Flores-Soto, M.E. Effect of inhibition of fatty acid amide hydrolase on MPTP-induced dopaminergic neuronal damage. Neurologia 2017. [Google Scholar] [CrossRef]
- Celorrio, M.; Fernández-Suárez, D.; Rojo-Bustamante, E.; Echeverry-Alzate, V.; Ramírez, M.J.; Hillard, C.J.; López-Morenoa, J.A.; Maldonado, R.; Oyarzábal, J.; Franco, R.; et al. Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson’s disease. Brain Behav. Immun. 2016, 57, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Mnich, K.; Finn, D.P.; Dowd, E.; Gorman, A.M. Inhibition by anandamide of 6-hydroxydopamine-induced cell death in PC12 cells. Int. J. Cell Biol. 2010, 2010, 818497. [Google Scholar] [CrossRef]
- Mounsey, R.B.; Mustafa, S.; Robinson, L.; Ross, R.A.; Riedel, G.; Pertwee, R.G.; Tesimann, P. Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. Exp. Neurol. 2015, 273, 36–44. [Google Scholar] [CrossRef]
- Hracskó, Z.; Baranyi, M.; Csölle, C.; Gölöncsér, F.; Madarász, E.; Kittel, A.; Sperlágh, B. Lack of neuroprotection in the absence of P2X7 receptors in toxin-induced animal models of Parkinson’s disease. Mol. Neurodegener. 2011, 6, 28. [Google Scholar] [CrossRef]
- Di Marzo, V.; Hill, M.P.; Bisogno, T.; Crossman, A.R.; Brotchie, J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000, 14, 1432–1438. [Google Scholar]
- Freestone, P.S.; Guatteo, E.; Piscitelli, F.; di Marzo, V.; Lipski, J.; Mercuri, N.B. Glutamate spillover drives endocannabinoid production and inhibits GABAergic transmission in the Substantia Nigra pars compacta. Neuropharmacology 2014, 79, 467–475. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 2007, 445, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Wang, L.; Yang, C. High Performance Liquid Chromatography-Mass Spectrometry (LC-MS) Based Quantitative Lipidomics Study of Ganglioside-NANA-3 Plasma to Establish Its Association with Parkinson’s Disease Patients. Med. Sci. Monit. 2017, 23, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.B.; Perotte, A.J.; Zhou, B.; Liong, C.; Shorr, E.J.; Marder, K.S.; et al. Elevated GM3 plasma concentration in idiopathic Parkinson’s disease: A lipidomic analysis. PLoS ONE 2017, 12, e0172348. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Tippireddy, S.; Feriante, J.; Woltjer, R.L. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 2018, 13, e0191815. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Halliday, G.M.; et al. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 2011, 6, e17299. [Google Scholar] [CrossRef] [PubMed]
- Simón-Sánchez, J.; van Hilten, J.J.; van de Warrenburg, B.; Post, B.; Berendse, H.W.; Arepalli, S.; Hernandez, D.G.; de Bie, R.M.; Velseboar, D.; Scheffer, H.; et al. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur. J. Hum. Genet. 2011, 19, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Song, W.; Huang, R.; Chen, K.; Zhao, B.; Li, J.; Yang, Y.; Shang, H.F. GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson’s disease in a Chinese population. J. Clin. Neurosci. 2013, 20, 880–883. [Google Scholar] [CrossRef]
- Sakane, F.; Mizuno, S.; Takahashi, D.; Sakai, H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018, 67, 101–108. [Google Scholar] [CrossRef]
- Guo, X.; Song, W.; Chen, K.; Chen, X.; Zheng, Z.; Cao, B.; Huang, R.; Zhao, B.; Wu, Y.; Shang, H.F. The serum lipid profile of Parkinson’s disease patients: A study from China. Int. J. Neurosci. 2015, 125, 838–844. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, H.; Tian, Y.; Xu, F.; Chen, X.; Wang, K. Reduced serum levels of triglyceride, very low density lipoprotein cholesterol and apolipoprotein B in Parkinson’s disease patients. PLoS ONE 2013, 8, e75743. [Google Scholar] [CrossRef] [PubMed]
- Gregório, M.L.; Pinhel, M.A.S.; Sado, C.L.; Longo, G.S.; Oliveira, F.N.; Amorim, G.S.; Nakazone, M.A.; Florim, G.M.; Mazeti, C.M.; Martins, D.P.; et al. Impact of genetic variants of apolipoprotein E on lipid profile in patients with Parkinson’s disease. Biomed. Res. Int. 2013, 2013, 641515. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Cassani, E.; Barichella, M.; Spadafranca, A.; Caccialanza, R.; Bertoli, S.; Battezzati, A.; Pezzoli, G. Low cardiometabolic risk in Parkinson’s disease is independent of nutritional status, body composition and fat distribution. Clin. Nutr. 2012, 31, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Sääksjärvi, K.; Knekt, P.; Männistö, S.; Lyytinen, J.; Heliövaara, M. Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease. Park. Relat. Disord. 2015, 21, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Vikdahl, M.; Bäckman, L.; Johansson, I.; Forsgren, L.; Håglin, L. Cardiovascular risk factors and the risk of Parkinson’s disease. Eur. J. Clin. Nutr. 2015, 69, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Scigliano, G.; Musicco, M.; Soliveri, P.; Piccolo, I.; Ronchetti, G.; Girotti, F. Reduced risk factors for vascular disorders in Parkinson disease patients: A case-control study. Stroke 2006, 37, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Hishikawa, N.; Shang, J.; Sato, K.; Nakano, Y.; Morihara, R.; Ohta, Y.; Yamashita, T.; Abe, K. Peripheral arterial endothelial dysfunction of neurodegenerative diseases. J. Neurol. Sci. 2016, 366, 94–99. [Google Scholar] [CrossRef]
- Ya, L.; Lu, Z. Differences in ABCA1 R219K Polymorphisms and Serum Indexes in Alzheimer and Parkinson Diseases in Northern China. Med. Sci. Monit. 2017, 23, 4591–4600. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Guerreiro, P.S.; Coelho, J.E.; Sousa-Lima, I.; Macedo, P.; Lopes, L.V.; Outeiro, T.F.; Pais, T.F. Mutant A53T α-Synuclein Improves Rotarod Performance Before Motor Deficits and Affects Metabolic Pathways. Neuromol. Med. 2017, 19, 113–121. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, R.; Zhang, Y.; Qiao, M.; Liu, L.; Jing, P.; Wang, L.; Liu, J.; Gao, Y. An activated sympathetic nervous system affects white adipocyte differentiation and lipolysis in a rat model of Parkinson’s disease. J. Neurosci. Res. 2015, 93, 350–360. [Google Scholar] [CrossRef]
- He, Q.; Wang, M.; Petucci, C.; Gardell, S.J.; Han, X. Rotenone induces reductive stress and triacylglycerol deposition in C2C12 cells. Int. J. Biochem. Cell Biol. 2013, 45, 2749–2755. [Google Scholar] [CrossRef]
- Sere, Y.Y.; Regnacq, M.; Colas, J.; Berges, T. A Saccharomyces cerevisiae strain unable to store neutral lipids is tolerant to oxidative stress induced by α-synuclein. Free Radic. Biol. Med. 2010, 49, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B.; Murphy, D.D.; Grider, T.; Rueter, S.; Brasaemle, D.; Nussbaum, R.L. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J. Biol. Chem. 2002, 277, 6344–6352. [Google Scholar] [CrossRef]
- Sánchez Campos, S.; Alza, N.P.; Salvador, G.A. Lipid metabolism alterations in the neuronal response to A53T α-synuclein and Fe-induced injury. Arch. Biochem. Biophys. 2018, 655, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B.; Vanni, S.; Shindou, H.; Ferreira, T. From zero to six double bonds: Phospholipid unsaturation and organelle function. Trends Cell Biol. 2015, 25, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bohdanowicz, M.; Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 2013, 93, 69–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Tamura, Y.; Roy, M.; Adachi, Y.; Iijima, M.; Sesaki, H. Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy. Cell. Mol. Life Sci. 2014, 71, 3767–3778. [Google Scholar] [CrossRef]
- Boss, W.F.; Im, Y.J. Phosphoinositide signaling. Annu. Rev. Plant Biol. 2012, 63, 409–429. [Google Scholar] [CrossRef]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef]
- Kay, J.G.; Grinstein, S. Phosphatidylserine-mediated cellular signaling. Adv. Exp. Med. Biol. 2013, 991, 177–193. [Google Scholar] [CrossRef]
- Musille, P.M.; Kohn, J.A.; Ortlund, E.A. Phospholipid—Driven gene regulation. FEBS Lett. 2013, 587, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Y.; Wang, X. Phosphatidic acid-mediated signaling. Adv. Exp. Med. Biol. 2013, 991, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.R.; Kassas, N.; Bader, M.F.; Vitale, N. Phosphatidic acid in neuronal development: A node for membrane and cytoskeleton rearrangements. Biochimie 2014, 107, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Frohman, M.A. Mitochondria: Signaling with phosphatidic acid. Int. J. Biochem. Cell Biol. 2012, 44, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hess, S.K.; Heinrich, F.; Lee, J.C. Molecular details of α-synuclein membrane association revealed by neutrons and photons. J. Phys. Chem. B 2015, 119, 4812–4823. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Interaction of human alpha-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J. Biol. Chem. 2000, 275, 34393–34398. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Sasai, H.; Kume, A.; Takahashi, D.; Satoh, M.; Kado, S.; Sakane, F. Dioleoyl-phosphatidic acid selectively binds to α-synuclein and strongly induces its aggregation. FEBS Lett. 2017, 591, 784–791. [Google Scholar] [CrossRef]
- Soper, J.H.; Kehm, V.; Burd, C.G.; Bankaitis, V.A.; Lee, V.M.Y. Aggregation of α-synuclein in S. cerevisiae is associated with defects in endosomal trafficking and phospholipid biosynthesis. J. Mol. Neurosci. 2011, 43, 391–405. [Google Scholar] [CrossRef]
- Holemans, T.; Sørensen, D.M.; van Veen, S.; Martin, S.; Hermans, D.; Kemmer, G.C.; Van den Haute, C.; Baekelandt, V.; Günther Pomorski, T.; Agostinis, P.; et al. A lipid switch unlocks Parkinson’s disease-associated ATP13A2. Proc. Natl. Acad. Sci. USA 2015, 112, 9040–9045. [Google Scholar] [CrossRef]
- Martin, S.; van Veen, S.; Holemans, T.; Demirsoy, S.; van den Haute, C.; Baekelandt, V.; Agostinis, P.; Eggermont, J.; Vangheluwe, P. Protection against Mitochondrial and Metal Toxicity Depends on Functional Lipid Binding Sites in ATP13A2. Park. Dis. 2016, 2016, 9531917. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Gomez, H.R.; Singh, J.; Meyers, C.; Chen, W.; Gorbatyuk, O.S.; Muzyczka, N. The Lipase Activity of Phospholipase D2 is Responsible for Nigral Neurodegeneration in a Rat Model of Parkinson’s Disease. Neuroscience 2018, 377, 174–183. [Google Scholar] [CrossRef]
- Binder, B.Y.K.; Williams, P.A.; Silva, E.A.; Leach, J.K. Lysophosphatidic Acid and Sphingosine-1-Phosphate: A Concise Review of Biological Function and Applications for Tissue Engineering. Tissue Eng. Part B Rev. 2015, 21, 531–542. [Google Scholar] [CrossRef]
- Sheng, X.; Yung, Y.C.; Chen, A.; Chun, J. Lysophosphatidic acid signalling in development. Development 2015, 142, 1390–1395. [Google Scholar] [CrossRef]
- Aikawa, S.; Hashimoto, T.; Kano, K.; Aoki, J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem. 2015, 157, 81–89. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhao, E.Y.; Zhuang, W.X.; Sun, F.X.; Han, H.L.; Han, H.R.; Lin, Z.J.; Pan, Z.F.; Qu, M.H.; Zeng, X.W.; et al. LPA signaling is required for dopaminergic neuron development and is reduced through low expression of the LPA1 receptor in a 6-OHDA lesion model of Parkinson’s disease. Neurol. Sci. 2015, 36, 2027–2033. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, M.; Oh, S.; Nah, S.Y.; Cho, I.H. Multi-Target Protective Effects of Gintonin in 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Mediated Model of Parkinson’s Disease via Lysophosphatidic Acid Receptors. Front. Pharmacol. 2018, 9, 515. [Google Scholar] [CrossRef]
- Vance, J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- Guedes, L.C.; Chan, R.B.; Gomes, M.A.; Conceição, V.A.; Machado, R.B.; Soares, T.; Xu, Y.; Gaspar, P.; Carriço, J.A.; Alacaly, R.N.; et al. Serum lipid alterations in GBA-associated Parkinson’s disease. Park. Relat. Disord. 2017, 44, 58–65. [Google Scholar] [CrossRef]
- Riekkinen, P.; Rinne, U.K.; Pelliniemi, T.T.; Sonninen, V. Interaction between dopamine and phospholipids. Studies of the substantia nigra in Parkinson disease patients. Arch. Neurol. 1975, 32, 25–27. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Choi, H.; Chevalier, A.; Hogan, D.; Akgoc, Z.; Schneider, J.S. Sex-Related Abnormalities in Substantia Nigra Lipids in Parkinson’s Disease. ASN Neuro 2018, 10. [Google Scholar] [CrossRef]
- Ross, B.M.; Mamalias, N.; Moszczynska, A.; Rajput, A.H.; Kish, S.J. Elevated activity of phospholipid biosynthetic enzymes in substantia nigra of patients with Parkinson’s disease. Neuroscience 2001, 102, 899–904. [Google Scholar] [CrossRef]
- Jo, E.; McLaurin, J.; Yip, C.M.; St George-Hyslop, P.; Fraser, P.E. alpha-Synuclein membrane interactions and lipid specificity. J. Biol. Chem. 2000, 275, 34328–34334. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, S.D.; Hulleman, J.D.; Dutseva, E.A.; Antonenko, Y.N.; Rochet, J.C.; Cramer, W.A. Helical alpha-synuclein forms highly conductive ion channels. Biochemistry 2007, 46, 14369–14379. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Liou, L.C.; Ren, Q.; Zhang, Z.; Caldwell, G.A.; Witt, S.N. Phosphatidylethanolamine deficiency disrupts α-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, E3976–E3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Xu, C.; Barron, A.; Galiano, F.; Patel, D.; Lee, Y.J.; Caldwell, G.A.; Caldwell, K.A.; Witt, S.N. Chemical Compensation of Mitochondrial Phospholipid Depletion in Yeast and Animal Models of Parkinson’s Disease. PLoS ONE 2016, 11, e0164465. [Google Scholar] [CrossRef]
- Lee, E.S.; Charlton, C.G. 1-Methyl-4-phenyl-pyridinium increases S-adenosyl-L-methionine dependent phospholipid methylation. Pharmacol. Biochem. Behav. 2001, 70, 105–114. [Google Scholar] [CrossRef]
- Lee, E.S.Y.; Chen, H.; Charlton, C.G.; Soliman, K.F.A. The role of phospholipid methylation in 1-methyl-4-phenyl-pyridinium ion (MPP+)-induced neurotoxicity in PC12 cells. Neurotoxicology 2005, 26, 945–957. [Google Scholar] [CrossRef]
- Leventis, P.A.; Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010, 39, 407–427. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Lobasso, S.; Tanzarella, P.; Vergara, D.; Maffia, M.; Cocco, T.; Corcelli, A. Lipid profiling of parkin-mutant human skin fibroblasts. J. Cell. Physiol. 2017, 232, 3540–3551. [Google Scholar] [CrossRef]
- Valadas, J.S.; Esposito, G.; Vandekerkhove, D.; Miskiewicz, K.; Deaulmerie, L.; Raitano, S.; Seibler, P.; Lein, C.; Verstreken, P. ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease. Neuron 2018, 98, 1155–1169.e6. [Google Scholar] [CrossRef]
- Wei, L.; Sun, C.; Lei, M.; Li, G.; Yi, L.; Luo, F.; Li, Y.; Ding, L.; Liu, Z.; Li, S.; et al. Activation of Wnt/β-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 2013, 49, 105–115. [Google Scholar] [CrossRef]
- Lupescu, A.; Jilani, K.; Zbidah, M.; Lang, F. Induction of apoptotic erythrocyte death by rotenone. Toxicology 2012, 300, 132–137. [Google Scholar] [CrossRef]
- González-Polo, R.A.; Niso-Santano, M.; Ortíz-Ortíz, M.A.; Gómez-Martín, A.; Morán, J.M.; García-Rubio, L.; Francisco-Mocillo, J.; Zaragoza, C.; Soler, G.; Fuentes, J.M. Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol. Sci. 2007, 97, 448–458. [Google Scholar] [CrossRef]
- Ye, S.; Koon, H.K.; Fan, W.; Xu, Y.; Wei, W.; Xu, C.; Cai, J. Effect of a Traditional Chinese Herbal Medicine Formulation on Cell Survival and Apoptosis of MPP+-Treated MES 23.5 Dopaminergic Cells. Park. Dis. 2017, 2017, 4764212. [Google Scholar] [CrossRef]
- Flower, T.R.; Chesnokova, L.S.; Froelich, C.A.; Dixon, C.; Witt, S.N. Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson’s disease. J. Mol. Biol. 2005, 351, 1081–1100. [Google Scholar] [CrossRef]
- Emmrich, J.V.; Hornik, T.C.; Neher, J.J.; Brown, G.C. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013, 280, 5030–5038. [Google Scholar] [CrossRef]
- Almandoz-Gil, L.; Lindström, V.; Sigvardson, J.; Kahle, P.J.; Lannfelt, L.; Ingelsson, M.; Bergström, J. Mapping of Surface-Exposed Epitopes of In Vitro and In Vivo Aggregated Species of Alpha-Synuclein. Cell. Mol. Neurobiol. 2017, 37, 1217–1226. [Google Scholar] [CrossRef]
- Bartels, T.; Kim, N.C.; Luth, E.S.; Selkoe, D.J. N-alpha-acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS ONE 2014, 9, e103727. [Google Scholar] [CrossRef]
- Araki, K.; Sugawara, K.; Hayakawa, E.H.; Ubukawa, K.; Kobayashi, I.; Wakui, H.; Takahasi, N.; Sawada, K.; Mochizuki, H.; Nunomura, W. The localization of α-synuclein in the process of differentiation of human erythroid cells. Int. J. Hematol. 2018, 108, 130–138. [Google Scholar] [CrossRef]
- Hu, R.; Diao, J.; Li, J.; Tang, Z.; Li, X.; Leitz, J.; Long, J.; Liu, J.; Yu, D.; Zhao, Q. Intrinsic and membrane-facilitated α-synuclein oligomerization revealed by label-free detection through solid-state nanopores. Sci. Rep. 2016, 6, 20776. [Google Scholar] [CrossRef]
- Lou, X.; Kim, J.; Hawk, B.J.; Shin, Y.K. α-Synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking. Biochem. J. 2017, 474, 2039–2049. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef]
- Treede, I.; Braun, A.; Sparla, R.; Kühnel, M.; Giese, T.; Turner, J.R.; Anes, E.; Kulaksiz, H.; Füllekrug, J.; Stremmel, W.; et al. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 2007, 282, 27155–27164. [Google Scholar] [CrossRef]
- Lagace, T.A. Phosphatidylcholine: Greasing the Cholesterol Transport Machinery. Lipid Insights 2015, 8 (Suppl. 1), 65–73. [Google Scholar] [CrossRef]
- Marcucci, H.; Paoletti, L.; Jackowski, S.; Banchio, C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem. 2010, 285, 25382–25393. [Google Scholar] [CrossRef]
- Li, T.; Tang, W.; Zhang, L. Monte Carlo cross-validation analysis screens pathway cross-talk associated with Parkinson’s disease. Neurol. Sci. 2016, 37, 1327–1333. [Google Scholar] [CrossRef]
- Farmer, K.; Smith, C.A.; Hayley, S.; Smith, J. Major Alterations of Phosphatidylcholine and Lysophosphotidylcholine Lipids in the Substantia Nigra Using an Early Stage Model of Parkinson’s Disease. Int. J. Mol. Sci. 2015, 16, 18865–18877. [Google Scholar] [CrossRef]
- Stöckl, M.; Fischer, P.; Wanker, E.; Herrmann, A. Alpha-synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J. Mol. Biol. 2008, 375, 1394–1404. [Google Scholar] [CrossRef]
- Jiang, Z.; de Messieres, M.; Lee, J.C. Membrane remodeling by α-synuclein and effects on amyloid formation. J. Am. Chem. Soc. 2013, 135, 15970–15973. [Google Scholar] [CrossRef]
- Di Pasquale, E.; Fantini, J.; Chahinian, H.; Maresca, M.; Taïeb, N.; Yahi, N. Altered ion channel formation by the Parkinson’s-disease-linked E46K mutant of alpha-synuclein is corrected by GM3 but not by GM1 gangliosides. J. Mol. Biol. 2010, 397, 202–218. [Google Scholar] [CrossRef]
- O’Leary, E.I.; Jiang, Z.; Strub, M.P.; Lee, J.C. Effects of phosphatidylcholine membrane fluidity on the conformation and aggregation of N-terminally acetylated α-synuclein. J. Biol. Chem. 2018, 293, 11195–11205. [Google Scholar] [CrossRef]
- Hollie, N.I.; Cash, J.G.; Matlib, M.A.; Wortman, M.; Basford, J.E.; Abplanalp, W.; Hui, D.Y. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta 2014, 1841, 888–895. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F.; et al. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef]
- Hung, N.D.; Sok, D.E.; Kim, M.R. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Inflamm. Res. 2012, 61, 473–483. [Google Scholar] [CrossRef]
- Lee, E.S.Y.; Chen, H.; Shepherd, K.R.; Lamango, N.S.; Soliman, K.F.A.; Charlton, C.G. Inhibitory effects of lysophosphatidylcholine on the dopaminergic system. Neurochem. Res. 2004, 29, 1333–1342. [Google Scholar] [CrossRef]
- Lee, E.S.Y.; Soliman, K.F.A.; Charlton, C.G. Lysophosphatidylcholine decreases locomotor activities and dopamine turnover rate in rats. Neurotoxicology 2005, 26, 27–38. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Jope, R.S. Phosphoinositide signaling in human brain. Prog. Neurobiol. 1996, 50, 255–273. [Google Scholar] [CrossRef]
- Chalimoniuk, M.; Snoek, G.T.; Adamczyk, A.; Małecki, A.; Strosznajder, J.B. Phosphatidylinositol transfer protein expression altered by aging and Parkinson disease. Cell. Mol. Neurobiol. 2006, 26, 1153–1166. [Google Scholar] [CrossRef]
- Cockcroft, S. The diverse functions of phosphatidylinositol transfer proteins. Curr. Top. Microbiol. Immunol. 2012, 362, 185–208. [Google Scholar] [CrossRef]
- Lee, E.N.; Lee, S.Y.; Lee, D.; Kim, J.; Paik, S.R. Lipid interaction of alpha-synuclein during the metal-catalyzed oxidation in the presence of Cu2+ and H2O2. J. Neurochem. 2003, 84, 1128–1142. [Google Scholar] [CrossRef]
- Narayanan, V.; Guo, Y.; Scarlata, S. Fluorescence studies suggest a role for alpha-synuclein in the phosphatidylinositol lipid signaling pathway. Biochemistry 2005, 44, 462–470. [Google Scholar] [CrossRef]
- Sekar, S.; Taghibiglou, C. Elevated nuclear phosphatase and tensin homolog (PTEN) and altered insulin signaling in substantia nigral region of patients with Parkinson’s disease. Neurosci. Lett. 2018, 666, 139–143. [Google Scholar] [CrossRef]
- Demirsoy, S.; Martin, S.; Motamedi, S.; van Veen, S.; Holemans, T.; Van den Haute, C.; Jordanova, A.; Baekelandt, V.; Vangheluwe, P.; Agostinis, P. ATP13A2/PARK9 regulates endo-/lysosomal cargo sorting and proteostasis through a novel PI(3, 5)P2-mediated scaffolding function. Hum. Mol. Genet. 2017, 26, 1656–1669. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Jensen, P.H.; Marsh, D. Association of alpha-synuclein and mutants with lipid membranes: Spin-label ESR and polarized IR. Biochemistry 2006, 45, 3386–3395. [Google Scholar] [CrossRef]
- Jiang, Z.; Flynn, J.D.; Teague, W.E.; Gawrisch, K.; Lee, J.C. Stimulation of α-synuclein amyloid formation by phosphatidylglycerol micellar tubules. Biochim. Biophys. Acta 2018. [Google Scholar] [CrossRef]
- Bédard, L.; Lefèvre, T.; Morin-Michaud, É.; Auger, M. Besides fibrillization: Putative role of the peptide fragment 71-82 on the structural and assembly behavior of α-synuclein. Biochemistry 2014, 53, 6463–6472. [Google Scholar] [CrossRef]
- Pandey, A.P.; Haque, F.; Rochet, J.C.; Hovis, J.S. Clustering of alpha-synuclein on supported lipid bilayers: Role of anionic lipid, protein, and divalent ion concentration. Biophys. J. 2009, 96, 540–551. [Google Scholar] [CrossRef]
- Stefanovic, A.N.D.; Claessens, M.M.A.E.; Blum, C.; Subramaniam, V. Alpha-synuclein amyloid oligomers act as multivalent nanoparticles to cause hemifusion in negatively charged vesicles. Small 2015, 11, 2257–2262. [Google Scholar] [CrossRef]
- Van Rooijen, B.D.; Claessens, M.M.A.E.; Subramaniam, V. Lipid bilayer disruption by oligomeric alpha-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim. Biophys. Acta 2009, 1788, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Van Rooijen, B.D.; Claessens, M.M.A.E.; Subramaniam, V. Membrane Permeabilization by Oligomeric α-Synuclein: In Search of the Mechanism. PLoS ONE 2010, 5, e14292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Qin, Z.J.; Hu, D.; Munishkina, L.A.; Fink, A.L. Alpha-synuclein can function as an antioxidant preventing oxidation of unsaturated lipid in vesicles. Biochemistry 2006, 45, 8135–8142. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Phoon, C.K.L.; Schlame, M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Cardiolipin and mitochondrial function in health and disease. Antioxid. Redox. Signal. 2014, 20, 1925–1953. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 2014, 1837, 408–417. [Google Scholar] [CrossRef]
- Vos, M.; Geens, A.; Böhm, C.; Deaulmerie, L.; Swerts, J.; Rossi, M.; Craessaerts, K.; Leites, E.P.; Seibler, P.; Rakovic, A.; et al. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency. J. Cell Biol. 2017, 216, 695–708. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Winnica, D.E.; Kapralova, V.I.; Kapralov, A.A.; Tyurin, V.A.; Kagan, V.E. LC/MS characterization of rotenone induced cardiolipin oxidation in human lymphocytes: Implications for mitochondrial dysfunction associated with Parkinson’s disease. Mol. Nutr. Food Res. 2013, 57, 1410–1422. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Polimova, A.M.; Maciel, E.; Tyurin, V.A.; Kapralova, V.I.; Winnica, D.E.; Vikulina, A.S.; Domigues, M.R.; McCoy, J.; Samders, L.H.; et al. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: Implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic. Res. 2015, 49, 681–691. [Google Scholar] [CrossRef]
- Zigoneanu, I.G.; Yang, Y.J.; Krois, A.S.; Haque, E.; Pielak, G.J. Interaction of α-synuclein with vesicles that mimic mitochondrial membranes. Biochim. Biophys. Acta 2012, 1818, 512–519. [Google Scholar] [CrossRef]
- Robotta, M.; Gerding, H.R.; Vogel, A.; Hauser, K.; Schildknecht, S.; Karreman, C.; Leist, M.; Subramaniam, V.; Drescher, M. Alpha-synuclein binds to the inner membrane of mitochondria in an α-helical conformation. Chembiochem 2014, 15, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.; Bamm, V.V.; Stykel, M.G.; Coackley, C.L.; Humphries, K.M.; Jamieson-Williams, R.; Ambasudhan, R.; Mosser, D.D.; Lipton, S.A.; Harauz, G.; et al. Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 2018, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nemani, V.M.; Azarbal, F.; Skibinski, G.; Levy, J.M.; Egami, K.; Munushkina, L.; Zhang, J.; Gardner, B.; Wakabayashi, J.; et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 2011, 286, 20710–20726. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Du, T.; Wang, X.; Duan, C.; Gao, G.; Zhang, J.; Lu, L.; Yang, H. α-Synuclein amino terminus regulates mitochondrial membrane permeability. Brain Res. 2014, 1591, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, Z.; Lu, L.; Duan, C.; Wang, X.; Yang, H. Morphological analysis of mitochondria for evaluating the toxicity of α-synuclein in transgenic mice and isolated preparations by atomic force microscopy. Biomed. Pharmacother. 2017, 96, 1380–1388. [Google Scholar] [CrossRef]
- Bayir, H.; Kapralov, A.A.; Jiang, J.; Huang, Z.; Tyurina, Y.Y.; Tyurin, V.A.; Zhao, Q.; Belikova, N.A.; Vlasova, I.I.; Maeda, A.; et al. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: Protection against apoptosis versus delayed oxidative stress in Parkinson disease. J. Biol. Chem. 2009, 284, 15951–15969. [Google Scholar] [CrossRef]
- Ghio, S.; Kamp, F.; Cauchi, R.; Giese, A.; Vassallo, N. Interaction of α-synuclein with biomembranes in Parkinson’s disease—Role of cardiolipin. Prog. Lipid Res. 2016, 61, 73–82. [Google Scholar] [CrossRef]
- Chu, C.T.; Bayır, H.; Kagan, V.E. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: Implications for Parkinson disease. Autophagy 2014, 10, 376–378. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Xu, Y.H.; Barnes, S.; Sun, Y.; Grabowski, G.A. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J. Lipid Res. 2010, 51, 1643–1675. [Google Scholar] [CrossRef]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef]
- Martin, R.; Sospedra, M. Sphingosine-1 phosphate and central nervous system. Curr. Top. Microbiol. Immunol. 2014, 378, 149–170. [Google Scholar] [CrossRef]
- Taguchi, Y.V.; Liu, J.; Ruan, J.; Pacheco, J.; Zhang, X.; Abbasi, J.; Keutzer, J.; Mistry, P.K.; Chandra, S.S. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 2017, 37, 9617–9631. [Google Scholar] [CrossRef]
- Motyl, J.; Wencel, P.L.; Cieślik, M.; Strosznajder, R.P.; Strosznajder, J.B. Alpha-synuclein alters differently gene expression of Sirts, PARPs and other stress response proteins: Implications for neurodegenerative disorders. Mol. Neurobiol. 2018, 55, 727–740. [Google Scholar] [CrossRef]
- Zhang, L.; Okada, T.; Badawy, S.M.M.; Hirai, C.; Kajimoto, T.; Nakamura, S.I. Extracellular α-synuclein induces sphingosine 1-phosphate receptor subtype 1 uncoupled from inhibitory G-protein leaving β-arrestin signal intact. Sci. Rep. 2017, 7, 44248. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.M.; Okada, T.; Kajimoto, T.; Hirase, M.; Matovelo, S.A.; Nakamura, S.; Yoshida, D.; Ijuin, T.; Nakamura, S.I. Extracellular α-synuclein drives sphingosine 1-phosphate receptor subtype 1 out of lipid rafts, leading to impaired inhibitory G-protein signaling. J. Biol. Chem. 2018, 293, 8208–8216. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Kanagaraj, N.; Dheen, S.T.; Tay, S.S.W. Sphingosine kinase 2 and sphingosine-1-phosphate promotes mitochondrial function in dopaminergic neurons of mouse model of Parkinson’s disease and in MPP+-treated MN9D cells in vitro. Neuroscience 2015, 290, 636–648. [Google Scholar] [CrossRef]
- Pyszko, J.A.; Strosznajder, J.B. The key role of sphingosine kinases in the molecular mechanism of neuronal cell survival and death in an experimental model of Parkinson’s disease. Folia Neuropathol. 2014, 52, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Pyszko, J.; Strosznajder, J.B. Sphingosine kinase 1 and sphingosine-1-phosphate in oxidative stress evoked by 1-methyl-4-phenylpyridinium (MPP+) in human dopaminergic neuronal cells. Mol. Neurobiol. 2014, 50, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yang, X.; Yang, L.; Li, M.; Wood, K.; Liu, Q.; Zhu, X. Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease. FASEB J. 2017, 31, 172–179. [Google Scholar] [CrossRef]
- Mencarelli, C.; Martinez-Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell. Mol. Life Sci. 2013, 70, 181–203. [Google Scholar] [CrossRef]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Kogot-Levin, A.; Saada, A. Ceramide and the mitochondrial respiratory chain. Biochimie 2014, 100, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Maetzler, W.; Haughey, N.J.; Bandaru, V.V.R.; Savica, R.; Deuschle, C.; Gasser, T.; Hauser, A.K.; Gräber-Sultan, S.; Schleicher, E.; et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: A pilot study. PLoS ONE 2013, 8, e73094. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Hammond, D.; Perera, G.; Dobson-Stone, C.; Mueller, N.; Pickford, R.; Kim, W.S.; Kwok, J.B.; Lewis, S.J.G.; Halliday, G.M.; et al. Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci. Rep. 2018, 8, 15446. [Google Scholar] [CrossRef]
- Murphy, K.E.; Gysbers, A.M.; Abbott, S.K.; Tayebi, N.; Kim, W.S.; Sidransky, E.; Cooper, A.; Garner, B.; Halliday, G.M. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 2014, 137 Pt 3, 834–848. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, S.; Burbulla, L.F.; Krainc, D. Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function. Hum. Mol. Genet. 2018, 27, 1972–1988. [Google Scholar] [CrossRef]
- Abbott, S.K.; Li, H.; Muñoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef]
- Halliday, G.M.; McCann, H. The progression of pathology in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2010, 1184, 188–195. [Google Scholar] [CrossRef]
- Lin, G.; Lee, P.T.; Chen, K.; Mao, D.; Tan, K.L.; Zuo, Z.; Lin, W.W.; Wang, L.; Bellen, H.J. Phospholipase PLA2G6, a Parkinsonism-Associated Gene, Affects Vps26 and Vps35, Retromer Function, and Ceramide Levels, Similar to α-Synuclein Gain. Cell Metab. 2018. [Google Scholar] [CrossRef]
- Ferrazza, R.; Cogo, S.; Melrose, H.; Bubacco, L.; Greggio, E.; Guella, G.; Civiero, L.; Plotegher, N. LRRK2 deficiency impacts ceramide metabolism in brain. Biochem. Biophys. Res. Commun. 2016, 478, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Torres-Odio, S.; Key, J.; Hoepken, H.H.; Canet-Pons, J.; Valek, L.; Roller, B.; Walter, M.; Morales-Gordo, B.; Meierhofer, D.; Harter, P.N.; et al. Progression of pathology in PINK1-deficient mouse brain from splicing via ubiquitination, ER stress, and mitophagy changes to neuroinflammation. J. Neuroinflamm. 2017, 14, 154. [Google Scholar] [CrossRef]
- Arboleda, G.; Cárdenas, Y.; Rodríguez, Y.; Morales, L.C.; Matheus, L.; Arboleda, H. Differential regulation of AKT, MAPK and GSK3β during C2-ceramide-induced neuronal death. Neurotoxicology 2010, 31, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Martinez, T.N.; Chen, X.; Bandyopadhyay, S.; Merrill, A.H.; Tansey, M.G. Ceramide sphingolipid signaling mediates Tumor Necrosis Factor (TNF)-dependent toxicity via caspase signaling in dopaminergic neurons. Mol. Neurodegener. 2012, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- France-Lanord, V.; Brugg, B.; Michel, P.P.; Agid, Y.; Ruberg, M. Mitochondrial free radical signal in ceramide-dependent apoptosis: A putative mechanism for neuronal death in Parkinson’s disease. J. Neurochem. 1997, 69, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, G.; Waters, C.; Gibson, R. Inhibition of caspases but not of calpains temporarily protect against C2-ceramide-induced death of CAD cells. Neurosci. Lett. 2007, 421, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.A.; Ancolio, K.; Checler, F. Wild-type but not Parkinson’s disease-related ala-53→Thr mutant alpha -synuclein protects neuronal cells from apoptotic stimuli. J. Biol. Chem. 2000, 275, 24065–24069. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mora, R.M.; Arboleda, H.; Arboleda, G. PINK1 overexpression protects against C2-ceramide-induced CAD cell death through the PI3K/AKT pathway. J. Mol. Neurosci. 2012, 47, 582–594. [Google Scholar] [CrossRef]
- Rojas-Charry, L.; Cookson, M.R.; Niño, A.; Arboleda, H.; Arboleda, G. Downregulation of Pink1 influences mitochondrial fusion-fission machinery and sensitizes to neurotoxins in dopaminergic cells. Neurotoxicology 2014, 44, 140–148. [Google Scholar] [CrossRef]
- Jaramillo-Gómez, J.; Niño, A.; Arboleda, H.; Arboleda, G. Overexpression of DJ-1 protects against C2-ceramide-induced neuronal death through activation of the PI3K/AKT pathway and inhibition of autophagy. Neurosci. Lett. 2015, 603, 71–76. [Google Scholar] [CrossRef]
- Jung, J.S.; Shin, K.O.; Lee, Y.M.; Shin, J.A.; Park, E.M.; Jeong, J.; Kim, D.H.; Choi, J.W.; Kim, H.S. Anti-inflammatory mechanism of exogenous C2 ceramide in lipopolysaccharide-stimulated microglia. Biochim. Biophys. Acta 2013, 1831, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, X.; Duan, C.; Lu, L.; Yang, H. Alpha-synuclein overexpression increases phospho-protein phosphatase 2A levels via formation of calmodulin/Src complex. Neurochem. Int. 2013, 63, 180–194. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, M.; Du, T.; Duan, C.; Gao, G.; Yang, H. The novel mechanism of rotenone-induced α-synuclein phosphorylation via reduced protein phosphatase 2A activity. Int. J. Biochem. Cell Biol. 2016, 75, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.F. Sphingolipids in inflammation: Pathological implications and potential therapeutic targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef]
- Norris, G.H.; Blesso, C.N. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Aβ Accumulation. Oxid. Med. Cell. Longev. 2015, 2015, 346783. [Google Scholar] [CrossRef]
- Tommasino, C.; Marconi, M.; Ciarlo, L.; Matarrese, P.; Malorni, W. Autophagic flux and autophagosome morphogenesis require the participation of sphingolipids. Apoptosis 2015, 20, 645–657. [Google Scholar] [CrossRef]
- Foo, J.N.; Liany, H.; Bei, J.X.; Yu, X.-Q.; Liu, J.; Au, W.L.; Prakash, K.M.; Tan, L.C.; Tan, E.K. Rare lysosomal enzyme gene SMPD1 variant (p.R591C) associates with Parkinson’s disease. Neurobiol. Aging 2013, 34, 2890.e13-5. [Google Scholar] [CrossRef]
- Mao, C.Y.; Yang, J.; Wang, H.; Zhang, S.Y.; Yang, Z.H.; Luo, H.Y.; Li, F.; Shi, M.; Liu, Y.T.; Zhuang, Z.P.; et al. SMPD1 variants in Chinese Han patients with sporadic Parkinson’s disease. Park. Relat. Disord. 2017, 34, 59–61. [Google Scholar] [CrossRef]
- Kim, W.S.; Halliday, G.M. Changes in sphingomyelin level affect alpha-synuclein and ABCA5 expression. J. Park. Dis. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Den Jager, W.A. Sphingomyelin in Lewy inclusion bodies in Parkinson’s disease. Arch. Neurol. 1969, 21, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Gegg, M.E.; Sweet, L.; Wang, B.H.; Shihabuddin, L.S.; Sardi, S.P.; Schapira, A.H.V. No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov. Disord. 2015, 30, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef] [PubMed]
- Kurup, R.K.; Kurup, P.A. Hypothalamic digoxin-mediated model for Parkinson’s disease. Int. J. Neurosci. 2003, 113, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Sun, Y.; Shacka, J.J.; Auray-Blais, C. Tandem Mass Spectrometry Multiplex Analysis of Glucosylceramide and Galactosylceramide Isoforms in Brain Tissues at Different Stages of Parkinson Disease. Anal. Chem. 2016, 88, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Gegg, M.E.; Schapira, A.H.V. The role of glucocerebrosidase in Parkinson disease pathogenesis. FEBS J. 2018. [Google Scholar] [CrossRef]
- Marshall, M.S.; Bongarzone, E.R. Beyond Krabbe’s disease: The potential contribution of galactosylceramidase deficiency to neuronal vulnerability in late-onset synucleinopathies. J. Neurosci. Res. 2016, 94, 1328–1332. [Google Scholar] [CrossRef]
- Kim, S.; Yun, S.P.; Lee, S.; Umanah, G.E.; Bandaru, V.V.R.; Yin, X.; Rhee, P.; Karuppagounder, S.S.; Kwon, S.H.; Lee, H.; et al. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc. Natl. Acad. Sci. USA 2018, 115, 798–803. [Google Scholar] [CrossRef]
- Zunke, F.; Moise, A.C.; Belur, N.R.; Gelyana, E.; Stojkovska, I.; Dzaferbegovic, H.; Toker, N.J.; Jeon, S.; Fredriksen, K.; Mazzulli, J.R. Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron 2017, 97, 92–107.e10. [Google Scholar] [CrossRef]
- Xu, Y.H.; Sun, Y.; Ran, H.; Quinn, B.; Witte, D.; Grabowski, G.A. Accumulation and distribution of α-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol. Genet. Metab. 2011, 102, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Fujikake, N.; Takeuchi, T.; Kohyama-Koganeya, A.; Nakajima, K.; Hirabayashi, Y.; Wada, K.; Nagai, Y. Glucocerebrosidase deficiency accelerates the accumulation of proteinase K-resistant α-synuclein and aggravates neurodegeneration in a Drosophila model of Parkinson’s disease. Hum. Mol. Genet. 2015, 24, 6675–6686. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Xu, Y.H.; Sun, Y.; Knight, A.L.; McLean, P.J.; Caldwell, G.A.; Sidransky, E.; Grabowski, G.A.; Krainc, D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 2011, 146, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Sardi, S.P.; Viel, C.; Clarke, J.; Treleaven, C.M.; Richards, A.M.; Park, H.; Olszewski, M.A.; Dodge, J.C.; Marshall, J.; Makino, E.; et al. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc. Natl. Acad. Sci. USA 2017, 114, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Noelker, C.; Lu, L.; Höllerhage, M.; Vulinovic, F.; Sturn, A.; Roscher, R.; Höglinger, G.U.; Hirsch, E.C.; Oertel, W.H.; Alvarez-Fisher, D.; et al. Glucocerebrosidase deficiency and mitochondrial impairment in experimental Parkinson disease. J. Neurol. Sci. 2015, 356, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.J.; Huebecker, M.; Brekk, O.R.; Moloney, E.B.; Rocha, E.M.; Priestman, D.A.; Platt, F.M.; Isacson, O. Glycosphingolipid levels and glucocerebrosidase activity are altered in normal aging of the mouse brain. Neurobiol. Aging 2018, 67, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.T.; Ariga, T.; Yanagisawa, M. Structures, biosynthesis, and functions of gangliosides—An overview. J. Oleo Sci. 2011, 60, 537–544. [Google Scholar] [CrossRef]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; McJarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef]
- Schnaar, R.L. Gangliosides of the Vertebrate Nervous System. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Kulkarni, N.; Ledeen, R.W. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 2012, 90, 1997–2008. [Google Scholar] [CrossRef]
- Schneider, J.S. Altered expression of genes involved in ganglioside biosynthesis in substantia nigra neurons in Parkinson’s disease. PLoS ONE 2018, 13, e0199189. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Cambi, F.; Gollomp, S.M.; Kuwabara, H.; Brašić, J.R.; Leiby, B.; Sendek, S.; Wong, D.F. GM1 ganglioside in Parkinson’s disease: Pilot study of effects on dopamine transporter binding. J. Neurol. Sci. 2015, 356, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Gollomp, S.M.; Sendek, S.; Colcher, A.; Cambi, F.; Du, W. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J. Neurol. Sci. 2013, 324, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Sendek, S.; Daskalakis, C.; Cambi, F. GM1 ganglioside in Parkinson’s disease: Results of a five year open study. J. Neurol. Sci. 2010, 292, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S. GM1 ganglioside in the treatment of Parkinson’s disease. Ann. N. Y. Acad. Sci. 1998, 845, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Roeltgen, D.P.; Mancall, E.L.; Chapas-Crilly, J.; Rothblat, D.S.; Tatarian, G.T. Parkinson’s disease: Improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology 1998, 50, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Roeltgen, D.P.; Rothblat, D.S.; Chapas-Crilly, J.; Seraydarian, L.; Rao, J. GM1 ganglioside treatment of Parkinson’s disease: An open pilot study of safety and efficacy. Neurology 1995, 45, 1149–1154. [Google Scholar] [CrossRef]
- Schneider, J.S.; Seyfried, T.N.; Choi, H.S.; Kidd, S.K. Intraventricular Sialidase Administration Enhances GM1 Ganglioside Expression and Is Partially Neuroprotective in a Mouse Model of Parkinson’s Disease. PLoS ONE 2015, 10, e0143351. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Y.; Fang, X.; Lu, Y.; Li, J.; Zhang, J.; Deng, X.; Li, S. The possible mechanism of Parkinson’s disease progressive damage and the preventive effect of GM1 in the rat model induced by 6-hydroxydopamine. Brain Res. 2014, 1592, 73–81. [Google Scholar] [CrossRef]
- Goettl, V.M.; Wemlinger, T.A.; Duchemin, A.M.; Neff, N.H.; Hadjiconstantinou, M. GM1 ganglioside restores dopaminergic neurochemical and morphological markers in aged rats. Neuroscience 1999, 92, 991–1000. [Google Scholar] [CrossRef]
- Emborg, M.E.; Colombo, J.A. Long-term MPTP-treated monkeys are resistant to GM1 systemic therapy. Mol. Chem. Neuropathol. 1994, 21, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pope-Coleman, A.; Schneider, J.S. Effects of Chronic GM1 Ganglioside Treatment on Cognitieve and Motor Deficits in a Slowly Progressing Model of Parkinsonism in Non-Human Primates. Restor. Neurol. Neurosci. 1998, 12, 255–266. [Google Scholar] [PubMed]
- Herrero, M.T.; Perez-Otaño, I.; Oset, C.; Kastner, A.; Hirsch, E.C.; Agid, Y.; Luguin, M.R.; Obeso, J.A.; Del Río, J. GM-1 ganglioside promotes the recovery of surviving midbrain dopaminergic neurons in MPTP-treated monkeys. Neuroscience 1993, 56, 965–972. [Google Scholar] [CrossRef]
- Rothblat, D.S.; Schneider, J.S. Effects of GM1 ganglioside treatment on dopamine innervation of the striatum of MPTP-treated mice. Ann. N. Y. Acad. Sci. 1998, 845, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Kean, A.; DiStefano, L. GM1 ganglioside rescues substantia nigra pars compacta neurons and increases dopamine synthesis in residual nigrostriatal dopaminergic neurons in MPTP-treated mice. J. Neurosci. Res. 1995, 42, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kastner, A.; Herrero, M.T.; Hirsch, E.C.; Guillen, J.; Luquin, M.R.; Javoy-Agid, F.; Obeso, J.A.; Agid, Y. Decreased tyrosine hydroxylase content in the dopaminergic neurons of MPTP-intoxicated monkeys: Effect of levodopa and GM1 ganglioside therapy. Ann. Neurol. 1994, 36, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.T.; Kastner, A.; Perez-Otaño, I.; Hirsch, E.C.; Luquin, M.R.; Javoy-Agid, F.; Del Río, J.; Obeso, J.A.; Agid, Y. Gangliosides and parkinsonism. Neurology 1993, 43, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Pope, A.; Simpson, K.; Taggart, J.; Smith, M.G.; DiStefano, L. Recovery from experimental parkinsonism in primates with GM1 ganglioside treatment. Science 1992, 256, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S. MPTP-induced parkinsonism: Acceleration of biochemical and behavioral recovery by GM1 ganglioside treatment. J. Neurosci. Res. 1992, 31, 112–119. [Google Scholar] [CrossRef]
- Fazzini, E.; Durso, R.; Davoudi, H.; Szabo, G.K.; Albert, M.L. GM1 gangliosides alter acute MPTP-induced behavioral and neurochemical toxicity in mice. J. Neurol. Sci. 1990, 99, 59–68. [Google Scholar] [CrossRef]
- Gupta, M.; Schwarz, J.; Chen, X.L.; Roisen, F.J. Gangliosides prevent MPTP toxicity in mice—An immunocytochemical study. Brain Res. 1990, 527, 330–334. [Google Scholar] [CrossRef]
- Schneider, J.S.; Yuwiler, A. GM1 ganglioside treatment promotes recovery of striatal dopamine concentrations in the mouse model of MPTP-induced parkinsonism. Exp. Neurol. 1989, 105, 177–183. [Google Scholar] [CrossRef]
- Hadjiconstantinou, M.; Mariani, A.P.; Neff, N.H. GM1 ganglioside-induced recovery of nigrostriatal dopaminergic neurons after MPTP: An immunohistochemical study. Brain Res. 1989, 484, 297–303. [Google Scholar] [CrossRef]
- Ba, X. Therapeutic effects of GM1 on Parkinson’s disease in rats and its mechanism. Int. J. Neurosci. 2016, 126, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Lundius, E.G.; Vukojevic, V.; Hertz, E.; Stroth, N.; Cederlund, A.; Hiraiwa, M.; Terenius, L.; Svennigsson, P. GPR37 protein trafficking to the plasma membrane regulated by prosaposin and GM1 gangliosides promotes cell viability. J. Biol. Chem. 2014, 289, 4660–4673. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, K.S.; Lee, S.B.; Ryu, J.S.; Chung, K.C.; Choo, Y.K.; Jou, I.; Kim, J.; Park, S.M. On the mechanism of internalization of alpha-synuclein into microglia: Roles of ganglioside GM1 and lipid raft. J. Neurochem. 2009, 110, 400–411. [Google Scholar] [CrossRef]
- Grey, M.; Dunning, C.J.; Gaspar, R.; Grey, C.; Brundin, P.; Sparr, E.; Linse, S. Acceleration of α-synuclein aggregation by exosomes. J. Biol. Chem. 2015, 290, 2969–2982. [Google Scholar] [CrossRef]
- Martinez, Z.; Zhu, M.; Han, S.; Fink, A.L. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry 2007, 46, 1868–1877. [Google Scholar] [CrossRef]
- Garten, M.; Prévost, C.; Cadart, C.; Gautier, R.; Bousset, L.; Melki, R.; Bassereau, P.; Vanni, S. Methyl-branched lipids promote the membrane adsorption of α-synuclein by enhancing shallow lipid-packing defects. Phys. Chem. Chem. Phys. 2015, 17, 15589–15597. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Kulkarni, N.; Amin, R.; Ledeen, R.W. Mice lacking major brain gangliosides develop parkinsonism. Neurochem. Res. 2011, 36, 1706–1714. [Google Scholar] [CrossRef]
- Suzuki, K.; Iseki, E.; Togo, T.; Yamaguchi, A.; Katsuse, O.; Katsuyama, K.; Kanzaki, S.; Shiozaki, K.; Kawanishi, C.; Yamashita, S.; et al. Neuronal and glial accumulation of alpha- and beta-synucleins in human lipidoses. Acta Neuropathol. 2007, 114, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Akkhawattanangkul, Y.; Maiti, P.; Xue, Y.; Aryal, D.; Wetsel, W.C.; Hamilton, D.; Fowler, S.C.; McDonald, M.P. Targeted deletion of GD3 synthase protects against MPTP-induced neurodegeneration. Genes Brain Behav. 2017, 16, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Shin, W.H.; Kim, J.; Joe, E.H.; Lee, Y.B.; Cho, K.G.; Oh, Y.J.; Kim, S.U.; Jun, B.K. Trisialoganglioside GT1b induces in vivo degeneration of nigral dopaminergic neurons: Role of microglia. Glia 2002, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Fujita, M.; Nakai, M.; Waragai, M.; Sekigawa, A.; Sugama, S.; Takenouchi, T.; Masliah, E.; Hashimoto, M. Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies. Am. J. Pathol. 2009, 174, 1891–1909. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Finkielstein, C.V.; Capelluto, D.G.S. The enigmatic role of sulfatides: New insights into cellular functions and mechanisms of protein recognition. Adv. Exp. Med. Biol. 2013, 991, 27–40. [Google Scholar] [CrossRef]
- Antelmi, E.; Rizzo, G.; Fabbri, M.; Capellari, S.; Scaglione, C.; Martinelli, P. Arylsulphatase A activity in familial parkinsonism: A pathogenetic role? J. Neurol. 2014, 261, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, P.; Ippoliti, M.; Montanari, M.; Martinelli, A.; Mochi, M.; Giuliani, S.; Sangiorgi, S. Arylsulphatase A (ASA) activity in parkinsonism and symptomatic essential tremor. Acta Neurol. Scand. 1994, 89, 171–174. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, J.; McKeel, D.W.; Han, X. Specificity and potential mechanism of sulfatide deficiency in Alzheimer’s disease: An electrospray ionization mass spectrometric study. Cell. Mol. Biol. (Noisy-le-grand) 2003, 49, 809–818. [Google Scholar]
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Hannich, J.T.; Umebayashi, K.; Riezman, H. Distribution and Functions of Sterols and Sphingolipids. Cold Spring Harb. Perspect. Biol. 2011, 3, a004762. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.M.; Smith-Weller, T.; Franklin, G.M.; Longstreth, W.T.; Swanson, P.D.; Checkoway, H. Dietary fats, cholesterol and iron as risk factors for Parkinson’s disease. Park. Relat. Disord. 2009, 15, 47–52. [Google Scholar] [CrossRef]
- Johnson, C.C.; Gorell, J.M.; Rybicki, B.A.; Sanders, K.; Peterson, E.L. Adult nutrient intake as a risk factor for Parkinson’s disease. Int. J. Epidemiol. 1999, 28, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Sanderson, W.T.; Burchfiel, C.M.; Kashon, M.; Sharp, D.S.; Masaki, K.H.; Curb, J.D.; Petrovitch, H. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: Recent findings from the Honolulu-Asia Aging Study. J. Neurol. 2003, 250 (Suppl. 3), III30–III39. [Google Scholar] [CrossRef]
- Wang, A.; Lin, Y.; Wu, Y.; Zhang, D. Macronutrients intake and risk of Parkinson’s disease: A meta-analysis. Geriatr. Gerontol. Int. 2015, 15, 606–616. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, M.; Sterling, N.W.; Du, G.; Lewis, M.M.; Yao, T.; Mailman, R.B.; Li, R.; Huang, X. Circulating Cholesterol Levels May Link to the Factors Influencing Parkinson’s Risk. Front. Neurol. 2017, 8, 501. [Google Scholar] [CrossRef]
- Kirbas, A.; Kirbas, S.; Cure, M.C.; Tufekci, A. Paraoxonase and arylesterase activity and total oxidative/anti-oxidative status in patients with idiopathic Parkinson’s disease. J. Clin. Neurosci. 2014, 21, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Nakamura, Y.; Kiyozuka, T.; Aoyagi, J.; Hirayama, T.; Nagata, R.; Ito, H.; Iwamoto, K.; Murata, K.; Yoshii, Y.; et al. Serological profiles of urate, paraoxonase-1, ferritin and lipid in Parkinson’s disease: Changes linked to disease progression. Neurodegener. Dis. 2011, 8, 252–258. [Google Scholar] [CrossRef]
- Huang, X.; Alonso, A.; Guo, X.; Umbach, D.M.; Lichtenstein, M.L.; Ballantyne, C.M.; Mailman, R.B.; Mosley, T.H.; Chen, H. Statins, plasma cholesterol, and risk of Parkinson’s disease: A prospective study. Mov. Disord. 2015, 30, 552–559. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J. Neurol. Sci. 2010, 293, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.C.; Chen, H.; Schwarzschild, M.; Ascherio, A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007, 69, 1688–1695. [Google Scholar] [CrossRef]
- De Lau, L.M.L.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M.B. Serum cholesterol levels and the risk of Parkinson’s disease. Am. J. Epidemiol. 2006, 164, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Rozani, V.; Gurevich, T.; Giladi, N.; El-Ad, B.; Tsamir, J.; Hemo, B.; Peretz, C. Higher serum cholesterol and decreased Parkinson’s disease risk: A statin-free cohort study. Mov. Disord. 2018, 33, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Auinger, P.; Eberly, S.; Oakes, D.; Schwarzschild, M.; Ascherio, A.; Mailman, R.; Chen, H.; Parkinson Study Group DATATOP Investigators. Serum cholesterol the progression of Parkinson’s disease: Results from DATATOP. PLoS ONE 2011, 6, e22854. [Google Scholar] [CrossRef] [PubMed]
- Gudala, K.; Bansal, D.; Muthyala, H. Role of serum cholesterol in Parkinson’s disease: A meta-analysis of evidence. J. Park. Dis. 2013, 3, 363–370. [Google Scholar] [CrossRef]
- Sterling, N.W.; Lichtenstein, M.; Lee, E.Y.; Lewis, M.M.; Evans, A.; Eslinger, P.J.; Du, G.; Gao, X.; Chen, H.; Kong, L.; et al. Higher Plasma LDL-Cholesterol is Associated with Preserved Executive and Fine Motor Functions in Parkinson’s Disease. Aging Dis. 2016, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Ahlskog, J.E.; Rocca, W.A. Metabolic markers or conditions preceding Parkinson’s disease: A case-control study. Mov. Disord. 2012, 27, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Banerjee, B.D.; Bala, K.; Mitrabasu Dung Dung, A.A.; Chhillar, N. APOE and LRPAP1 gene polymorphism and risk of Parkinson’s disease. Neurol. Sci. 2014, 35, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Trautmann, E.; Sixel-Döring, F.; Wicke, T.; Ebentheuer, J.; Schaumburg, M.; Lang, E.; Focke, N.K.; Kumar, K.R.; Lohmann, K.; et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013, 81, 1226–1234. [Google Scholar] [CrossRef]
- Hu, G.; Antikainen, R.; Jousilahti, P.; Kivipelto, M.; Tuomilehto, J. Total cholesterol and the risk of Parkinson disease. Neurology 2008, 70, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Cassani, E.; Barichella, M.; Caccialanza, R.; Pezzoli, G. Anthropometric indices of fat distribution and cardiometabolic risk in Parkinson’s disease. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Musanti, R.; Parati, E.; Lamperti, E.; Ghiselli, G. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem. Med. Metab. Biol. 1993, 49, 133–142. [Google Scholar] [CrossRef]
- Shulman, J.M.; Yu, L.; Buchman, A.S.; Evans, D.A.; Schneider, J.A.; Bennett, D.A.; De Jager, P.L. Association of Parkinson Disease Risk Loci With Mild Parkinsonian Signs in Older Persons. JAMA Neurol. 2014, 71, 429. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Li, M.; Liu, N.; Li, X.; Ren, Y.; Luo, X. The Polymorphism of SREBF1 Gene rs11868035 G/A Is Associated with susceptibility to Parkinson’s disease in a Chinese Population. Int. J. Neurosci. 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, B.; Wu, Y.; Chen, Y.; Wei, Q.; Ou, R.; Yang, J.; Chen, X.; Zhao, B.; Song, W.; et al. Association analysis of SNP rs11868035 in SREBF1 with sporadic Parkinson’s disease, sporadic amyotrophic lateral sclerosis and multiple system atrophy in a Chinese population. Neurosci. Lett. 2018, 664, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hasson, S.A.; Fogel, A.I.; Wang, C.; MacArthur, R.; Guha, R.; Heman-Ackah, S.; Martin, S.; Youle, R.J.; Inglese, J. Chemogenomic profiling of endogenous PARK2 expression using a genome-edited coincidence reporter. ACS Chem. Biol. 2015, 10, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Stevens, M.V.; Akter, M.H.; Rusk, S.E.; Huang, R.J.; Cohen, A.; Noguchi, A.; Springer, D.; Bocharov, A.V.; Eggerman, T.L.; et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Investig. 2011, 121, 3701–3712. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamane, T.; Takahashi-Niki, K.; Kato, I.; Niki, T.; Goldberg, M.S.; Shen, J.; Ishimoto, K.; Doi, T.; Iguchi-Ariga, S.M. Transcriptional activation of low-density lipoprotein receptor gene by DJ-1 and effect of DJ-1 on cholesterol homeostasis. PLoS ONE 2012, 7, e38144. [Google Scholar] [CrossRef]
- Kim, J.M.; Cha, S.H.; Choi, Y.R.; Jou, I.; Joe, E.H.; Park, S.M. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci. Rep. 2016, 6, 28823. [Google Scholar] [CrossRef]
- Kyung, J.W.; Kim, J.M.; Lee, W.; Ha, T.Y.; Cha, S.H.; Chung, K.H.; Choi, D.J.; Joi, I.; Song, W.K.; Joe, E.H.; et al. DJ-1 deficiency impairs synaptic vesicle endocytosis and reavailability at nerve terminals. Proc. Natl. Acad. Sci. USA 2018, 115, 1629–1634. [Google Scholar] [CrossRef]
- Magalhaes, J.; Gegg, M.E.; Migdalska-Richards, A.; Doherty, M.K.; Whitfield, P.D.; Schapira, A.H.V. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: Relevance to Parkinson disease. Hum. Mol. Genet. 2016, 25, 3432–3445. [Google Scholar] [CrossRef]
- Cha, S.H.; Choi, Y.R.; Heo, C.H.; Kang, S.J.; Joe, E.H.; Jou, I.; Kim, H.M.; Park, S.M. Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: Implications for Parkinson’s disease. Mol. Neurodegener. 2015, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, P.; Orgaz, L.; Bueno-Gil, G.; Espadas, I.; Rodríguez-Traver, E.; Kulisevsky, J.; Gutierrez, A.; Dávila, J.C.; González-Polo, R.A.; Fuentes, J.M.; et al. N370S-GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson’s disease. Mov. Disord. 2017, 32, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.A.S.; Dave, K.D.; Frasier, M.A.; Sherer, T.B.; Greeley, M.; Beck, M.J.; Varsho, J.S.; Parker, G.A.; Moore, C.; Churchill, M.J.; et al. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS ONE 2013, 8, e80705. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, I.; Nath, S.; Bornefall, P.; Giraldo, A.M.V.; Öllinger, K. Impact of high cholesterol in a Parkinson’s disease model: Prevention of lysosomal leakage versus stimulation of α-synuclein aggregation. Eur. J. Cell Biol. 2017, 96, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Dehay, B.; Bezard, E.; Garcia-Ladona, F.J. U18666A, an activator of sterol regulatory element binding protein pathway, modulates presynaptic dopaminergic phenotype of SH-SY5Y neuroblastoma cells. Synapse 2017, 71, e21980. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Morin, N.; Rouillard, C.; Di Paolo, T. Membrane cholesterol removal and replenishment affect rat and monkey brain monoamine transporters. Neuropharmacology 2018, 133, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Choudhury, A.; Kumar, S.; Giri, A.; Sandhir, R.; Borah, A. Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson’s disease: Involvement of mitochondrial dysfunctions and oxidative stress. PLoS ONE 2017, 12, e0171285. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Choudhury, A.; Chandra Boruah, D.; Devi, R.; Bhattacharya, P.; Choudhury, M.D.; Borach, A. Hypercholesterolemia causes psychomotor abnormalities in mice and alterations in cortico-striatal biogenic amine neurotransmitters: Relevance to Parkinson’s disease. Neurochem. Int. 2017, 108, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Dutta, A.; Phukan, B.C.; Mazumder, M.K.; Justin-Thenmozhi, A.; Manivasagam, T.; Bhattacharya, P.; Borah, A. Accumulation of Cholesterol and Homocysteine in the Nigrostriatal Pathway of Brain Contributes to the Dopaminergic Neurodegeneration in Mice. Neuroscience 2018, 388, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Raju, A.; Jaisankar, P.; Borah, A.; Mohanakumar, K.P. 1-Methyl-4-Phenylpyridinium-Induced Death of Differentiated SH-SY5Y Neurons Is Potentiated by Cholesterol. Ann. Neurosci. 2018, 24, 243–251. [Google Scholar] [CrossRef]
- Fantini, J.; Carlus, D.; Yahi, N. The fusogenic tilted peptide (67-78) of α-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta 2011, 1808, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Beyer, K. Binding of alpha-synuclein affects the lipid packing in bilayers of small vesicles. J. Biol. Chem. 2006, 281, 9251–9259. [Google Scholar] [CrossRef] [PubMed]
- Van Maarschalkerweerd, A.; Vetri, V.; Vestergaard, B. Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett. 2015, 589, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Shvadchak, V.V.; Falomir-Lockhart, L.J.; Yushchenko, D.A.; Jovin, T.M. Specificity and kinetics of alpha-synuclein binding to model membranes determined with fluorescent excited state intramolecular proton transfer (ESIPT) probe. J. Biol. Chem. 2011, 286, 13023–13032. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.E.; Gysbers, A.M.; Abbott, S.K.; Spiro, A.S.; Furuta, A.; Cooper, A.; Garner, B.; Kabuta, T.; Halliday, G.M. Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov. Disord. 2015, 30, 1639–1647. [Google Scholar] [CrossRef]
- Bar-On, P.; Crews, L.; Koob, A.O.; Mizuno, H.; Adame, A.; Spencer, B.; Masliah, E. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J. Neurochem. 2008, 105, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Di Scala, C.; Yahi, N.; Boutemeur, S.; Flores, A.; Rodriguez, L.; Chahinian, H.; Fantini, J. Common molecular mechanism of amyloid pore formation by Alzheimer’s β-amyloid peptide and α-synuclein. Sci. Rep. 2016, 6, 28781. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Yahi, N. The driving force of alpha-synuclein insertion and amyloid channel formation in the plasma membrane of neural cells: Key role of ganglioside- and cholesterol-binding domains. Adv. Exp. Med. Biol. 2013, 991, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bate, C.; Williams, A. α-Synuclein-induced synapse damage in cultured neurons is mediated by cholesterol-sensitive activation of cytoplasmic phospholipase A2. Biomolecules 2015, 5, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, P.; Rockenstein, E.; Adame, A.; Ho, G.; Hashimoto, M.; Masliah, E. Effects of the cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synucleinopathy. J. Neurochem. 2006, 98, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.L.; Troyer, M.D.; Nakamura, K.; Kubo, S.; Anthony, M.D.; Edwards, R.H. Lipid rafts mediate the synaptic localization of alpha-synuclein. J. Neurosci. 2004, 24, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.H.T.; Halliday, G.M.; Kim, W.S. α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules 2017, 22, 1769. [Google Scholar] [CrossRef] [PubMed]
- Leftin, A.; Job, C.; Beyer, K.; Brown, M.F. Solid-state 13C NMR reveals annealing of raft-like membranes containing cholesterol by the intrinsically disordered protein α-Synuclein. J. Mol. Biol. 2013, 425, 2973–2987. [Google Scholar] [CrossRef]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Chesselet, M.F.; Fleming, S.; Mortazavi, F.; Meurers, B. Strengths and limitations of genetic mouse models of Parkinson’s disease. Park. Relat. Disord. 2008, 14 (Suppl. 2), S84–S87. [Google Scholar] [CrossRef]
- Sheng, Z.; Jia, X.; Kang, M. Statin use and risk of Parkinson’s disease: A meta-analysis. Behav. Brain Res. 2016, 309, 29–34. [Google Scholar] [CrossRef]
- Friedman, B.; Lahad, A.; Dresner, Y.; Vinker, S. Long-term statin use and the risk of Parkinson’s disease. Am. J. Manag. Care 2013, 19, 626–632. [Google Scholar]
- Gao, X.; Simon, K.C.; Schwarzschild, M.A.; Ascherio, A. Prospective study of statin use and risk of Parkinson disease. Arch. Neurol. 2012, 69, 380–384. [Google Scholar] [CrossRef]
- Wolozin, B.; Wang, S.W.; Li, N.C.; Lee, A.; Lee, T.A.; Kazis, L.E. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007, 5, 20. [Google Scholar] [CrossRef]
- Huang, X.; Chen, H.; Miller, W.C.; Mailman, R.B.; Woodard, J.L.; Chen, P.C.; Xiang, D.; Murrow, R.M.; Wand, Y.Z.; Poole, C. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov. Disord. 2007, 22, 22. [Google Scholar] [CrossRef]
- Wahner, A.D.; Bronstein, J.M.; Bordelon, Y.M.; Ritz, B. Statin use and the risk of Parkinson disease. Neurology 2008, 70, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Rozani, V.; Giladi, N.; El-Ad, B.; Gurevich, T.; Tsamir, J.; Hemo, B.; Peretz, C. Statin adherence and the risk of Parkinson’s disease: A population-based cohort study. PLoS ONE 2017, 12, e0175054. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Manthripragada, A.D.; Qian, L.; Schernhammer, E.; Wermuth, L.; Olsen, J.; Friss, S. Statin use and Parkinson’s disease in Denmark. Mov. Disord. 2010, 25, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Jick, S.S.; Meier, C.R. Use of statins and the risk of Parkinson’s disease: A retrospective case-control study in the UK. Drug Saf. 2008, 31, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sterling, N.W.; Kong, L.; Lewis, N.M.; Mailman, R.B.; Chen, H.; Leslie, D.; Huang, X. Statins may facilitate Parkinson’s disease: Insight gained from a large, national claims database. Mov Disord 2017, 32, 913–917. [Google Scholar] [CrossRef]
- Bykov, K.; Yoshida, K.; Weisskopf, M.G.; Gagne, J.J. Confounding of the association between statins and Parkinson disease: Systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2017, 26, 294–300. [Google Scholar] [CrossRef]
- Marques, N.F.; Castro, A.A.; Mancini, G.; Rocha, F.L.; Santos, A.R.S.; Prediger, R.D.; De Bem, A.F.; Tasca, C.I. Atorvastatin Prevents Early Oxidative Events and Modulates Inflammatory Mediators in the Striatum Following Intranasal 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) Administration in Rats. Neurotox. Res. 2018, 33, 549–559. [Google Scholar] [CrossRef]
- Yan, J.Q.; Ma, Y.J.; Sun, J.C.; Bai, S.F.; Huang, L.N. Neuroprotective effect of lovastatin by inhibiting NMDA receptor1 in 6-hydroxydopamine treated PC12 cells. Int. J. Clin. Exp. Med. 2014, 7, 3313–3319. [Google Scholar]
- Jiang, P.; Gan, M.; Lin, W.L.; Yen, S.H.C. Nutrient deprivation induces α-synuclein aggregation through endoplasmic reticulum stress response and SREBP2 pathway. Front. Aging Neurosci. 2014, 6, 268. [Google Scholar] [CrossRef]
- Koob, A.O.; Ubhi, K.; Paulsson, J.F.; Kelly, J.; Rockenstein, E.; Mante, M.; Adame, A.; Masliah, E. Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Exp. Neurol. 2010, 221, 267–274. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Long, L.; Yan, J.Q.; Wei, L.; Pan, M.Q.; Gao, H.M.; Zhou, P.; Liu, M.; Zhu, C.S.; Tang, B.S.; et al. Simvastatin induces neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3 pathway and anti-inflammatory responses. CNS Neurosci. Ther. 2013, 19, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, N.; Gupta, A.; Kalonia, H.; Mishra, J. Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res. 2012, 1471, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Roy, A.; Matras, J.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2009, 29, 13543–13556. [Google Scholar] [CrossRef] [PubMed]
- Selley, M.L. Simvastatin prevents 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain. Res. 2005, 1037, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, X.N.; Wang, L.; Yenari, M.A.; Ying, W.; Goh, B.C.; Lee, H.S.; Wilder-Smith, E.P.; Wong, P.T. Effects of high dose of simvastatin on levels of dopamine and its reuptake in prefrontal cortex and striatum among SD rats. Neurosci. Lett. 2006, 408, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Kreisler, A.; Gelé, P.; Wiart, J.F.; Lhermitte, M.; Destée, A.; Bordet, R. Lipid-lowering drugs in the MPTP mouse model of Parkinson’s disease: Fenofibrate has a neuroprotective effect, whereas bezafibrate and HMG-CoA reductase inhibitors do not. Brain Res. 2007, 1135, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Schirris, T.J.J.; Renkema, G.H.; Ritschel, T.; Voermans, N.C.; Bilos, A.; van Engelen, B.G.M.; Brandt, U.; Koopman, W.J.; Beyrath, J.D.; Rodenburg, R.J.; et al. Statin-Induced Myopathy Is Associated with Mitochondrial Complex III Inhibition. Cell Metab. 2015, 22, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Mori, F.; Tanji, K.; Miki, Y.; Yamada, M.; Kakita, A.; Takahasi, H.; Utsumi, J.; Sasaki, H.; Wakabayashi, K. Isopentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathology 2015, 35, 432–440. [Google Scholar] [CrossRef]
- Kabuto, H.; Yamanushi, T.T.; Janjua, N.; Takayama, F.; Mankura, M. Effects of squalene/squalane on dopamine levels, antioxidant enzyme activity, and fatty acid composition in the striatum of Parkinson’s disease mouse model. J. Oleo Sci. 2013, 62, 21–28. [Google Scholar] [CrossRef]
- Lim, L.; Jackson-Lewis, V.; Wong, L.C.; Shui, G.H.; Goh, A.X.H.; Kesavapany, S.; Jenner, A.M.; Fivaz, M.; Przedborski, S.; Wenk, M.R. Lanosterol induces mitochondrial uncoupling and protects dopaminergic neurons from cell death in a model for Parkinson’s disease. Cell Death Differ. 2012, 19, 416–427. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, A.; Jana, A.; Liu, X.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson’s disease. PLoS ONE 2012, 7, e38113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Glukhova, S.A.; Caldwell, K.A.; Caldwell, G.A. NCEH-1 modulates cholesterol metabolism and protects against α-synuclein toxicity in a C. elegans model of Parkinson’s disease. Hum. Mol. Genet. 2017, 26, 3823–3836. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Lövgren-Sandblom, A.; Leoni, V.; Meaney, S.; Brodin, L.; Salveson, L.; Winge, K.; Pålhagen, S.; Svenningsson, P. Oxysterols and Parkinson’s disease: Evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci. Lett. 2013, 555, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Holley, A.E.; Flitter, W.D.; Slater, T.F.; Wells, F.R.; Daniel, S.E.; Lee, A.J.; Jenner, P.; Marsden, C.D. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: An HPLC and ESR study. Mov. Disord. 1994, 9, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Patra, K.; Boxer, A.L.; Svenningsson, P. 24S-Hydroxycholesterol Correlates with Tau and Is Increased in Cerebrospinal Fluid in Parkinson’s Disease and Corticobasal Syndrome. Front. Neurol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Monaco, A.; Pedone, C.; Tessitore, A.; De Mase, A.; Tedeschi, G.; Netti, P.A.; Abrescia, P. The level of 24-hydroxycholesteryl esters decreases in plasma of patients with Parkinson’s disease. Neurosci. Lett. 2018, 672, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Rantham Prabhakara, J.P.; Feist, G.; Thomasson, S.; Thompson, A.; Schommer, E.; Ghribi, O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on tyrosine hydroxylase and alpha-synuclein in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1722–1729. [Google Scholar] [CrossRef]
- Marwarha, G.; Rhen, T.; Schommer, T.; Ghribi, O. The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors—Relevance to Parkinson’s disease. J. Neurochem. 2011, 119, 1119–1136. [Google Scholar] [CrossRef]
- Cheng, D.; Kim, W.S.; Garner, B. Regulation of alpha-synuclein expression by liver X receptor ligands in vitro. Neuroreport 2008, 19, 1685–1689. [Google Scholar] [CrossRef]
- Schommer, J.; Marwarha, G.; Schommer, T.; Flick, T.; Lund, J.; Ghribi, O. 27-Hydroxycholesterol increases α-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci. 2018, 19, 17. [Google Scholar] [CrossRef]
- Emanuelsson, I.; Norlin, M. Protective effects of 27- and 24-hydroxycholesterol against staurosporine-induced cell death in undifferentiated neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2012, 525, 44–48. [Google Scholar] [CrossRef]
- Theofilopoulos, S.; Wang, Y.; Kitambi, S.S.; Sacchetti, P.; Sousa, K.M.; Bodin, K.; Kirk, J.; Saltó, C.; Gustafsson, M.; Toledo, E.M.; et al. Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. Nat. Chem. Biol. 2013, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, P.; Sousa, K.M.; Hall, A.C.; Liste, I.; Steffensen, K.R.; Theofilopoulos, S.; Parish, C.L.; Hazenber, C.; Richter, L.A.; Hovatta, O.; et al. Liver X receptors and oxysterols promote ventral midbrain neurogenesis in vivo and in human embryonic stem cells. Cell Stem Cell 2009, 5, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014, 52, 1695–1727. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.R.; Berlyand, Y.; Xie, S.X.; Alcalay, R.N.; Chahine, L.M.; Chen-Plotkin, A.S. Plasma apolipoprotein A1 associates with age at onset and motor severity in early Parkinson’s disease patients. Mov. Disord. 2015, 30, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.R.; Li, K.; Unger, T.L.; Gallagher, M.D.; Van Deerlin, V.M.; Agarwal, P.; Leverenz, J.; Roberts, J.; Samii, A.; Gross, R.G.; et al. Lower plasma apolipoprotein A1 levels are found in Parkinson’s disease and associate with apolipoprotein A1 genotype. Mov. Disord. 2015, 30, 805–812. [Google Scholar] [CrossRef]
- Lu, W.; Wan, X.; Liu, B.; Rong, X.; Zhu, L.; Li, P.; Li, J.; Wang, L.; Cui, L.; Wang, X. Specific changes of serum proteins in Parkinson’s disease patients. PLoS ONE 2014, 9, e95684. [Google Scholar] [CrossRef]
- Qiang, J.K.; Wong, Y.C.; Siderowf, A.; Hurtig, H.I.; Xie, S.X.; Lee, V.M.Y.; Trojanowski, J.Q.; Yearout, D.; Leverenz, J.; Montine, T.J.; et al. Plasma apolipoprotein A1 as a biomarker for Parkinson disease. Ann. Neurol. 2013, 74, 119–127. [Google Scholar] [CrossRef]
- Cassani, E.; Cereda, E.; Barichella, M.; Madio, C.; Cancello, R.; Caccialanza, R.; Zini, M.; Cilia, R.; Pezzoli, G. Cardiometabolic factors and disease duration in patients with Parkinson’s disease. Nutrition 2013, 29, 1331–1335. [Google Scholar] [CrossRef]
- Kawata, M.; Nemoto, Y.; Asahina, M.; Moroo, I.; Shinomiya, M.; Yamada, T. Risk factors for cerebral arteriosclerosis in Parkinson’s disease. Park. Relat. Disord. 1996, 2, 75–79. [Google Scholar] [CrossRef]
- Du, G.; Lewis, M.M.; Shaffer, M.L.; Chen, H.; Yang, Q.X.; Mailman, R.B.; Huang, X. Serum cholesterol and nigrostriatal R2* values in Parkinson’s disease. PLoS ONE 2012, 7, e35397. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Abbott, R.D.; Petrovitch, H.; Mailman, R.B.; Ross, G.W. Low LDL cholesterol and increased risk of Parkinson’s disease: Prospective results from Honolulu-Asia Aging Study. Mov. Disord. 2008, 23, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjærg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef] [PubMed]
- Andican, G.; Konukoglu, D.; Bozluolcay, M.; Bayülkem, K.; Firtiına, S.; Burcak, G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol. Belg. 2012, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Williams, R.J.; Matin, R.; Iversen, L.; Rice-Evans, C.A. Phenolic antioxidants attenuate neuronal cell death following uptake of oxidized low-density lipoprotein. Free Radic. Biol. Med. 2000, 29, 1222–1233. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Tumanov, S.; Kamphorst, J.J. Recent advances in expanding the coverage of the lipidome. Curr. Opin. Biotechnol. 2017, 43, 127–133. [Google Scholar] [CrossRef]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Piomelli, D.; Astarita, G.; Rapaka, R. A neuroscientist’s guide to lipidomics. Nat. Rev. Neurosci. 2007, 8, 743–754. [Google Scholar] [CrossRef]
- Andersen, O.S.; Koeppe, R.E. Bilayer thickness and membrane protein function: An energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Rohrbough, J.; Broadie, K. Lipid regulation of the synaptic vesicle cycle. Nat. Rev. Neurosci. 2005, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Galvagnion, C.; Brown, J.W.P.; Ouberai, M.M.; Flagmeier, P.; Vendruscolo, M.; Buell, A.K.; Sparr, E.; Dobson, C.M. Chemical properties of lipids strongly affect the kinetics of the membrane-induced aggregation of α-synuclein. Proc. Natl. Acad. Sci. USA 2016, 113, 7065–7070. [Google Scholar] [CrossRef]
- Esposito, G.; Ana Clara, F.; Verstreken, P. Synaptic vesicle trafficking and Parkinson’s disease. Dev. Neurobiol. 2012, 72, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.A.; Ingrassia, A.; de Menezes, R.X.; van Kesteren, R.E.; Rozemuller, A.J.M.; Heutink, P.; van de Berg, W.D. Evidence for Immune Response, Axonal Dysfunction and Reduced Endocytosis in the Substantia Nigra in Early Stage Parkinson’s Disease. PLoS ONE 2015, 10, e0128651. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Panov, A.; Orynbayeva, Z.; Vavilin, V.; Lyakhovich, V. Fatty acids in energy metabolism of the central nervous system. Biomed. Res. Int. 2014, 2014, 472459. [Google Scholar] [CrossRef]
- Ebert, D.; Haller, R.G.; Walton, M.E. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 2003, 23, 5928–5935. [Google Scholar] [CrossRef]
- Rustam, Y.H.; Reid, G.E. Analytical Challenges and Recent Advances in Mass Spectrometry Based Lipidomics. Anal. Chem. 2018, 90, 374–397. [Google Scholar] [CrossRef]
- Bou Khalil, M.; Hou, W.; Zhou, H.; Elisma, F.; Swayne, L.A.; Blanchard, A.P.; Yao, Z.; Bennett, S.A.; Figeys, D. Lipidomics era: Accomplishments and challenges. Mass Spectrom. Rev. 2010, 29, 877–929. [Google Scholar] [CrossRef]
- Hu, P.; Fabyanic, E.; Kwon, D.Y.; Tang, S.; Zhou, Z.; Wu, H. Dissecting Cell-Type Composition and Activity-Dependent Transcriptional State in Mammalian Brains by Massively Parallel Single-Nucleus RNA-Seq. Mol. Cell 2017, 68, 1006–1015.e7. [Google Scholar] [CrossRef] [PubMed]
- Rubakhin, S.S.; Romanova, E.V.; Nemes, P.; Sweedler, J.V. Profiling metabolites and peptides in single cells. Nat. Methods 2011, 8, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hoovield, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef]
- Caesar, R.; Nygren, H.; Orešič, M.; Bäckhed, F. Interaction between dietary lipids and gut microbiota regulates hepatic cholesterol metabolism. J. Lipid Res. 2016, 57, 474–481. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Minato, T.; Maeda, T.; Fujisawa, Y.; Tsuji, H.; Nomoto, K.; Ohno, K.; Hirayama, M. Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS ONE 2017, 12, e0187307. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Barichella, M.; Cancello, R.; Cavanna, F.; Iorio, L.; Cereda, E.; Bolliri, C.; Zampella Maria, P.; Bianchi, F.; Cestaro, B.; et al. Increased urinary indoxyl sulfate (indican): New insights into gut dysbiosis in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Lista, S.; Khachaturian, Z.S.; Rujescu, D.; Garaci, F.; Dubois, B.; Hampel, H. Application of Systems Theory in Longitudinal Studies on the Origin and Progression of Alzheimer’s Disease. Methods Mol. Biol. 2016, 1303, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Dehairs, J.; Derua, R.; Rueda-Rincon, N.; Swinnen, J.V. Lipidomics in drug development. Drug Discov. Today Technol. 2015, 13, 33–38. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).