Vitamin B6 and Its Role in Cell Metabolism and Physiology
Abstract
:1. Introduction
2. Complex Organization of PLP Synthases
3. Regulation of Salvage Pathway Genes
4. Regulation of De Novo VitB6 Synthesis Genes
5. Transport and Distribution of VitB6
6. Vitamin B6 and Its Involvement in Cellular Metabolism
6.1. VitB6 Involvement in Protein Folding
6.2. VitB6 Involvement in Amino Acid Biosynthesis
6.3. VitB6 and Degradation of Cellular Storage Compounds
6.4. VitB6 and Its Relevance in Tetrapyrrole Biosynthesis
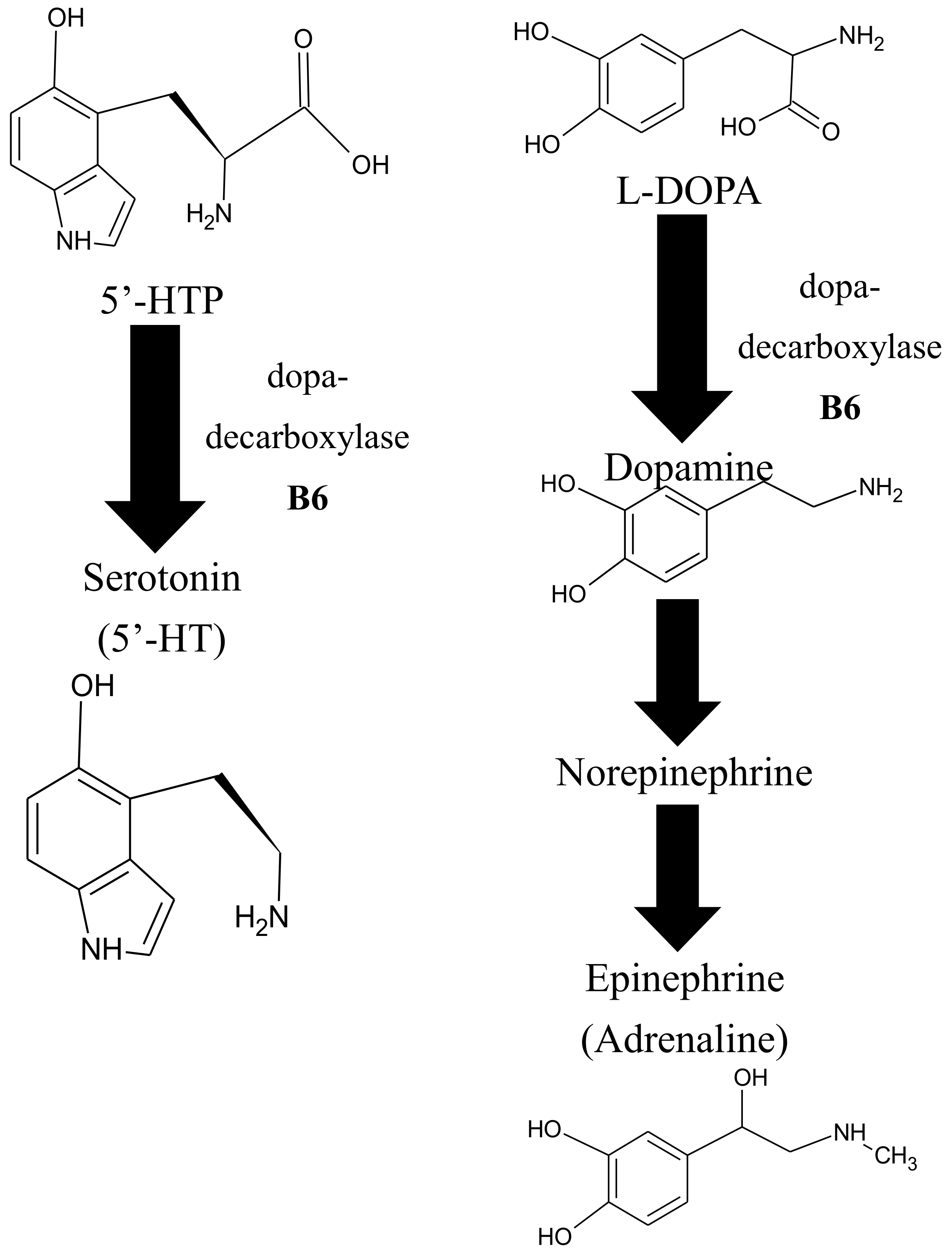
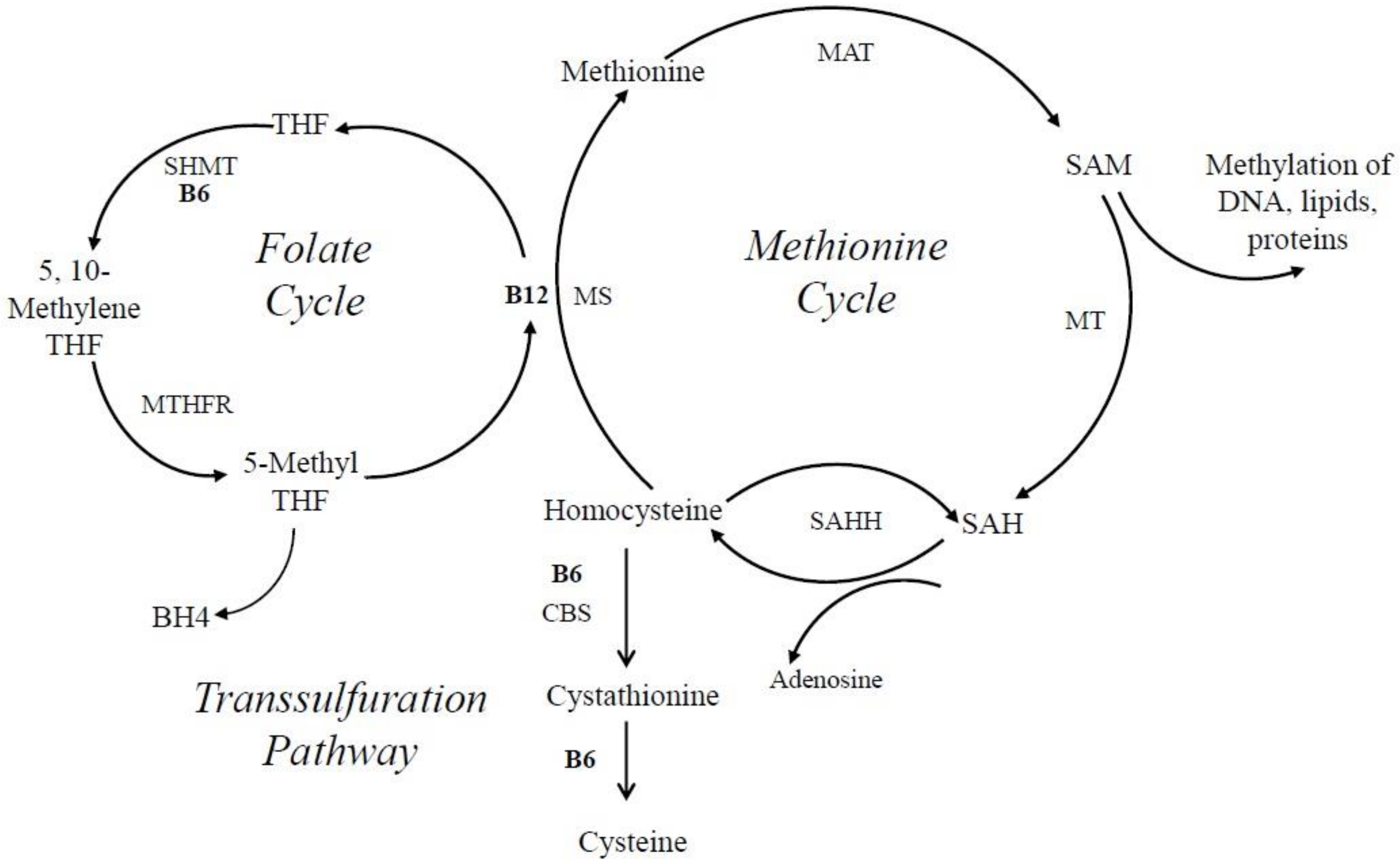
6.5. VitB6 and Its Role in Neurotransmitter Biosynthesis
7. VitB6 Requirements in Human Nutrition
8. VitB6 and the Potential to Develop New Medical Drugs
9. Conclusions
Funding
Acknowledgement
Conflicts of Interest
Abbreviations
| AASA | L-aminoadipate-semialdehyde |
| AAT | aspartate aminotransferase |
| ABC | ATP BINDING CASSETTE |
| ABRE | abscisic acid-responsive elements |
| ACC | 1-aminocyclopropane-1-carboxilic acid |
| ALP | alkaline phosphatase |
| ALS | acetolactate synthase |
| BCAA | branched-chain amino acid |
| BCAT | branched-chain amino acid transaminase |
| BH4 | tetrahydrobiopterin |
| CBS | cystathionine-β-synthase |
| DAP | deoxyxylose 5′-phosphate |
| DXP | deoxyxylose 5′-phosphate |
| ERE | ethylene-responsive elements |
| FMN | flavin mononucleotide |
| GAP | glyceraldehyde-3-phosphate |
| GLN | L-glutamine |
| GLU | L-glutamate |
| HKT | 3-hydroxykynurenine transaminase |
| HTH | helix-turn-helix |
| MAT | methionine adenosyltransferase |
| MS | methionine synthase |
| MT | methyltransferase |
| MTHFR | methylenetetrahydrofolate reductase |
| NR | nitrate reductase |
| NUP1 | NICOTINE UPTAKE PERMEASE 1 |
| ODX | ornithine decarboxylase |
| P6C | 1-piperideine-6-carboxylic acid |
| PDE | pyridoxine-dependent epilepsy |
| PDXA | Pyridoxine Biosynthesis Proteins A |
| PDXH | pyridoxine/pyridoxamine 5′-phosphate oxidase |
| PDXJ | pyridoxine 5′-phosphate synthase |
| PDXK | pyridoxal kinase |
| PHT | 4-phosphohydroxy-L-threonine |
| PL | pyridoxal |
| PLK | pyridoxal kinase specific to PL |
| PLP | pyridoxal 5′-phosphate |
| PLR | pyridoxal reductase |
| PM | pyridoxamine |
| PMP | pyridoxamine 5′-phosphate |
| PN | pyridoxine |
| PNP | pyridoxine 5′-phosphate |
| PNPox | pyridoxine 5′-phosphate oxidase |
| PPL | pyridoxyllysine |
| PUP | purine permease |
| RDA | recommended dietary allowances |
| RIP | ribose-5-phosphate |
| ROS | reactive oxygen species |
| RUP | ribulose-5-phosphate |
| SAH | S-adenosylhomocysteine |
| SAHH | S-adenosylhomocysteine hydrolase |
| SAM | S-adenosylmethionine |
| SHMT | serine hydroxymethyltransferase |
| THF | tetrahydrofolate |
| UL | tolerable upper intake levels |
| vitB12 | vitamin B12 |
| vitB6 | vitamin B6 |
References
- Hellmann, H.; Mooney, S. Vitamin B6: A molecule for human health? Mol. Basel Switz. 2010, 15, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Birch, T.W.; György, P.; Harris, L.J. The vitamin B(2) complex. Differentiation of the antiblacktongue and the “P.-P.” factors from lactoflavin and vitamin B(6) (so-called “rat pellagra” factor). Parts I-VI. Biochem. J. 1935, 29, 2830–2850. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.; Leuendorf, J.-E.; Hendrickson, C.; Hellmann, H. Vitamin B6: A Long Known Compound of Surprising Complexity. Molecules 2009, 14, 329–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.B.; George, F.; Audhya, T. Abnormally high plasma levels of vitamin B6 in children with autism not taking supplements compared to controls not taking supplements. J. Altern. Complement. Med. (N. Y. NY) 2006, 12, 59–63. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Singh, S.K.; Roy, S.; Sengupta, D.N. An insight into the sequential, structural and phylogenetic properties of banana 1-aminocyclopropane-1-carboxylate synthase 1 and study of its interaction with pyridoxal-5′-phosphate and aminoethoxyvinylglycine. J. Biosci. 2010, 35, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Seijo, J.A.; Ruzanski, C.; Krucewicz, K.; Meier, S.; Hägglund, P.; Svensson, B.; Palcic, M.M. Functional and structural characterization of plastidic starch phosphorylase during barley endosperm development. PLoS ONE 2017, 12, e0175488. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.Y.; Saito, H.; Katsuki, H. Effects of vitamin B6 and its related compounds on survival of cultured brain neurons. Neurosci. Res. 1995, 24, 61–65. [Google Scholar] [CrossRef]
- Plecko, B.; Stöckler, S. Vitamin B6 dependent seizures. Can. J. Neurol. Sci. 2009, 36 (Suppl. 2), S73–S77. [Google Scholar] [PubMed]
- Tsang, E.W.T.; Hu, Z.; Chang, Q.; McGregor, D.I.; Keller, W.A. Expression of a Brassic napus glutamate 1-semialdehyde aminotransferase in Escherichia coli and characterization of the recombinant protein. Protein Expr. Purif. 2003, 29, 193–201. [Google Scholar] [CrossRef]
- Ercan-Fang, N.; Taylor, M.R.; Treadway, J.L.; Levy, C.B.; Genereux, P.E.; Gibbs, E.M.; Rath, V.L.; Kwon, Y.; Gannon, M.C.; Nuttall, F.Q. Endogenous effectors of human liver glycogen phosphorylase modulate effects of indole-site inhibitors. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E366–E372. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Drewke, C.; Klein, M.; Clade, D.; Arenz, A.; Müller, R.; Leistner, E. 4-O-phosphoryl-L-threonine, a substrate of the pdxC(serC) gene product involved in vitamin B6 biosynthesis. FEBS Lett. 1996, 390, 179–182. [Google Scholar] [CrossRef]
- Notheis, C.; Drewke, C.; Leistner, E. Purification and characterization of the pyridoxol-5′-phosphate:oxygen oxidoreductase (deaminating) from Escherichia coli. Biochim. Biophys. Acta 1995, 1247, 265–271. [Google Scholar] [CrossRef]
- Mittenhuber, G. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J. Mol. Microbiol. Biotechnol. 2001, 3, 1–20. [Google Scholar] [PubMed]
- Tambasco-Studart, M.; Tews, I.; Amrhein, N.; Fitzpatrick, T.B. Functional analysis of PDX2 from Arabidopsis, a glutaminase involved in vitamin B6 biosynthesis. Plant Physiol. 2007, 144, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Tambasco-Studart, M.; Titiz, O.; Raschle, T.; Forster, G.; Amrhein, N.; Fitzpatrick, T.B. Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. USA 2005, 102, 13687–13692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titiz, O.; Tambasco-Studart, M.; Warzych, E.; Apel, K.; Amrhein, N.; Laloi, C.; Fitzpatrick, T.B. PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. Cell Mol. Biol. 2006, 48, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Bernhardt, A.; Leuendorf, J.E.; Drewke, C.; Lytovchenko, A.; Mujahed, N.; Gurgui, C.; Frommer, W.B.; Leistner, E.; Fernie, A.R.; et al. Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals the critical function of the PDX1 protein family in metabolism, development, and vitamin B6 biosynthesis. Plant Cell 2006, 18, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Boycheva, S.; Dominguez, A.; Rolcik, J.; Boller, T.; Fitzpatrick, T.B. Consequences of a Deficit in Vitamin B6 Biosynthesis de Novo for Hormone Homeostasis and Root Development in Arabidopsis. Plant Physiol. 2015, 167, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Nakamura, Y.; Dong, Y.-X.; Nikawa, J.; Sueda, S. Pyridoxine biosynthesis in yeast: Participation of ribose 5-phosphate ketol-isomerase. Biochem. J. 2004, 379, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Jochmann, N.; Götker, S.; Tauch, A. Positive transcriptional control of the pyridoxal phosphate biosynthesis genes pdxST by the MocR-type regulator PdxR of Corynebacterium glutamicum ATCC 13032. Microbiology (Read. Engl.) 2011, 157, 77–88. [Google Scholar] [CrossRef] [PubMed]
- El Qaidi, S.; Yang, J.; Zhang, J.-R.; Metzger, D.W.; Bai, G. The vitamin B6 biosynthesis pathway in Streptococcus pneumoniae is controlled by pyridoxal 5′-phosphate and the transcription factor PdxR and has an impact on ear infection. J. Bacteriol. 2013, 195, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Kita, M.; Katsuragi, T.; Ogasawara, N.; Tani, Y. yaaD and yaaE are involved in vitamin B6 biosynthesis in Bacillus subtilis. J. Biosci. Bioeng. 2002, 93, 309–312. [Google Scholar] [CrossRef]
- Apostolakos, D.; Birge, E.A. A thermosensitive pdxJ mutation affecting vitamin B6 biosynthesis in Escherichia coli K-12. Curr. Microbiol. 1979, 2, 39. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, L. Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J. 2005, 44, 396–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschke, M.; Boycheva, S.; Crèvecoeur, M.; Nunes-Nesi, A.; Witt, S.; Fernie, A.R.; Amrhein, N.; Fitzpatrick, T.B. Enhanced levels of vitamin B(6) increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J. 2011, 66, 414–432. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Danehower, D.; Daub, M.E. Vitamer Levels, Stress Response, Enzyme Activity, and Gene Regulation of Arabidopsis Lines Mutant in the Pyridoxine/Pyridoxamine 5′-Phosphate Oxidase (PDX3) and the Pyridoxal Kinase (SOS4) Genes Involved in the Vitamin B6 Salvage Pathway. Plant Physiol. 2007, 145, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tsui, H.C.; Man, T.K.; Winkler, M.E. Identification and function of the pdxY gene, which encodes a novel pyridoxal kinase involved in the salvage pathway of pyridoxal 5′-phosphate biosynthesis in Escherichia coli K-12. J. Bacteriol. 1998, 180, 1814–1821. [Google Scholar] [PubMed]
- Tang, L.; Li, M.-H.; Cao, P.; Wang, F.; Chang, W.-R.; Bach, S.; Reinhardt, J.; Ferandin, Y.; Galons, H.; Wan, Y.; et al. Crystal structure of pyridoxal kinase in complex with roscovitine and derivatives. J. Biol. Chem. 2005, 280, 31220–31229. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin B(6) salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta 2011, 1814, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, G.; Winkler, M.E. Identification of the pdxK gene that encodes pyridoxine (vitamin B6) kinase in Escherichia coli K-12. FEMS Microbiol. Lett. 1996, 141, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.K.; Au, K.; Cherney, M.M.; Garen, C.; James, M.N.G. The molecular structure of Rv2074, a probable pyridoxine 5′-phosphate oxidase from Mycobacterium tuberculosis, at 1.6 angstroms resolution. Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Musayev, F.N.; Schirch, V. Structure of Escherichia coli pyridoxine 5′-phosphate oxidase in a tetragonal crystal form: Insights into the mechanistic pathway of the enzyme. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Herrero, S.; González, E.; Gillikin, J.W.; Vélëz, H.; Daub, M.E. Identification and characterization of a pyridoxal reductase involved in the vitamin B6 salvage pathway in Arabidopsis. Plant Mol. Biol. 2011, 76, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.; Nocek, B.; Brown, G.; Makarova, K.S.; Flick, R.; Wolf, Y.I.; Khusnutdinova, A.; Evdokimova, E.; Jin, K.; Tan, K.; et al. Functional Diversity of Haloacid Dehalogenase Superfamily Phosphatases from Saccharomyces cerevisiae: Biochemical, structural, and evolutionary insights. J. Biol. Chem. 2015, 290, 18678–18698. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, Ø.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, M.C.; Sampson, D.A.; Skala, J.H.; Gietzen, D.W.; Grier, R.E. Evaluation of vitamin B-6 status and function of rats fed excess pyridoxine. J. Nutr. 1989, 119, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H.; Horiike, K.; McCormick, D.B. Evidence for the regulation of pyridoxal 5′-phosphate formation in liver by pyridoxamine (pyridoxine) 5′-phosphate oxidase. Biochem. Biophys. Res. Commun. 1978, 83, 984–990. [Google Scholar] [CrossRef]
- Narisawa, S.; Wennberg, C.; Millán, J.L. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J. Pathol. 2001, 193, 125–133. [Google Scholar] [CrossRef]
- Whyte, M.P.; Mahuren, J.D.; Vrabel, L.A.; Coburn, S.P. Markedly increased circulating pyridoxal-5′-phosphate levels in hypophosphatasia. Alkaline phosphatase acts in vitamin B6 metabolism. J. Clin. Investig. 1985, 76, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P.; Mahuren, J.D.; Fedde, K.; Cole, F.; McCabe, E.R.B.; Coburn, S.P. Perinatal hypophosphatasia: Tissue levels of vitamin B6 are unremarkable despite markedly increased circulating concentrations of pyridoxal-5′-phosphate. Evidence for an ectoenzyme role for tissue-nonspecific alkaline phosphatase. J. Clin. Investig. 1988, 81, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Zhang, L.; Du, W.; Zhuang, X. A nutritional conditional lethal mutant due to pyridoxine 5′-phosphate oxidase deficiency in Drosophila melanogaster. G3 Genes Genomes Genet. 2014, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Barbosa, J.M.; Wu, H.; Locy, R.D.; Singh, N.K. Identification of a pyridoxine (pyridoxamine) 5′-phosphate oxidase from Arabidopsis thaliana. FEBS Lett. 2007, 581, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xiong, L.; Stevenson, B.; Lu, T.; Zhu, J.-K. The Arabidopsis salt overly sensitive 4 Mutants Uncover a Critical Role for Vitamin B6 in Plant Salt Tolerance. Plant Cell 2002, 14, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Colinas, M.; Eisenhut, M.; Tohge, T.; Pesquera, M.; Fernie, A.R.; Weber, A.P.M.; Fitzpatrick, T.B. Balancing of B6 Vitamers Is Essential for Plant Development and Metabolism in Arabidopsis. Plant Cell 2016, 28, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.R.; Leech, R.M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972, 50, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueschhoff, E.E.; Gillikin, J.W.; Sederoff, H.W.; Daub, M.E. The SOS4 pyridoxal kinase is required for maintenance of vitamin B6-mediated processes in chloroplasts. Plant Physiol. Biochem. 2013, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Brown, W.C.; Harms, E.; Smith, J.L. Crystal Structures Capture Three States in the Catalytic Cycle of a Pyridoxal Phosphate (PLP) Synthase. J. Biol. Chem. 2015, 290, 5226–5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Burgner, J.W.; Harms, E.; Belitsky, B.R.; Smith, J.L. A new arrangement of (beta/alpha)8 barrels in the synthase subunit of PLP synthase. J. Biol. Chem. 2005, 280, 27914–27923. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, M.; Raschle, T.; Mazurkiewicz, J.; Rippe, K.; Sinning, I.; Fitzpatrick, T.B.; Tews, I. Structure of a bacterial pyridoxal 5′-phosphate synthase complex. Proc. Natl. Acad. Sci. USA 2006, 103, 19284–19289. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R. Physical and enzymological interaction of Bacillus subtilis proteins required for de novo pyridoxal 5′-phosphate biosynthesis. J. Bacteriol. 2004, 186, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Zein, F.; Zhang, Y.; Kang, Y.-N.; Burns, K.; Begley, T.P.; Ealick, S.E. Structural insights into the mechanism of the PLP synthase holoenzyme from Thermotoga maritima. Biochemistry (Mosc.) 2006, 45, 14609–14620. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, M.; Strohmeier, M.; Windeisen, V.; Wallner, S.; Deller, S.; Rippe, K.; Sinning, I.; Macheroux, P.; Tews, I. X-ray crystal structure of Saccharomyces cerevisiae Pdx1 provides insights into the oligomeric nature of PLP synthases. FEBS Lett. 2009, 583, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.C.; Kaufmann, M.; Roux, C.; Fitzpatrick, T.B. Structural definition of the lysine swing in Arabidopsis thaliana PDX1: Intermediate channeling facilitating vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, E5821–E5829. [Google Scholar] [CrossRef] [PubMed]
- Guédez, G.; Hipp, K.; Windeisen, V.; Derrer, B.; Gengenbacher, M.; Böttcher, B.; Sinning, I.; Kappes, B.; Tews, I. Assembly of the eukaryotic PLP-synthase complex from Plasmodium and activation of the Pdx1 enzyme. Structure 2012, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Windeisen, V.; Zhang, Y.; Guédez, G.; Weber, S.; Strohmeier, M.; Hanes, J.W.; Royant, A.; Evans, G.; Sinning, I.; et al. Lysine relay mechanism coordinates intermediate transfer in vitamin B6 biosynthesis. Nat. Chem. Biol. 2017, 13, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Moccand, C.; Boycheva, S.; Surriabre, P.; Tambasco-Studart, M.; Raschke, M.; Kaufmann, M.; Fitzpatrick, T.B. The Pseudoenzyme PDX1.2 Boosts Vitamin B6 Biosynthesis under Heat and Oxidative Stress in Arabidopsis. J. Biol. Chem. 2014, 289, 8203–8216. [Google Scholar] [CrossRef] [PubMed]
- Leuendorf, J.E.; Mooney, S.L.; Chen, L.; Hellmann, H.A. Arabidopsis thaliana PDX1.2 is critical for embryo development and heat shock tolerance. Planta 2014, 240, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Denslow, S.A.; Rueschhoff, E.E.; Daub, M.E. Regulation of the Arabidopsis thaliana vitamin B6 biosynthesis genes by abiotic stress. Plant Physiol. Biochem. 2007, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dezfulian, M.H.; Foreman, C.; Jalili, E.; Pal, M.; Dhaliwal, R.K.; Roberto, D.K.A.; Imre, K.M.; Kohalmi, S.E.; Crosby, W.L. Acetolactate synthase regulatory subunits play divergent and overlapping roles in branched-chain amino acid synthesis and Arabidopsis development. BMC Plant Biol. 2017, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Amorim Franco, T.M.; Hegde, S.; Blanchard, J.S. Chemical Mechanism of the Branched-Chain Aminotransferase IlvE from Mycobacterium tuberculosis. Biochemistry (Mosc.) 2016, 55, 6295–6303. [Google Scholar] [CrossRef] [PubMed]
- Prunetti, L.; El Yacoubi, B.; Schiavon, C.R.; Kirkpatrick, E.; Huang, L.; Bailly, M.; ElBadawi-Sidhu, M.; Harrison, K.; Gregory, J.F.; Fiehn, O.; et al. Evidence That COG0325 Proteins are involved in PLP Homeostasis. Microbiology (Read. Engl.) 2016, 162, 694–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tramonti, A.; Milano, T.; Nardella, C.; di Salvo, M.L.; Pascarella, S.; Contestabile, R. Salmonella typhimurium PtsJ is a novel MocR-like transcriptional repressor involved in regulating the vitamin B6 salvage pathway. FEBS J. 2017, 284, 466–484. [Google Scholar] [CrossRef] [PubMed]
- Milano, T.; Contestabile, R.; Lo, A.P.; Ciccozzi, M.; Pascarella, S. The aspartate aminotransferase-like domain of Firmicutes MocR transcriptional regulators. Comput. Biol. Chem. 2015, 58, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, H.; Yao, L.; Zhang, J.; Huang, L. Effect of exogenous hormones on transcription levels of pyridoxal 5′-phosphate biosynthetic enzymes in the silkworm (Bombyx mori). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 194–195, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Locy, R.D.; Goertzen, L.R.; Rashotte, A.M.; Si, Y.; Kang, K.; Singh, N.K. Expression, in vivo localization and phylogenetic analysis of a pyridoxine 5′-phosphate oxidase in Arabidopsis thaliana. Plant Physiol. Biochem. 2011, 49, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Samsatly, J.; Chamoun, R.; Gluck-Thaler, E.; Jabaji, S. Genes of the de novo and Salvage Biosynthesis Pathways of Vitamin B6 are Regulated under Oxidative Stress in the Plant Pathogen Rhizoctonia solani. Front. Microbiol. 2015, 6, 1429. [Google Scholar] [CrossRef] [PubMed]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Wojtaszek, P. Oxidative burst: An early plant response to pathogen infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Di, M.S.; Hunt, S.; Schirch, V. Expression, purification, and kinetic constants for human and Escherichia coli pyridoxal kinases. Protein Expr. Purif. 2004, 36, 300–306. [Google Scholar] [CrossRef]
- Li, M.; Kwok, F.; Chang, W.; Liu, S.; Lo, S.C.L.; Zhang, J.; Jiang, T.; Liang, D. Conformational Changes in the Reaction of Pyridoxal Kinase. J. Biol. Chem. 2004, 279, 17459–17465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musayev, F.N.; di Salvo, M.L.; Ko, T.-P.; Gandhi, A.K.; Goswami, A.; Schirch, V.; Safo, M.K. Crystal Structure of human pyridoxal kinase: Structural basis of M+ and M2+ activation. Protein Sci. Publ. Protein Soc. 2007, 16, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Gong, Y.; Tang, L.; Leung, Y.-C.; Jiang, T. Crystal structure of human pyridoxal kinase. J. Struct. Biol. 2006, 154, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Musayev, F.N.; di Salvo, M.L.; Hunt, S.; Claude, J.-B.; Schirch, V. Crystal structure of pyridoxal kinase from the Escherichia coli pdxK gene: Implications for the classification of pyridoxal kinases. J. Bacteriol. 2006, 188, 4542–4552. [Google Scholar] [CrossRef] [PubMed]
- Musayev, F.N.; Di Salvo, M.L.; Ko, T.-P.; Schirch, V.; Safo, M.K. Structure and properties of recombinant human pyridoxine 5′-phosphate oxidase. Protein Sci. Publ. Protein Soc. 2003, 12, 1455–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Winkler, M.E. Kinetic limitation and cellular amount of pyridoxine (pyridoxamine) 5′-phosphate oxidase of Escherichia coli K-12. J. Bacteriol. 1995, 177, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.-F.; di Salvo, M.; Schirch, V. Distribution of B6 Vitamers in Escherichia coli as Determined by Enzymatic Assay. Anal. Biochem. 2001, 298, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Churchich, J.E.; Zaiden, E.; Kwok, F. Brain pyridoxine-5-phosphate oxidase. Modulation of its catalytic activity by reaction with pyridoxal 5-phosphate and analogs. J. Biol. Chem. 1987, 262, 12013–12017. [Google Scholar] [PubMed]
- Choi, J.-D.; Bowers-Komro, M.; Davis, M.D.; Edmondson, D.; Mccormick, D. Kinetic properties of pyridoxamie (pyridoxine) 5′-phosphate oxidase from rabbit liver. J. Biol. Chem. 1983, 258, 840–845. [Google Scholar] [PubMed]
- Pease, A.J.; Roa, B.R.; Luo, W.; Winkler, M.E. Positive growth rate-dependent regulation of the pdxA, ksgA, and pdxB genes of Escherichia coli K-12. J. Bacteriol. 2002, 184, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Finkel, S.E.; Johnson, R.C. The Fis protein: It’s not just for DNA inversion anymore. Mol. Microbiol. 1992, 6, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, H.; Nilsson, L.; Bosch, L. The mechanism of trans-activation of the Escherichia coli operon thrU(tufB) by the protein FIS. A model. Nucleic Acids Res. 1992, 20, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.L.; Fuge, E.K.; Padilla, P.A.; Werner-Washburne, M. A stationary-phase gene in Saccharomyces cerevisiae is a member of a novel, highly conserved gene family. J. Bacteriol. 1996, 178, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- Tramonti, A.; Fiascarelli, A.; Milano, T.; di Salvo, M.L.; Nogués, I.; Pascarella, S.; Contestabile, R. Molecular mechanism of PdxR—A transcriptional activator involved in the regulation of vitamin B6 biosynthesis in the probiotic bacterium Bacillus clausii. FEBS J. 2015, 282, 2966–2984. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R. Role of PdxR in the activation of vitamin B6 biosynthesis in Listeria monocytogenes. Mol. Microbiol. 2014, 92, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Milano, T.; Angelaccio, S.; Tramonti, A.; Salvo, D.; Luigi, M.; Contestabile, R.; Pascarella, S. A Bioinformatics Analysis Reveals a Group of MocR Bacterial Transcriptional Regulators Linked to a Family of Genes Coding for Membrane Proteins. Available online: https://www.hindawi.com/journals/bri/2016/4360285/abs/ (accessed on 11 June 2018).
- Oka, T. Modulation of gene expression by vitamin B6. Nutr. Res. Rev. 2001, 14, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Cake, M.H.; DiSorbo, D.M.; Litwack, G. Effect of pyridoxal phosphate on the DNA binding site of activated hepatic glucocorticoid receptor. J. Biol. Chem. 1978, 253, 4886–4891. [Google Scholar] [PubMed]
- Allgood, V.E.; Cidlowski, J.A. Vitamin B6 modulates transcriptional activation by multiple members of the steroid hormone receptor superfamily. J. Biol. Chem. 1992, 267, 3819–3824. [Google Scholar] [PubMed]
- Somvanshi, V.S.; Sloup, R.E.; Crawford, J.M.; Martin, A.R.; Heidt, A.J.; Kim, K.; Clardy, J.; Ciche, T.A. A single promoter inversion switches Photorhabdus between pathogenic and mutualistic states. Science 2012, 337, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, V.S.; Kaufmann-Daszczuk, B.; Kim, K.-S.; Mallon, S.; Ciche, T.A. Photorhabdus phase variants express a novel fimbrial locus, mad, essential for symbiosis. Mol. Microbiol. 2010, 77, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Benabdellah, K.; Azcón-Aguilar, C.; Valderas, A.; Speziga, D.; Fitzpatrick, T.B.; Ferrol, N. GintPDX1 encodes a protein involved in vitamin B6 biosynthesis that is up-regulated by oxidative stress in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2009, 184, 682–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antelmann, H.; Bernhardt, J.; Schmid, R.; Mach, H.; Völker, U.; Hecker, M. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 1997, 18, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Brosché, M.; Schuler, M.A.; Kalbina, I.; Connor, L.; Strid, A. Gene regulation by low level UV-B radiation: Identification by DNA array analysis. Photochem. Photobiol. Sci. 2002, 1, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dawes, I.W.; Roe, J.H. Adaptive response of Schizosaccharomyces pombe to hydrogen peroxide and menadione. Microbiology (Read. Engl.) 1995, 141 Pt 12, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Padilla, P.A.; Fuge, E.K.; Crawford, M.E.; Errett, A.; Werner-Washburne, M. The highly conserved, coregulated SNO and SNZ gene families in Saccharomyces cerevisiae respond to nutrient limitation. J. Bacteriol. 1998, 180, 5718–5726. [Google Scholar] [PubMed]
- Roy, S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal. Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-L.; Ma, N.-N.; Meng, X.; Zhang, S.; Wang, J.-R.; Chai, S.; Meng, Q.-W. A novel tomato MYC-type ICE1-like transcription factor, SlICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiol. Biochem. 2013, 73, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Johnson, J.M.; Hieno, A.; Tokizawa, M.; Nomoto, M.; Tada, Y.; Godfrey, R.; Obokata, J.; Sherameti, I.; Yamamoto, Y.Y.; et al. High REDOX RESPONSIVE TRANSCRIPTION FACTOR1 Levels Result in Accumulation of Reactive Oxygen Species in Arabidopsis thaliana Shoots and Roots. Mol. Plant 2015, 8, 1253–1273. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Sharoni, A.M.; Satoh, K.; Kumar, A.; Leung, H.; Kikuchi, S. Comparative transcriptome profiles of the WRKY gene family under control, hormone-treated, and drought conditions in near-isogenic rice lines reveal differential, tissue specific gene activation. J. Plant Physiol. 2014, 171, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Hellmann, H.; Schmidt, R.; Willmitzer, L.; Frommer, W.B. Identification of mutants in metabolically regulated gene expression. Plant J. Cell Mol. Biol. 1997, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.W. Intracellular trafficking of the pyridoxal cofactor. Implications for health and metabolic disease. Arch. Biochem. Biophys. 2016, 592, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolz, J.; Wöhrmann, H.J.P.; Vogl, C. Amiloride uptake and toxicity in fission yeast are caused by the pyridoxine transporter encoded by bsu1+ (car1+). Eukaryot. Cell 2005, 4, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.; Vielreicher, M. Tpn1p, the plasma membrane vitamin B6 transporter of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 18990–18996. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Staub, O.; Hummler, E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: Importance in the control of blood pressure and hypertension. FEBS Lett. 2013, 587, 1929–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, L.; Bernhardt, R.; Bernhardt, I. Nongenomic effect of aldosterone on ion transport pathways of red blood cells. Cell. Physiol. Biochem. 2008, 22, 269–278. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Olivieri, O.; Girelli, D.; Lupo, A.; Bernich, P.; Corrocher, R. Red blood cell cation transports in uraemic anaemia: Evidence for an increased K/Cl co-transport activity. Effects of dialysis and erythropoietin treatment. Eur. J. Clin. Investig. 1995, 25, 762–768. [Google Scholar] [CrossRef]
- Said, Z.M.; Subramanian, V.S.; Vaziri, N.D.; Said, H.M. Pyridoxine uptake by colonocytes: A specific and regulated carrier-mediated process. Am. J. Physiol. Cell Physiol. 2008, 294, C1192–C1197. [Google Scholar] [CrossRef] [PubMed]
- Bürkle, L.; Cedzich, A.; Döpke, C.; Stransky, H.; Okumoto, S.; Gillissen, B.; Kühn, C.; Frommer, W.B. Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J. Cell Mol. Biol. 2003, 34, 13–26. [Google Scholar] [CrossRef]
- Kato, K.; Shitan, N.; Shoji, T.; Hashimoto, T. Tobacco NUP1 transports both tobacco alkaloids and vitamin B6. Phytochemistry 2015, 113, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Szydlowski, N.; Bürkle, L.; Pourcel, L.; Moulin, M.; Stolz, J.; Fitzpatrick, T.B. Recycling of pyridoxine (vitamin B6) by PUP1 in Arabidopsis. Plant J. Cell Mol. Biol. 2013, 75, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.; LaBrie, D.A.; Wagner, R.P. Amino acid synthesis by the mitochondria of Neurospora crassa: I. Dependence on respiration of mitochondria. Arch. Biochem. Biophys. 1969, 134, 401–407. [Google Scholar] [CrossRef]
- Whittaker, M.M.; Penmatsa, A.; Whittaker, J.W. The Mtm1p carrier and pyridoxal 5′-phosphate cofactor trafficking in yeast mitochondria. Arch. Biochem. Biophys. 2015, 568, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodionov, D.A.; Hebbeln, P.; Eudes, A.; ter Beek, J.; Rodionova, I.A.; Erkens, G.B.; Slotboom, D.J.; Gelfand, M.S.; Osterman, A.L.; Hanson, A.D.; et al. A novel class of modular transporters for vitamins in prokaryotes. J. Bacteriol. 2009, 191, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cellini, B.; Montioli, R.; Oppici, E.; Astegno, A.; Borri Voltattorni, C. The chaperone role of the pyridoxal 5′-phosphate and its implications for rare diseases involving B6-dependent enzymes. Clin. Biochem. 2014, 47, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cellini, B.; Bertoldi, M.; Montioli, R.; Laurents, D.V.; Paiardini, A.; Voltattorni, C.B. Dimerization and Folding Processes of Treponema denticola Cystalysin: The Role of Pyridoxal 5′-Phosphate. Biochemistry (Mosc.) 2006, 45, 14140–14154. [Google Scholar] [CrossRef] [PubMed]
- Deu, E.; Kirsch, J.F. Cofactor-directed reversible denaturation pathways: The cofactor-stabilized Escherichia coli aspartate aminotransferase homodimer unfolds through a pathway that differs from that of the apoenzyme. Biochemistry (Mosc.) 2007, 46, 5819–5829. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.N.; Bhakuni, V. Characterization of pyridoxal 5′- phosphate-binding domain and folding intermediate of Bacillus subtilis serine hydroxymethyltransferase: An autonomous folding domain. J. Biochem. (Tokyo) 2008, 144, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.; Hellmann, H. Vitamin B6: Killing two birds with one stone? Phytochemistry 2010, 71, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Leuendorf, J.E.; Osorio, S.; Szewczyk, A.; Fernie, A.R.; Hellmann, H. Complex assembly and metabolic profiling of Arabidopsis thaliana plants overexpressing vitamin B6 biosynthesis proteins. Mol. Plant 2010, 3, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, S.; Sagong, H.-Y.; Son, H.F.; Jin, K.S.; Kim, I.-K.; Kim, K.-J. Structural basis for cytokinin production by LOG from Corynebacterium glutamicum. Sci. Rep. 2016, 6, 31390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriechbaumer, V.; Botchway, S.W.; Hawes, C. Localization and interactions between Arabidopsis auxin biosynthetic enzymes in the TAA/YUC-dependent pathway. J. Exp. Bot. 2016, 67, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Ose, T.; Yao, M.; Tokiwano, T.; Honma, M.; Watanabe, N.; Tanaka, I. Structural and enzymatic properties of 1-aminocyclopropane-1-carboxylate deaminase homologue from Pyrococcus horikoshii. J. Mol. Biol. 2004, 341, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Li, J.; Sánchez-López, Á.M.; Baroja-Fernández, E.; Muñoz, F.J.; Ovecka, M.; Almagro, G.; Montero, M.; Ezquer, I.; Etxeberria, E.; et al. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol. Adv. 2014, 32, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossmann, J.; Lloyd, J. Understanding and influencing starch biochemistry. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 141–196. [Google Scholar] [CrossRef] [PubMed]
- David, E.S.; Crerar, M.M. Quantitation of muscle glycogen phosphorylase mRNA and enzyme amounts in adult rat tissues. Biochim. Biophys. Acta BBA Gen. Subj. 1986, 880, 78–90. [Google Scholar] [CrossRef]
- Shin, Y.S. Glycogen storage disease: Clinical, biochemical, and molecular heterogeneity. Semin. Pediatr. Neurol. 2006, 13, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Oikonomakos, N.G. Glycogen phosphorylase as a molecular target for type 2 diabetes therapy. Curr. Protein Pept. Sci. 2002, 3, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Immenschuh, S.; Vijayan, V.; Janciauskiene, S.; Gueler, F. Heme as a Target for Therapeutic Interventions. Front. Pharmacol. 2017, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Ankar, A.; Bhimji, S.S. Vitamin, B12 (Cobalamin), Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Taniguchi, M.; Lindsey, J.S. Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Voloshin, R.A.; Korol’kova, D.V.; Tomo, T.; Shen, J.-R. Chlorophylls d and f and Their Role in Primary Photosynthetic Processes of Cyanobacteria. Biochem. Biokhimiia 2016, 81, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hedtke, B.; Alawady, A.; Albacete, A.; Kobayashi, K.; Melzer, M.; Roitsch, T.; Masuda, T.; Grimm, B. Deficiency in riboflavin biosynthesis affects tetrapyrrole biosynthesis in etiolated Arabidopsis tissue. Plant Mol. Biol. 2012, 78, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Haust, H.L.; Poon, H.C.; Carson, R.; VanDeWetering, C.; Peter, F. Protoporphyrinaemia and decreased activities of 5-aminolevulinic acid dehydrase and uroporphyrinogen I synthetase in erythrocytes of a Vitamin B6-deficient epileptic boy given valproic acid and carbamazepine. Clin. Biochem. 1989, 22, 201–211. [Google Scholar] [CrossRef]
- Davison, K.M.; Kaplan, B.J. Nutrient intakes are correlated with overall psychiatric functioning in adults with mood disorders. Can. J. Psychiatry 2012, 57, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Herbison, C.E.; Hickling, S.; Allen, K.L.; O’Sullivan, T.A.; Robinson, M.; Bremner, A.P.; Huang, R.-C.; Beilin, L.J.; Mori, T.A.; Oddy, W.H. Low intake of B-vitamins is associated with poor adolescent mental health and behaviour. Prev. Med. 2012, 55, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaner, G.; Soylu, M.; Yüksel, N.; Inanç, N.; Ongan, D.; Başmısırlı, E. Evaluation of Nutritional Status of Patients with Depression. BioMed Res. Int. 2015, 2015, 521481. [Google Scholar] [CrossRef] [PubMed]
- Rechenberg, K. Nutritional Interventions in Clinical Depression. Clin. Psychol. Sci. 2016, 4, 144–162. [Google Scholar] [CrossRef]
- Goldstein, D.S. Adrenal responses to stress. Cell. Mol. Neurobiol. 2010, 30, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Obeid, A.I.; Johnson, L.; Potts, J.; Mookherjee, S.; Eich, R.H. Fluid therapy in severe systemic reaction to radiopaque dye. Ann. Intern. Med. 1975, 83, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Obeid, R. Hyperhomocysteinemia and response of methionine cycle intermediates to vitamin treatment in renal patients. Clin. Chem. Lab. Med. 2005, 43, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Purves-Tyson, T.D.; Owens, S.J.; Rothmond, D.A.; Halliday, G.M.; Double, K.L.; Stevens, J.; McCrossin, T.; Shannon Weickert, C. Putative presynaptic dopamine dysregulation in schizophrenia is supported by molecular evidence from post-mortem human midbrain. Transl. Psychiatry 2017, 7, e1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Kozielska, M.; Pilla Reddy, V.; Vermeulen, A.; Barton, H.A.; Grimwood, S.; de Greef, R.; Groothuis, G.M.M.; Danhof, M.; Proost, J.H. Translational Modeling in Schizophrenia: Predicting Human Dopamine D2 Receptor Occupancy. Pharm. Res. 2016, 33, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, G.; Nunes, G.; Bezerra, E.M.; da Costa, R.F.; Martins, A.; Caetano, E.W.S.; Freire, V.N.; Gottfried, C. Antipsychotic haloperidol binding to the human dopamine D3 receptor: Beyond docking through QM/MM refinement toward the design of improved schizophrenia medicines. ACS Chem. Neurosci. 2014, 5, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.M.; Raschke, J.; Emborg, M.E. Cell-based therapies for Parkinson’s disease: Past, present, and future. Antioxid. Redox Signal. 2009, 11, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Keniston, R.C.; Enriquez, J.I.; McNamee, G.A. Depression of vitamin B6 levels due to dopamine. Vet. Hum. Toxicol. 1991, 33, 118–121. [Google Scholar] [PubMed]
- Rawson, K.S.; Dixon, D.; Nowotny, P.; Ricci, W.M.; Binder, E.F.; Rodebaugh, T.L.; Wendleton, L.; Doré, P.; Lenze, E.J. Association of functional polymorphisms from brain-derived neurotrophic factor and serotonin-related genes with depressive symptoms after a medical stressor in older adults. PLoS ONE 2015, 10, e0120685. [Google Scholar] [CrossRef] [PubMed]
- Bundeff, A.W.; Woodis, C.B. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann. Pharmacother. 2014, 48, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Ridgewell, A.; Ashwin, C. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proc. Biol. Sci. 2009, 276, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A. The Public Health Impact of Major Depression: A Call for Interdisciplinary Prevention Efforts. Prev. Sci. 2011, 12, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.F.; Ward, M.; Tracey, F.; Hoey, L.; Molloy, A.M.; Pentieva, K.; McNulty, H. B-Vitamin Intake and Biomarker Status in Relation to Cognitive Decline in Healthy Older Adults in a 4-Year Follow-Up Study. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-H.; Chang, Y.-P.; Yeh, W.-T.; Guei, Y.-S.; Lin, B.-F.; Wei, I.-L.; Yang, F.L.; Liaw, Y.-P.; Chen, K.-J.; Chen, W.J. Co-occurrence of anemia, marginal vitamin B6, and folate status and depressive symptoms in older adults. J. Geriatr. Psychiatry Neurol. 2012, 25, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Abali, E.E.; Skacel, N.E.; Celikkaya, H.; Hsieh, Y.-C. Regulation of human dihydrofolate reductase activity and expression. Vitam. Horm. 2008, 79, 267–292. [Google Scholar] [CrossRef] [PubMed]
- Cortese, C.; Motti, C. MTHFR gene polymorphism, homocysteine and cardiovascular disease. Public Health Nutr. 2001, 4, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Kamen, B. Folate and antifolate pharmacology. Semin. Oncol. 1997, 24 (Suppl. 18), S18-30–S18-39. [Google Scholar]
- Brown, R.R.; Rose, D.P.; Leklem, J.E.; Linkswiler, H.M. Effects of oral contraceptives on tryptophan metabolism and vitamin B6 requirements in women. Acta Vitaminol. Enzymol. 1975, 29, 151–157. [Google Scholar] [PubMed]
- Fryar-Williams, S. Fundamental Role of Methylenetetrahydrofolate Reductase 677 C→ T Genotype and Flavin Compounds in Biochemical Phenotypes for Schizophrenia and Schizoaffective Psychosis. Front. Psychiatry 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, R.A.; Nicolia, V.; Fiorenza, M.T.; Scarpa, S.; Fuso, A. S-Adenosylmethionine and Superoxide Dismutase 1 Synergistically Counteract Alzheimer’s Disease Features Progression in TgCRND8 Mice. Antioxid. Basel Switz. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Mansoor, M.A.; Pentieva, K.D.; Hamer, M.; Mishra, G.D. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of People Aged 65 Years and Over. Br. J. Nutr. 2010, 104, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.K.; Gordon, R.K.; Tal, J.; Zeng, G.C.; Doctor, B.P.; Pardhasaradhi, K.; McCann, P.P. S-Adenosylmethionine and methylation. FASEB J. 1996, 10, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Brocardo, P.S.; Budni, J.; Kaster, M.P.; Santos, A.R.S.; Rodrigues, A.L.S. Folic acid administration produces an antidepressant-like effect in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 2008, 54, 464–473. [Google Scholar] [CrossRef] [PubMed]
- di Salvo, M.L.; Safo, M.K.; Contestabile, R. Biomedical aspects of pyridoxal 5′-phosphate availability. Front. Biosci. Elite Ed. 2012, 4, 897–913. [Google Scholar] [PubMed]
- Morris, M.S.; Picciano, M.F.; Jacques, P.F.; Selhub, J. Plasma pyridoxal 5′-phosphate in the US population: The National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Pentieva, K.D.; Prentice, A.; Mansoor, M.A.; Finch, S. Plasma pyridoxal phosphate and pyridoxic acid and their relationship to plasma homocysteine in a representative sample of British men and women aged 65 years and over. Br. J. Nutr. 1999, 81, 191–201. [Google Scholar] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- Brown, M.J.; Beier, K. Vitamin, B6 (Pyridoxine), Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Glória, L.; Cravo, M.; Camilo, M.E.; Resende, M.; Cardoso, J.N.; Oliveira, A.G.; Leitão, C.N.; Mira, F.C. Nutritional deficiencies in chronic alcoholics: Relation to dietary intake and alcohol consumption. Am. J. Gastroenterol. 1997, 92, 485–489. [Google Scholar] [PubMed]
- Lin, G.W. Effect of ethanol and vitamin B6 deficiency on pyridoxal 5-phosphate levels and fetal growth in rat. Alcohol. Clin. Exp. Res. 1989, 13, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Göbert, A.; Franke, S.; Ott, U.; Gerth, J.; Müller, A.; Stein, G.; Bitsch, R.; Wolf, G. Vitamin B6 metabolism in chronic kidney disease--relation to transsulfuration, advanced glycation and cardiovascular disease. Nephron Clin. Pract. 2010, 114, c38–c46. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.S.; Williams, H.; Stabej, P.L.Q.; James, C.; Ocaka, L.; Bacchelli, C.; Footitt, E.J.; Boyd, S.; Cleary, M.A.; Mills, P.B.; et al. Seizures Due to a KCNQ2 Mutation: Treatment with Vitamin B6. JIMD Rep. 2016, 27, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G.; Lee, Y.; Shin, H.; Kang, K.; Park, J.-M.; Kim, B.-K.; Kwon, O.; Lee, J.-J. Seizures Related to Vitamin B6 Deficiency in Adults. J. Epilepsy Res. 2015, 5, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, S.; Skidmore, C.T.; Sperling, M.R. B-vitamin deficiency in patients treated with antiepileptic drugs. Epilepsy Behav. 2012, 24, 341–344. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.B. Present Knowledge in Nutrition Volume I; Bowman, B.A., Russel, R., Eds.; International Life Sciences Institue: Washington, DC, USA, 2006. [Google Scholar]
- Apeland, T.; Mansoor, M.A.; Pentieva, K.; McNulty, H.; Strandjord, R.E. Fasting and Post-Methionine Loading Concentrations of Homocysteine, Vitamin B2, and Vitamin B6 in Patients on Antiepileptic Drugs. Clin. Chem. 2003, 49, 1005–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagianos, K.; Bernstein, C.N. Homocysteinemia and B vitamin status among adult patients with inflammatory bowel disease: A one-year prospective follow-up study. Inflamm. Bowel Dis. 2012, 18, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Hess, O.M. Homocysteine and B vitamins. Handb. Exp. Pharmacol. 2005, 325–338. [Google Scholar]
- Merrill, A.H.; Henderson, J.M. Diseases Associated with Defects in Vitamin B6 Metabolism or Utilization. Annu. Rev. Nutr. 1987, 7, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Striano, S.; Andria, G. Electroencephalographic abnormalities in homocystinuria due to cystathionine synthase deficiency. Clin. Neurol. Neurosurg. 1983, 85, 165–168. [Google Scholar] [CrossRef]
- Toriyama, T.; Matsuo, S.; Fukatsu, A.; Takahashi, H.; Sato, K.; Mimuro, N.; Kawahara, H. Effects of high-dose vitamin B6 therapy on microcytic and hypochromic anemia in hemodialysis patients. Nihon Jinzo Gakkai Shi 1993, 35, 975–980. [Google Scholar] [PubMed]
- Merete, C.; Falcon, L.M.; Tucker, K.L. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J. Am. Coll. Nutr. 2008, 27, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Malouf, R.; Grimley Evans, J. The effect of vitamin B6 on cognition. Cochrane Database Syst. Rev. 2003, CD004393. [Google Scholar] [CrossRef]
- Qian, B.; Shen, S.; Zhang, J.; Jing, P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.; Girija, A.S. Pyridoxine-dependent seizures: A review. Indian Pediatr. 2003, 40, 633–638. [Google Scholar] [PubMed]
- Schaumburg, H.; Kaplan, J.; Windebank, A.; Vick, N.; Rasmus, S.; Pleasure, D.; Brown, M.J. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N. Engl. J. Med. 1983, 309, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Parry, G.J.; Bredesen, D.E. Sensory neuropathy with low-dose pyridoxine. Neurology 1985, 35, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Zeris, S.; Kothari, M.J. Elevated B6 levels and peripheral neuropathies. Electromyogr. Clin. Neurophysiol. 2008, 48, 219–223. [Google Scholar] [PubMed]
- Baer, R.L. Cutaneous skin changes probably due to pyridoxine abuse. J. Am. Acad. Dermatol. 1984, 10, 527–528. [Google Scholar] [CrossRef]
- Friedman, M.A.; Resnick, J.S.; Baer, R.L. Subepidermal vesicular dermatosis and sensory peripheral neuropathy caused by pyridoxine abuse. J. Am. Acad. Dermatol. 1986, 14, 915–917. [Google Scholar] [CrossRef]
- De Zegher, F.; Przyrembel, H.; Chalmers, R.A.; Wolff, E.D.; Huijmans, J.G.M. Successful treatment of infantile type I primary hyperoxaluria complicated by pyridoxine toxicity. Lancet 1985, 326, 392–393. [Google Scholar] [CrossRef]
- Clayton, P.T. B6-responsive disorders: A model of vitamin dependency. J. Inherit. Metab. Dis. 2006, 29, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Amadasi, A.; Bertoldi, M.; Contestabile, R.; Bettati, S.; Cellini, B.; di Salvo, M.L.; Borri-Voltattorni, C.; Bossa, F.; Mozzarelli, A. Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr. Med. Chem. 2007, 14, 1291–1324. [Google Scholar] [CrossRef] [PubMed]
- Kronenberger, T.; Lindner, J.; Meissner, K.A.; Zimbres, F.M.; Coronado, M.A.; Sauer, F.M.; Schettert, I.; Wrenger, C. Vitamin B6-dependent enzymes in the human malaria parasite Plasmodium falciparum: A druggable target? BioMed Res. Int. 2014, 2014, 108516. [Google Scholar] [CrossRef] [PubMed]
- Schnell, R.; Sriram, D.; Schneider, G. Pyridoxal-phosphate dependent mycobacterial cysteine synthases: Structure, mechanism and potential as drug targets. Biochim. Biophys. Acta 2015, 1854, 1175–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, P. Pyridoxine-dependent and pyridoxine-responsive seizures. Dev. Med. Child Neurol. 2001, 43, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Stockler, S.; Plecko, B.; Gospe, S.M.; Coulter-Mackie, M.; Connolly, M.; van Karnebeek, C.; Mercimek-Mahmutoglu, S.; Hartmann, H.; Scharer, G.; Struijs, E.; et al. Pyridoxine dependent epilepsy and antiquitin deficiency: Clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol. Genet. Metab. 2011, 104, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.B.; Struys, E.; Jakobs, C.; Plecko, B.; Baxter, P.; Baumgartner, M.; Willemsen, M.A.A.P.; Omran, H.; Tacke, U.; Uhlenberg, B.; et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat. Med. 2006, 12, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Pena, I.A.; Roussel, Y.; Daniel, K.; Mongeon, K.; Johnstone, D.; Mendes, H.W.; Bosma, M.; Saxena, V.; Lepage, N.; Chakraborty, P.; et al. Pyridoxine-Dependent Epilepsy in Zebrafish Caused by Aldh7a1 Deficiency. Genetics 2017, 207, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Elstner, M.; Morris, C.M.; Heim, K.; Lichtner, P.; Bender, A.; Mehta, D.; Schulte, C.; Sharma, M.; Hudson, G.; Goldwurm, S.; et al. Single-cell expression profiling of dopaminergic neurons combined with association analysis identifies pyridoxal kinase as Parkinson’s disease gene. Ann. Neurol. 2009, 66, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Guella, I.; Asselta, R.; Tesei, S.; Zini, M.; Pezzoli, G.; Duga, S. The PDXK rs2010795 variant is not associated with Parkinson disease in Italy. Ann. Neurol. 2010, 67, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Vilariño-Güell, C.; Wider, C.; Aasly, J.O.; White, L.R.; Rajput, A.; Rajput, A.H.; Lynch, T.; Krygowska-Wajs, A.; Jasinska-Myga, B.; Opala, G.; et al. Association of pyridoxal kinase and Parkinson disease. Ann. Neurol. 2010, 67, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Ehrenshaft, M.; Chung, K.R.; Jenns, A.E.; Daub, M.E. Functional characterization of SOR1, a gene required for resistance to photosensitizing toxins in the fungus Cercospora nicotianae. Curr. Genet. 1999, 34, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Ehrenshaft, M.; Jenns, A.E.; Chung, K.R.; Daub, M.E. SOR1, a gene required for photosensitizer and singlet oxygen resistance in Cercospora fungi, is highly conserved in divergent organisms. Mol. Cell 1998, 1, 603–609. [Google Scholar] [CrossRef]
- Daub, M.E.; Ehrenshaft, M. The photoactivated Cercospora toxin Cercosporin: Contributions to Plant Disease and Fundamental Biology. Annu. Rev. Phytopathol. 2000, 38, 461–490. [Google Scholar] [CrossRef] [PubMed]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Bilski, P.; Li, M.Y.; Chignell, C.F.; Daub, M.E. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 9374–9378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Jin, X.; Ouyang, Z.; Li, X.; Liu, B.; Huang, L.; Hong, Y.; Zhang, H.; Song, F.; Li, D. Vitamin B6 contributes to disease resistance against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea in Arabidopsis thaliana. J. Plant. Physiol. 2015, 175, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yoshiga, T.; Hasegawa, K. Activated and inactivated immune responses in Caenorhabditis elegans against Photorhabdus luminescens TT01. SpringerPlus 2014, 3, 274. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yoshiga, T.; Hasegawa, K. Involvement of Vitamin B6 Biosynthesis Pathways in the Insecticidal Activity of Photorhabdus luminescens. Appl. Environ. Microbiol. 2016, 82, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Grubman, A.; Phillips, A.; Thibonnier, M.; Kaparakis-Liaskos, M.; Johnson, C.; Thiberge, J.-M.; Radcliff, F.J.; Ecobichon, C.; Labigne, A.; de Reuse, H.; et al. Vitamin B6 is required for full motility and virulence in Helicobacter pylori. mBio 2010, 1, e00112-10. [Google Scholar] [CrossRef] [PubMed]
- Dick, T.; Manjunatha, U.; Kappes, B.; Gengenbacher, M. Vitamin B6 biosynthesis is essential for survival and virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2010, 78, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Cui, Y.; Li, Z.; Chen, L.; Liu, S. New Targets and Cofactors for the Transcription Factor LrpA from Mycobacterium tuberculosis. DNA Cell Biol. 2016, 35, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, M.; Vogelzang, A.; Schuerer, S.; Lazar, D.; Kaiser, P.; Kaufmann, S.H.E. Dietary Pyridoxine Controls Efficacy of Vitamin B6 -Auxotrophic Tuberculosis Vaccine Bacillus Calmette-Guérin ΔureC::hly Δpdx1 in Mice. mBio 2014, 5, e01262-14. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2015. Available online: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (accessed on 27 December 2017).
- Mayer, J.E. Delivering golden rice to developing countries. J. AOAC Int. 2007, 90, 1445–1449. [Google Scholar] [PubMed]
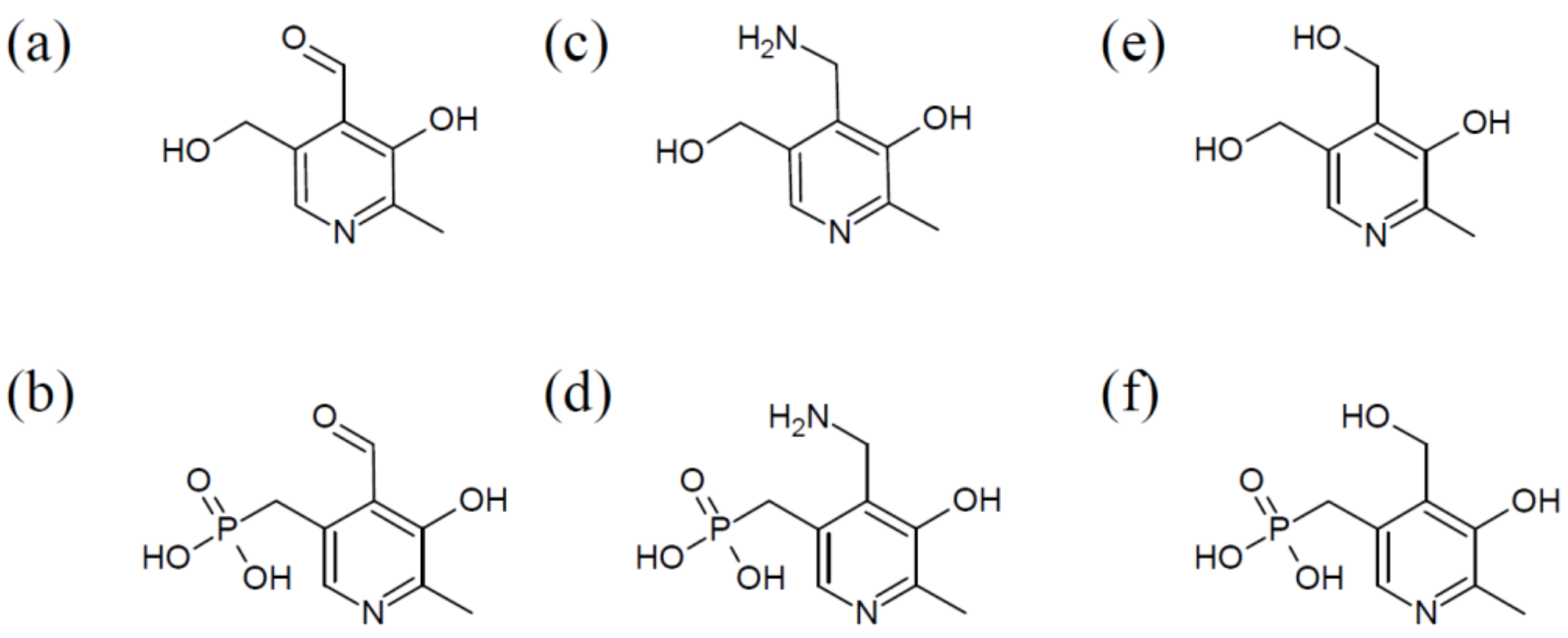
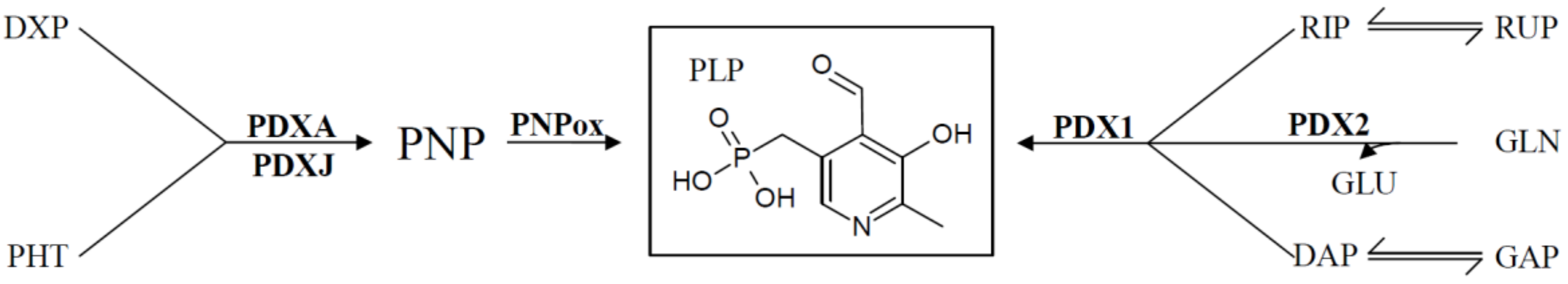
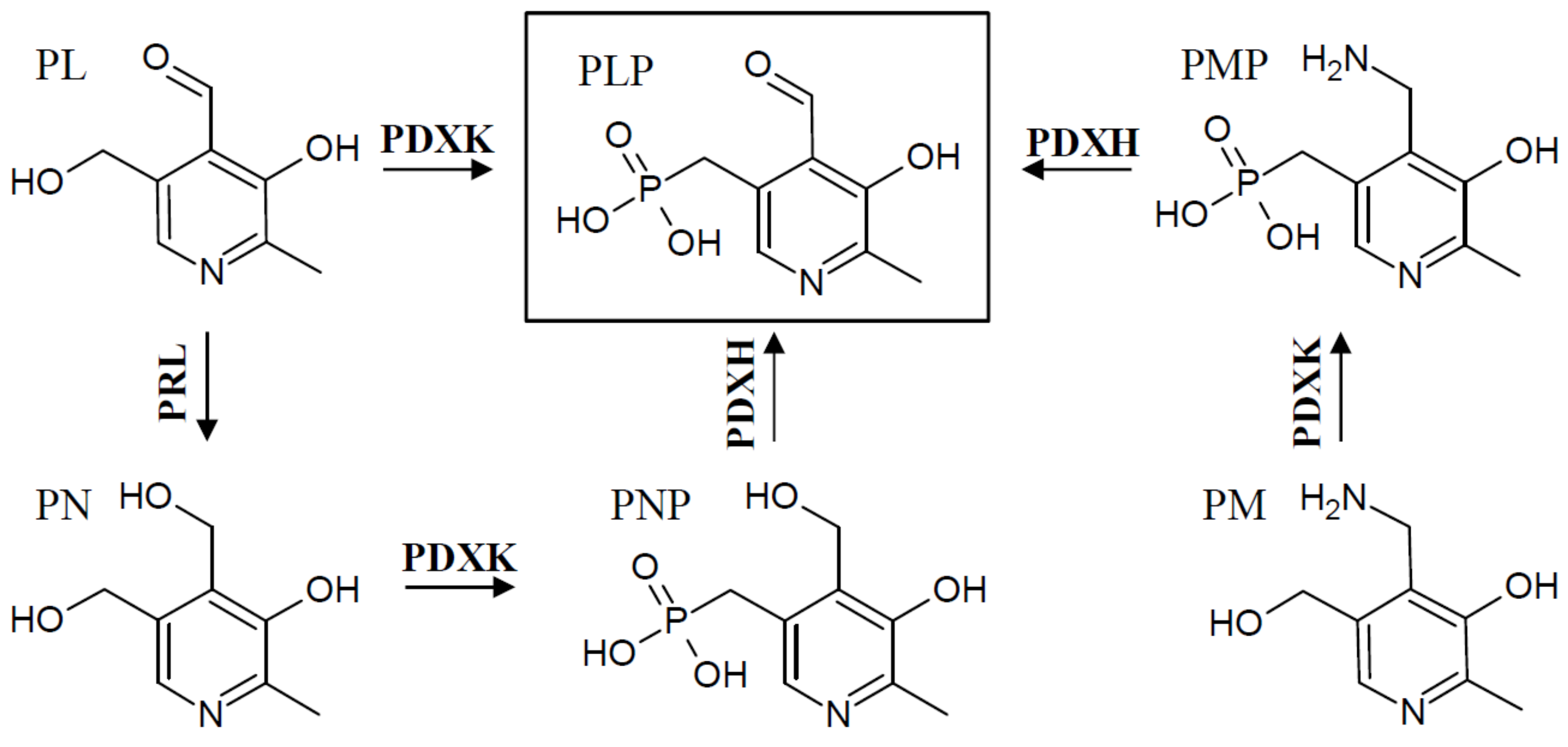
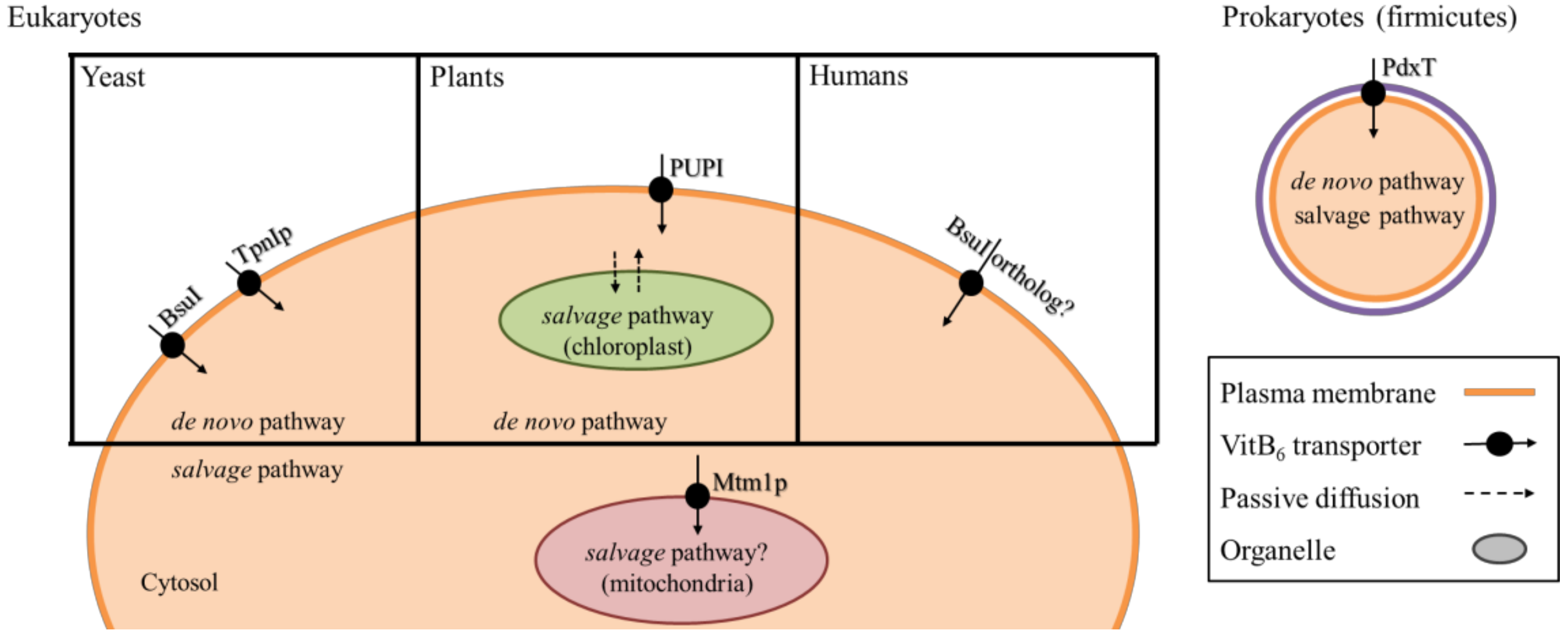
| Food Source | VitB6 [mg/100 g] |
|---|---|
| Crude Rice Bran | 4.07 |
| Vegetable Oil Spread (60% Fat) | 3.75 |
| Raw Garlic | 1.235 |
| Cooked Chicken Breast | 1.157 |
| Cooked Beef Liver | 1.083 |
| Roasted Pistachio Nuts | 1.07 |
| Cooked Yellow Fin Tuna | 1.04 |
| Top Round Boneless Steak | 0.891 |
| Cooked Sockeye Salmon | 0.83 |
| Potato Chips | 0.8 |
| Roasted Hazelnuts | 0.62 |
| Baked Potato | 0.614 |
| American Cheese | 0.567 |
| Flaxseeds | 0.47 |
| Feta Cheese | 0.424 |
| Raw Bananas | 0.367 |
| Raw Avocado | 0.257 |
| Hard-boiled Egg | 0.121 |
| American Cheddar | 0.12 |
| Dried Pine Nuts | 0.09 |
| Milk (2% Fat) | 0.051 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. https://doi.org/10.3390/cells7070084
Parra M, Stahl S, Hellmann H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells. 2018; 7(7):84. https://doi.org/10.3390/cells7070084
Chicago/Turabian StyleParra, Marcelina, Seth Stahl, and Hanjo Hellmann. 2018. "Vitamin B6 and Its Role in Cell Metabolism and Physiology" Cells 7, no. 7: 84. https://doi.org/10.3390/cells7070084





