Chlamydomonas Basal Bodies as Flagella Organizing Centers
Abstract
:1. Introduction
2. Basal Body Structure and Composition in C. reinhardtii
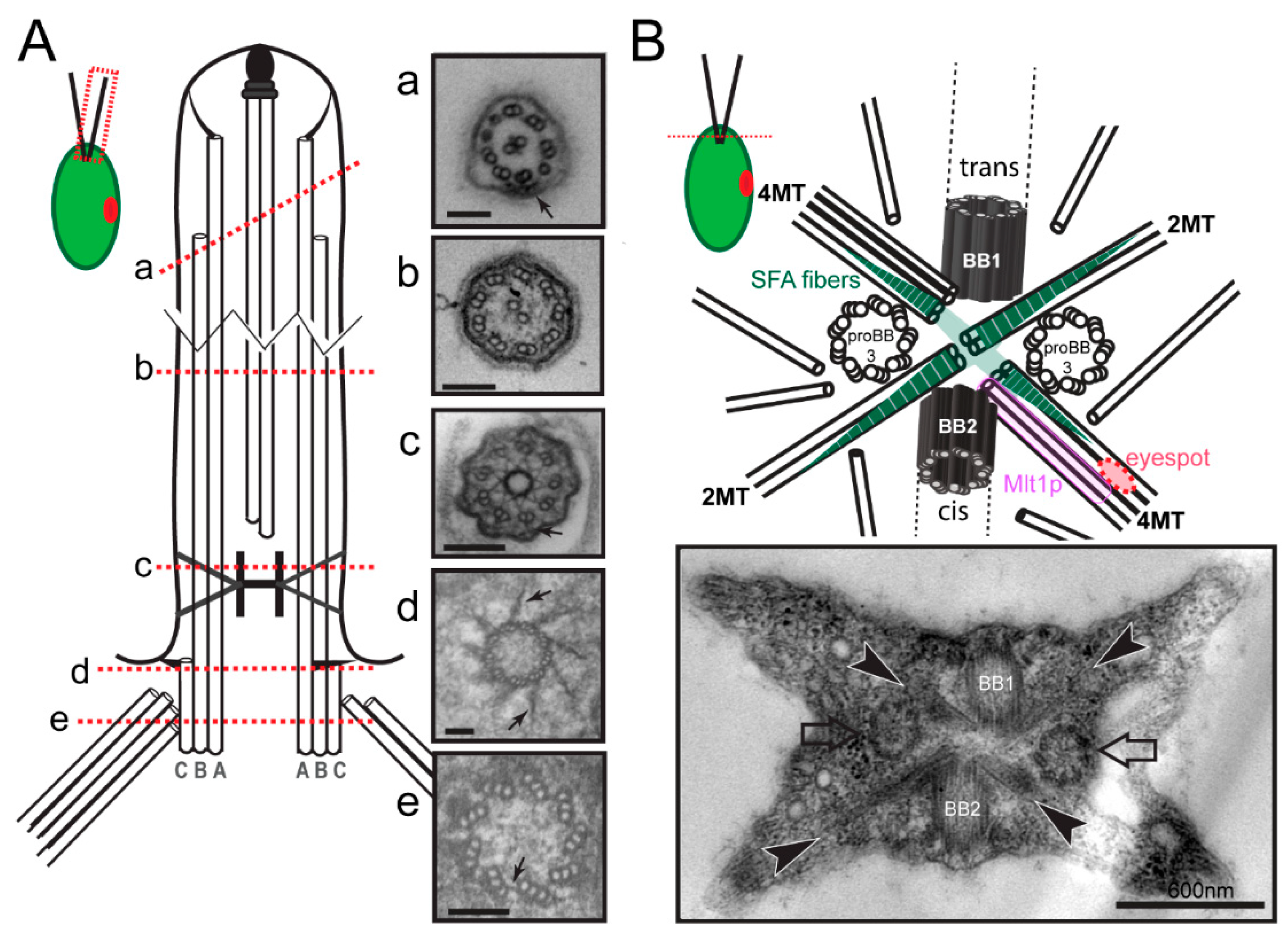
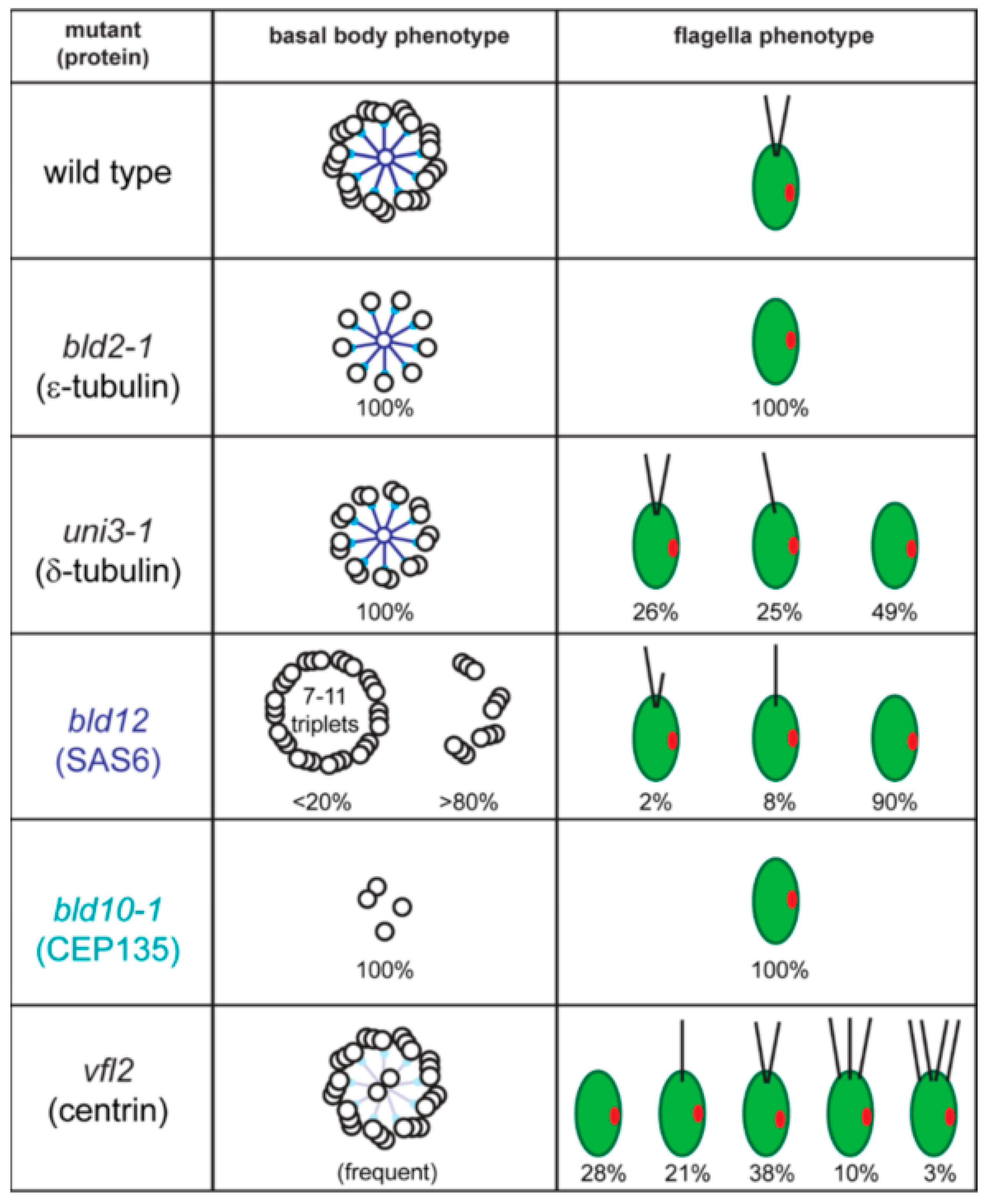
2.1. Basal Bodies
2.2. The Basal Body Scaffold
3. Basal Body-Associated Structures
3.1. Microtubular Roots
3.2. Fibrous Roots
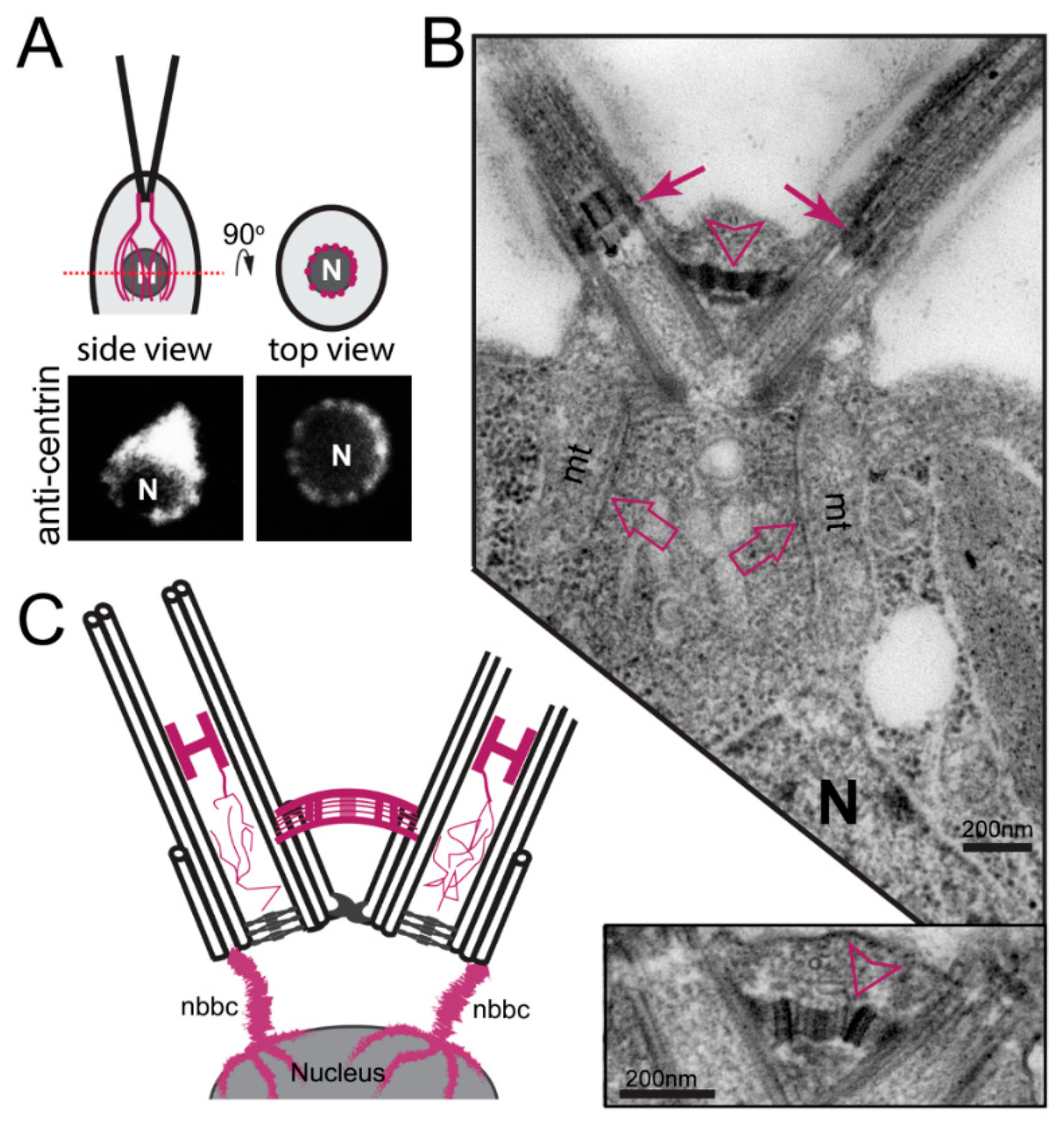
3.2.1. Centrin-Based Structures
3.2.2. Striated Fiber Assemblin (SFA) Fibers
4. The Basal Bodies in Mitosis
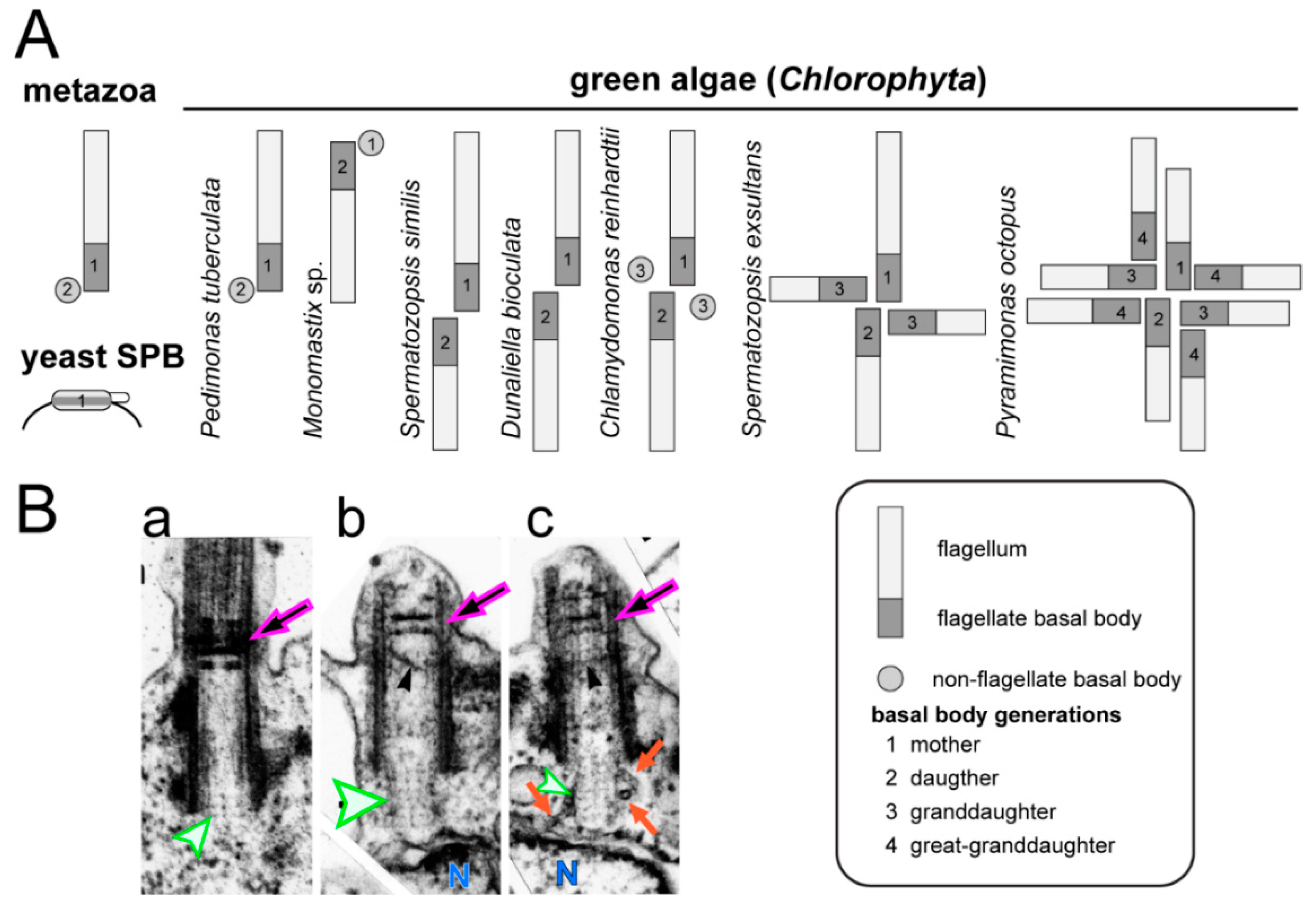
4.1. Cell Cycle and Mitosis of C. reinhardtii and Other Green Algae
4.2. The Basal Body and Flagellar Developmental Cycle
5. Basal Bodies as Organizing Centers for Flagella
5.1. Templating of the Axoneme
5.2. The Transition Zone
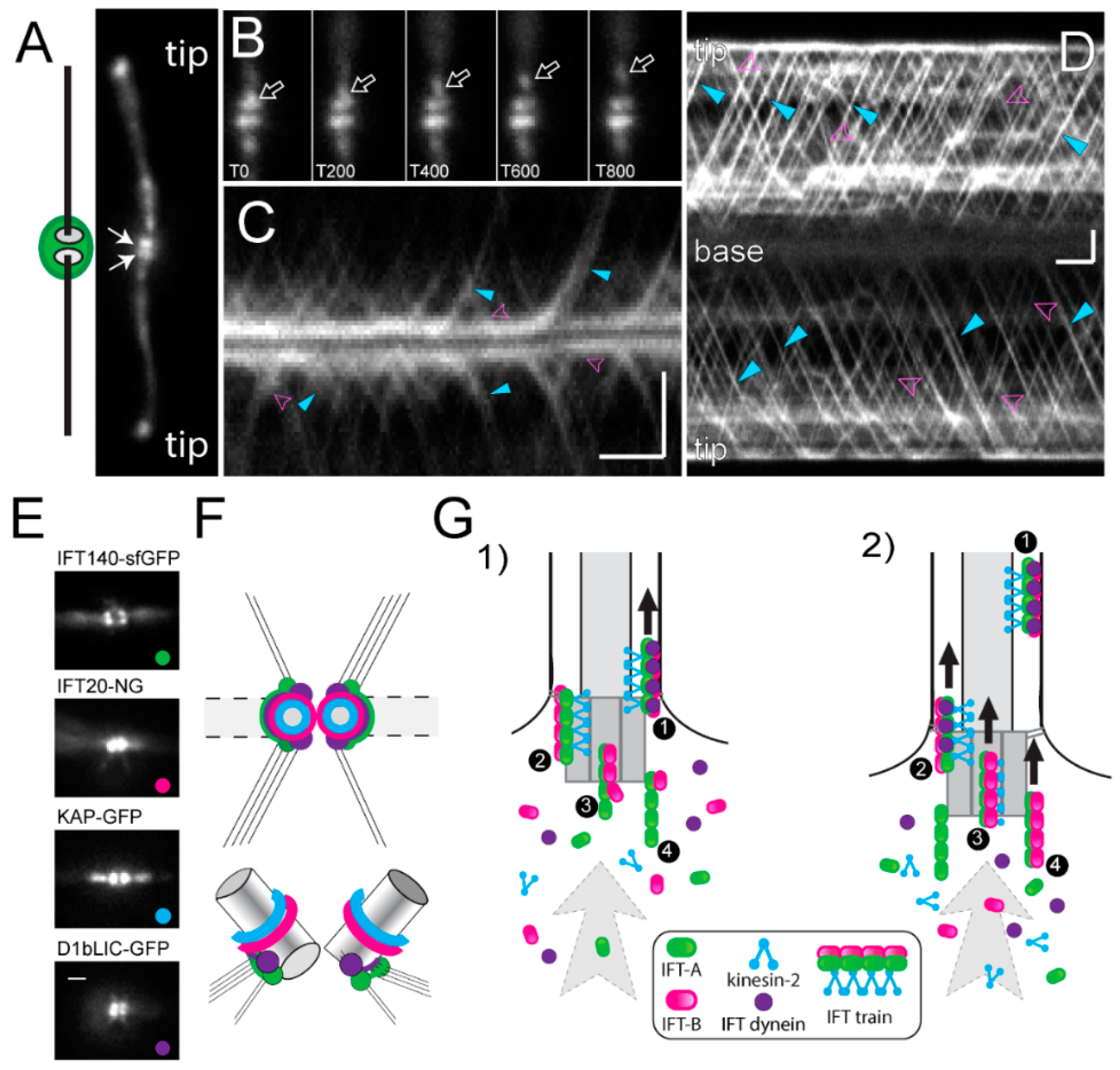
5.3. Recruitment of Intraflagellar Transport (IFT) Proteins and IFT Train Assembly
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | central pair |
| TZ | transition zone |
| POC | proteome of the centriole |
| NPHP | nephronophthisis |
| GFP | green fluorescent protein |
| IFT | intraflagellar transport |
References
- Satir, P. CILIA: Before and after. Cilia 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Witman, G.B. Cilia and Diseases. Bioscience 2014, 64, 1126–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawcett, D.W.; Porter, K.R. A study of the fine structure of ciliated epithelia. J. Morphol. 1954, 94, 221–281. [Google Scholar] [CrossRef]
- Musgrave, A.; de Wildt, P.; van Etten, I.; Pijst, H.; Scholma, C.; Kooyman, R.; Homan, W.; van den Ende, H. Evidence for a functional membrane barrier in the transition zone between the flagellum and cell body of Chlamydomonas eugametos gametes. Planta 1986, 167, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Dentler, W.L. Structures linking the tips of ciliary and flagellar microtubules to the membrane. J. Cell Sci. 1980, 42, 207–220. [Google Scholar] [PubMed]
- O’Toole, E.T.; Dutcher, S.K. Site-specific basal body duplication in Chlamydomonas. Cytoskeleton (Hoboken) 2014, 71, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Ehler, L.L.; Dutcher, S.K. Pharmacological and genetic evidence for a role of rootlet and phycoplast microtubules in the positioning and assembly of cleavage furrows in Chlamydomonas reinhardtii. Cell Motil. Cytoskelet. 1998, 40, 193–207. [Google Scholar] [CrossRef]
- Marshall, W.F.; Rosenbaum, J.L. How centrioles work: Lessons from green yeast. Curr. Opin. Cell Biol. 2000, 12, 119–125. [Google Scholar] [CrossRef]
- Snell, W.J.; Dentler, W.L.; Haimo, L.T.; Binder, L.I.; Rosenbaum, J.L. Assembly of Chick Brain Tubulin onto Isolated Basal Bodies of Chlamydomonas reinhardi. Science 1974, 185, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.R. The basal bodies of Chlamydomonas reinhardtii. Formation from probasal bodies, isolation, and partial characterization. J. Cell Biol. 1975, 65, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.C.; Romijn, E.P.; Zamora, I.; Yates, J.R., 3rd; Marshall, W.F. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr. Biol. 2005, 15, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- McKean, P.G.; Baines, A.; Vaughan, S.; Gull, K. Gamma-tubulin functions in the nucleation of a discrete subset of microtubules in the eukaryotic flagellum. Curr. Biol. 2003, 13, 598–602. [Google Scholar] [CrossRef]
- Ruiz, F.; Beisson, J.; Rossier, J.; Dupuis-Williams, P. Basal body duplication in Paramecium requires gamma-tubulin. Curr. Biol. 1999, 9, 43–46. [Google Scholar] [CrossRef]
- Silflow, C.D.; Liu, B.; LaVoie, M.; Richardson, E.A.; Palevitz, B.A. Gamma-tubulin in Chlamydomonas: Characterization of the gene and localization of the gene product in cells. Cell Motil. Cytoskelet. 1999, 42, 285–297. [Google Scholar] [CrossRef]
- Guichard, P.; Chrétien, D.; Marco, S.; Tassin, A.M. Procentriole assembly revealed by cryo-electron tomography. EMBO J. 2010, 29, 1565–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, E.T.; Giddings, T.H.; McIntosh, J.R.; Dutcher, S.K. Three-dimensional Organization of Basal Bodies from Wild-Type and δ-Tubulin Deletion Strains of Chlamydomonas reinhardtii. Mol. Biol. Cell 2003, 14, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, S.K.; Trabuco, E.C. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes delta-tubulin, a new member of the tubulin superfamily. Mol. Biol. Cell 1998, 9, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ramanis, Z.; Dutcher, S.K.; Luck, D.J. Uniflagellar mutants of Chlamydomonas: Evidence for the role of basal bodies in transmission of positional information. Cell 1982, 29, 745–753. [Google Scholar] [CrossRef]
- Goodenough, U.W.; StClair, H.S. BALD-2: A mutation affecting the formation of doublet and triplet sets of microtubules in Chlamydomonas reinhardtii. J. Cell Biol. 1975, 66, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, S.K.; Morrissette, N.S.; Preble, A.M.; Rackley, C.; Stanga, J. Epsilon-tubulin is an essential component of the centriole. Mol. Biol. Cell 2002, 13, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Preble, A.M.; Giddings, T.H., Jr.; Dutcher, S.K. Extragenic bypass suppressors of mutations in the essential gene BLD2 promote assembly of basal bodies with abnormal microtubules in Chlamydomonas reinhardtii. Genetics 2001, 157, 163–181. [Google Scholar] [PubMed]
- Dutcher, S.K. The tubulin fraternity: Alpha to eta. Curr. Opin. Cell Biol. 2001, 13, 49–54. [Google Scholar] [CrossRef]
- Garreau de Loubresse, N.; Ruiz, F.; Beisson, J.; Klotz, C. Role of delta-tubulin and the C-tubule in assembly of Paramecium basal bodies. BMC Cell Biol. 2001, 2, 4. [Google Scholar] [CrossRef]
- Chang, P.; Stearns, T. Delta-tubulin and epsilon-tubulin: Two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat. Cell Biol. 2000, 2, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Geimer, S.; Teltenkotter, A.; Plessmann, U.; Weber, K.; Lechtreck, K.F. Purification and characterization of basal apparatuses from a flagellate green alga. Cell Motil. Cytoskelet. 1997, 37, 72–85. [Google Scholar] [CrossRef]
- Dammermann, A.; Muller-Reichert, T.; Pelletier, L.; Habermann, B.; Desai, A.; Oegema, K. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell 2004, 7, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Leidel, S.; Delattre, M.; Cerutti, L.; Baumer, K.; Gonczy, P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 2005, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Hiraki, M.; Kamiya, R.; Hirono, M. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 2007, 17, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Vakonakis, I.; Olieric, N.; Hilbert, M.; Keller, D.; Olieric, V.; Bortfeld, M.; Erat, M.C.; Fluckiger, I.; Gonczy, P.; et al. Structural basis of the 9-fold symmetry of centrioles. Cell 2011, 144, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Guichard, P.; Hamel, V.; Le Guennec, M.; Banterle, N.; Iacovache, I.; Nemcikova, V.; Fluckiger, I.; Goldie, K.N.; Stahlberg, H.; Levy, D.; et al. Cell-free reconstitution reveals centriole cartwheel assembly mechanisms. Nat. Commun. 2017, 8, 14813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guichard, P.; Hamel, V.; Gonczy, P. The Rise of the Cartwheel: Seeding the Centriole Organelle. Bioessays 2018, 40, e1700241. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, M.; Noga, A.; Frey, D.; Hamel, V.; Guichard, P.; Kraatz, S.H.; Pfreundschuh, M.; Hosner, S.; Fluckiger, I.; Jaussi, R.; et al. SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture. Nat. Cell Biol. 2016, 18, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraki, M.; Nakazawa, Y.; Kamiya, R.; Hirono, M. Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr. Biol. 2007, 17, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Lefebvre, P.A.; Kamiya, R.; Hirono, M. Bld10p, a novel protein essential for basal body assembly in Chlamydomonas: Localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. J. Cell Biol. 2004, 165, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, J.; Hergert, P.; Delouvée, A.; Euteneuer, U.; Formstecher, E.; Khodjakov, A.; Bornens, M. hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 2009, 185, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meehl, J.B.; Bayless, B.A.; Giddings, T.H., Jr.; Pearson, C.G.; Winey, M. Tetrahymena Poc1 ensures proper intertriplet microtubule linkages to maintain basal body integrity. Mol. Biol. Cell 2016, 27, 2394–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, C.G.; Osborn, D.P.; Giddings, T.H., Jr.; Beales, P.L.; Winey, M. Basal body stability and ciliogenesis requires the conserved component Poc1. J. Cell Biol. 2009, 187, 905–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, L.C.; Geimer, S.; Romijn, E.; Yates, J., 3rd; Zamora, I.; Marshall, W.F. Molecular architecture of the centriole proteome: The conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol. Biol. Cell 2009, 20, 1150–1166. [Google Scholar] [CrossRef] [PubMed]
- Ringo, D.L. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 1967, 33, 543–571. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Liu, Y.; Yang, P.; Kner, P.; Lechtreck, K.F. Single-particle imaging reveals intraflagellar transport-independent transport and accumulation of EB1 in Chlamydomonas flagella. Mol. Biol. Cell 2016, 27, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Visetsouk, M.; Mynlieff, M.; Qin, H.; Lechtreck, K.F.; Yang, P. H+- and Na+- elicited rapid changes of the microtubule cytoskeleton in the biflagellated green alga Chlamydomonas. eLife 2017, 6, e26002. [Google Scholar] [CrossRef] [PubMed]
- Mittelmeier, T.M.; Thompson, M.D.; Lamb, M.R.; Lin, H.; Dieckmann, C.L. MLT1 links cytoskeletal asymmetry to organelle placement in chlamydomonas. Cytoskeleton (Hoboken) 2015, 72, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, M.R.; Dutcher, S.K.; Worley, C.K.; Dieckmann, C.L. Eyespot-Assembly Mutants in Chlamydomonas reinhardtii. Genetics 1999, 153, 721–729. [Google Scholar] [PubMed]
- Geimer, S.; Lechtreck, K.F.; Melkonian, M. A Novel Basal Apparatus Protein of 90 kD (BAp90) from the Flagellate Green Alga Spermatozopsis similis is a Component of the Proximal Plates and Identifies the d-(dexter) Surface of the Basal Body. Protist 1998, 149, 173–184. [Google Scholar] [CrossRef]
- Geimer, S.; Melkonian, M. The ultrastructure of the Chlamydomonas reinhardtii basal apparatus: Identification of an early marker of radial asymmetry inherent in the basal body. J. Cell Sci. 2004, 117, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.L. Roots. J. Eukaryot. Microbiol. 1998, 45, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, M. Ultrastructural aspects of basal body associated fibrous structures in green algae: A critical review. Biosystems 1980, 12, 85–104. [Google Scholar] [CrossRef]
- Lechtreck, K.-F.; Melkonian, M. An update on fibrous flagellar roots in green algae. Protoplasma 1991, 164, 38–44. [Google Scholar] [CrossRef]
- Tourbez, M.; Firanescu, C.; Yang, A.; Unipan, L.; Duchambon, P.; Blouquit, Y.; Craescu, C.T. Calcium-dependent self-assembly of human centrin 2. J. Biol. Chem. 2004, 279, 47672–47680. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.J.; Daly, O.M.; Morrison, C.G. Such small hands: The roles of centrins/caltractins in the centriole and in genome maintenance. Cell. Mol. Life Sci. 2012, 69, 2979–2997. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, J.V. Sfi1p has conserved centrin-binding sites and an essential function in budding yeast spindle pole body duplication. J. Cell Biol. 2003, 162, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, J.L. Centrosomes: Sfi1p and Centrin Unravel a Structural Riddle. Curr. Biol. 2004, 14, R27–R29. [Google Scholar] [CrossRef] [PubMed]
- Gogendeau, D.; Beisson, J.; de Loubresse, N.G.; Le Caer, J.P.; Ruiz, F.; Cohen, J.; Sperling, L.; Koll, F.; Klotz, C. An Sfi1p-like centrin-binding protein mediates centrin-based Ca2+-dependent contractility in Paramecium tetraurelia. Eukaryot. Cell 2007, 6, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.L.; Floyd, G.L. Calcium-induced contraction of the rhizoplast of a quadriflagellate green alga. Science 1978, 202, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Mengersen, A.; Lee, V.D. Molecular cloning of cDNA for caltractin, a basal body-associated Ca2+-binding protein: Homology in its protein sequence with calmodulin and the yeast CDC31 gene product. J. Cell Biol. 1988, 107, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.D.; Stapleton, M.; Huang, B. Genomic structure of Chlamydomonas caltractin. Evidence for intron insertion suggests a probable genealogy for the EF-hand superfamily of proteins. J. Mol. Biol. 1991, 221, 175–191. [Google Scholar] [CrossRef]
- Salisbury, J.L.; Baron, A.T.; Sanders, M.A. The centrin-based cytoskeleton of Chlamydomonas reinhardtii: Distribution in interphase and mitotic cells. J. Cell Biol. 1988, 107, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Geimer, S.; Melkonian, M. Centrin scaffold in Chlamydomonas reinhardtii revealed by immunoelectron microscopy. Eukaryot. Cell 2005, 4, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.L.; Salisbury, J.; Jarvik, J.W. A nucleus-basal body connector in Chlamydomonas reinhardtii that may function in basal body localization or segregation. J. Cell Biol. 1985, 101, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.L.; Sanders, M.A.; Harpst, L. Flagellar Root Contraction and Nuclear-Movement during Flagellar Regeneration in Chlamydomonas-Reinhardtii. J. Cell Biol. 1987, 105, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Grunow, A.; Lechtreck, K.F. Mitosis in Dunaliella bioculata (Chlorophyta): Centrin but not basal bodies are at the spindle poles. J. Phycol. 2001, 37, 1030–1043. [Google Scholar] [CrossRef]
- Hayashi, M.; Yagi, T.; Yoshimura, K.; Kamiya, R. Real-time observation of Ca2+-induced basal body reorientation in Chlamydomonas. Cell Motil. Cytoskelet. 1998, 41, 49–56. [Google Scholar] [CrossRef]
- McFadden, G.I.; Schulze, D.; Surek, B.; Salisbury, J.L.; Melkonian, M. Basal body reorientation mediated by a Ca2+-modulated contractile protein. J. Cell Biol. 1987, 105, 903–912. [Google Scholar] [CrossRef] [PubMed]
- LeDizet, M.; Piperno, G. The light chain p28 associates with a subset of inner dynein arm heavy chains in Chlamydomonas axonemes. Mol. Biol. Cell 1995, 6, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.L.; Adler, S.A.; Spanier, J.G.; Jarvik, J.W. Nucleus-basal body connector in Chlamydomonas: Evidence for a role in basal body segregation and against essential roles in mitosis or in determining cell polarity. Cell Motil. Cytoskelet. 1989, 14, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Taillon, B.E.; Adler, S.A.; Suhan, J.P.; Jarvik, J.W. Mutational analysis of centrin: An EF-hand protein associated with three distinct contractile fibers in the basal body apparatus of Chlamydomonas. J. Cell Biol. 1992, 119, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Kuchka, M.R.; Jarvik, J.W. Analysis of flagellar size control using a mutant of Chlamydomonas reinhardtii with a variable number of flagella. J. Cell Biol. 1982, 92, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.F.; Vucica, Y.; Rosenbaum, J.L. Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr. Biol. 2001, 11, 308–317. [Google Scholar] [CrossRef]
- Koblenz, B.; Schoppmeier, J.; Grunow, A.; Lechtreck, K.F. Centrin deficiency in Chlamydomonas causes defects in basal body replication, segregation and maturation. J. Cell Sci. 2003, 116, 2635–2646. [Google Scholar] [CrossRef] [PubMed]
- Jarvik, J.W.; Suhan, J.P. The role of the flagellar transition region: Inferences from the analysis of a Chlamydomonas mutant with defective transition region structures. J. Cell Sci. 1991, 99, 731–740. [Google Scholar]
- Lechtreck, K.F.; Silflow, C.D. SF-assemblin in Chlamydomonas: Sequence conservation and localization during the cell cycle. Cell Motil. Cytoskelet. 1997, 36, 190–201. [Google Scholar] [CrossRef]
- Goodenough, U.W.; Weiss, R.L. Interrelationships between microtubules, a striated fiber, and the gametic mating structure of Chlamydomonas reinhardi. J. Cell Biol. 1978, 76, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F.; Melkonian, M. Striated microtubule-associated fibers: Identification of assemblin, a novel 34-kD protein that forms paracrystals of 2-nm filaments in vitro. J. Cell Biol. 1991, 115, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F.; Frins, S.; Bilski, J.; Teltenkotter, A.; Weber, K.; Melkonian, M. The cruciated microtubule-associated fibers of the green alga Dunaliella bioculata consist of a 31 kDa SF-assemblin. J. Cell Sci. 1996, 109 Pt 4, 827–835. [Google Scholar] [PubMed]
- Francia, M.E.; Jordan, C.N.; Patel, J.D.; Sheiner, L.; Demerly, J.L.; Fellows, J.D.; de Leon, J.C.; Morrissette, N.S.; Dubremetz, J.-F.; Striepen, B. Cell Division in Apicomplexan Parasites Is Organized by a Homolog of the Striated Rootlet Fiber of Algal Flagella. PLoS Biol. 2012, 10, e1001444. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F. Striated fiber assemblin in apicomplexan parasites. Mol. Biochem. Parasitol. 2003, 128, 95–99. [Google Scholar] [CrossRef]
- Harper, J.D.; Thuet, J.; Lechtreck, K.F.; Hardham, A.R. Proteins related to green algal striated fiber assemblin are present in stramenopiles and alveolates. Protoplasma 2009, 236, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Geisler, N.; Plessmann, U.; Bremerich, A.; Lechtreck, K.F.; Melkonian, M. SF-assemblin, the structural protein of the 2-nm filaments from striated microtubule associated fibers of algal flagellar roots, forms a segmented coiled coil. J. Cell Biol. 1993, 121, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palm, D.; Weiland, M.; McArthur, A.G.; Winiecka-Krusnell, J.; Cipriano, M.J.; Birkeland, S.R.; Pacocha, S.E.; Davids, B.; Gillin, F.; Linder, E.; et al. Developmental changes in the adhesive disk during Giardia differentiation. Mol. Biochem. Parasitol. 2005, 141, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F. Analysis of striated fiber formation by recombinant SF-assemblin in vitro. J. Mol. Biol. 1998, 279, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F.; Rostmann, J.; Grunow, A. Analysis of Chlamydomonas SF-assemblin by GFP tagging and expression of antisense constructs. J. Cell Sci. 2002, 115, 1511–1522. [Google Scholar] [PubMed]
- Iftode, F.; Fleury-Aubusson, A. Structural inheritance in Paramecium: Ultrastructural evidence for basal body and associated rootlets polarity transmission through binary fission. Biol. Cell 2003, 95, 39–51. [Google Scholar] [CrossRef]
- Lechtreck, K.F.; Grunow, A. Evidence for a direct role of nascent basal bodies during spindle pole initiation in the green alga Spermatozopsis similis. Protist 1999, 150, 163–181. [Google Scholar] [CrossRef]
- Schoppmeier, J.; Lechtreck, K.F. Localization of p210-related proteins in green flagellates and analysis of flagellar assembly in the green alga Dunaliella bioculata with monoclonal anti-p210. Protoplasma 2002, 220, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.F.; Bonney, S.; Kronenberg, Z.; Clarissa, C.; Yandell, M.; Elde, N.C.; Jerka-Dziadosz, M.; Giddings, T.H.; Frankel, J.; Pearson, C.G. DisAp-dependent striated fiber elongation is required to organize ciliary arrays. J. Cell Biol. 2014, 207, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, F.R.; Umen, J.G. The Chlamydomonas cell cycle. Plant J. 2015, 82, 370–392. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.R.; Huang, K.; Diener, D.R.; Rosenbaum, J.L. The cilium secretes bioactive ectosomes. Curr. Biol. 2013, 23, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Coss, R.A. Mitosis in Chlamydomonas reinhardtii basal bodies and the mitotic apparatus. J. Cell Biol. 1974, 63, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, G.; Kamiya, R.; Hirono, M. Scaffolding function of the Chlamydomonas procentriole protein CRC70, a member of the conserved Cep70 family. J. Cell Sci. 2011, 124, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Werner, W.K. Assembly and fate of basal bodies in the colourless phytoflagellate Polytoma papillatum. Biol. Cell 1992, 76, 193–200. [Google Scholar]
- Gaffal, K.P.; Elgammal, S. Elucidation of the Enigma of the Metaphase Band of Chlamydomonas-Reinhardtii. Protoplasma 1990, 156, 139–148. [Google Scholar] [CrossRef]
- Johnson, U.G.; Porter, K.R. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J. Cell Biol. 1968, 38, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Beech, P.L.; Heimann, K.; Melkonian, M. Development of the Flagellar Apparatus during the Cell-Cycle in Unicellular Algae. Protoplasma 1991, 164, 23–37. [Google Scholar] [CrossRef]
- Hori, T.; Moestrup, O. Ultrastructure of the Flagellar Apparatus in Pyramimonas-Octopus (Prasinophyceae). 1. Axoneme Structure and Numbering of Peripheral Doublets Triplets. Protoplasma 1987, 138, 137–148. [Google Scholar] [CrossRef]
- Melkonian, M.; Reize, I.B.; Preisig, H.R. Maturation of a Flagellum/Basal Body Requires More than One Cell Cycle in Algal Flagellates: Studies on Nephroselmis Olivacea (Prasinophyceae); Springer: Berlin/Heidelberg, Germany, 1987; pp. 102–113. [Google Scholar]
- Kamiya, R.; Witman, G.B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 1984, 98, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Schoppmeier, J.; Lechtreck, K.F. Flagellar regeneration in Spermatozopsis similis (Chlorophyta). J. Phycol. 2003, 39, 918–922. [Google Scholar] [CrossRef]
- Lechtreck, K.-F.; Reize, I.B.; Melkonian, M. The Cytoskeleton of the Naked Green Flagellate Spermatozopsis Similis (Chlorophyta): Flagellar and Basal Body Developmental Cycle1. J. Phycol. 1997, 33, 254–265. [Google Scholar] [CrossRef]
- Wetherbee, R.; Platt, S.J.; Beech, P.L.; Pickett-Heaps, J.D. Flagellar transformation in the heterokont Epipyxis pulchra (Chrysophyceae): Direct observations using image enhanced light microscopy. Protoplasma 1988, 145, 47–54. [Google Scholar] [CrossRef]
- Lange, B.M.; Gull, K. A molecular marker for centriole maturation in the mammalian cell cycle. J. Cell Biol. 1995, 130, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Gonzalo, F.R.; Reiter, J.F. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J. Cell Biol. 2012, 197, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanek, L.; Pigino, G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 2016, 352, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Liu, D.; Kastritis, P.L.; Basu, K.; Hsu, T.C.; Yang, S.; Bui, K.H. Subnanometre-resolution structure of the doublet microtubule reveals new classes of microtubule-associated proteins. Nat. Commun. 2017, 8, 15035. [Google Scholar] [CrossRef] [PubMed]
- Comartin, D.; Gupta, G.D.; Fussner, E.; Coyaud, E.; Hasegan, M.; Archinti, M.; Cheung, S.W.; Pinchev, D.; Lawo, S.; Raught, B.; et al. CEP120 and SPICE1 cooperate with CPAP in centriole elongation. Curr. Biol. 2013, 23, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, M.R.; Xie, Z.; Stearns, T. Cep120 is asymmetrically localized to the daughter centriole and is essential for centriole assembly. J. Cell Biol. 2010, 191, 331–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piasecki, B.P.; LaVoie, M.; Tam, L.W.; Lefebvre, P.A.; Silflow, C.D. The Uni2 phosphoprotein is a cell cycle regulated component of the basal body maturation pathway in Chlamydomonas reinhardtii. Mol. Biol. Cell 2008, 19, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, B.P.; Silflow, C.D. The UNI1 and UNI2 genes function in the transition of triplet to doublet microtubules between the centriole and cilium in Chlamydomonas. Mol. Biol. Cell 2009, 20, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.A.; Salisbury, J.L. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J. Cell Biol. 1989, 108, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.K.Y.; Marshall, W.F. Self-repairing cells: How single cells heal membrane ruptures and restore lost structures. Science 2017, 356, 1022–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Gao, K.; Zheng, S.; Zhu, X.; Liang, Y.; Pan, J. Calmodulin regulates a TRP channel (ADF1) and phospholipase C (PLC) to mediate elevation of cytosolic calcium during acidic stress that induces deflagellation in Chlamydomonas. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dymek, E.E.; Smith, E.F. PF19 encodes the p60 catalytic subunit of katanin and is required for assembly of the flagellar central apparatus in Chlamydomonas. J. Cell Sci. 2012, 125, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Lohret, T.A.; McNally, F.J.; Quarmby, L.M. A Role for Katanin-mediated Axonemal Severing during Chlamydomonas Deflagellation. Mol. Biol. Cell 1998, 9, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.D.; Hilton, L.K.; Diener, D.R.; Rasi, M.Q.; Mahjoub, M.R.; Rosenbaum, J.L.; Quarmby, L.M. Centrioles are freed from cilia by severing prior to mitosis. Cytoskeleton (Hoboken) 2010, 67, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diener, D.R.; Lupetti, P.; Rosenbaum, J.L. Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins. Curr. Biol. 2015, 25, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Gasiewski, J.A.; Eagan, M.K.; Garcia, G.A.; Hurtado, S.; Chang, M.J. From Gatekeeping to Engagement: A Multicontextual, Mixed Method Study of Student Academic Engagement in Introductory STEM Courses. Res. High. Educ. 2012, 53, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Gilula, N.B.; Satir, P. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 1972, 53, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.L.; Goodenough, D.A.; Goodenough, U.W. Membrane particle arrays associated with the basal body and with contractile vacuole secretion in Chlamydomonas. J. Cell Biol. 1977, 72, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Awata, J.; Takada, S.; Standley, C.; Lechtreck, K.F.; Bellve, K.D.; Pazour, G.J.; Fogarty, K.E.; Witman, G.B. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J. Cell Sci. 2014, 127, 4714–4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craige, B.; Tsao, C.C.; Diener, D.R.; Hou, Y.; Lechtreck, K.F.; Rosenbaum, J.L.; Witman, G.B. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 2010, 190, 927–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechtreck, K.F.; Gould, T.J.; Witman, G.B. Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia 2013, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Ariyoshi, T.; Noga, A.; Kamiya, R.; Hirono, M. Space-dependent formation of central pair microtubules and their interactions with radial spokes. PLoS ONE 2014, 9, e110513. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.R. The flagellar central pair apparatus. In The Chlamydomonas Sourcebook; Elsevier: New York, NY, USA, 2009; pp. 235–252. [Google Scholar]
- Witman, G.B. The Site of In Vivo Assembly of Flagellar Microtubules. Ann. N. Y. Acad. Sci. 1975, 253, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.L.; Child, F.M. Flagellar regeneration in protozoan flagellates. J. Cell Biol. 1967, 34, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Agrin, N.; Leszyk, J.; Witman, G.B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 2005, 170, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechtreck, K.F. IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem. Sci. 2015, 40, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Taschner, M.; Lorentzen, E. The Intraflagellar Transport Machinery. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Kozminski, K.G.; Beech, P.L.; Rosenbaum, J.L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 1995, 131, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Piperno, G.; Siuda, E.; Henderson, S.; Segil, M.; Vaananen, H.; Sassaroli, M. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J. Cell Biol. 1998, 143, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.G.; Diener, D.R.; Himelblau, A.L.; Beech, P.L.; Fuster, J.C.; Rosenbaum, J.L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998, 141, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Pigino, G.; Geimer, S.; Lanzavecchia, S.; Paccagnini, E.; Cantele, F.; Diener, D.R.; Rosenbaum, J.L.; Lupetti, P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J. Cell Biol. 2009, 187, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentler, W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J. Cell Biol. 2005, 170, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.B.; Geimer, S.; Rosenbaum, J.L. Dissecting the molecular mechanisms of intraflagellar transport in chlamydomonas. Curr. Biol. 2006, 16, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, E.; Paccagnini, E.; Cantele, F.; Gentile, M.; Dini, D.; Fino, F.; Diener, D.; Mencarelli, C.; Lupetti, P. Two classes of short intraflagellar transport train with different 3D structures are present in Chlamydomonas flagella. J. Cell Sci. 2016, 129, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.T.; Gao, C.; Lucker, B.F.; Cole, D.G.; Mitchell, D.R. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 2008, 183, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Deane, J.A.; Cole, D.G.; Seeley, E.S.; Diener, D.R.; Rosenbaum, J.L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001, 11, 1586–1590. [Google Scholar] [CrossRef]
- Richey, E.A.; Qin, H. Dissecting the sequential assembly and localization of intraflagellar transport particle complex B in Chlamydomonas. PLoS ONE 2012, 7, e43118. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, M.; Scholz, D.; Geimer, S. Chapter Fourteen—Electron Microscopy of Flagella, Primary Cilia, and Intraflagellar Transport in Flat-Embedded Cells. In Methods in Enzymology; Marshall, W.F., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 524, pp. 243–263. [Google Scholar]
- Taschner, M.; Kotsis, F.; Braeuer, P.; Kuehn, E.W.; Lorentzen, E. Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. J. Cell Biol. 2014, 207, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taschner, M.; Weber, K.; Mourao, A.; Vetter, M.; Awasthi, M.; Stiegler, M.; Bhogaraju, S.; Lorentzen, E. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 2016, 35, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Katoh, Y.; Nakayama, K. Intraflagellar transport-A complex mediates ciliary entry and retrograde trafficking of ciliary G protein-coupled receptors. Mol. Biol. Cell 2017, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Terada, M.; Nishijima, Y.; Takei, R.; Nozaki, S.; Hamada, H.; Nakayama, K. Overall Architecture of the Intraflagellar Transport (IFT)-B Complex Containing Cluap1/IFT38 as an Essential Component of the IFT-B Peripheral Subcomplex. J. Biol. Chem. 2016, 291, 10962–10975. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wan, L.; Taschner, M.; Cheng, X.; Lorentzen, E.; Huang, K. Intraflagellar transport protein IFT52 recruits IFT46 to the basal body and flagella. J. Cell Sci. 2017, 130, 1662–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.M.; Cochran, D.A.; Craige, B.; Kubo, T.; Witman, G.B. Assembly of IFT Trains at the Ciliary Base Depends on IFT74. Curr. Biol. 2015, 25, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Pazour, G.J.; Witman, G.B. A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Mol. Biol. Cell 2004, 15, 4382–4394. [Google Scholar] [CrossRef] [PubMed]
- Reck, J.; Schauer, A.M.; VanderWaal Mills, K.; Bower, R.; Tritschler, D.; Perrone, C.A.; Porter, M.E. The role of the dynein light intermediate chain in retrograde IFT and flagellar function in Chlamydomonas. Mol. Biol. Cell 2016, 27, 2404–2422. [Google Scholar] [CrossRef] [PubMed]
- Taschner, M.; Lorentzen, A.; Mourão, A.; Collins, T.; Freke, G.M.; Moulding, D.; Basquin, J.; Jenkins, D.; Lorentzen, E. Crystal structure of intraflagellar transport protein 80 reveals a homo-dimer required for ciliogenesis. eLife 2018, 7, e33067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingfield, J.L.; Mengoni, I.; Bomberger, H.; Jiang, Y.Y.; Walsh, J.D.; Brown, J.M.; Picariello, T.; Cochran, D.A.; Zhu, B.; Pan, J.; et al. IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. eLife 2017, 6, e26609. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Qin, H.; Follit, J.A.; Pazour, G.J.; Rosenbaum, J.L.; Witman, G.B. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007, 176, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Pang, Y.; Wu, Q.; Hu, Z.; Han, X.; Xu, Y.; Deng, H.; Pan, J. FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev. Cell 2014, 30, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Hagiya, Y.; Kubo, T.; Takei, R.; Katoh, Y.; Nakayama, K. RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol. Biol. Cell 2017, 28, 1652–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanie, T.; Abbott, K.L.; Mooney, N.A.; Plowey, E.D.; Demeter, J.; Jackson, P.K. The CEP19-RABL2 GTPase Complex Binds IFT-B to Initiate Intraflagellar Transport at the Ciliary Base. Dev. Cell 2017, 42, 22–36.e12. [Google Scholar] [CrossRef] [PubMed]
- Wren, K.N.; Craft, J.M.; Tritschler, D.; Schauer, A.; Patel, D.K.; Smith, E.F.; Porter, M.E.; Kner, P.; Lechtreck, K.F. A differential cargo loading model of ciliary length regulation by IFT. Curr. Boil. 2013, 23. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.; Shih, S.M.; Bower, R.; Tritschler, D.; Porter, M.E.; Yildiz, A. Dynamics of the IFT machinery at the ciliary tip. eLife 2017, 6, e28606. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wingfield, J.L.; Lechtreck, K.-F. Chlamydomonas Basal Bodies as Flagella Organizing Centers. Cells 2018, 7, 79. https://doi.org/10.3390/cells7070079
Wingfield JL, Lechtreck K-F. Chlamydomonas Basal Bodies as Flagella Organizing Centers. Cells. 2018; 7(7):79. https://doi.org/10.3390/cells7070079
Chicago/Turabian StyleWingfield, Jenna Lynne, and Karl-Ferdinand Lechtreck. 2018. "Chlamydomonas Basal Bodies as Flagella Organizing Centers" Cells 7, no. 7: 79. https://doi.org/10.3390/cells7070079
APA StyleWingfield, J. L., & Lechtreck, K.-F. (2018). Chlamydomonas Basal Bodies as Flagella Organizing Centers. Cells, 7(7), 79. https://doi.org/10.3390/cells7070079




