Sub-Cellular Localization of Metalloproteinases in Megakaryocytes
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
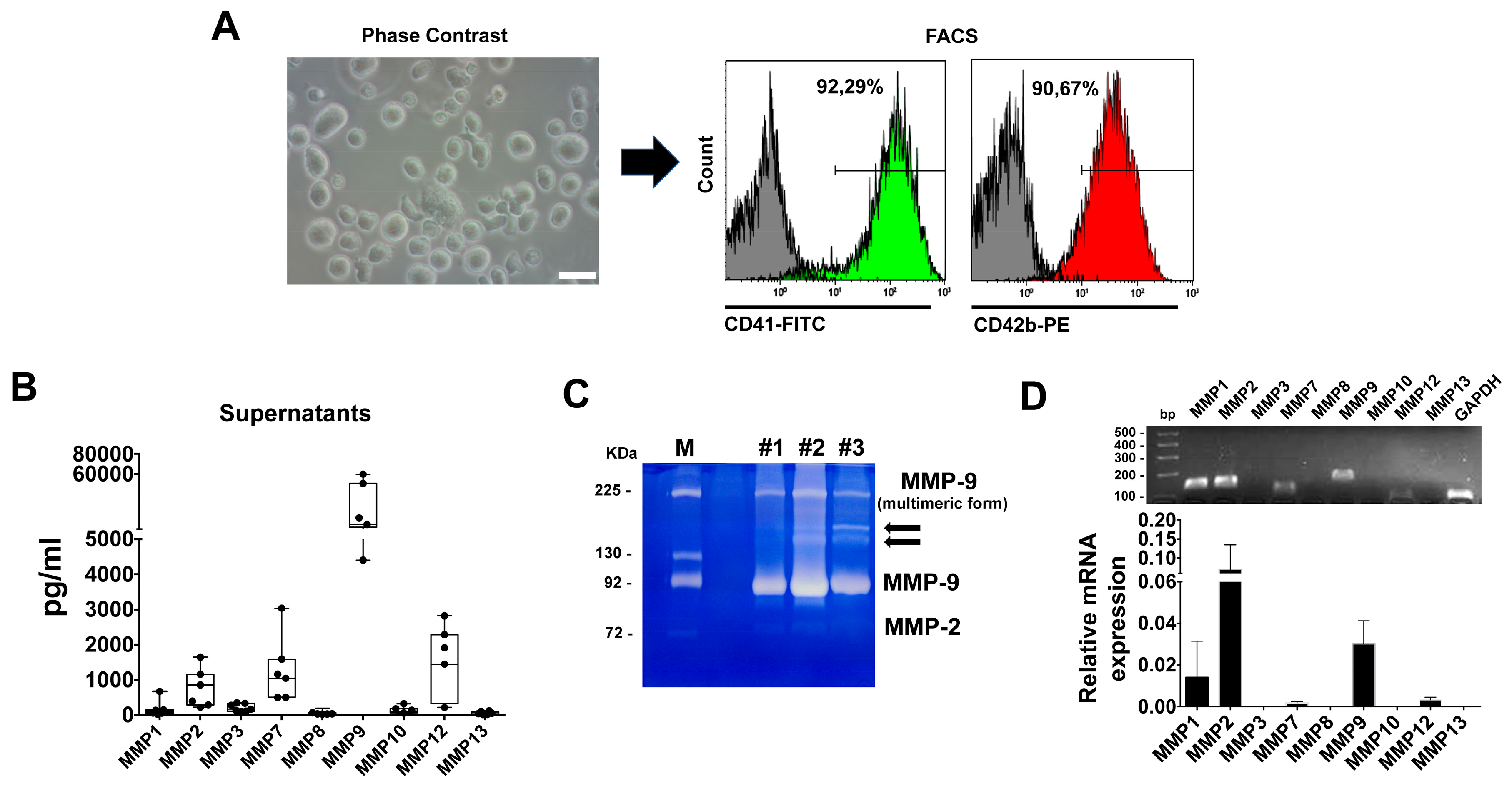
2.2. Flow Cytometry
2.3. Real Time PCR
2.4. Zymography
2.5. Cell Fractionation
2.6. MMPs Multiplex Array
2.7. Immunofluorescence
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malara, A.; Currao, M.; Gruppi, C.; Celesti, G.; Viarengo, G.; Buracchi, C.; Laghi, L.; Kaplan, D.L.; Balduini, A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Gruppi, C.; Celesti, G.; Abbonante, V.; Viarengo, G.; Laghi, L.; De Marco, L.; Muro, A.F.; Balduini, A. Alternatively spliced fibronectin extra domain a is required for hemangiogenic recovery upon bone marrow chemotherapy. Haematologica 2018, 103, e42–e45. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Abbonante, V.; Di Buduo, C.A.; Tozzi, L.; Currao, M.; Balduini, A. The secret life of a megakaryocyte: Emerging roles in bone marrow homeostasis control. Cell Mol. Life Sci. 2015, 72, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, M.; Yang, W.; Gao, A.; Yin, X.; Hu, L.; Wang, X.; Xu, J.; Hao, S.; Cheng, T.; et al. Megakaryocyte-derived excessive transforming growth factor β1 inhibits proliferation of normal hematopoietic stem cells in acute myeloid leukemia. Exp. Hematol. 2018, 60, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Bruns, I.; Lucas, D.; Pinho, S.; Ahmed, J.; Lambert, M.P.; Kunisaki, Y.; Scheiermann, C.; Schiff, L.; Poncz, M.; Bergman, A.; et al. Megakaryocytes regulate hematopoietic stem cell quiescence through cxcl4 secretion. Nat. Med. 2014, 20, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Perry, J.M.; Marshall, H.; Venkatraman, A.; Qian, P.; He, X.C.; Ahamed, J.; Li, L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014, 20, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; Di Buduo, C.A.; Gruppi, C.; Malara, A.; Gianelli, U.; Celesti, G.; Anselmo, A.; Laghi, L.; Vercellino, M.; Visai, L.; et al. Thrombopoietin/TGF-β1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells 2016, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Gruppi, C.; Rebuzzini, P.; Visai, L.; Perotti, C.; Moratti, R.; Balduini, C.; Tira, M.E.; Balduini, A. Megakaryocyte-matrix interaction within bone marrow: New roles for fibronectin and factor xiii-a. Blood 2011, 117, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; Chitalia, V.; Rosti, V.; Leiva, O.; Matsuura, S.; Balduini, A.; Ravid, K. Upregulation of lysyl oxidase and adhesion to collagen of human megakaryocytes and platelets in primary myelofibrosis. Blood 2017, 130, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Bagnara, G.P.; Papa, S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells 2006, 24, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Lane, W.J.; Dias, S.; Hattori, K.; Heissig, B.; Choy, M.; Rabbany, S.Y.; Wood, J.; Moore, M.A.; Rafii, S. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood 2000, 96, 4152–4159. [Google Scholar] [PubMed]
- Choi, W.S.; Jeon, O.H.; Kim, H.H.; Kim, D.S. Mmp-2 regulates human platelet activation by interacting with integrin alphaiibbeta3. J. Thromb. Haemost. 2008, 6, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Falcinelli, E.; Loffredo, F.; Cimmino, G.; Corazzi, T.; Forte, L.; Guglielmini, G.; Momi, S.; Golino, P. Platelets release matrix metalloproteinase-2 in the coronary circulation of patients with acute coronary syndromes: Possible role in sustained platelet activation. Eur. Heart J. 2011, 32, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, G.; Appolloni, V.; Momi, S.; De Groot, P.G.; Battiston, M.; De Marco, L.; Falcinelli, E.; Gresele, P. Matrix metalloproteinase-2 enhances platelet deposition on collagen under flow conditions. J. Thromb. Haemost. 2016, 115, 333–343. [Google Scholar]
- Wrzyszcz, A.; Wozniak, M. On the origin of matrix metalloproteinase-2 and -9 in blood platelets. Platelets 2012, 23, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Medda, V. Differential expression of MMP-2 and MMP-9 activity in megakaryocytes and platelets. Blood 2011, 118, 6470–6471. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, J.; Block, A.; Le Bousse-Kerdiles, M.C.; Lepreux, S.; Nurden, P.; Ripoche, J.; Nurden, A.T. Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: A novel organization for these secreted proteins. Exp. Hematol. 2009, 37, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, L.; Tolley, N.D.; Michetti, N.; Bury, L.; Weyrich, A.S.; Gresele, P. Megakaryocytes differentially sort mrnas for matrix metalloproteinases and their inhibitors into platelets: A mechanism for regulating synthetic events. Blood 2011, 118, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta 2017, 1864, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.C.; Cheung, S.T.; Fan, S.T. Atypical localization of membrane type 1-matrix metalloproteinase in the nucleus is associated with aggressive features of hepatocellular carcinoma. Mol. Carcinog. 2007, 46, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Medda, V. Nuclear localization of matrix metalloproteinases. Prog. Histochem. Cytochem. 2012, 47, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Aldonyte, R.; Brantly, M.; Block, E.; Patel, J.; Zhang, J. Nuclear localization of active matrix metalloproteinase-2 in cigarette smoke-exposed apoptotic endothelial cells. Exp. Lung Res. 2009, 35, 59–75. [Google Scholar] [PubMed]
- Kwan, J.A.; Schulze, C.J.; Wang, W.; Leon, H.; Sariahmetoglu, M.; Sung, M.; Sawicka, J.; Sims, D.E.; Sawicki, G.; Schulz, R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (adp-ribose) polymerase (parp) in vitro. FASEB J. 2004, 18, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.F.; Robinson, D.R.; Rosenbaum, J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. 2006, 169, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the ctgf/ccn2 gene. Mol. Cell. Biol. 2008, 28, 2391–2413. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Luchetti, F.; Canonico, B.; Falcieri, E.; Papa, S. Measurements, zymographic analysis, and characterization of matrix metalloproteinase-2 and -9 in healthy human umbilical cord blood. Clin. Chem. 2004, 50, 1715–1717. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Bose, P.; Leong-Quong, R.Y.; Fujita, D.J.; Riabowol, K. Reap: A two minute cell fractionation method. BMC Res. Notes 2010, 3, 294. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Mosti, G.; Croce, L.; Raffetto, J.D.; Mannello, F. Chronic venous disease—Part II: Proteolytic biomarkers in wound healing. Biochim. Biophys. Acta 2016, 1862, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Tschesche, H.; Zölzer, V.; Triebel, S.; Bartsch, S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur. J. Biochem. 2001, 268, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Shirvaikar, N.; Reca, R.; Jalili, A.; Marquez-Curtis, L.; Lee, S.F.; Ratajczak, M.Z.; Janowska-Wieczorek, A. Cfu-megakaryocytic progenitors expanded ex vivo from cord blood maintain their in vitro homing potential and express matrix metalloproteinases. Cytotherapy 2008, 10, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Matrisian, L.M. Matrilysin: An epithelial matrix metalloproteinase with potentially novel functions. Int. J. Biochem. Cell Biol. 1996, 28, 123–136. [Google Scholar] [CrossRef]
- Dunsmore, S.E.; Saarialho-Kere, U.K.; Roby, J.D.; Wilson, C.L.; Matrisian, L.M.; Welgus, H.G.; Parks, W.C. Matrilysin expression and function in airway epithelium. J. Clin. Invest. 1998, 102, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Park, P.W.; Wilson, C.L.; Parks, W.C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002, 111, 635–646. [Google Scholar] [CrossRef]
- Qu, P.; Yan, C.; Du, H. Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood 2011, 117, 4476–4489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, Y.; Wei, G.; Hu, W.; Li, L.; Lu, S.; Meng, Z. Matrix metalloproteinase12 facilitated platelet activation by shedding carcinoembryonic antigen related cell adhesion molecule1. Biochem. Biophys. Res. Commun. 2017, 486, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Kile, B.T. The role of apoptosis in megakaryocytes and platelets. Br. J. Haematol. 2014, 165, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Hartwig, J.H.; Italiano, J.E. The biogenesis of platelets from megakaryocyte proplatelets. J. Clin. Investig. 2005, 115, 3348–3354. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malara, A.; Ligi, D.; Di Buduo, C.A.; Mannello, F.; Balduini, A. Sub-Cellular Localization of Metalloproteinases in Megakaryocytes. Cells 2018, 7, 80. https://doi.org/10.3390/cells7070080
Malara A, Ligi D, Di Buduo CA, Mannello F, Balduini A. Sub-Cellular Localization of Metalloproteinases in Megakaryocytes. Cells. 2018; 7(7):80. https://doi.org/10.3390/cells7070080
Chicago/Turabian StyleMalara, Alessandro, Daniela Ligi, Christian A. Di Buduo, Ferdinando Mannello, and Alessandra Balduini. 2018. "Sub-Cellular Localization of Metalloproteinases in Megakaryocytes" Cells 7, no. 7: 80. https://doi.org/10.3390/cells7070080
APA StyleMalara, A., Ligi, D., Di Buduo, C. A., Mannello, F., & Balduini, A. (2018). Sub-Cellular Localization of Metalloproteinases in Megakaryocytes. Cells, 7(7), 80. https://doi.org/10.3390/cells7070080






