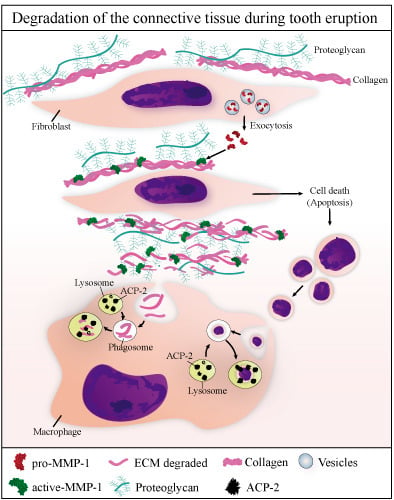
Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars
Abstract
1. Introduction
2. Materials and Methods
2.1. Light Microscopy
2.2. Collagen Content Measurement in the Eruptive Pathway
2.3. Immunohistochemical Detection of MMP-1
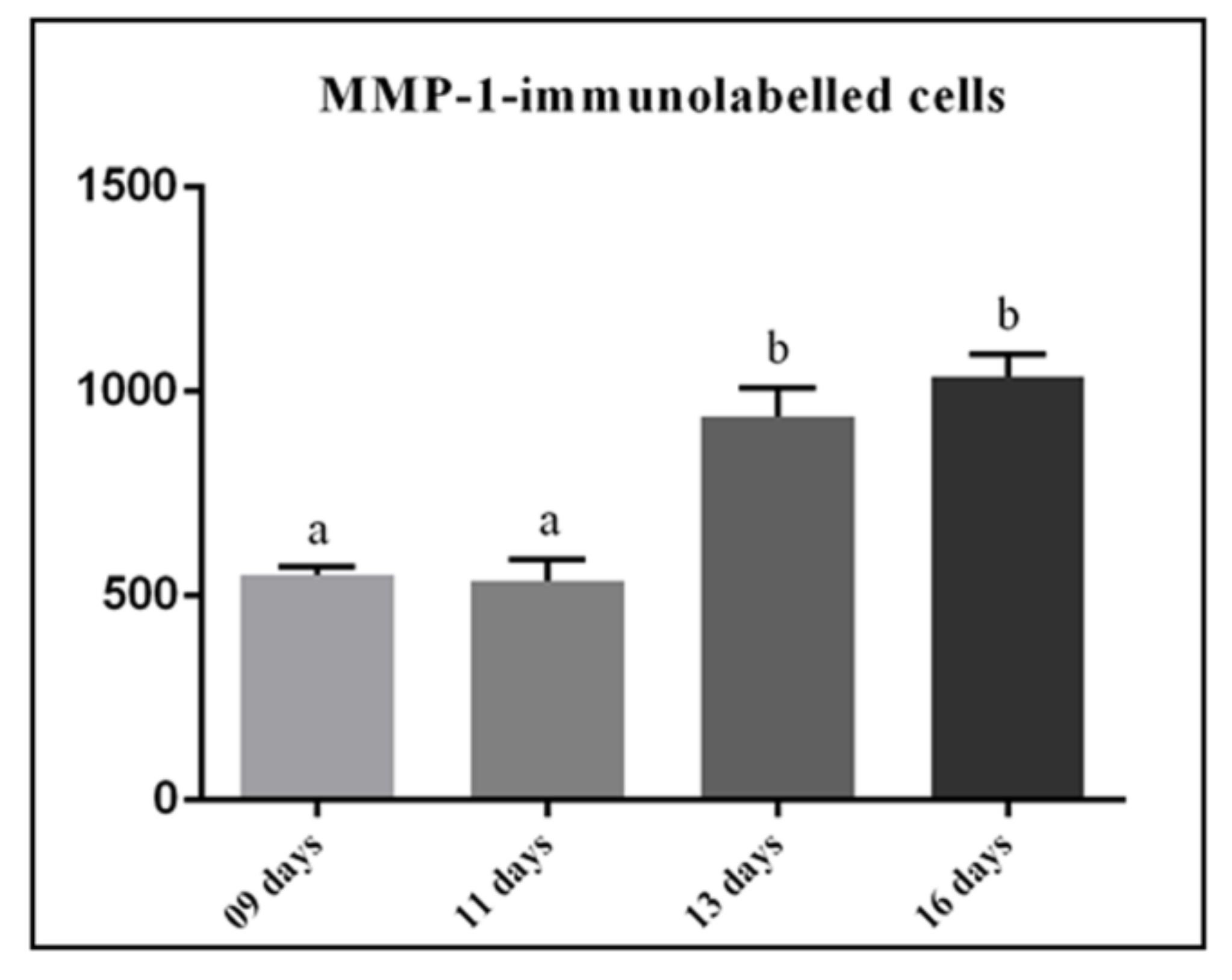
2.4. Numerical Density of MMP-1-Immunolabeled Cells
2.5. Immunohistochemical Detection of Acid Phosphatase (ACP-2)
2.6. Protein Extraction and Western Blot for MMP-1 and ACP-2
2.7. Transmission Electron Microscopy
2.8. Ultrastructural Localization of Acid Phosphatase Activity
2.9. Statistical Analysis
3. Results
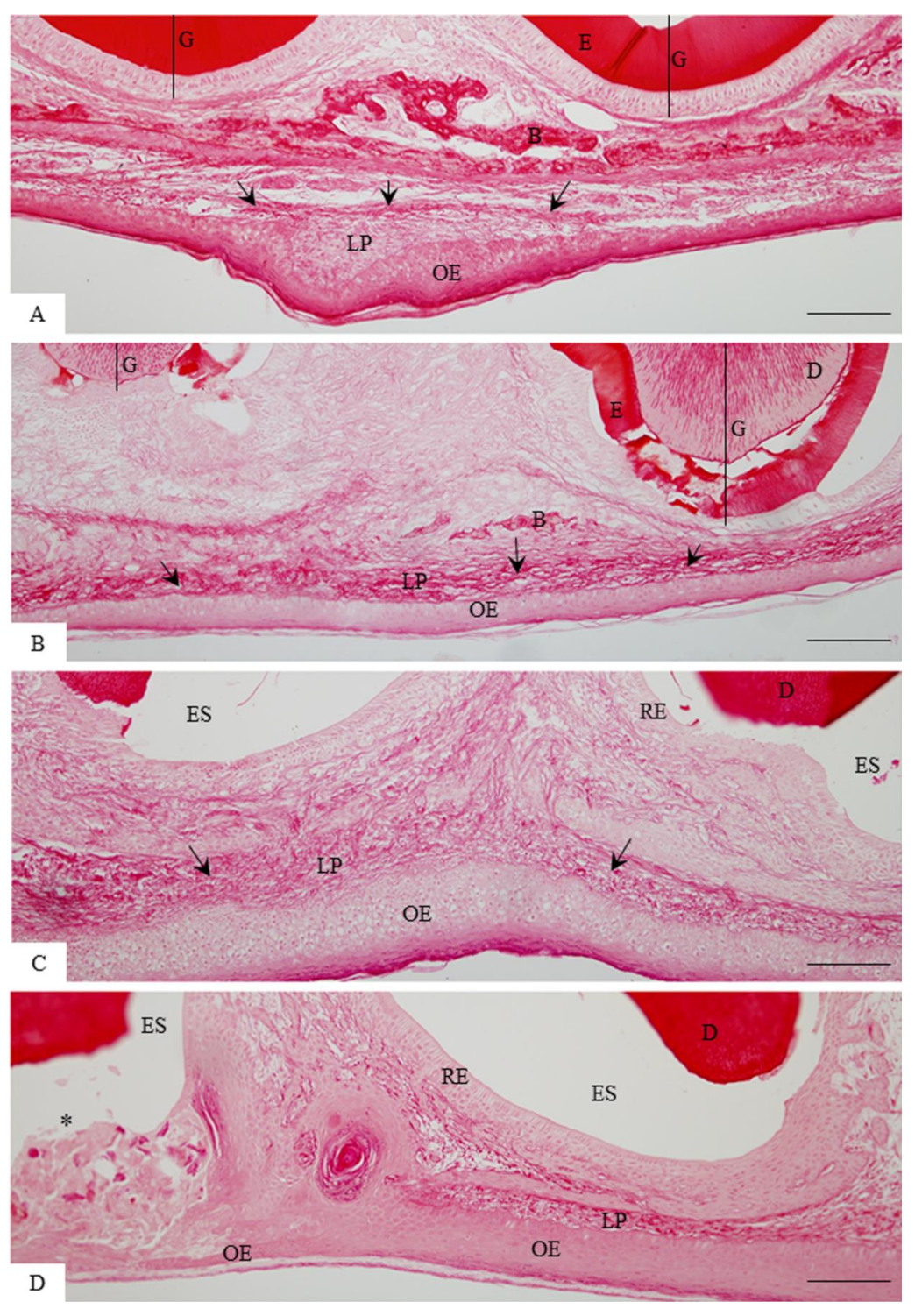
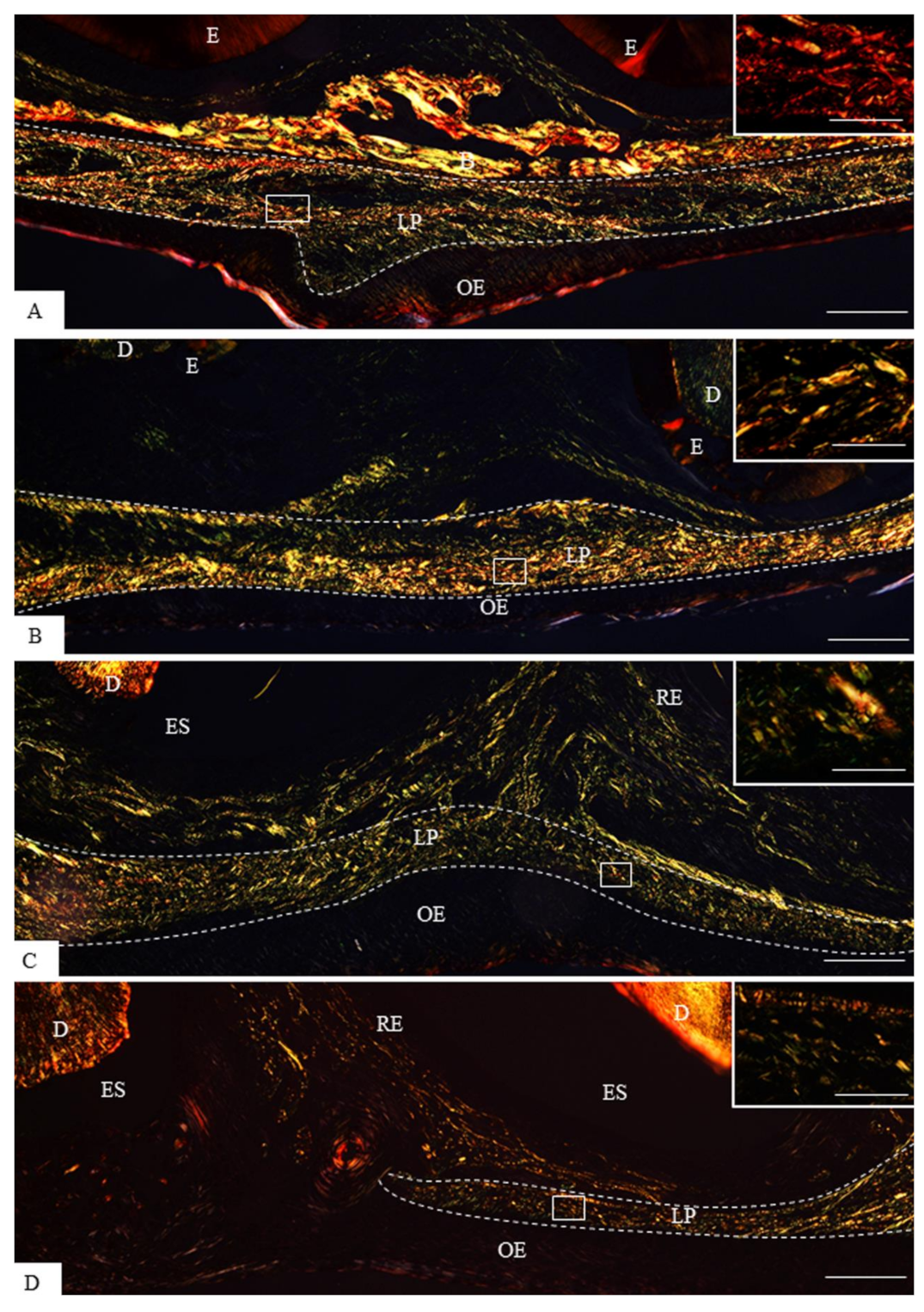
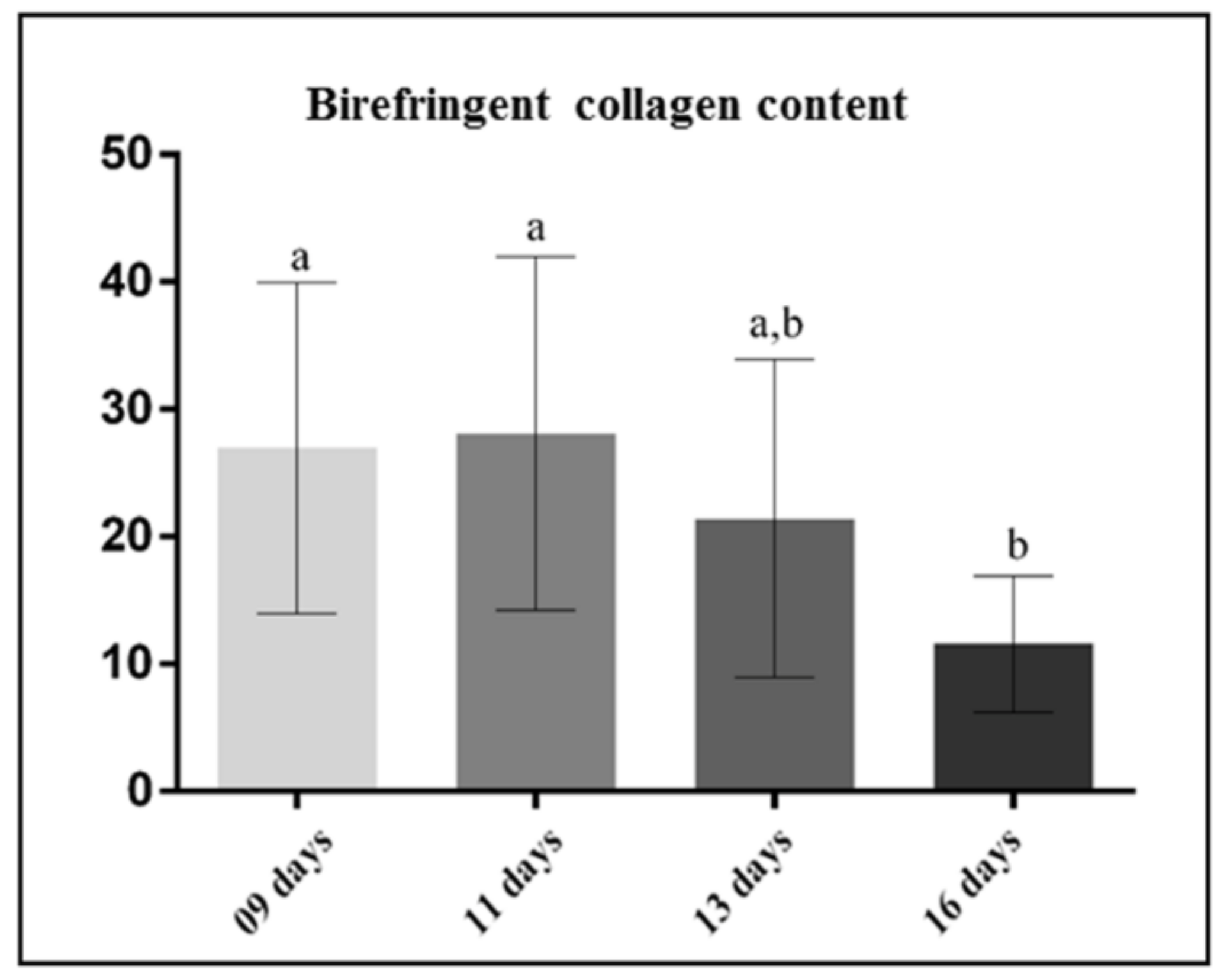
3.1. Morphological Findings and Content of Birefringent Collagen
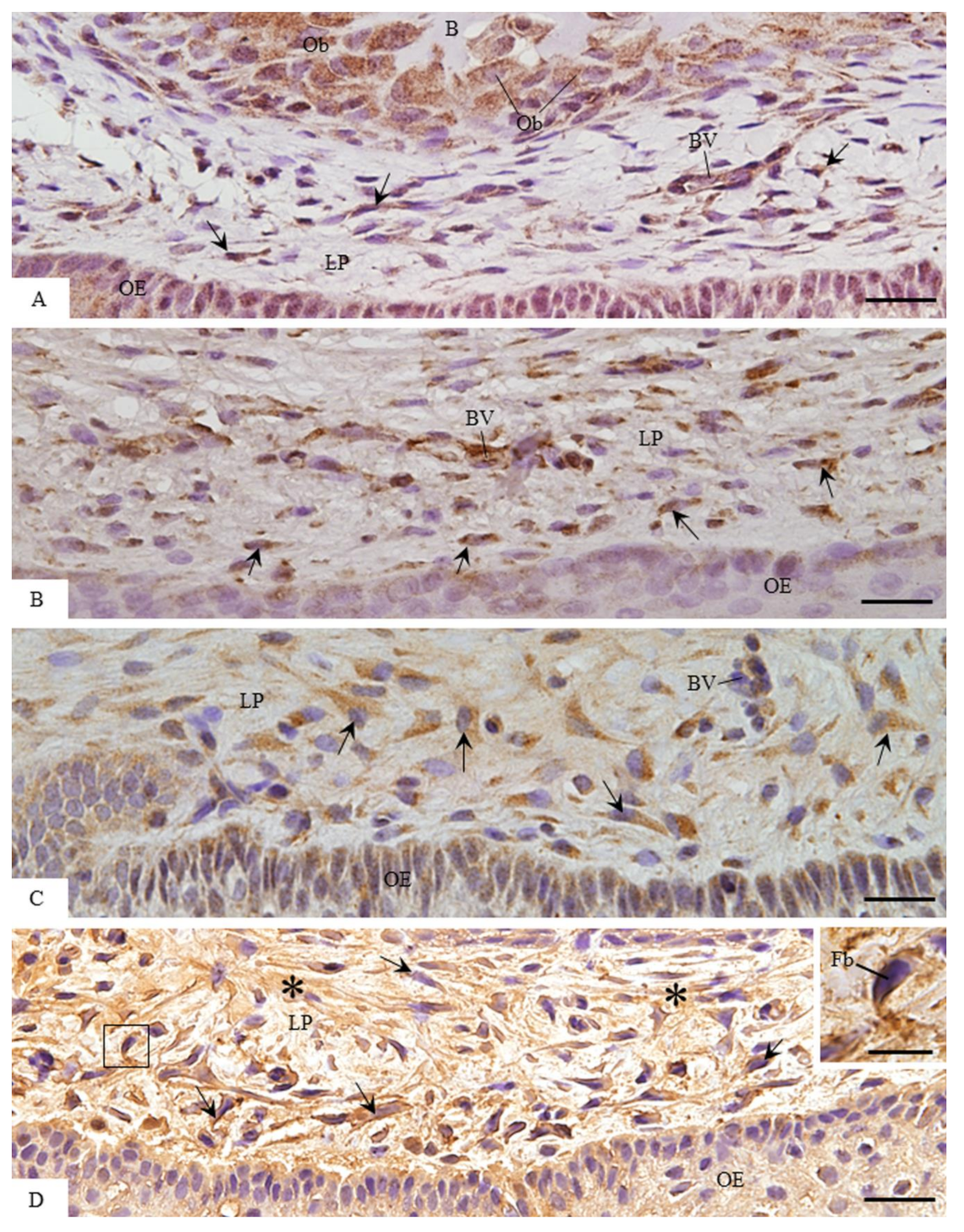
3.2. MMP-1 Immunoexpression in the Lamina Propria
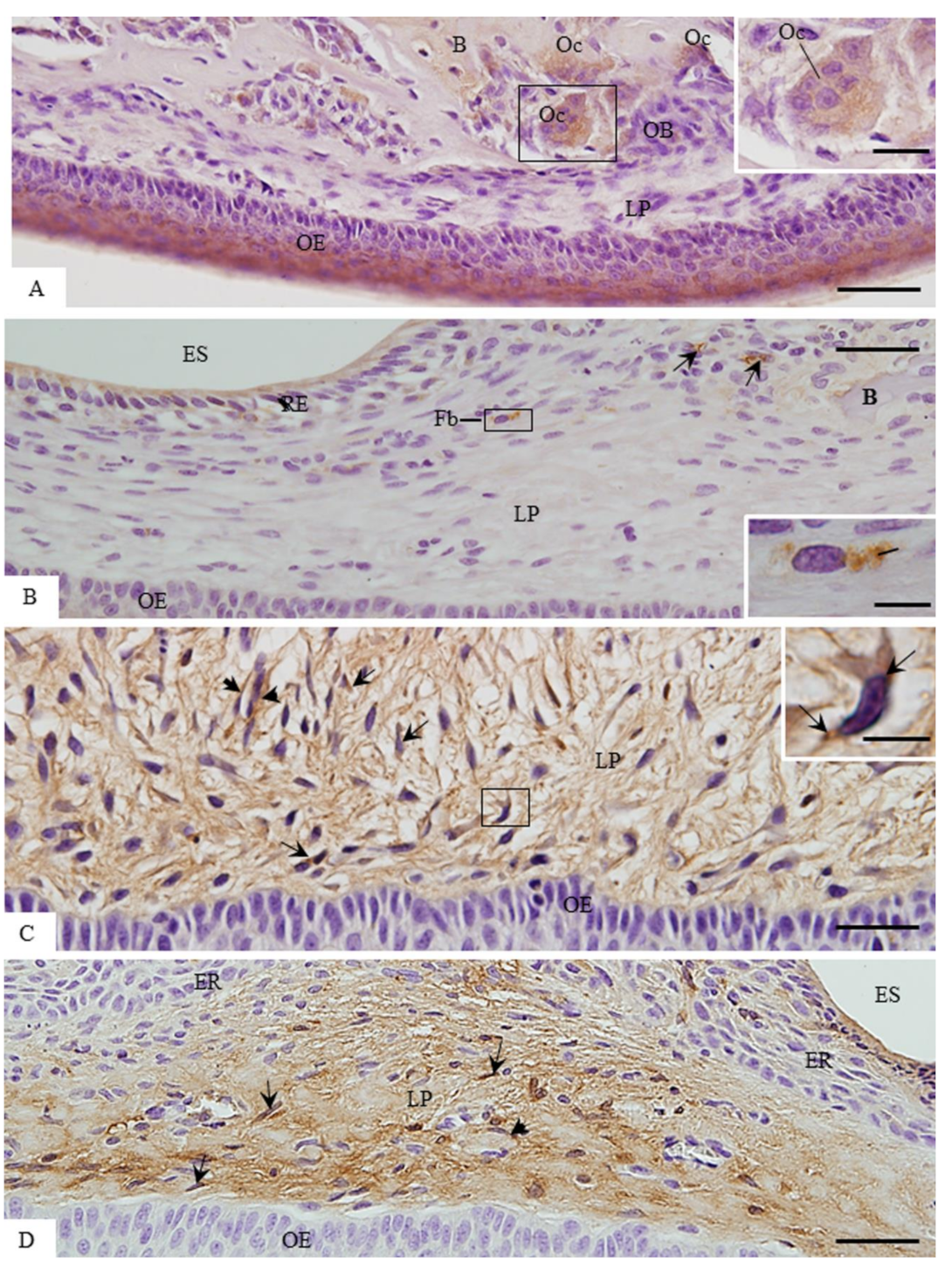
3.3. ACP-2 Immunoexpression in the Lamina Propria
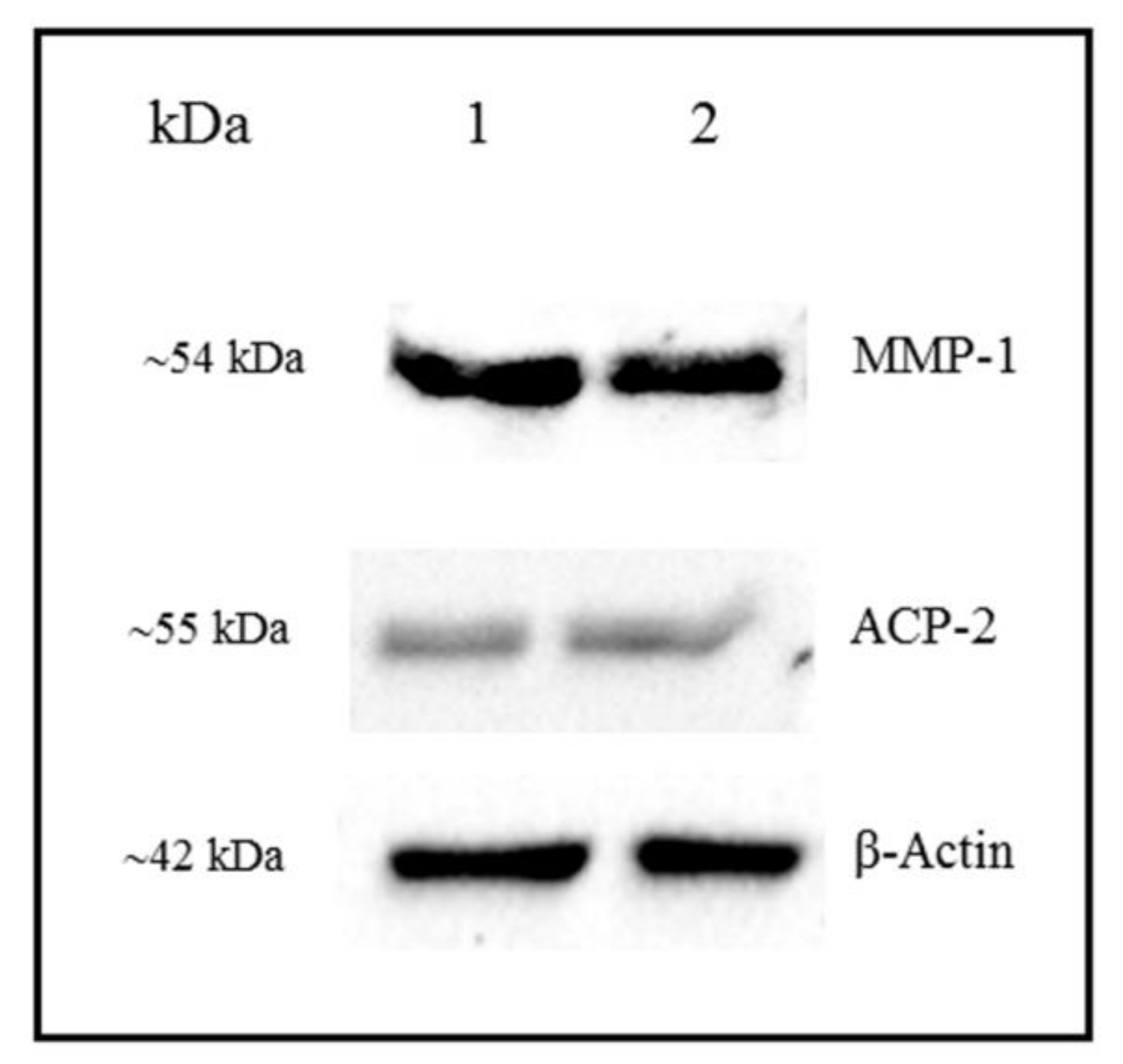
3.4. Detection of MMP-1 and ACP-2 by Western Blot
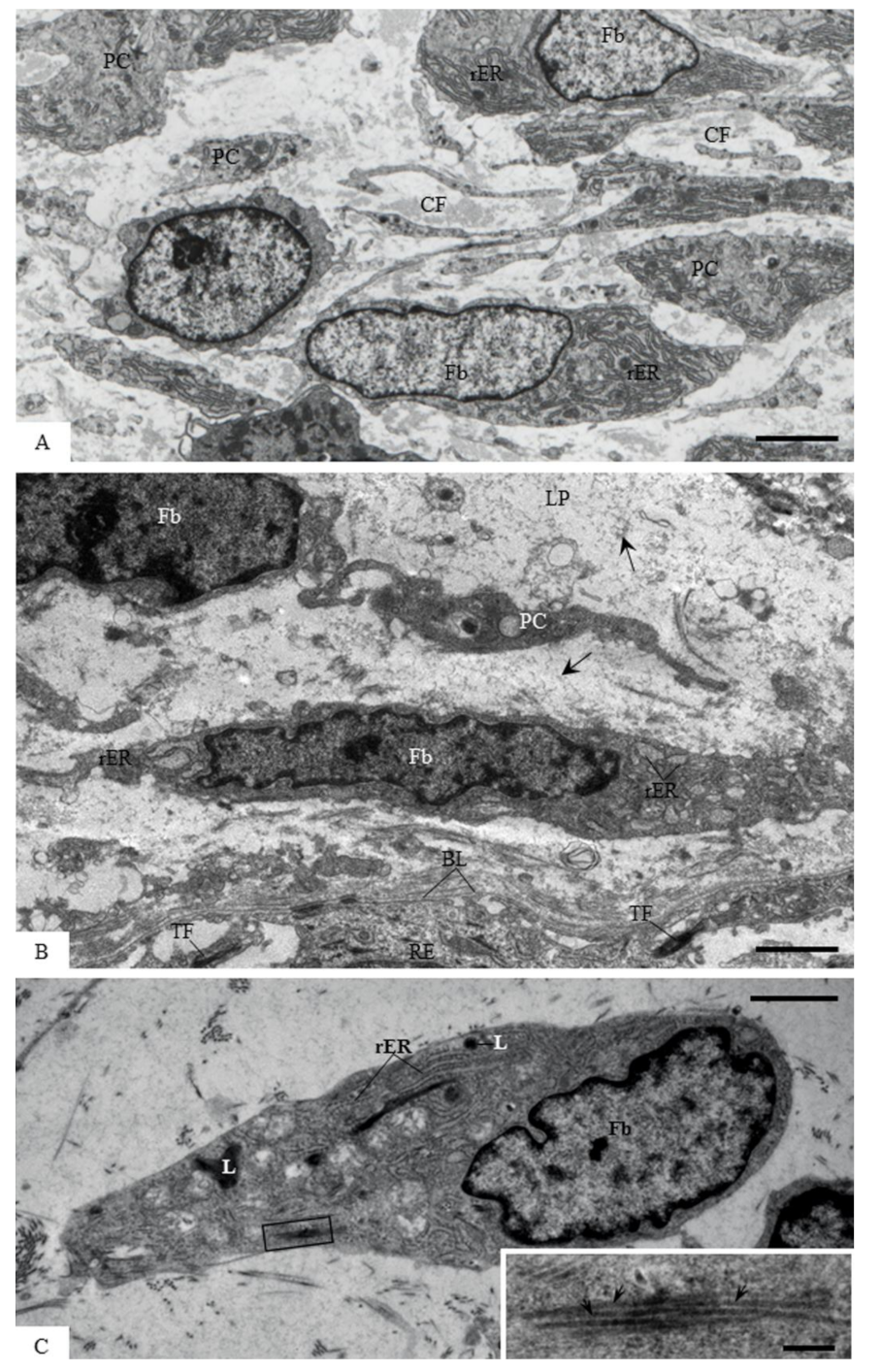
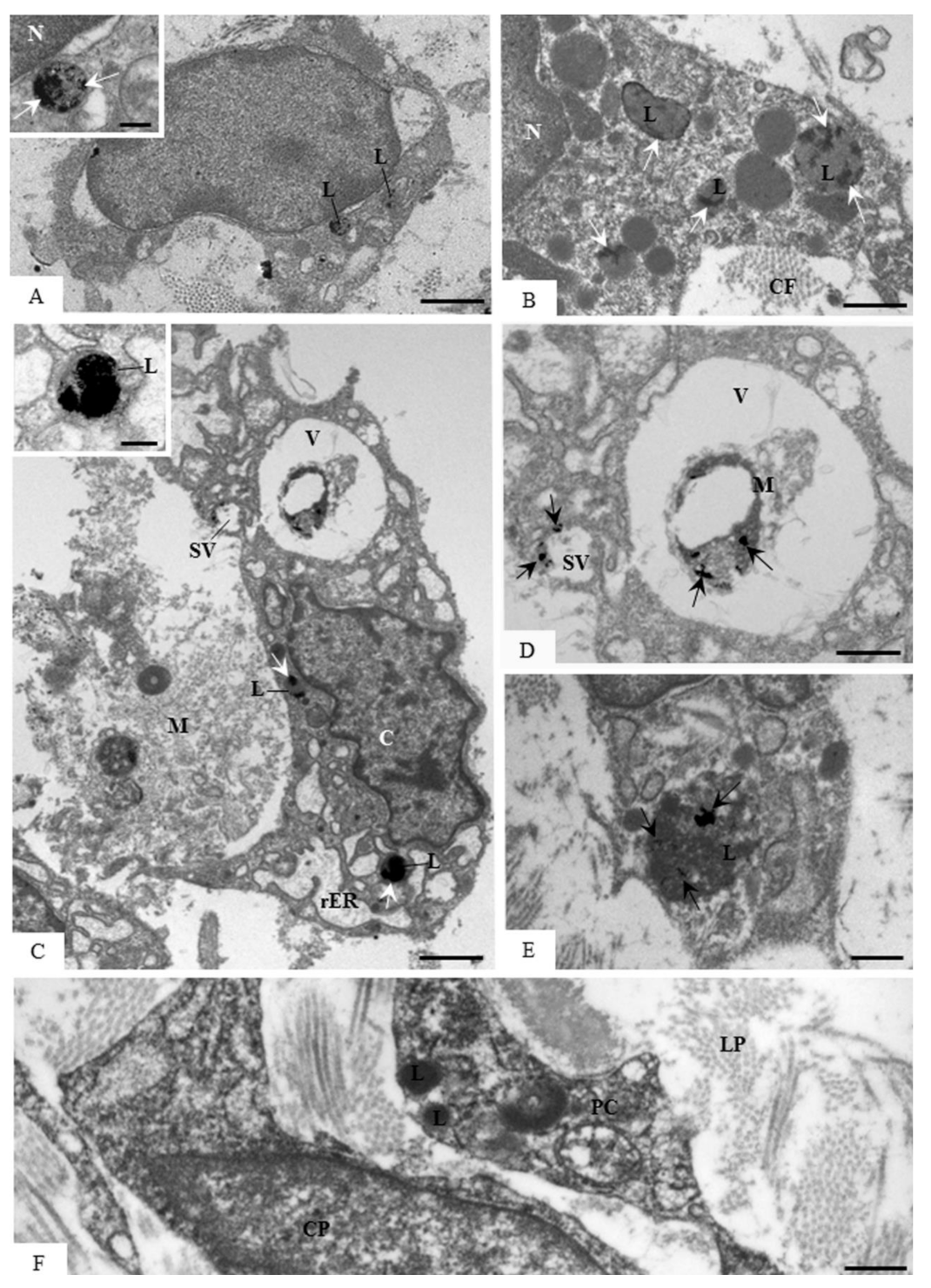
3.5. Ultrastructural Localization of Acid Phosphatase Activity
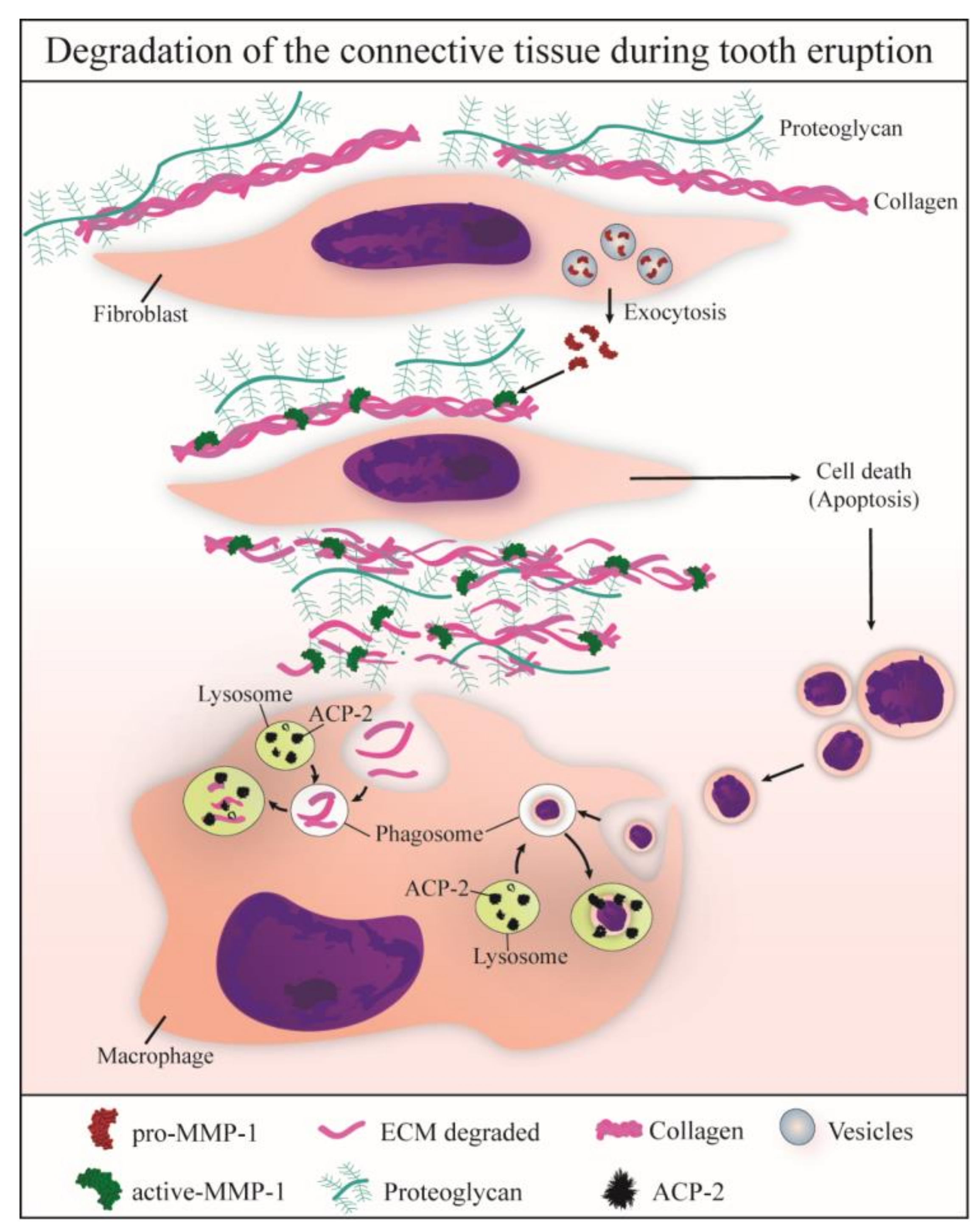
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurol, J. Impacted and ankylosed teeth: Why, when, and how to intervene. Am. J. Orthodont. Dent. Orthop. 2006, 129, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Loriato, L.B.; Machado, A.W.; Souki, B.Q.; Pereira, T.J. Late diagnosis of dentoalveolar ankylosis: Impact on effectiveness and efficiency of orthodontic treatment. Am. J. Orthodont. Dent. Orthop. 2009, 135, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Frazier-Bowers, S.A.; Puranik, C.P.; Mahaney, M.C. The etiology of eruption disorders—Further evidence of a ‘genetic paradigm’. Semin. Orthodont. 2010, 16, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kreiborg, S.; Jensen, B.L. Tooth formation and eruption—Lessons learnt from cleidocranial dysplasia. Eur. J. Oral Sci. 2018, 126, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Arul, A.S.; Arul, A.S.; Chitra, S. Erupted complex odontoma of the posterior maxilla: A rarity. J. Nat. Sci. Biol. Med. 2015, 6, S167–S169. [Google Scholar] [PubMed]
- Marks, S.C., Jr.; Schroeder, H.E. Tooth eruption: Theories and facts. Anat. Rec. 1996, 245, 374–393. [Google Scholar] [CrossRef]
- Kjær, I. Mechanism of human tooth eruption: Review article including a new theory for future studies on the eruption process. Scientifica 2014, 2014, 341905. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P. Tooth eruption without roots. J. Dent. Res. 2013, 92, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Cerri, P.S.; Pereira-Júnior, J.A.; Biselli, N.B.; Sasso-Cerri, E. Mast cells and MMP-9 in the lamina propria during eruption of rat molars: Quantitative and immunohistochemical evaluation. J. Anat. 2010, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- De Pizzol Júnior, J.P.; Sasso-Cerri, E.; Cerri, P.S. Apoptosis and reduced microvascular density of the lamina propria during tooth eruption in rats. J. Anat. 2015, 227, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E. Cellular and molecular basis of tooth eruption. Orthodont. Craniofac. Res. 2009, 12, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, S.; Wise, G.E. MyD88 expression in the rat dental follicle: Implications for osteoclastogenesis and tooth eruption. Eur. J. Oral Sci. 2010, 118, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, S.; Wise, G.E. Regulation of SFRP-1 expression in the rat dental follicle. Connect. Tissue Res. 2012, 53, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, B.; Simon, Y.; Jacques, J.; Hess, E.; Choi, Y.W.; Blin-Wakkach, C.; Mueller, C.; Berdal, A.; Lézot, F. Bone resorption control of tooth eruption and root morphogenesis: Involvement of the receptor activator of NF-κB (RANK). J. Cell. Physiol. 2011, 226, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Razavian, M.; Ye, Y.; Zhang, J.; Jung, J.J.; Toczek, J.; Gona, K.; Kim, H.Y.; Elias, J.A.; Lee, C.G.; et al. Matrix metalloproteinase-targeted imaging of lung inflammation and remodeling. J. Nucl. Med. 2017, 58, 138–143. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.A.; de Pizzol-Júnior, J.P.; Longhini, R.; Sasso-Cerri, E.; Cerri, P.S. Cimetidine reduces interleukin-6, matrix metalloproteinases-1 and -9 immunoexpression in the gingival mucosa of rat Molars with induced periodontal disease. J. Periodontol. 2017, 88, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Aalinkeel, R.; Nair, B.B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunol. Invest. 2011, 40, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Słowik, A. Matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in amyotrophic lateral sclerosis (ALS). J. Neural Transm. 2014, 121, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; White, L.A.; Schroen, D.J.; Benbow, U.; Brinckerhoff, C.E. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): Mechanisms that control enzyme activity, transcription, and mRNA stability. Crit. Rev. Eukaryot. Gene Expr. 1996, 6, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Brinckerhoff, C.E.; Rutter, J.L.; Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000, 6, 4823–4830. [Google Scholar] [PubMed]
- Bartlett, J.D.; Zhou, Z.; Skobe, Z.; Dobeck, J.M.; Tryggvason, K. Delayed tooth eruption in membrane type-1 matrix metalloproteinase deficient mice. Connect. Tissue Res. 2003, 44, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, M.H.; Chae, C.H.; Jung, Y.K.; Choi, J.Y. Downregulation of matrix metalloproteinases in hyperplastic dental follicles results in abnormal tooth eruption. BMB Rep. 2008, 41, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Bull, H.; Murray, P.G.; Thomas, D.; Fraser, A.M.; Nelson, P.N. Acid phosphatases. Mol. Pathol. 2002, 55, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Deporter, D.A.; Ten Cate, A.R. Fine structural localization of acid and alkaline phosphatase in collagen-containing vesicles of fibroblasts. J. Anat. 1973, 114, 457–461. [Google Scholar] [PubMed]
- Katz, S.G. Extracellular breakdown of collagen by mice decidual cells. A cytochemical and ultrastructural study. Biocell 2005, 29, 261–270. [Google Scholar] [PubMed]
- Cerri, P.S.; Freymüller, E.; Katchburian, E. Apoptosis in the early developing periodontium of rat molars. Anat. Rec. 2000, 258, 136–144. [Google Scholar] [CrossRef]
- Moss, D.W.; Raymond, F.D.; Wile, D.B. Clinical and biological aspects of acid phosphatase. Crit. Rev. Clin. Lab. Sci. 1995, 32, 431–467. [Google Scholar] [CrossRef] [PubMed]
- Suter, A.; Everts, V.; Boyde, A.; Jones, S.J.; Lüllmann-Rauch, R.; Hartmann, D.; Hayman, A.R.; Cox, T.M.; Evans, M.J.; Meister, T.; et al. Overlapping functions of lysosomal acid phosphatase (LAP) and tartrate-resistant acid phosphatase (Acp5) revealed by doubly deficient mice. Development 2001, 128, 4899–4910. [Google Scholar] [PubMed]
- Bailey, K.; Balaei, M.R.; Mannan, A.; Del Bigio, M.R.; Marzban, H. Purkinje cell compartmentation in the cerebellum of the lysosomal Acid phosphatase 2 mutant mouse (nax-naked-ataxia mutant mouse). PLoS ONE 2014, 9, e94327. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 7–15. [Google Scholar] [CrossRef]
- Marynka-Kalmani, K.; Treves, S.; Yafee, M.; Rachima, H.; Gafni, Y.; Cohen, M.A.; Pitaru, S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. 2010, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.L.; Czajka, C.A.; Oury, T.D.; Gilbert, T.W. Extracellular matrix powder protects against bleomycin-induced pulmonary fibrosis. Tissue Eng. Part A 2011, 17, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.; Whittaker, P. Collagen and picrosirius red staining: A polarized light assessment of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 2005, 22, 97–104. [Google Scholar]
- Koshimizu, J.Y.; Beltrame, F.L.; de Pizzol, J.P., Jr.; Cerri, P.S.; Caneguim, B.H.; Sasso-Cerri, E. NF-kB overexpression and decreased immunoexpression of AR in the muscular layer is related to structural damages and apoptosis in cimetidine-treated rat vas deferens. Reprod. Biol. Endocrinol. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Cerri, P.S. Osteoblasts engulf apoptotic bodies during alveolar bone formation in the rat maxilla. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 286, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Barka, T. Electron histochemical localization of acid phosphatase activity in the small intestine of mouse. J. Histochem. Cytochem. 1964, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, B.K.; Dumin, J.A.; Sudbeck, B.D.; Krane, S.M.; Welgus, H.G.; Parks, W.C. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J. Cell Biol. 1997, 137, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005, 166, 1555–1563. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.G.; Lichtinghagen, R.; Sommerhoff, C.P. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001, 21, 3821–3828. [Google Scholar] [PubMed]
- Inoue, T.; Yashiro, M.; Nishimura, S.; Maeda, K.; Sawada, T.; Ogawa, Y.; Sowa, M.; Chung, K.H. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int. J. Mol. Med. 1999, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, G.; Biondi, M.L.; Mangoni, J.; Leviti, S.; DeMonti, M.; Guagnellini, E.; Scorza, R. Matrix metalloproteinase-1 promoter polymorphism 1G/2G is correlated with colorectal cancer invasiveness. Clin. Cancer Res. 2001, 7, 2344–2346. [Google Scholar] [PubMed]
- Nikkola, J.; Vihinen, P.; Vlaykova, T.; Hahka-Kemppinen, M.; Kähäri, V.M.; Pyrhönen, S. High expression levels of collagenase-1 and stromelysin-1 correlate with shorter disease-free survival in human metastatic melanoma. Int. J. Cancer 2002, 97, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Piérard, G.E. Sirius red polarization method is useful to visualize the organization of connective tissues but not the molecular composition of their fibrous polymers. Matrix 1989, 9, 68–71. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Montes, G.S.; Sanchez, E.M. The influence of tissue section thickness on the study of collagen by the picrosirius-polarization method. Histochemistry 1982, 74, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tamayo, R.; Montfort, I. The susceptibility of hepatic collagen to homologous collagenase in human and experimental cirrhosis of the liver. Am. J. Pathol. 1980, 100, 427–442. [Google Scholar] [PubMed]
- Amălinei, C.; Căruntu, I.D.; Giuşcă, S.E.; Bălan, R.A. Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol. 2010, 51, 215–228. [Google Scholar] [PubMed]
- Butoi, E.; Gan, A.M.; Tucureanu, M.M.; Stan, D.; Macarie, R.D.; Constantinescu, C.; Calin, M.; Simionescu, M.; Manduteanu, I. Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim. Biophys. Acta 2016, 1863, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Du, G.L.; Chen, W.Y.; Li, X.N.; He, R.; Feng, P.F. Induction of MMP-1 and -3 by cyclical mechanical stretch is mediated by IL-6 in cultured fibroblasts of keratoconus. Mol. Med. Rep. 2017, 15, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 30, 21491–21494. [Google Scholar] [CrossRef]
- Huang, H.; Wise, G.E. Delay of tooth eruption in null mice devoid of the type I IL-1R gene. Eur. J. Oral. Sci. 2000, 108, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Prpic, V.; Pan, F.; Wise, G.E. TNF-alpha upregulates expression of BMP-2 and BMP-3 genes in the rat dental follicle—Implications for tooth eruption. Connect. Tissue Res. 2010, 51, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Ida-Yonemochi, H.; Noda, T.; Shimokawa, H.; Saku, T. Disturbed tooth eruption in osteopetrotic (op/op) mice: Histopathogenesis of tooth malformation and odontomas. J. Oral Pathol. Med. 2002, 31, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J. Clin. Invest. 1999, 103, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Chesler, N.C.; Ku, D.N.; Galis, Z.S. Transmural pressure induces matrix-degrading activity in porcine arteries ex vivo. Am. J. Physiol. 1999, 277, H2002–H2009. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Johnson, C.; Godin, D.; Magid, R.; Shipley, J.M.; Senior, R.M; Ivan, E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 2002, 91, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Suri, L.; Gagari, E.; Vastardis, H. Delayed tooth eruption: Pathogenesis, diagnosis, and treatment. A literature review. Am. J. Orthodont. Dent. Orthop. 2004, 126, 432–445. [Google Scholar] [CrossRef]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.B.; Brorson, S.H.; Stordalen, G.A.; Baekkevold, E.S.; Andersson, G.; Reinholt, F.P. Increased tartrate-resistant acid phosphatase expression in osteoblasts and osteocytes in experimental osteoporosis in rats. Calcif. Tissue Int. 2014, 94, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.T.; Knight, M.B.; Harakas, N.K.; Wittwer, A.J.; Feder, J. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal. Biochem. 1986, 152, 136–140. [Google Scholar] [CrossRef]
- Turner, R.R.; Beckstead, J.H.; Warnke, R.A.; Wood, G.S. Endothelial cell phenotypic diversity. In situ demonstration of immunologic and enzymatic heterogeneity that correlates with specific morphologic subtypes. Am. J. Clin. Pathol. 1987, 87, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.G. Demonstration of extracellular acid phosphatase activity in the involuting, antimesometrial decidua in fed and acutely fasted mice by combined cytochemistry and electron microscopy. Anat. Rec. 1998, 252, 1–7. [Google Scholar] [CrossRef]
- Faccioli, C.K.; Chedid, R.A.; Mori, R.H.; Amaral, A.C.; Franceschini-Vicentini, I.B.; Vicentini, C.A. Acid and alkaline phosphatase localization in the digestive tract mucosa of the Hemisorubim platyrhynchos. Acta Histochem. 2016, 118, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, N.; Jiao, X.; Rahimi-Balaei, M.; Amiri, S.; Mehr, S.E.; Yeganeh, B.; Marzban, H. Lysosomal acid phosphatase biosynthesis and dysfunction: A mini review focused on lysosomal enzyme dysfunction in brain. Curr. Mol. Med. 2016, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Geier, C.; Kreysing, J.; Boettcher, H.; Pohlmann, R.; von Figura, K. Localization of lysosomal acid phosphatase mRNA in mouse tissues. J. Histochem. Cytochem. 1992, 40, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lemanski, L.F.; Aldoroty, R. Role of acid phosphatase in the breakdown of yolk platelets in developing amphibian embryos. J. Morphol. 1977, 153, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Hartmann, D.; Lüllmann-Rauch, R.; Wolff, J.; Evers, M.; Köster, A.; Hetman, M.; von Figura, K.; Peters, C. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J. Biol. Chem. 1997, 272, 18628–18635. [Google Scholar] [CrossRef] [PubMed]
- Pipan, N.; Sterle, M. Cytochemical analysis of organelle degradation in phagosomes and apoptotic cells of the mucoid epithelium of mice. Histochemistry 1979, 59, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.J.; Anderson, T.J. Ultrastructural observations on cell death by apoptosis in the “resting” human breast. Virchows Arch. A Pathol. Anat. Histopathol. 1981, 393, 193–203. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, N.; Werb, Z.; Bissell, M.J. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. USA 1996, 93, 3509–3513. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pizzol Júnior, J.P.; Sasso-Cerri, E.; Cerri, P.S. Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars. Cells 2018, 7, 206. https://doi.org/10.3390/cells7110206
De Pizzol Júnior JP, Sasso-Cerri E, Cerri PS. Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars. Cells. 2018; 7(11):206. https://doi.org/10.3390/cells7110206
Chicago/Turabian StyleDe Pizzol Júnior, José Paulo, Estela Sasso-Cerri, and Paulo Sérgio Cerri. 2018. "Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars" Cells 7, no. 11: 206. https://doi.org/10.3390/cells7110206
APA StyleDe Pizzol Júnior, J. P., Sasso-Cerri, E., & Cerri, P. S. (2018). Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars. Cells, 7(11), 206. https://doi.org/10.3390/cells7110206