Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process
Abstract
1. Introduction
2. Materials and Methods
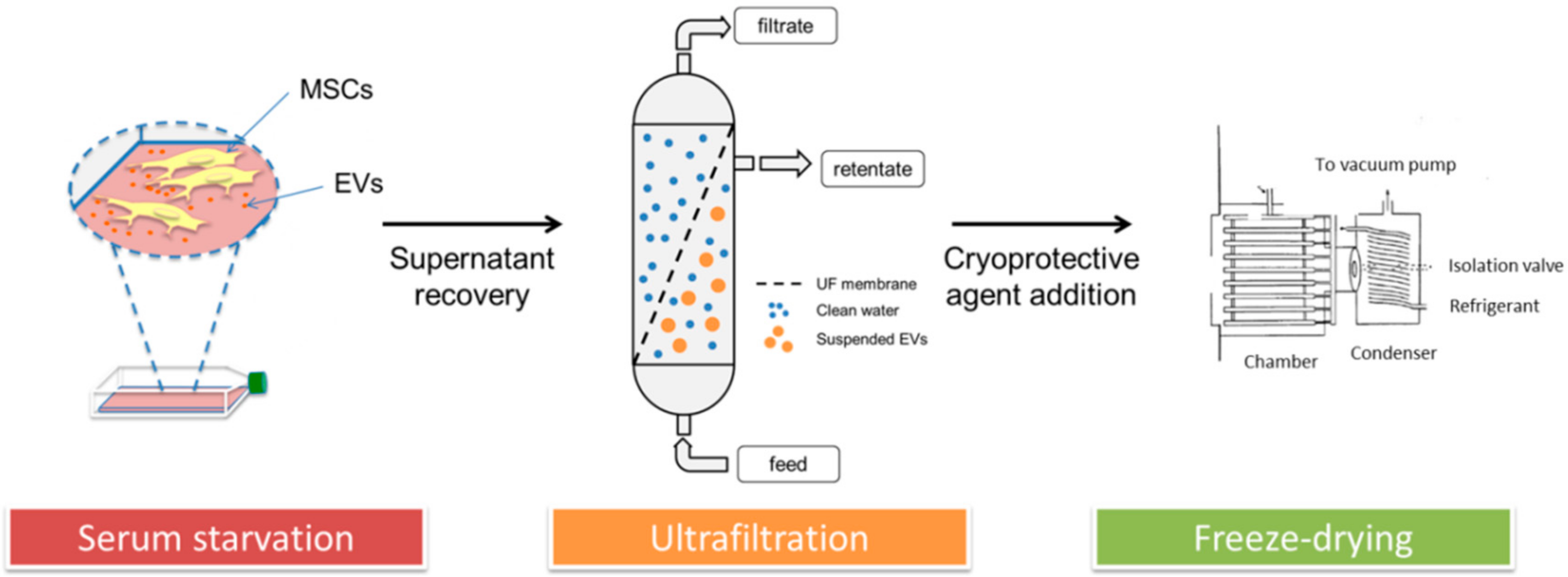
2.1. Lyo-Secretome Production Process in a GMP-Compliant Cell Factory
2.1.1. Isolation and Expansion of Human Adipose-Derived Mesenchymal Stromal Cells
2.1.2. MSC-Secretome Collection
2.1.3. MSC-Secretome Purification: Ultrafiltration
2.1.4. Secretome Freeze-Drying (FD)
2.1.5. Microbiological Control
2.2. Lyo-Secretome Characterization
2.2.1. Determination of Total Protein Content
2.2.2. Phospholipid Quantification by Nile Red Assay
2.2.3. BCA-Protein Assay and Nile Red Assay Validation
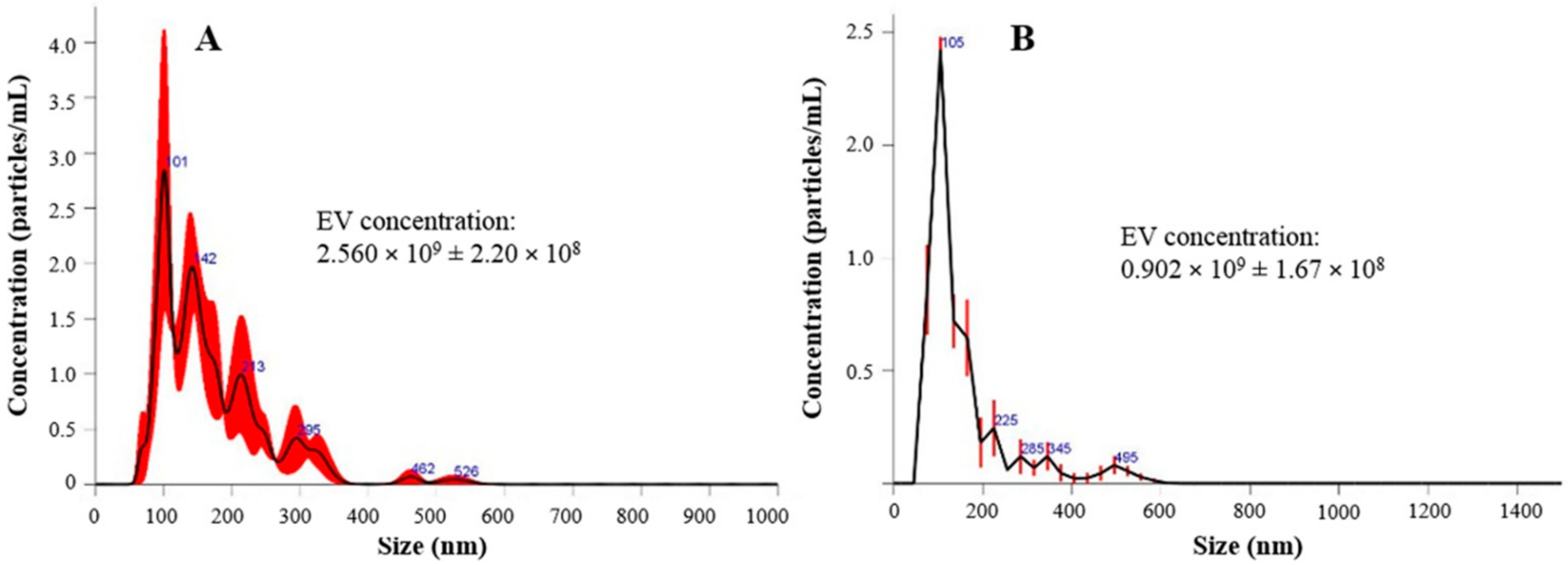
2.2.4. EV Particle Size Distribution
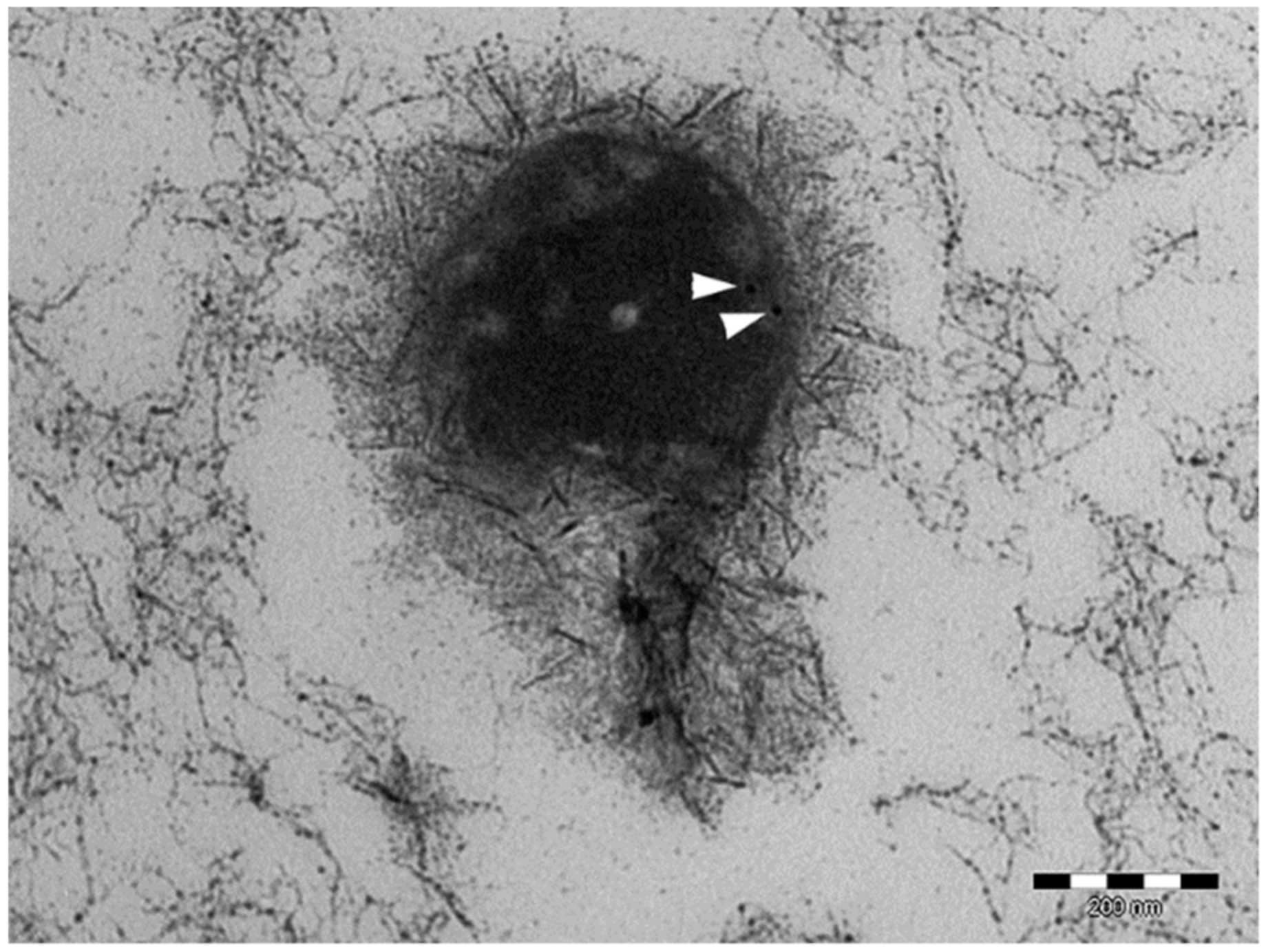
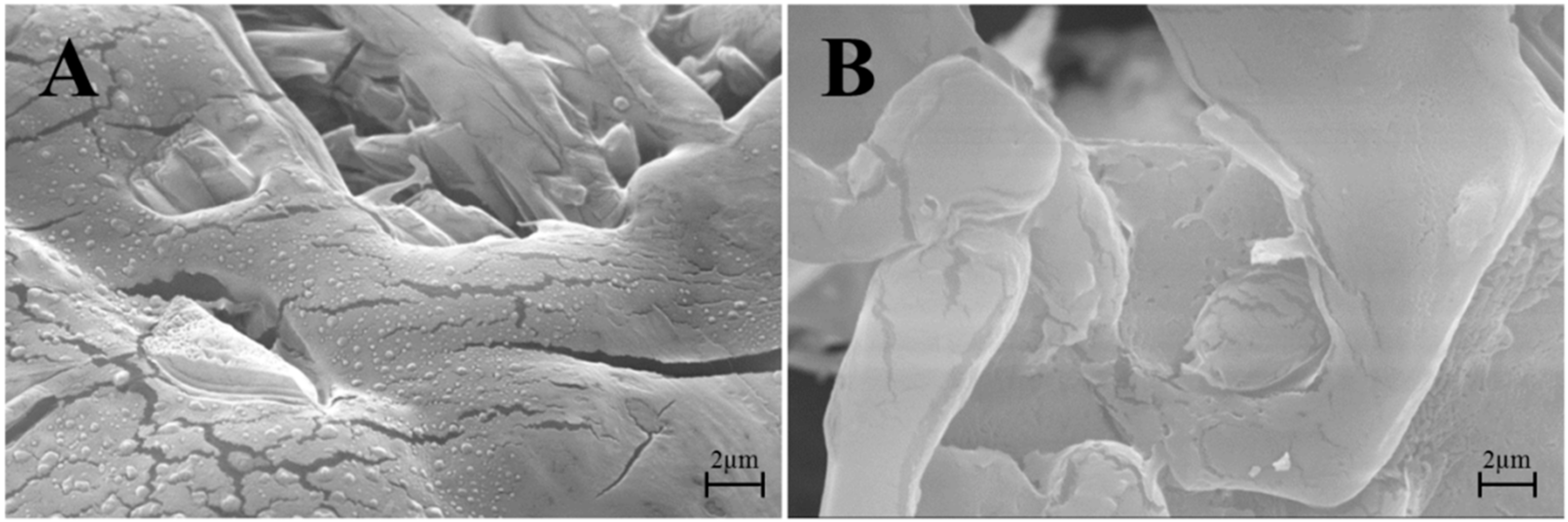
2.2.5. Ultrastructure Investigation by Transmission Electron Microscopy (TEM) and Morphology Investigation by Scanning Electron Microscopy (SEM)
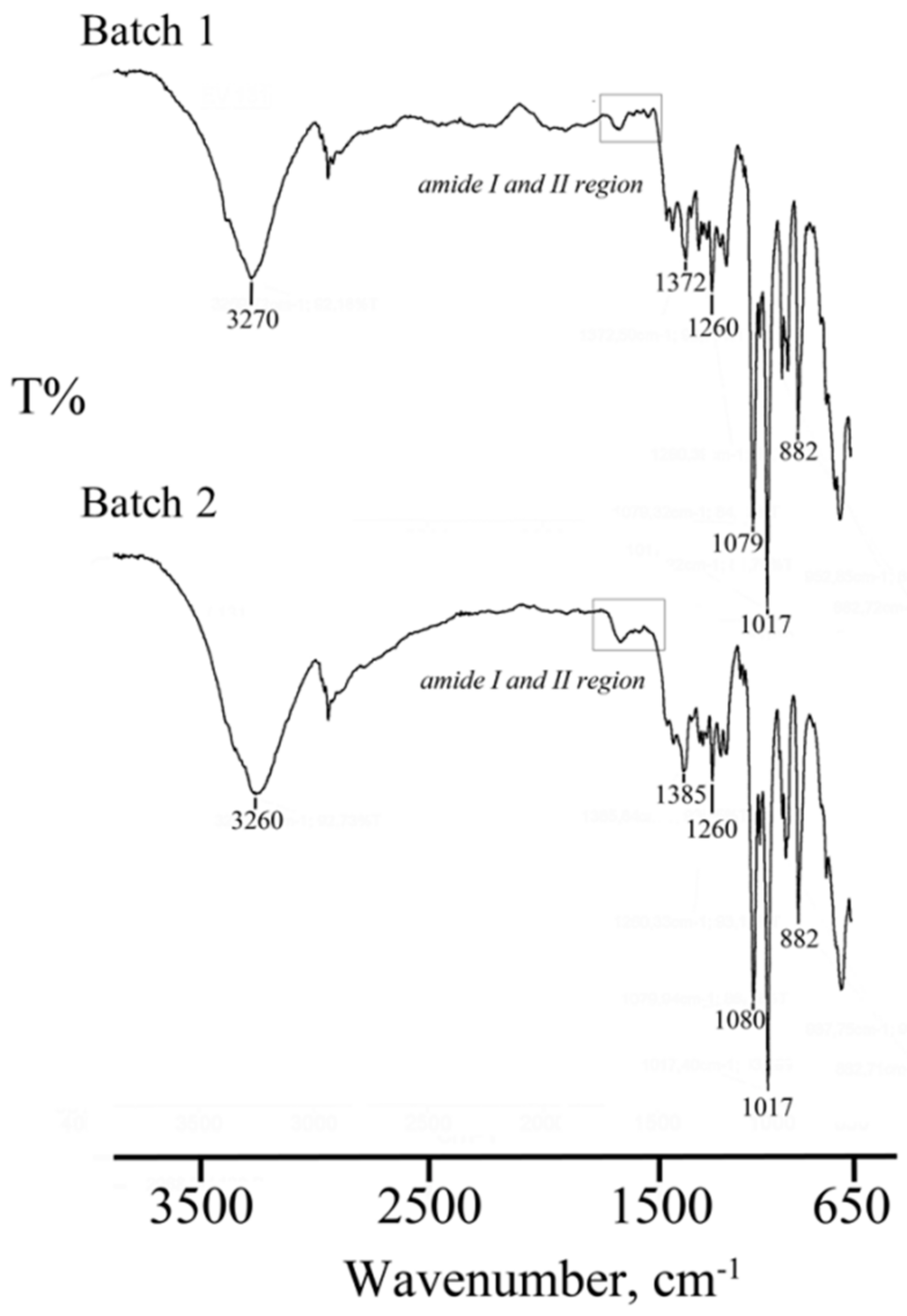
2.2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
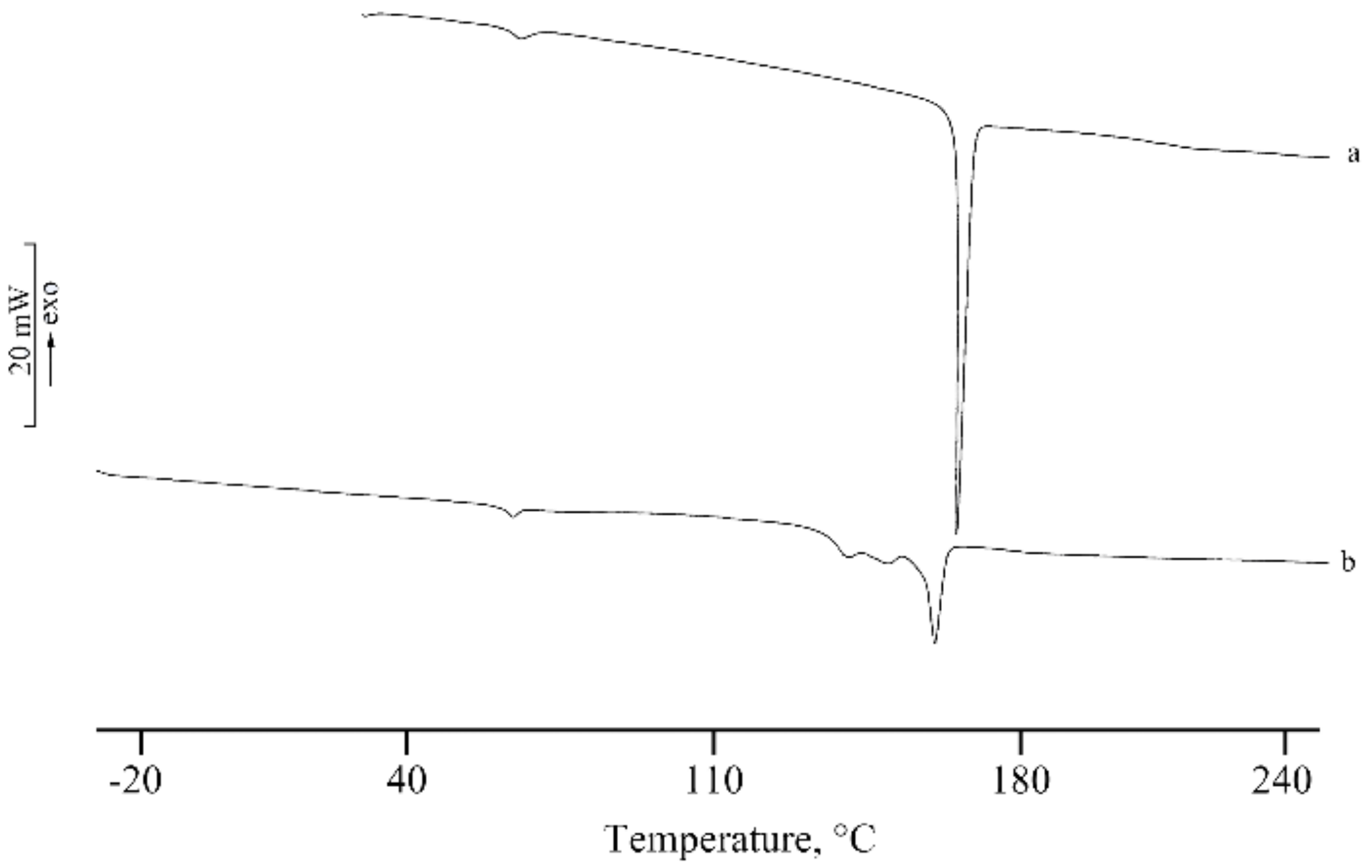
2.2.7. Thermal Characterization
2.2.8. Sample Preparation for Proteomic Analysis
2.2.9. LC-MS/MS Analysis
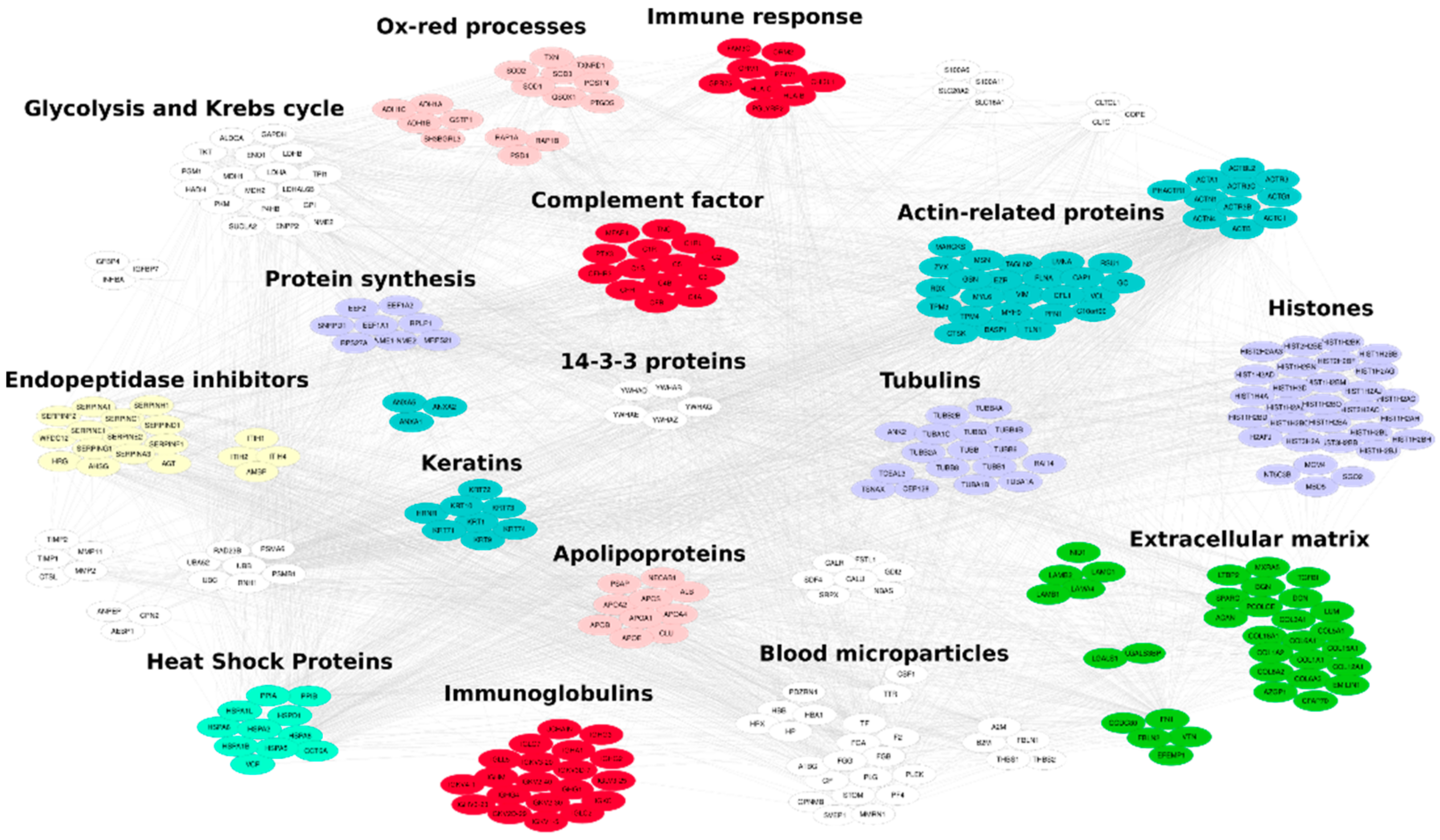
2.2.10. MS/MS Data Processing
2.3. Lyo-Secretome Safety Assays
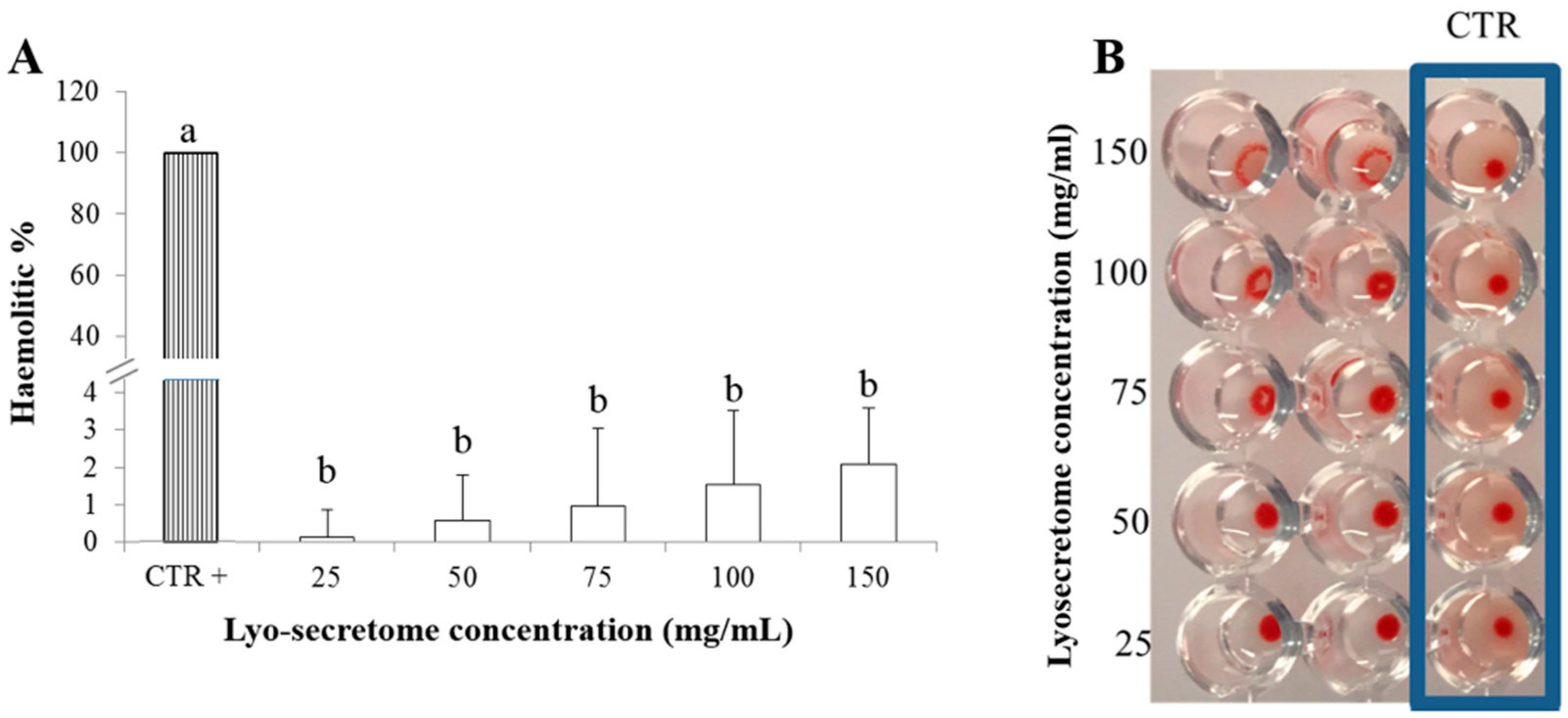
2.3.1. Blood Compatibility
2.3.2. Cytocompatibility Test
2.4. Lyo-Secretome Potency Assays
2.4.1. ROS-Scavenging Activity Assay
2.4.2. Oxidative Stress Protection
2.5. Statistical Analysis
3. Results
3.1. Lyo-Secretome Production
3.2. Lyo-Secretome Characterization
3.3. Lyo-Secretome Safety
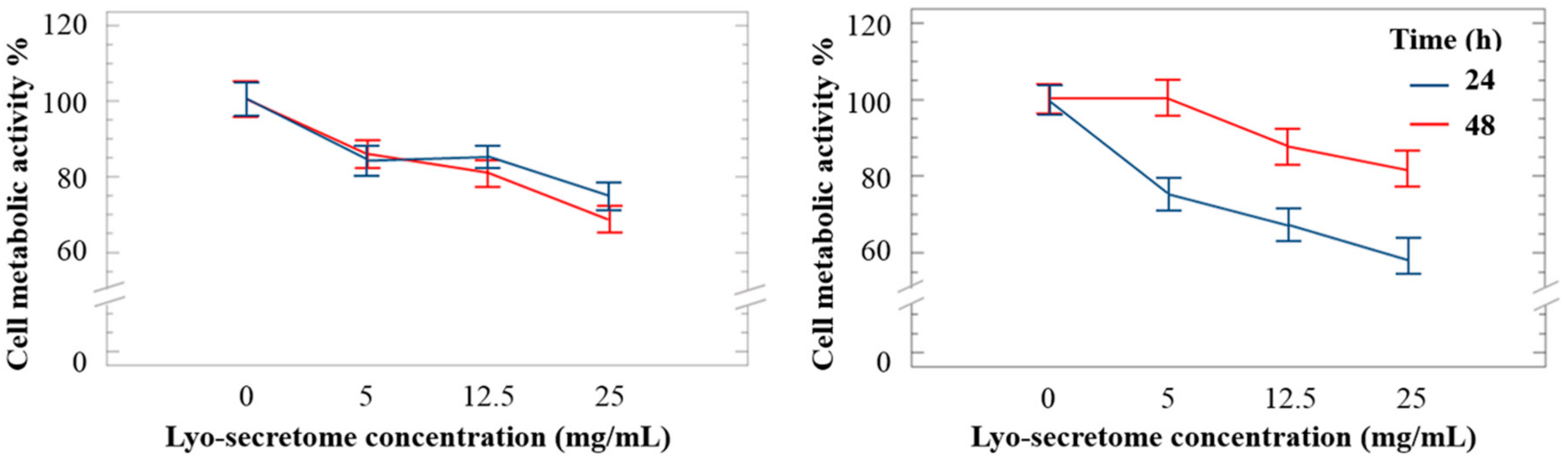
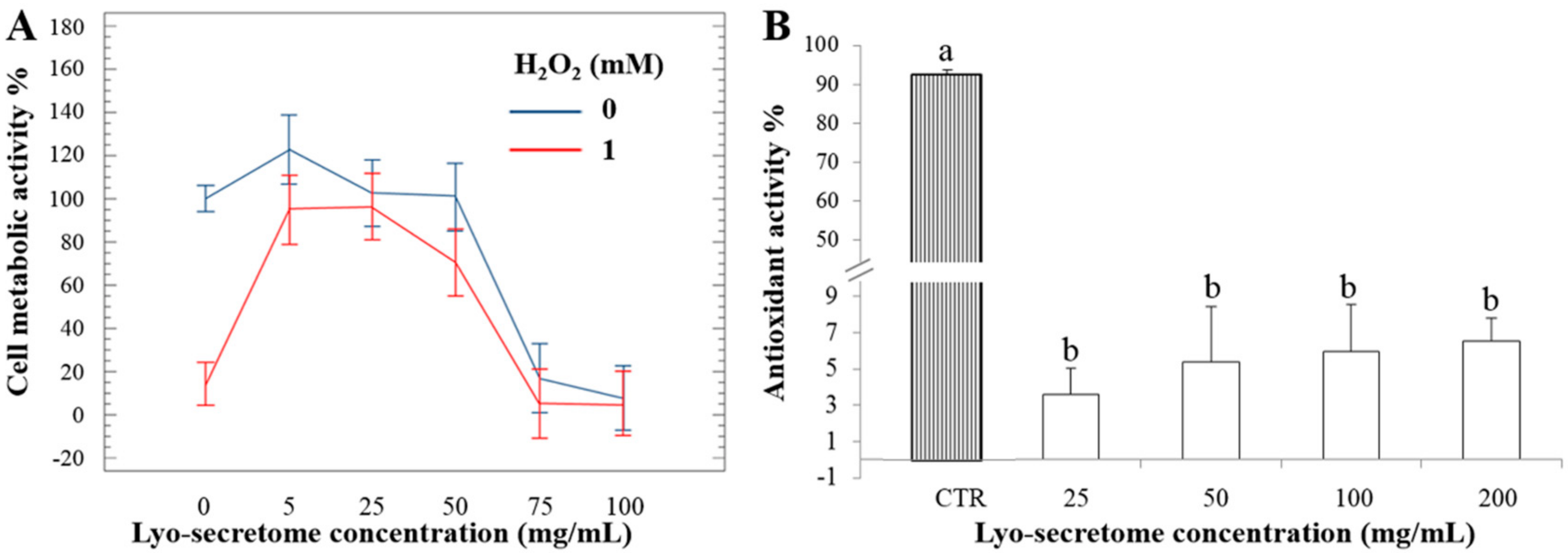
3.4. Lyo-Secretome Potency
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Del Fattore, A.; Muraca, M. The immunoregulatory activity of mesenchymal stem cells: ‘State of art’ and ‘future avenues’. Curr. Med. Chem. 2016, 23, 3014–3024. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.M.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.N.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by AKT-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Chlapanidas, T.; Perteghella, S.; Lucarelli, E.; Pascucci, L.; Brini, A.T.; Ferrero, I.; Marazzi, M.; Pessina, A.; Torre, M.L.; et al. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J. Control. Release 2017, 262, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.-C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98–110. [Google Scholar] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lei, D.; Ouyang, W.; Ren, J.; Li, H.; Hu, J.; Huang, S. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. BioMed Res. Int. 2014, 2014, 109389. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, R.A.; Nabil, I.; Attia, N.; Bary, A.A.; Razek, K.A.; Ahmed, T.A.; Elsayed, F. The effect of bone marrow-derived mesenchymal stem cells and their conditioned media topically-delivered in fibrin glue on chronic wound healing in rats. Tissue Eng. Part A 2015, 21, S96. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.-X.; He, A.-N.; Wang, J.-A.; Gui, C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J. Zhejiang Univ. Sci. B 2009, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, I.; Hwang, S.H.; Han, H.; Ha, H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res. Clin. Pract. 2012, 98, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. Msc-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular vesicles from mesenchymal stem cells activates vegf receptors and accelerates recovery of hindlimb ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.-L.; Lee, S.; Seo, K.-W.; Kang, K.-S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Huang, X.; Yu, W.; Chen, H.; Chen, Y.; Dai, Y.T. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 2018, 50, e12871. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Crivelli, B.; Catenacci, L.; Sorrenti, M.; Bruni, G.; Necchi, V.; Vigani, B.; Sorlini, M.; Torre, M.L.; Chlapanidas, T. Stem cell-extracellular vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. Int. J. Pharm. 2017, 520, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yuen, P.S.T.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. Exocarta as a resource for exosomal research. J. Extracell. Vesicles 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Bari, E.; Chlapanidas, T.; Sorlini, M.; De Girolamo, L.; Perucca Orfei, C.; Viganò, M.; Torre, M.L. Process for Isolating and Lyophilizing Extracellular Vesicles. PCT/IB2017/056591, 28 October 2016. [Google Scholar]
- Gaetani, P.; Torre, M.L.; Klinger, M.; Faustini, M.; Crovato, F.; Bucco, M.; Marazzi, M.; Chlapanidas, T.; Levi, D.; Tancioni, F.; et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: An in vitro reconstructed tissue in alginate capsules. Tissue Eng. Part A 2008, 14, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded mesenchymal stem cells for regenerative medicine: Yield in stromal vascular fraction from adipose tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Merino, A.M.; Hoogduijn, M.J.; Borras, F.E.; Franquesa, M. Therapeutic potential of extracellular vesicles. Front. Immunol. 2014, 5, 658. [Google Scholar] [CrossRef] [PubMed]
- Villani, S.; Marazzi, M.; Bucco, M.; Faustini, M.; Klinger, M.; Gaetani, P.; Crovato, F.; Vigo, D.; Caviggioli, F.; Torre, M.L. Statistical approach in alginate membrane formulation for cell encapsulation in a GMP-based cell factory. Acta Biomater. 2008, 4, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Mastracci, L.; Grillo, F.; Perteghella, S.; Preda, S.; Crivelli, B.; Antonioli, B.; Galuzzi, M.; Tosca, M.C.; Marazzi, M.; et al. Local biological effects of adipose stromal vascular fraction delivery systems after subcutaneous implantation in a murine model. J. Bioact. Compat. Polym. 2016, 31, 600–612. [Google Scholar] [CrossRef]
- Gronthos, S.; McCarty, R.; Mrozik, K.; Fitter, S.; Paton, S.; Menicanin, D.; Itescu, S.; Bartold, P.M.; Xian, C.; Zannettino, A.C.W. Heat shock protein-90 beta is expressed at the surface of multipotential mesenchymal precursor cells: Generation of a novel monoclonal antibody, STRO-4, with specificity for mesenchymal precursor cells from human and ovine tissues. Stem Cells Dev. 2009, 18, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Tabb, D.L. The sequest family tree. J. Am. Soc. Mass Spectrom. 2015, 26, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Overall, C.M.; Van Eyk, J.E.; Baker, M.S.; Palk, Y.-K.; Weintraub, S.T.; Lane, L.; Martens, L.; Vandenbrouck, Y.; Kusebauch, U.; et al. Human proteome project mass spectrometry data interpretation guidelines 2.1. J. Proteome Res. 2016, 15, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.-L.; Lotia, S.; Pico, A.R.; Bader, G.D.; Ideker, T. A travel guide to cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. Bingo: A cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Yan, I.K.; Parasramka, M.; Mohankumar, S.; Matsuda, A.; Patel, T. In vitro toxicology studies of extracellular vesicles. J. Appl. Toxicol. 2017, 37, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Saswati; Chakraborty, A.; Dash, S.P.; Panda, A.K.; Acharyya, R.; Biswas, A.; Mukhopadhyay, S.; Bhutia, S.K.; Crochet, A.; Patil, Y.P.; et al. Synthesis, X-ray structure and in vitro cytotoxicity studies of Cu(I/II) complexes of thiosemicarbazone: Special emphasis on their interactions with DNA. Dalton Trans. 2015, 44, 6140–6157. [Google Scholar]
- Nayak, D.; Boxi, A.; Ashe, S.; Thathapudi, N.C.; Nayak, B. Stavudine loaded gelatin liposomes for HIV therapy: Preparation, characterization and in vitro cytotoxic evaluation. Mater. Sci. Eng. C Mater. Boil. Appl. 2017, 73, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Farago, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ros-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Boil. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Tripodo, G.; Mastrogiacomo, M.; Mandracchia, D.; Trapani, A.; Farago, S.; et al. In vitro efficacy of silk sericin microparticles and platelet lysate for intervertebral disk regeneration. Int. J. Boil. Macromol. 2018, 118, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.L.; Lucarelli, E.; Guidi, S.; Ferrari, M.; Alessandri, G.; De Girolamo, L.; Pessina, A.; Ferrero, I.; Gruppo Italiano Staminali Mesenchimali (GISM). Ex vivo expanded mesenchymal stromal cell minimal quality requirements for clinical application. Stem Cells Dev. 2015, 24, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Milton, N.; Groleau, E.G.; Mishra, D.S.; Vansickle, R.E. Existence of a mannitol hydrate during freeze-drying and practical implications. J. Pharm. Sci. 1999, 88, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Suryanarayanan, R.; Botez, C.E.; Stephens, P.W. Characterization and crystal structure of D-mannitol hemihydrate. J. Pharm. Sci. 2004, 93, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marban, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Silva, A.M.; Teixeira, J.H.; Goncalves, R.M.; Almeida, M.I.; Barbosa, M.A.; Santos, S.G. Extracellular vesicles: Intelligent delivery strategies for therapeutic applications. J. Control. Release 2018, 289, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; Kumar, P.R.A.; Raj, D.K.; James, N.R. Nanogels based on alginic aldehyde and gelatin by inverse miniemulsion technique: Synthesis and characterization. Carbohydr. Polym. 2015, 119, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Mihaly, J.; Deak, R.; Szigyarto, I.C.; Bota, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and C-H stretching vibrations. Biochim. Biophys. Acta Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards therapeutic delivery of extracellular vesicles: Strategies for in vivo tracking and biodistribution analysis. Stem Cells Int. 2016, 2016, 5029619. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Dabla, P.K. Oxidative stress and antioxidants in hypertension-a current review. Curr. Hypertens. Rev. 2015, 11, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jo, S.-H.; Kim, W.H.; Kweon, O.-K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press. Res. 2016, 41, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, H.; Kemp, K.; Gray, E.; Hares, K.; Mallam, E.; Scolding, N.; Wilkins, A. Changes in expression of the antioxidant enzyme SOD3 occur upon differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Stem Cells Dev. 2012, 21, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C.W. Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int. 2016, 2016, 3924858. [Google Scholar] [CrossRef] [PubMed]
- Noone, C.; Kihm, A.; English, K.; O’Dea, S.; Mahon, B.P. IFN-gamma stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013, 22, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.-H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-gamma. Ebiomedicine 2018, 28, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.C.; Jeon, E.S.; Lee, I.H.; Kim, H.S.; Kim, M.B.; Kim, J.H. Tumor necrosis factor-alpha-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Investig. Dermatol. 2011, 131, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.; Jakob, M.; Ludwig, A.-K.; Giebel, B.; Lang, S.; Brandau, S. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010, 19, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhao, G.; Liu, L.; Liu, F.; Gong, W.; Liu, X.; Yang, L.; Wang, J.; Hou, Y. Pre-treatment with IL-1 beta enhances the efficacy of msc transplantation in DSS-induced colitis. Cell. Mol. Immunol. 2012, 9, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell. Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef] [PubMed]
| Batch n. | Cell Source Type | Cell Line | Total Cell Number | Cell Viability % |
|---|---|---|---|---|
| 1 | ASCs | AM16 | 85 × 106 | 98.2 |
| 2 | ASCs | AM21 | 82 × 106 | 96.6 |
| 3 | ASCs | AM18 | 121 × 106 | 98.0 |
| Batch n. | Yield (mg) | Cell Equivalent/mg (×106) | µg Protein/mg lyo-Secretome | µg Lipid/mg lyo-Secretome |
|---|---|---|---|---|
| 1 | 882 | 0.096 | 59.7 ± 2.053 a | 8.9 ± 0.137 a |
| 2 | 828 | 0.098 | 69.2 ± 1.332 b | 4.9 ± 0.304 b |
| 3 | 1115 | 0.104 | 21.7 ± 0.595 c | 1.9 ± 0.885 c |
| Batch | Mean Diameter | Mode (nm) | d10 (nm) | d50 (nm) | d90 (nm) | Particle Concentration Per mL/mg of Product |
|---|---|---|---|---|---|---|
| Before lyophilization | 171.8 ± 18.3 | 138.1 ± 38.8 | 97.7 ± 9.8 | 158.8 ± 21.1 | 282.1 ± 22.6 | 2.560 × 109 ± 2.20 × 108 |
| After lyophilization | 231.1 ± 10.2 | 152.5 ± 15.2 | 82.8 ± 2.0 | 172.8 ± 6.6 | 358.4 ± 33.6 | 0.902 × 109 ± 1.67 × 108 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, 190. https://doi.org/10.3390/cells7110190
Bari E, Perteghella S, Di Silvestre D, Sorlini M, Catenacci L, Sorrenti M, Marrubini G, Rossi R, Tripodo G, Mauri P, et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells. 2018; 7(11):190. https://doi.org/10.3390/cells7110190
Chicago/Turabian StyleBari, Elia, Sara Perteghella, Dario Di Silvestre, Marzio Sorlini, Laura Catenacci, Milena Sorrenti, Giorgio Marrubini, Rossana Rossi, Giuseppe Tripodo, Pierluigi Mauri, and et al. 2018. "Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process" Cells 7, no. 11: 190. https://doi.org/10.3390/cells7110190
APA StyleBari, E., Perteghella, S., Di Silvestre, D., Sorlini, M., Catenacci, L., Sorrenti, M., Marrubini, G., Rossi, R., Tripodo, G., Mauri, P., Marazzi, M., & Torre, M. L. (2018). Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells, 7(11), 190. https://doi.org/10.3390/cells7110190













