Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Pro-Oxidant Parameters
2.2. Antioxidant Parameters
2.2.1. Determination of Protein Antioxidants
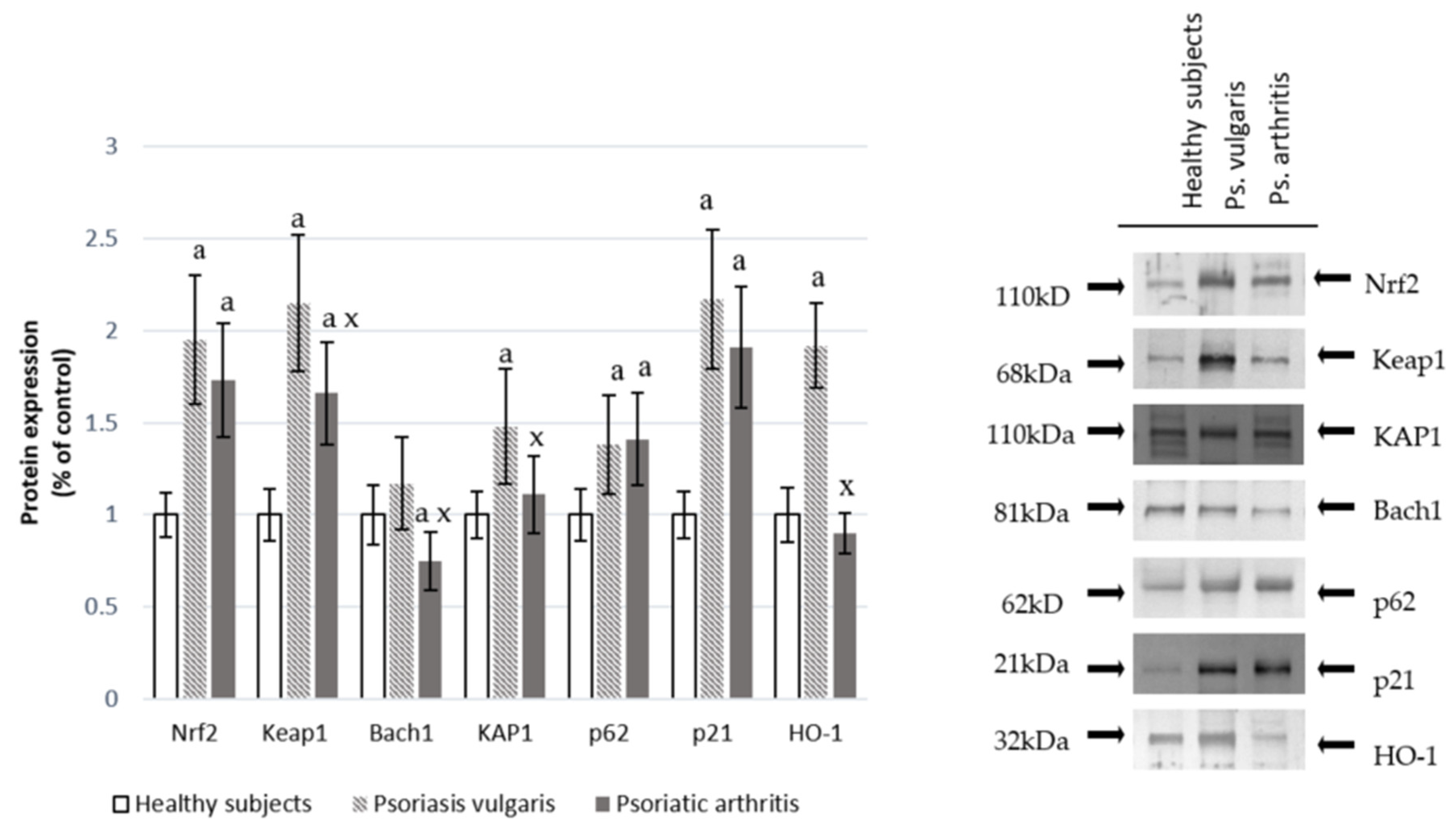
2.2.2. Nrf2 Pathway Parameters
2.2.3. Determination of Low Molecular Antioxidants
2.3. Phospholipid Metabolism
2.3.1. Lipidomic Analysis of Phospholipids
2.3.2. Phospholipid Profile
2.3.3. Determination of Phospholipid and Free Fatty Acids Profile
2.3.4. Determination of Enzymes Metabolizing Phospholipids
2.4. Lipid Peroxidation
2.5. Estimation of the Endocannabinoid System
2.6. Protein Modifications
Determination of Protein Oxidative Modifications
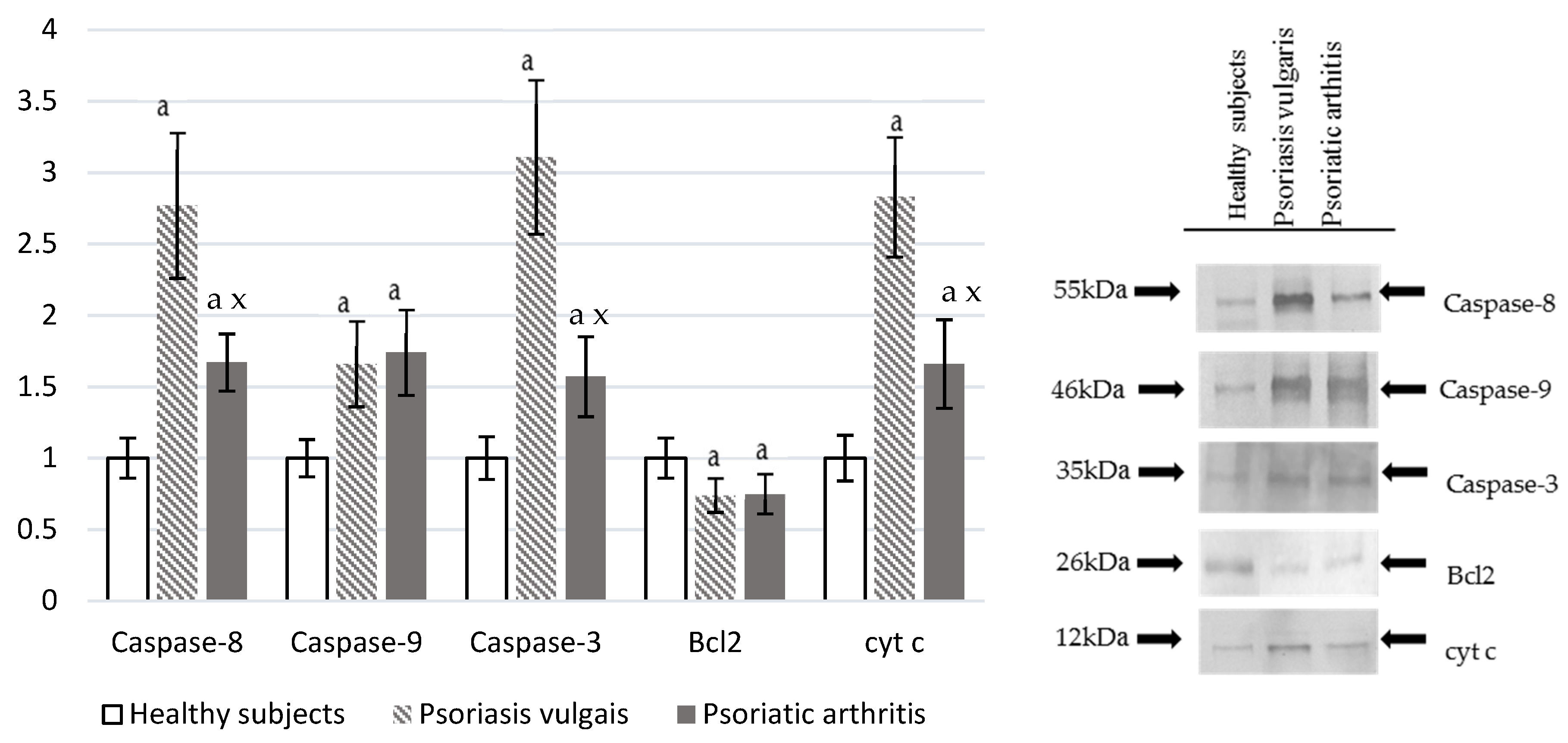
2.7. Expression of Pro-Apoptotic and Anti-Apoptotic Proteins in the Cells
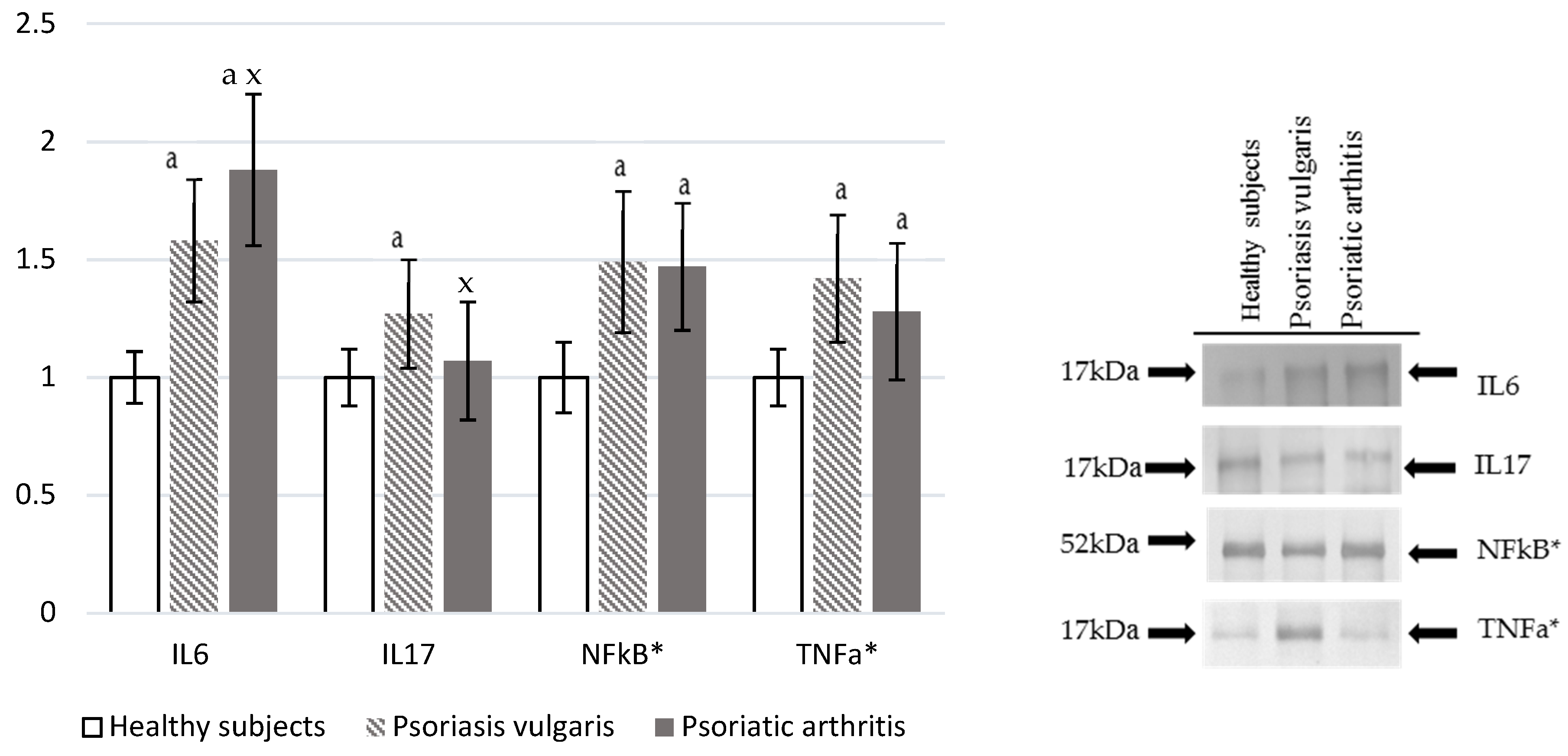
2.8. Pro-Inflammatory Factors
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Redox Balance
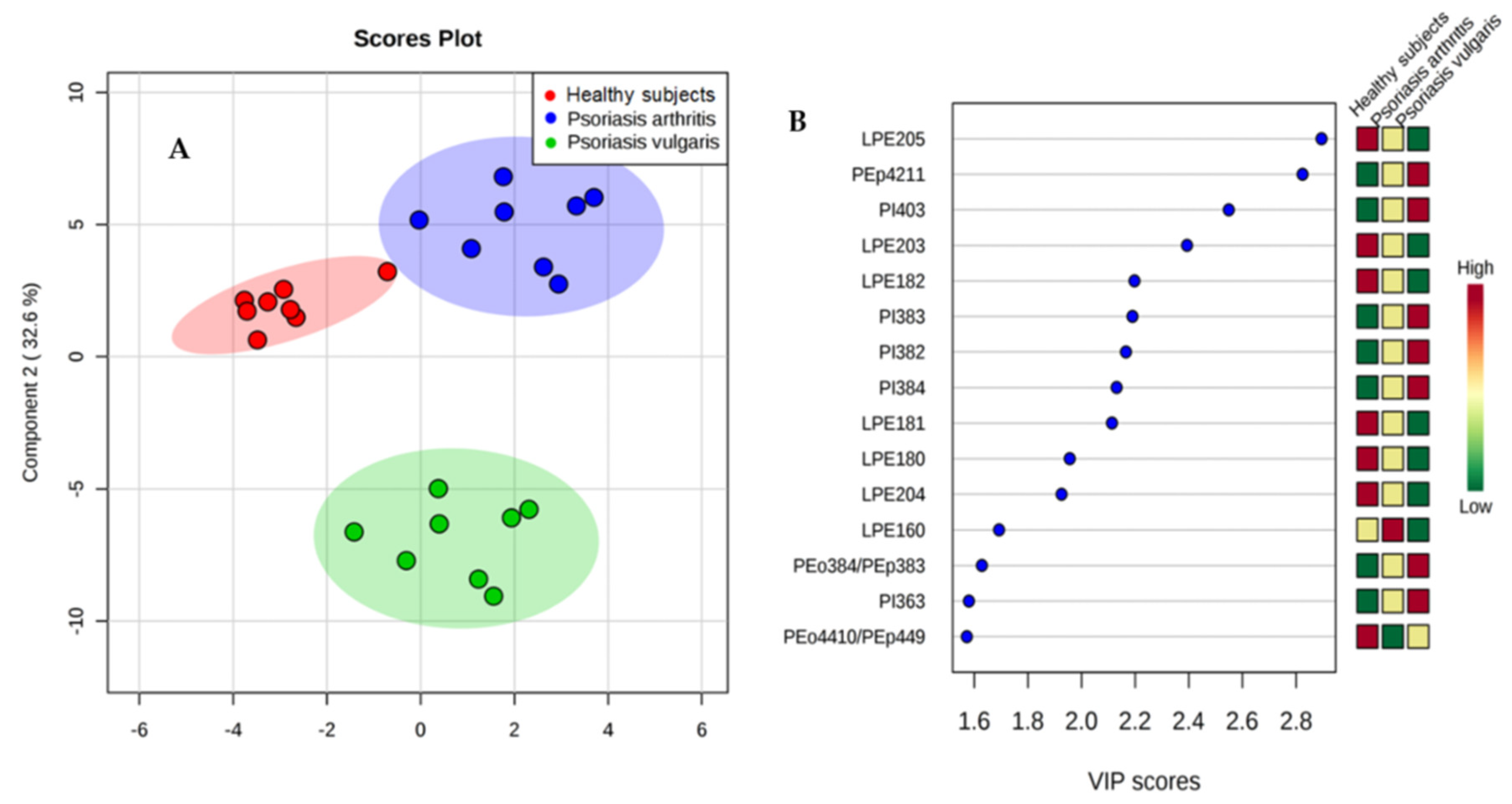
3.2. Phospholipid Metabolism
3.3. Fatty Acids Profile
3.4. Lipid Peroxidation
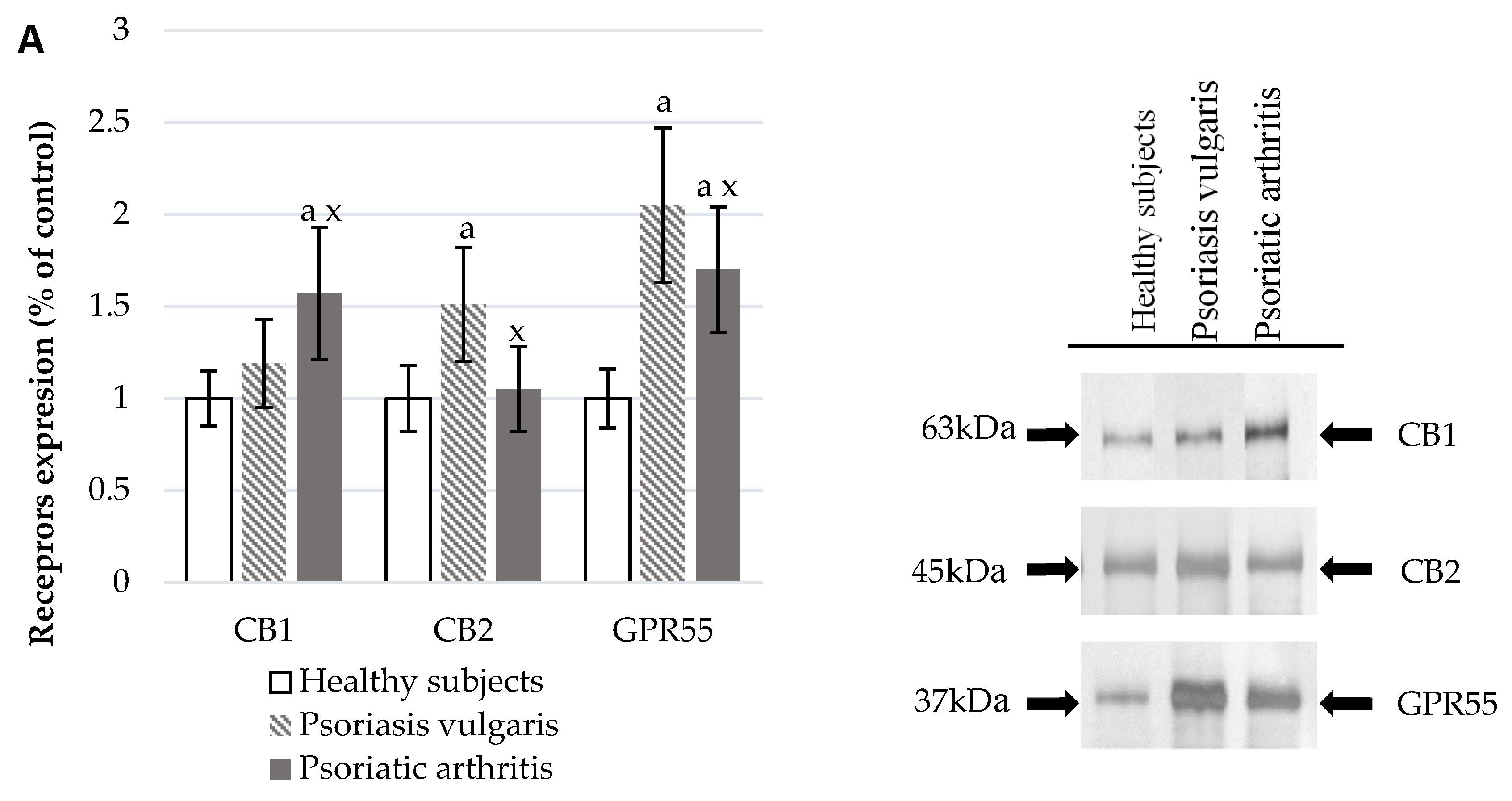
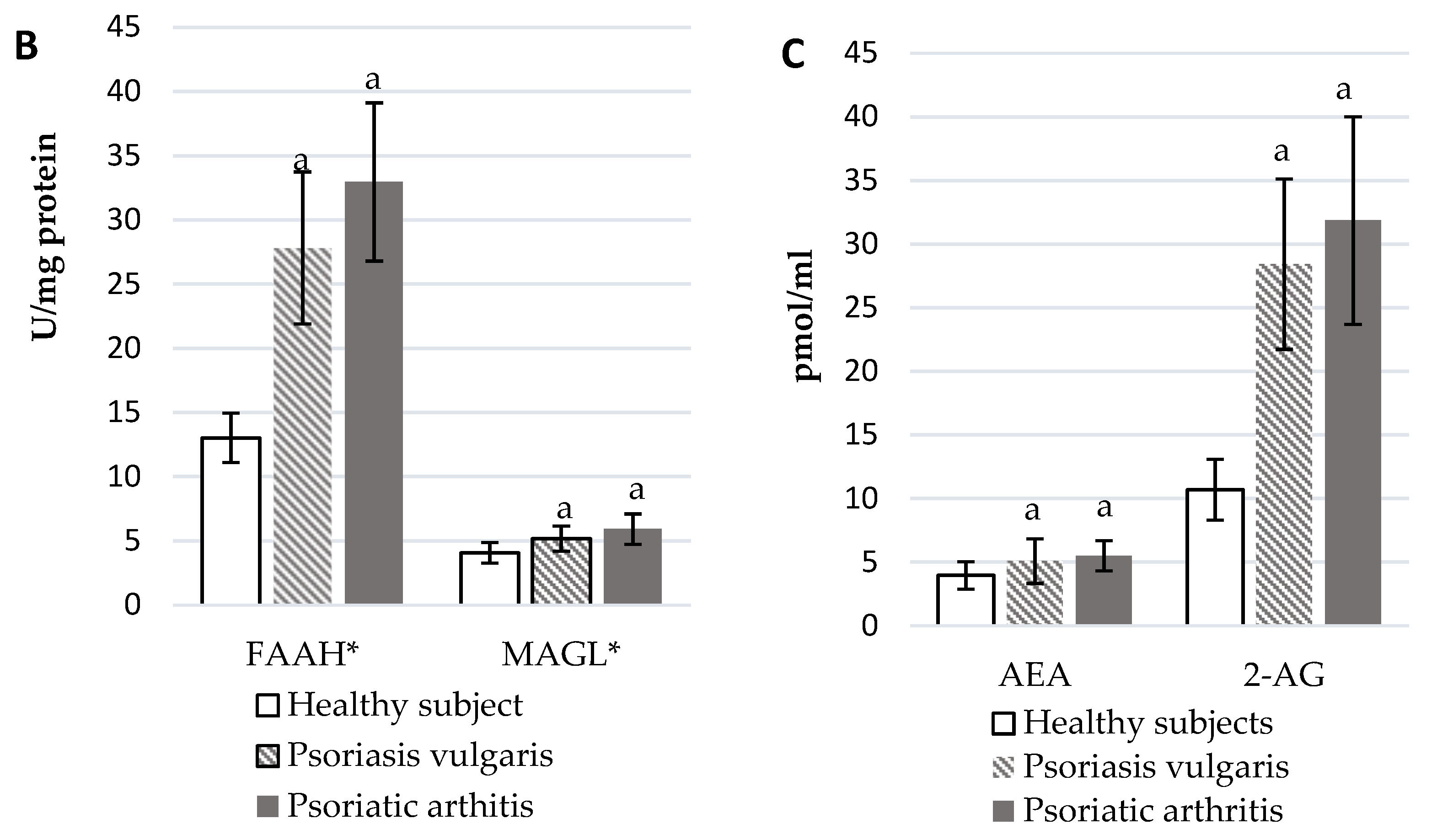
3.5. Endocannabinoid System
3.6. Protein Modifications
3.7. Pro-Inflammatory Mediators
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lipina, C.; Hundal, H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Nakagawa, Y.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Reactive Oxygen Species, Superoxide Dimutases, and PTEN-p53-AKT-MDM2 Signaling Loop Network in Mesenchymal Stem/Stromal Cells Regulation. Cells 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.; Guichardant, M.; Bernoud-Hubac, N.; Calzada, C.; Véricel, E. Oxygenation of polyunsaturated fatty acids and oxidative stress within blood platelets. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2018, 1863, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of Oxidative Stress in Various Stages of Psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Wiswedel, I. F(2)-isoprostanes: Sensitive biomarkers of oxidative stress in vitro and in vivo: A gas chromatography-mass spectrometric approach. Methods Mol. Biol. (Clifton NJ) 2009, 580, 3–16. [Google Scholar]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, X.; Huai, J.; Li, Y.; Cheng, C.; Bi, K.; Dai, R. Arachidonic acid metabonomics study for understanding therapeutic mechanism of Huo Luo Xiao Ling Dan on rat model of rheumatoid arthritis. J. Ethnopharmacol. 2018, 217, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Van Dross, R. Anandamide-induced endoplasmic reticulum stress and apoptosis are mediated by oxidative stress in non-melanoma skin cancer: Receptor-independent endocannabinoid signaling. Mol. Carcinog. 2016, 55, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.T.; Lee, J.H.; Borazjani, A.; Mangum, L.C.; Hou, X.; Ross, M.K. Oxyradical stress increases the biosynthesis of 2-arachidonoylglycerol: Involvement of NADPH oxidase. Am. J. Physiol. Cell Physiol. 2016, 311, C960–C974. [Google Scholar] [CrossRef] [PubMed]
- Eding, C.B.; Enerbäck, C. Involved and Uninvolved Psoriatic Keratinocytes Display a Resistance to Apoptosis that may Contribute to Epidermal Thickness. Acta Derm. Venereol. 2017, 97, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, P.; Villanova, F.; Nestle, F.O. Psoriasis. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Naik, H.B.; Natarajan, B.; Stansky, E.; Ahlman, M.A.; Teague, H.; Salahuddin, T.; Ng, Q.; Joshi, A.A.; Krishnamoorthy, P.; Dave, J.; et al. The Severity of Psoriasis Associates with Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Ansari, M.A.; Al-Harbi, M.M.; Alotaibi, M.R.; AlSaad, A.M.S.; Ahmad, S.F. Psoriasis-like inflammation leads to renal dysfunction via upregulation of NADPH oxidases and inducible nitric oxide synthase. Int. Immunopharmacol. 2017, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Cavaliere, A.; Palmery, M. Plasma total antioxidant capacity and peroxidation biomarkers in psoriasis. J. Biomed. Sci. 2016, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Borska, L.; Kremlacek, J.; Andrys, C.; Krejsek, J.; Hamakova, K.; Borsky, P.; Palicka, V.; Rehacek, V.; Malkova, A.; Fiala, Z. Systemic Inflammation, Oxidative Damage to Nucleic Acids, and Metabolic Syndrome in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Cipak, A.; Schaur, R.J.; Zarkovic, N. Pathophysiology of neutrophil-mediated extracellular redox reactions. Front. Biosci. Landmark Ed. 2016, 21, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3324. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Bae, J.H.; Kang, S.-G.; Cho, S.W.; Chun, D.-I.; Nam, S.M.; Kim, C.H.; Nam, H.S.; Lee, S.H.; Lee, S.H.; et al. Pro-oxidant status and Nrf2 levels in psoriasis vulgaris skin tissues and dimethyl fumarate-treated HaCaT cells. Arch. Pharm. Res. 2017, 40, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Prajda, N.; Weber, G. Malignant transformation-linked imbalance: Decreased xanthine oxidase activity in hepatomas. FEBS Lett. 1975, 59, 245–249. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic glutathione reductase. I. Purification and general kinetic properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Holmgren, A.; Bjornstedt, M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995, 252, 199–208. [Google Scholar] [PubMed]
- Lovell, M.A.; Xie, C.; Gabbita, S.P.; Markesbery, W.R. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radic. Biol. Med. 2000, 28, 418–427. [Google Scholar]
- Maeso, N.; García-Martínez, D.; Rupérez, F.J.; Cifuentes, A.; Barbas, C. Capillary electrophoresis of glutathione to monitor oxidative stress and response to antioxidant treatments in an animal model. J. Chromatogr. B 2005, 822, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Łuczaj, W.; Domingues, P.; Domingues, M.R.; Pancewicz, S.; Skrzydlewska, E. Phospholipidomic Analysis Reveals Changes in Sphingomyelin and Lysophosphatidylcholine Profiles in Plasma from Patients with Neuroborreliosis. Lipids 2017, 52, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Pulfer, M.; Murphy, R.C. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003, 22, 332–364. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis—AOCS Lipid Library. Available online: http://lipidlibrary.aocs.org/Analysis/content.cfm?ItemNumber=40374 (accessed on 12 March 2018).
- Reynolds, L.J.; Hughes, L.L.; Yu, L.; Dennis, E.A. 1-Hexadecyl-2-arachidonoylthio-2-deoxy-sn-glycero-3-phosphorylcholine as a substrate for the microtiterplate assay of human cytosolic phospholipase A2. Anal. Biochem. 1994, 217, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Aarsman, A.J.; Neys, F.W.; Van, H.; den, B. Catabolism of platelet-activating factor and its acyl analog. Differentiation of the activities of lysophospholipase and platelet-activating-factor acetylhydrolase. Eur. J. Biochem. 1991, 200, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kulmacz, R.J.; Lands, W.E. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins 1983, 25, 531–540. [Google Scholar] [CrossRef]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Coolen, S.a.J.; van Buuren, B.; Duchateau, G.; Upritchard, J.; Verhagen, H. Kinetics of biomarkers: Biological and technical validity of isoprostanes in plasma. Amino Acids 2005, 29, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Fam, S.S.; Murphey, L.J.; Terry, E.S.; Zackert, W.E.; Chen, Y.; Gao, L.; Pandalai, S.; Milne, G.L.; Roberts, L.J.; Porter, N.A.; et al. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 2002, 277, 36076–36084. [Google Scholar] [CrossRef] [PubMed]
- Gouveia-Figueira, S.; Nording, M.L. Development and validation of a sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of 15 endocannabinoids and related compounds in milk and other biofluids. Anal. Chem. 2014, 86, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, S.V.; Seki, E.; Osawa, Y.; Uchinami, H.; Cravatt, B.F.; Schwabe, R.F. Fatty acid amide hydrolase determines anandamide-induced cell death in the liver. J. Biol. Chem. 2006, 281, 10431–10438. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, N.M.; Deutsch, D.G. Assessment of a Spectrophotometric Assay for Monoacylglycerol Lipase Activity. AAPS J. 2010, 12, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Milkovic, L.; Bennett, S.J.; Griffiths, H.R.; Zarkovic, N.; Grune, T. Measurement of HNE-protein adducts in human plasma and serum by ELISA—Comparison of two primary antibodies. Redox Biol. 2013, 1, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Seada, L.S. Quantitation of bcl-2 protein in bladder cancer tissue by enzyme immunoassay: Comparison with Western blot and immunohistochemistry. Clin. Chem. 1998, 44, 1423–1429. [Google Scholar] [PubMed]
- Sancho, R.; Calzado, M.A.; Marzo, V.D.; Appendino, G.; Muñoz, E. Anandamide Inhibits Nuclear Factor-κB Activation through a Cannabinoid Receptor-Independent Pathway. Mol. Pharmacol. 2003, 63, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.S.L.; Jain, V.K.; Verma, P.; Yadav, J.P. Polymorphism of glutathione S-transferase M1 and T1 genes and susceptibility to psoriasis disease: A study from North India. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 39–44. [Google Scholar] [PubMed]
- Morales-González, J.A.; Madrigal-Santillán, E.; Morales-González, Á.; Bautista, M.; Gayosso-Islas, E.; Sánchez-Moreno, C. What is Known Regarding the Participation of Factor Nrf-2 in Liver Regeneration? Cells 2015, 4, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-S.; Yen, J.-H.; Kou, M.-C.; Wu, M.-J. Luteolin and Apigenin Attenuate 4-Hydroxy-2-Nonenal-Mediated Cell Death through Modulation of UPR, Nrf2-ARE and MAPK Pathways in PC12 Cells. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Wójcik, P.; Skrzydlewska, E. Proteomic plasma profile of psoriatic patients. J. Pharm. Biomed. Anal. 2018, 155, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Dimethyl Fumarate: A Review in Moderate to Severe Plaque Psoriasis. Drugs 2018, 78, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, M. A systematic review on the role of eicosanoid pathways in rheumatoid arthritis. Adv. Med. Sci. 2018, 63, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gindzienska-Sieskiewicz, E.; Jarocka-Karpowicz, I.; Andrisic, L.; Sierakowski, S.; Zarkovic, N.; Waeg, G.; Skrzydlewska, E. The onset of lipid peroxidation in rheumatoid arthritis: Consequences and monitoring. Free Radic. Res. 2016, 50, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chalimoniuk, M. Secretory phospholipase A2 and its role in oxidative stress and inflammation. Postep. Biochem. 2012, 58, 204–208. (In Polish) [Google Scholar] [PubMed]
- Fontana, L.; Giagulli, C.; Minuz, P.; Lechi, A.; Laudanna, C. 8-Iso-PGF2α Induces β2-Integrin–Mediated Rapid Adhesion of Human Polymorphonuclear Neutrophils: A Link Between Oxidative Stress and Ischemia/Reperfusion Injury. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.W.; Liu, G.Y.; Zhao, C.F.; Li, X.F.; Yang, X.Y. Differential expression of COX-2 in osteoarthritis and rheumatoid arthritis. Genet. Mol. Res. 2015, 14, 12872–12879. [Google Scholar] [CrossRef] [PubMed]
- Andringa, K.K.; Udoh, U.S.; Landar, A.; Bailey, S.M. Proteomic analysis of 4-hydroxynonenal (4-HNE) modified proteins in liver mitochondria from chronic ethanol-fed rats. Redox Biol. 2014, 2, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Sicińska, P.; Pytel, E.; Kurowska, J.; Koter-Michalak, M. Supplementation with omega fatty acids in various diseases. Postęp. Hig. Med. Dośw. 2015, 69, 838–852. [Google Scholar] [CrossRef]
- Hervouet, E.; Cheray, M.; Vallette, F.M.; Cartron, P.-F. DNA Methylation and Apoptosis Resistance in Cancer Cells. Cells 2013, 2, 545–573. [Google Scholar] [CrossRef] [PubMed]
- Staal, J.; Driege, Y.; Bekaert, T.; Demeyer, A.; Muyllaert, D.; Van Damme, P.; Gevaert, K.; Beyaert, R. T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1. EMBO J. 2011, 30, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. The endocannabinoid anandamide induces apoptosis of rat decidual cells through a mechanism involving ceramide synthesis and p38 MAPK activation. Apoptosis 2013, 18, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
| Analyzed Parameters | Healthy Subjects | Psoriasis Vulgaris | Psoriatic Arthritis |
|---|---|---|---|
| XO* U/mg protein | 18.8 ± 2.5 | 30.1 ± 5.3 a | 32.71 ± 5.73 a |
| NADPH oxidase* U/μg protein | 2.25 ± 0.28 | 3.21 ± 0.55 a | 3.92 ± 0.71 a |
| Cu,Zn-SOD U/mg protein | 3.91 ± 0.51 | 4.72 ± 0.81 a | 4.12 ± 0.53 x |
| GSH-Px mU/mg protein | 2.16 ± 0.47 | 1.86 ± 0.45 a | 2.17 ± 0.63 x |
| GSSG-R mU/mg protein | 0.73 ± 0.13 | 0.71 ± 0.14 | 0.68 ± 0.16 |
| Trx μg/mg protein | 6.45 ± 0.92 | 5.17 ± 0.98 a | 3.20 ± 0.89 a,x |
| TrxR U/mg protein | 0.97 ± 0.22 | 0.56 ± 0.17 a | 0.55 ± 0.13 a |
| GSH nmol/mL | 9.54 ± 1.24 | 6.35 ± 1.06 a | 6.19 ± 0.65 a |
| Vitamin C nmol/mL | 41.79 ± 8.69 | 28.22 ± 9.60 a | 22.82 ± 5.05 a,x |
| Vitamin E nmol/mL | 1.01 ± 0.24 | 0.79 ± 0.20 a | 0.80 ± 0.15 a |
| Vitamin A pmol/mL | 248.6 ± 22.7 | 228.8 ± 32.9 | 213.6 ± 35.2 a |
| Analyzed Parameters | Healthy Subjects | Psoriasis Vulgaris | Psoriatic Arthritis |
|---|---|---|---|
| Phospholipid LA (18:2) μmol/mL | 1.45 ± 0.21 | 1.24 ± 0.27 a | 1.11 ± 0.23 a,x |
| Phospholipid LA (18:3) μmol/mL | 29.95 ± 6.81 | 26.21 ± 7.96 a | 24.04 ± 7.82 a |
| Phospholipid AA (20:4) μmol/mL | 792.7 ± 124.9 | 739.6 ± 131.5 | 712.6 ± 143.9 a |
| Phospholipid DHA (22:6) μmol/mL | 292.4 ± 65.2 | 267.0 ± 71.4 | 253.5 ± 73.3 a |
| Free LA (18:2) nmol/mL | 17.46 ± 4.83 | 16.13 ± 5.31 | 15.09 ± 5.04 |
| Free AA (20:4) nmol/mL | 1.51 ± 0.32 | 1.32 ± 0.41 a | 1.09 ± 0.36 a,x |
| Free DHA (22:6) nmol/mL | 1.73 ± 0.41 | 1.43 ± 0.45 a | 1.20 ± 0.44 a,x |
| PLA2 nmol/mL/min | 9.18 ± 0.919 | 12.05 ± 1.59 a | 9.98 ± 1.48 a,x |
| PAH-AH nmol/mL/min | 30.27 ± 2.12 | 56.56 ± 10.49 a | 48.94 ± 7.53 a,x |
| COX-1 nmol/mL/min | 0.43 ± 0.07 | 0.63 ± 0.13 a | 0.54 ± 0.09 a,x |
| COX-2 nmol/mL/min | 0.17 ± 0.03 | 0.52 ± 0.14 a | 0.44 ± 0.09 a,x |
| Analyzed Parameters | Healthy Subjects | Psoriasis Vulgaris | Psoriatic Arthritis |
|---|---|---|---|
| 4-HNE nmol/mL | 8.90 ± 3.71 | 15.36 ± 7.81 a | 11.01 ± 1.86 a,x |
| Isoprostanes pmol/mL | 1.61 ± 0.37 | 3.41 ± 0.79 a | 3.84 ± 0.99 a |
| Neuroprostanes pmol/mL | 2.93 ± 0.61 | 6.56 ± 1.20 a | 6.42 ± 1.09 a |
| Tryptophan U/mg protein | 35.12 ± 5.36 | 26.84 ± 6.21 a | 24.79 ± 6.63 a |
| HNE–protein pmol/mg protein | 15.24 ± 3.62 | 21.59 ± 4.26 a | 17.45 ± 4.16 a,x |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. https://doi.org/10.3390/cells7100159
Ambrożewicz E, Wójcik P, Wroński A, Łuczaj W, Jastrząb A, Žarković N, Skrzydlewska E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells. 2018; 7(10):159. https://doi.org/10.3390/cells7100159
Chicago/Turabian StyleAmbrożewicz, Ewa, Piotr Wójcik, Adam Wroński, Wojciech Łuczaj, Anna Jastrząb, Neven Žarković, and Elżbieta Skrzydlewska. 2018. "Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients" Cells 7, no. 10: 159. https://doi.org/10.3390/cells7100159
APA StyleAmbrożewicz, E., Wójcik, P., Wroński, A., Łuczaj, W., Jastrząb, A., Žarković, N., & Skrzydlewska, E. (2018). Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells, 7(10), 159. https://doi.org/10.3390/cells7100159







