Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma
Abstract
1. Introduction
2. Results
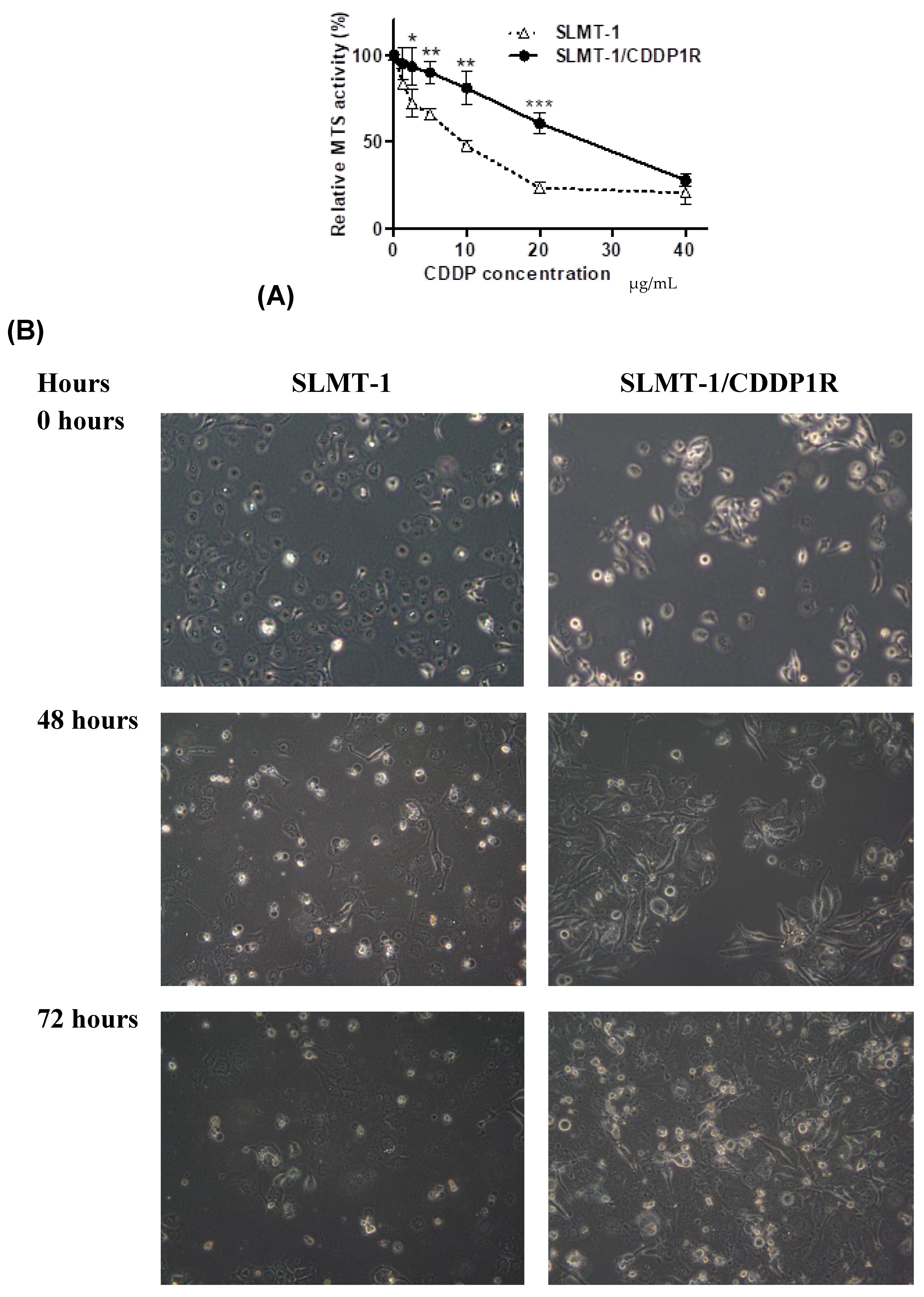
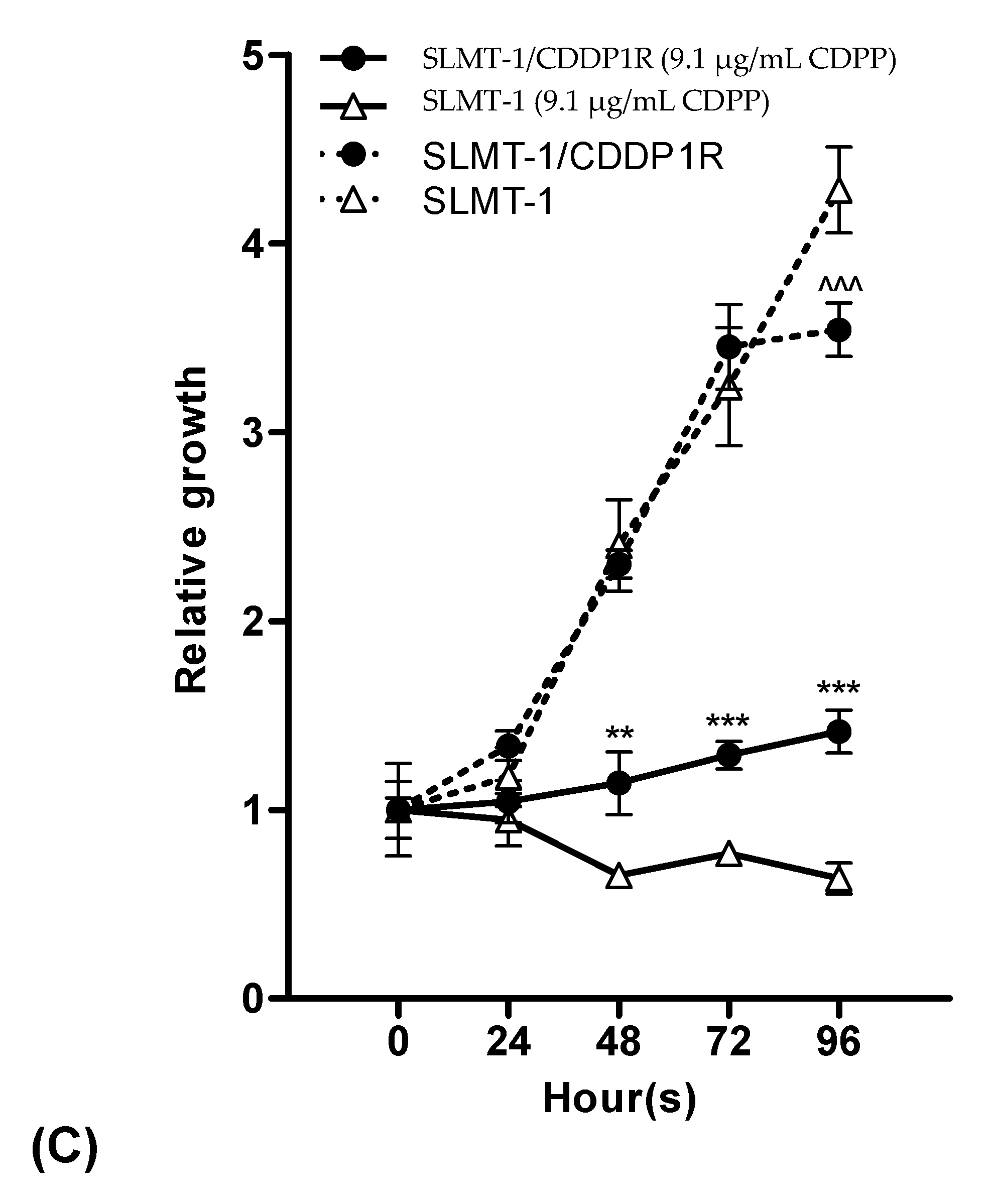
2.1. Evaluation forCisplatin-Resistance
2.2. Differentially Expressed Genes in SLMT-1 and SLMT-1/CDDP1R
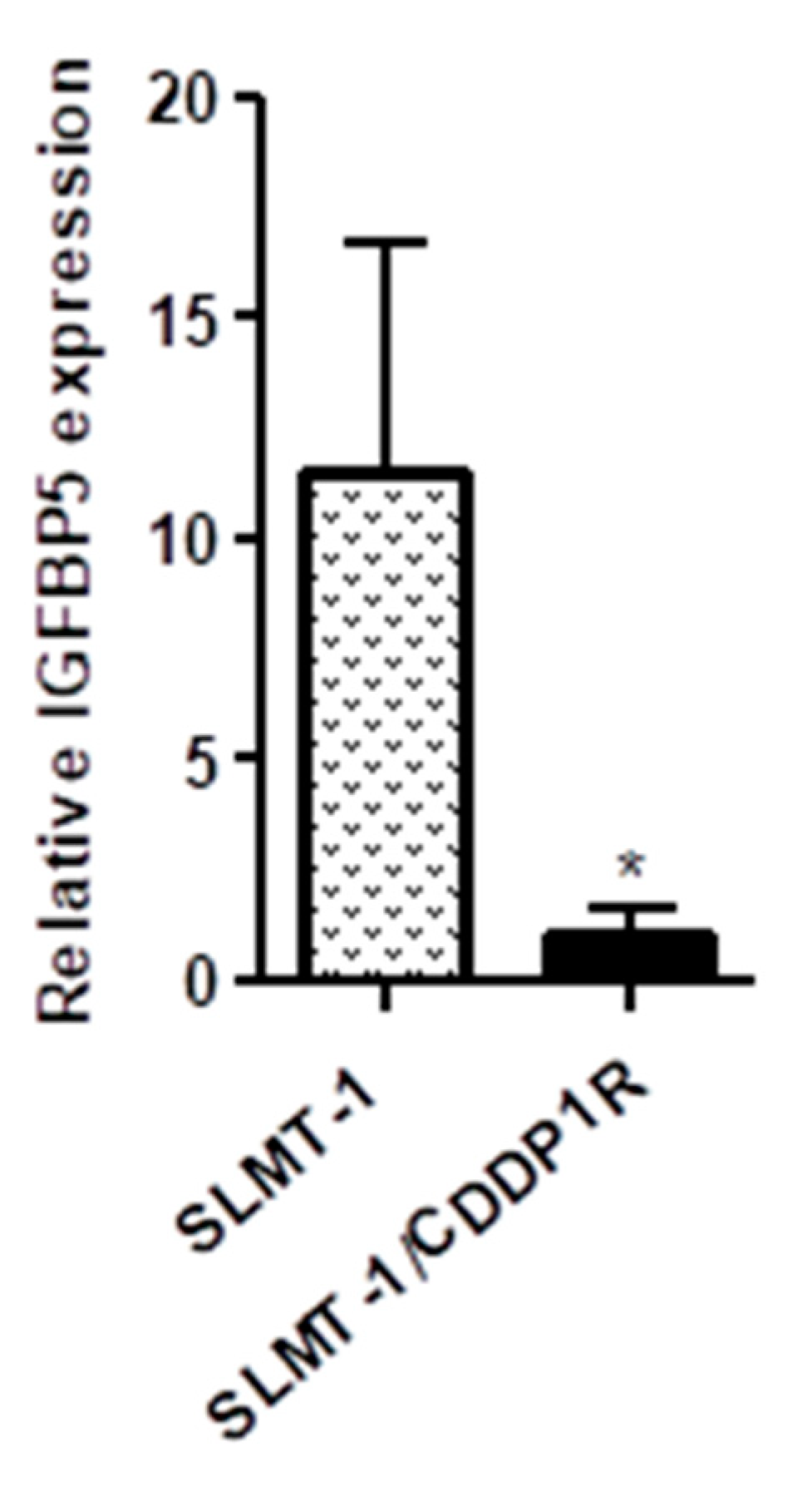
2.3. IGFBP-5 Downregulation AcquiresCisplatin-Resistance
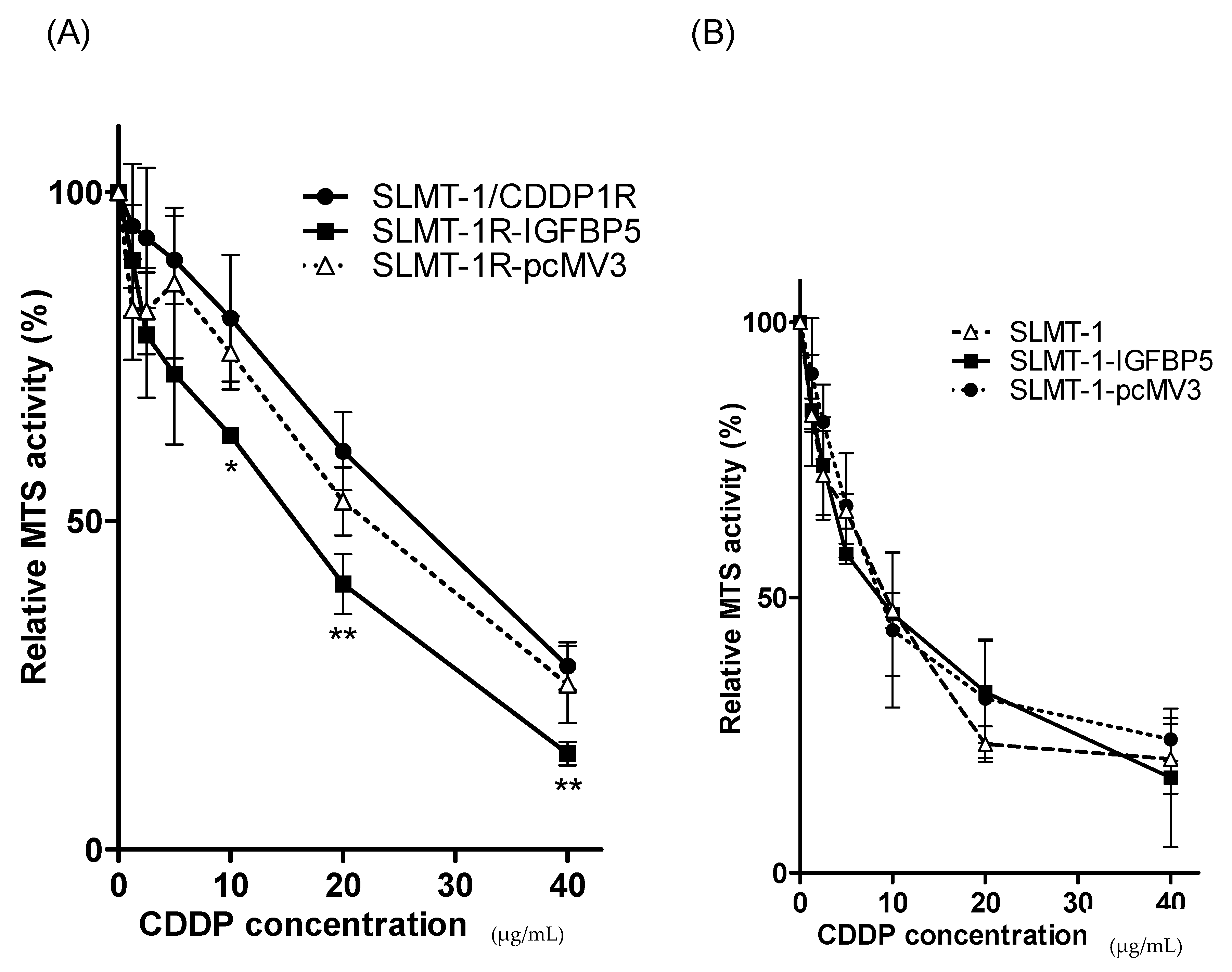
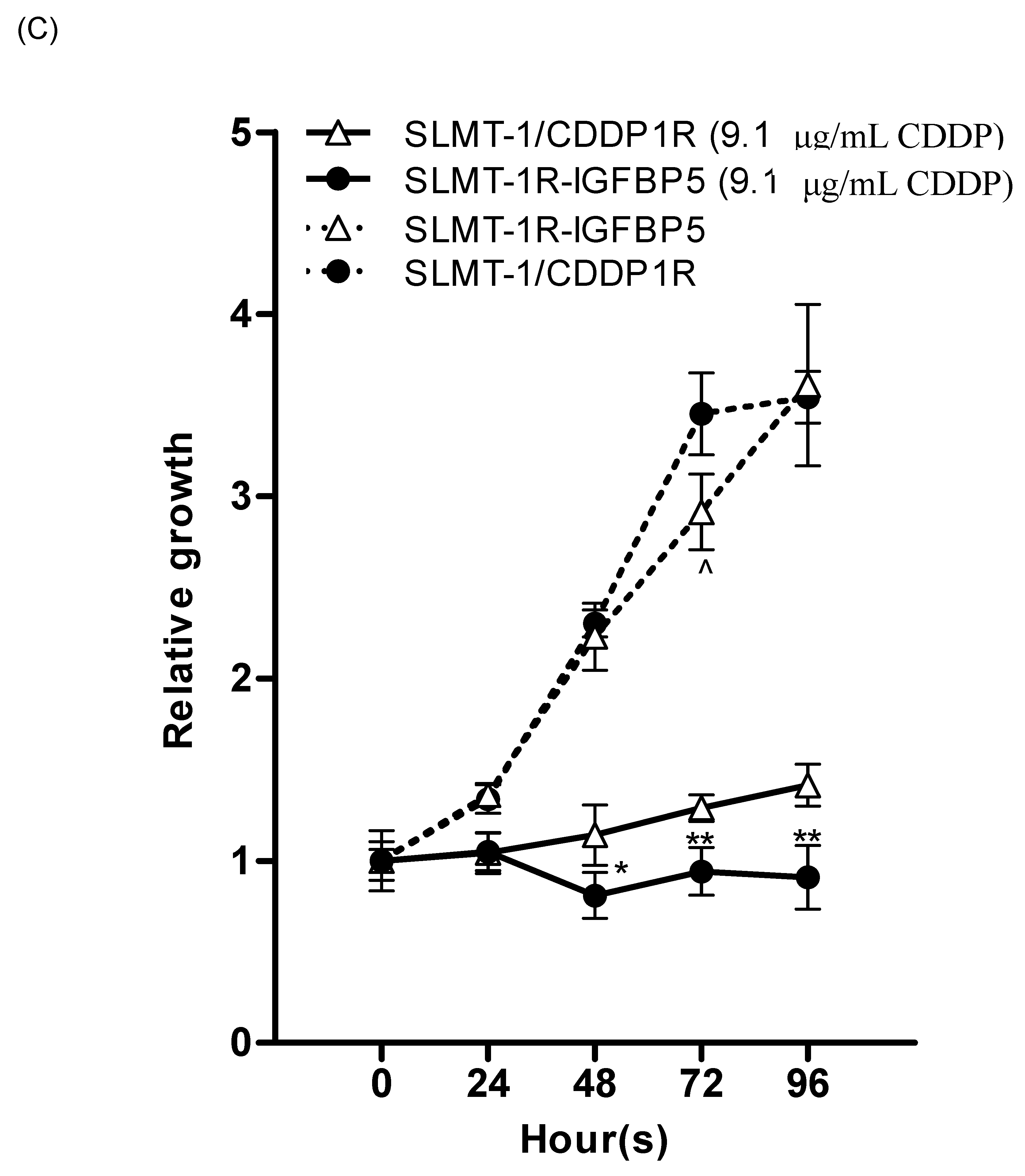
2.4. Upregulation of IGFBP5 Reverses Cisplatin-Resistance
3. Discussion
4. Material and Methods
4.1. Establishment of an ESCC Cell Line with Resistance to Cisplatin
4.2. Cell Proliferation Assay
4.3. Morphology Study
4.4. cDNA Microarray Analysis
4.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time PCR (qPCR)
4.6. Suppression of IGFBP5 Expression by RNA Interference
4.7. Restoring Expression Level of IGFBP5
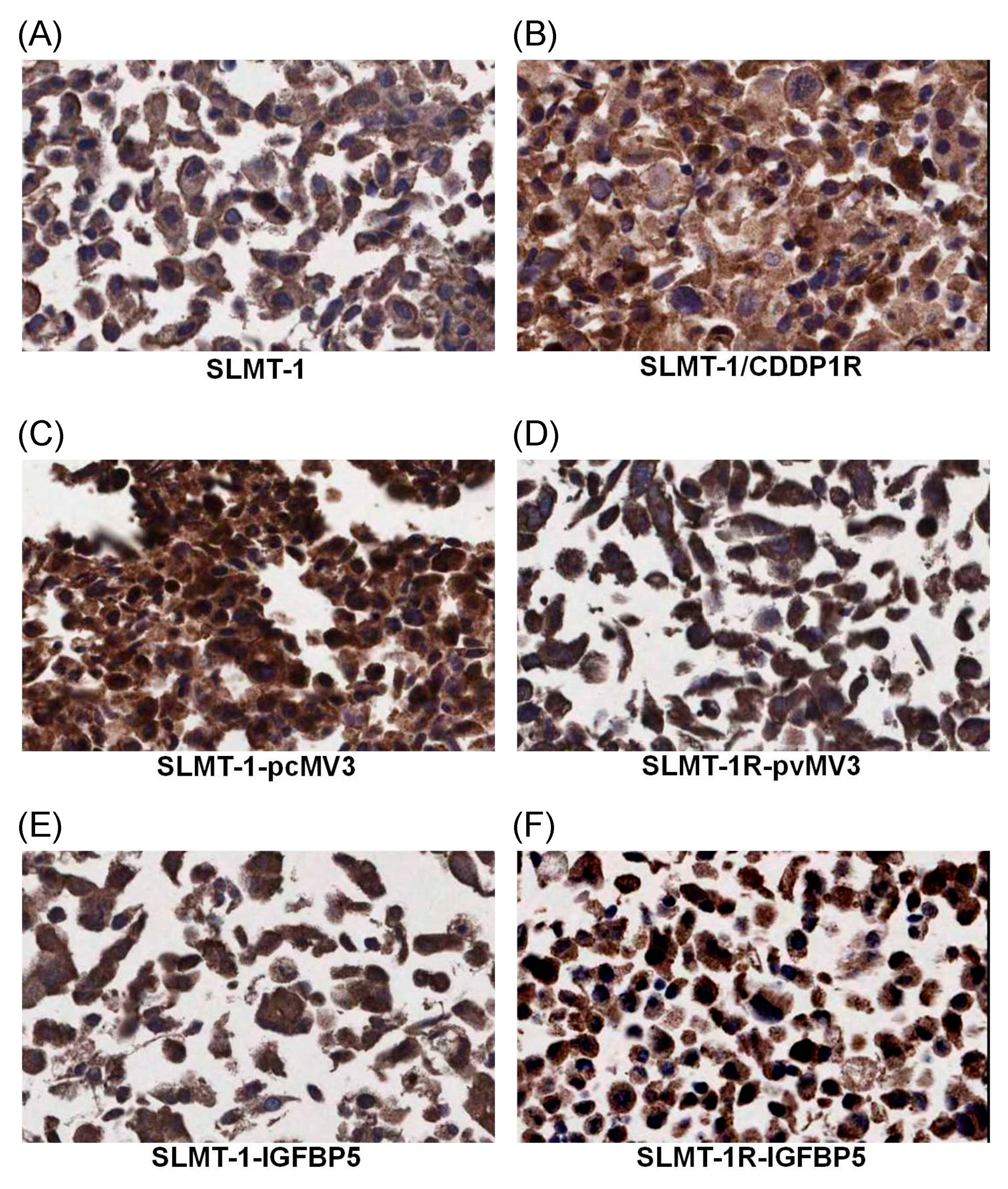
4.8. Evaluation of the Transfection Efficiency by Immunohistochemical Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Abbreviation
References
- Murphy, G.; McCormack, V.; Abedi-Ardekani, B.; Arnold, M.; Camargo, M.C.; Dar, N.A.; Dawsey, S.M.; Etemadi, A.; Fitzgerald, R.C.; Fleischer, D.E.; et al. International cancer seminars: A focus on esophageal squamous cell carcinoma. Ann. Oncol. 2017, 28, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Wei, Z.R.; Zhang, J.; Hu, W.; Ma, Z.F.; Liu, Q.H. Which factors are associated with extremely short-term survival after surgery in patients with esophageal squamous cell carcinoma? Asia-Pac. J. Clin. Oncol. 2016, 12, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Cao, K.; Xiao, Z.H.; Qiao, L.; Wang, X.Q.; Shang, B.; Jia, Y.; Wang, Z. VRK1 promotes cisplatin resistance by up-regulating c-MYC via c-Jun activation and serves as a therapeutic target in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 65642–65658. [Google Scholar] [CrossRef] [PubMed]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front Pharmacol. 2014, 5, 159. [Google Scholar] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, T.; Pan, J.; Shi, N.; Fan, Q.; Wang, L.; Lu, S.H. Assessment of insulin-like growth factor 1 receptor as an oncogene in esophageal squamous cell carcinoma and its potential implication in chemotherapy. Oncol. Rep. 2014, 32, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, N.; Servan, K.; Hildebrandt, B.; Pölitz, A.; von Jonquières, G.; Wolf-Kümmeth, S.; Napierski, I.; Hamacher, A.; Kassack, M.U.; Budczies, J. Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res. 2009, 69, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, S.; Torossian, A.; Speirs, C.K.; Schleicher, S.; Giacalone, N.J.; Carbone, D.P.; Zhao, Z.; Lu, B. Role of insulin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e563–e572. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; D’Costa, S.; Sills, R.C.; Kim, Y. Inhibition of the insulin-like growth factor 1 receptor pathway enhances the antitumor effect of cisplatin in human malignant mesothelioma cell lines. Cancer Lett. 2009, 278, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.G.; Zhou, Y.; Ying, K.J.; Ruan, W.J. IGFBP, a novel target of lung cancer? Clin. Chim. Acta 2017, 466, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Gershtein, E.S.; Isaeva, E.R.; Kushlinsky, D.N.; Korotkova, E.A.; Ermilova, V.D.; Laktionov, K.P.; Adamyan, L.V. Insulin-like growth factors (IGF) and IGF-binding proteins (IGFBP) in the serum of patients with ovarian tumors. Bull. Exp. Biol. Med. 2016, 160, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, S.K.; Idowu, O.; Wang, Z.L.; Liao, Z.; Yan, Z.J.; Mohammed, M.K.; Ye, J.X.; Wei, Q.; Wang, J.; Zhao, L.G.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Fann, C.S.J.; Su, W.H.; Wang, Y.C.; Weng, C.C.; Yu, C.J.; Hsu, C.L.; Hsieh, A.R.; Chien, R.N.; Chu, C.M.; et al. A genome-wide association study on chronic HBV infection and its clinical progression in male Han-Taiwanese. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.R.K.; Dupart, E.; Al-Sweel, N.; Chen, A.; Cawley, N.X.; Loh, Y.P. Carboxypeptidase E promotes cancer cell survival, but inhibits migration and invasion. Cancer Lett. 2013, 341, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, F.; Mabuchi, N.; Takamoto, M.; Sakurada, Y.; Yoneyama, S.; Kashiwagi, K.; Iijima, H.; Yamagata, Z.; Aihara, M.; Iwata, T.; et al. Genetic variant near PLXDC2 influences the risk of primary open-angle glaucoma by increasing intraocular pressure in the Japanese population. J. Glaucoma 2017, 26, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.O.; Wan, T.S.K.; Wong, N.; Pang, E.; Lam, K.Y.; Law, S.Y.; Chow, L.M.C.; Ma, E.S.K.; Chan, L.C.; Wong, J.; et al. Establishment and characterization of a new xenograft-derived human esophageal squamous cell carcinoma cell line SLMT-1 of Chinese origin. Cancer Genet. Cytogenet. 2001, 124, 36–41. [Google Scholar] [CrossRef]
- Teo, I.T.N.; Tang, J.C.O.; Chui, C.H.; Cheng, G.Y.M.; Yau, M.Y.C.; Lau, F.Y.; Wong, R.S.M.; Leung, T.W.T.; Cheung, F.; Ho, K.P.; et al. Superoxide anion is involved in the early apoptosis mediated by Gleditsia sinensis fruit extract. Int. J. Mol. Med. 2004, 13, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Chui, C.H.; Chan, S.W.; Kok, S.H.; Chan, D.; Tsoi, M.Y.; Leung, P.H.; Lam, A.K.; Chan, A.S.; Lam, K.H.; et al. Synthesis of 8-hydroxyquinoline derivatives as novel antitumor agents. ACS Med. Chem. Lett. 2013, 4, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Akkiprik, M.; Hu, L.M.; Sahin, A.; Hao, X.S.; Zhang, W. The subcellular localization of IGFBP5 affects its cell growth and migration functions in breast cancer. BMC Cancer 2009, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Gullu, G.; Peker, I.; Haholu, A.; Eren, F.; Kucukodaci, Z.; Gulec, B.; Baloglu, H.; Erzik, C.; Ozer, A.; Akkiprik, M. Clinical significance of miR-140-5p and miR-193b expression in patients with breast cancer and relationship to IGFBP5. Genet. Mol. Biol. 2015, 38, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krishnamurthy, S. Cellular responses to cisplatin-induced DNA damage. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kartalou, M.; Essigmann, J.M. Mechanisms of resistance to cisplatin. Mutation Res. Fundam. Mol. Mech. Mutagen. 2001, 478, 23–43. [Google Scholar] [CrossRef]
- Toshimitsu, H.; Hashimoto, K.; Tangoku, A.; Iizuka, N.; Yamamoto, K.; Kawauchi, S.; Furuya, T.; Oka, M.; Sasaki, K. Molecular signature linked to acquired resistance to cisplatin in esophageal cancer cells. Cancer Lett. 2004, 211, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gu, C.; Zhong, D.; Shi, L.; Kong, Y.; Zhou, Z.; Liu, S. Induction of autophagy counteracts the anticancer effect of cisplatin in human esophageal cancer cells with acquired drug resistance. Cancer Lett. 2014, 355, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Q.-Q.; Zhang, R.; Xi, M.; Liao, Y.-J.; Qian, D.; He, L.-R.; Zeng, Y.-X.; Xie, D.; Liu, M.-Z. The overexpression of IGFBP-3 is involved in the chemosensitivity of esophageal squamous cell carcinoma cells to nimotuzumab combined with cisplatin. Tumor Biol. 2012, 33, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Rzonca, S.; Malecki, M. Proapoptotic gene therapy and chemosensitivity of cancer cells. Wspolczesna Onkol. 2009, 13, 61–65. [Google Scholar]
- Clemmons, D.R. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol. Cell Endocrinol. 1998, 140, 19–24. [Google Scholar] [CrossRef]
- Imsumran, A.; Adachi, Y.; Yamamoto, H.; Li, R.; Wang, Y.; Min, Y.; Piao, W.; Nosho, K.; Arimura, Y.; Shinomura, Y. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis 2007, 28, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Ferté, C.; Loriot, Y.; Clémenson, C.; Commo, F.; Gombos, A.; Bibault, J.-E.; Fumagalli, I.; Hamama, S.; Auger, N.; Lahon, B. IGF-1R targeting increases the antitumor effects of DNA-damaging agents in SCLC model: An opportunity to increase the efficacy of standard therapy. Mol. Cancer Ther. 2013, 12, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Héron-Milhavet, L.; Karas, M.; Goldsmith, C.M.; Baum, B.J.; LeRoith, D. Insulin-like growth factor-I (IGF-I) receptor activation rescues UV-damaged cells through a p38 signaling pathway potential role of the IGF-I receptor in DNA repair. J. Biol. Chem. 2001, 276, 18185–18192. [Google Scholar] [CrossRef] [PubMed]
- Héron-Milhavet, L.; LeRoith, D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J. Biol. Chem. 2002, 277, 15600–15606. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Reed, E.; Li, Q.Q. Molecular basis of cellular response to cisplatin chemotherapy in non-small cell lung cancer (Review). Oncol. Rep. 2004, 12, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Chui, C.H.; Fatima, S.; Kok, S.H.; Pak, K.C.; Ou, T.M.; Hui, K.S.; Wong, M.M.; Wong, J.; Law, S. Inhibitory effects of Gleditsia sinensis fruit extract on telomerase activity and oncogenic expression in human esophageal squamous cell carcinoma. Int. J. Mol. Med. 2007, 19, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Law, F.; Chen, Y.; Wong, K.; Ying, J.; Tao, Q.; Langford, C.; Lee, P.; Law, S.; Cheung, R.; Chui, C. Identification of a novel tumor transforming gene GAEC1 at 7q22 which encodes a nuclear protein and is frequently amplified and overexpressed in esophageal squamous cell carcinoma. Oncogene 2007, 26, 5877–5888. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Tsoi, M.Y.T.; Di Liu, C.; Chan, S.H.; Law, S.Y.K.; Chan, K.W.; Chan, Y.P.; Gopalan, V.; Lam, A.K.Y.; Tang, J.C.O. Oncogene GAEC1 regulates CAPN10 expression which predicts survival in esophageal squamous cell carcinoma. World J.Gastroenterol. WJG 2013, 19, 2772. [Google Scholar] [CrossRef] [PubMed]
- Pun, I.H.Y.; Chan, D.; Chan, S.H.; Chung, P.Y.; Zhou, Y.Y.; Law, S.; Lam, A.K.Y.; Chui, C.H.; Chan, A.S.C.; Lam, K.H.; et al. Anti-cancer effects of a novel quinoline derivative 83b1 on human esophageal squamous cell carcinoma through down-regulation of COX-2 mRNA and PGE(2). Cancer Res. Treat. 2017, 49, 219–229. [Google Scholar] [CrossRef] [PubMed]


| Probe Set ID | Gene Title | Fold-Change |
|---|---|---|
| Genes downregulated in SLMT-1/CDDP1R | ||
| 211959_at | IGFBP5, insulin-like growth factor binding protein 5 | −43.48 |
| 212671_s_at | HLA-DQA2, major histocompatibility complex, class II, DQ alpha 2 (multiple annotations exist) | −22.45 |
| 201117_s_at | PE, carboxypeptidase E | −16.43 |
| 236297_at | CDNA FLJ45742fis, clone KIDNE2016327 | −14.09 |
| Gens upregulated in SLMT-1/CDDP1R | ||
| 1557636_a_at | LINC00520, long intergenic non-protein coding RNA 520 | 16.89 |
| 219836_at | SLITRK6, SLIT and NTRK-like family, member 6 | 13.75 |
| 205830_at | LOC100506377, uncharacterized LOC100506377 | 8.91 |
| 210229_s_at | COL15A1, collagen, type XV, alpha 1 | 8.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, D.; Zhou, Y.; Chui, C.H.; Lam, K.H.; Law, S.; Chan, A.S.-c.; Li, X.; Lam, A.K.-y.; Tang, J.C.O. Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma. Cells 2018, 7, 143. https://doi.org/10.3390/cells7100143
Chan D, Zhou Y, Chui CH, Lam KH, Law S, Chan AS-c, Li X, Lam AK-y, Tang JCO. Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma. Cells. 2018; 7(10):143. https://doi.org/10.3390/cells7100143
Chicago/Turabian StyleChan, Dessy, Yuanyuan Zhou, Chung Hin Chui, Kim Hung Lam, Simon Law, Albert Sun-chi Chan, Xingshu Li, Alfred King-yin Lam, and Johnny Cheuk On Tang. 2018. "Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma" Cells 7, no. 10: 143. https://doi.org/10.3390/cells7100143
APA StyleChan, D., Zhou, Y., Chui, C. H., Lam, K. H., Law, S., Chan, A. S.-c., Li, X., Lam, A. K.-y., & Tang, J. C. O. (2018). Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma. Cells, 7(10), 143. https://doi.org/10.3390/cells7100143







