Natural and Synthetic Modulators of the TRPM7 Channel
Abstract
:1. Functional Roles of TRPM7
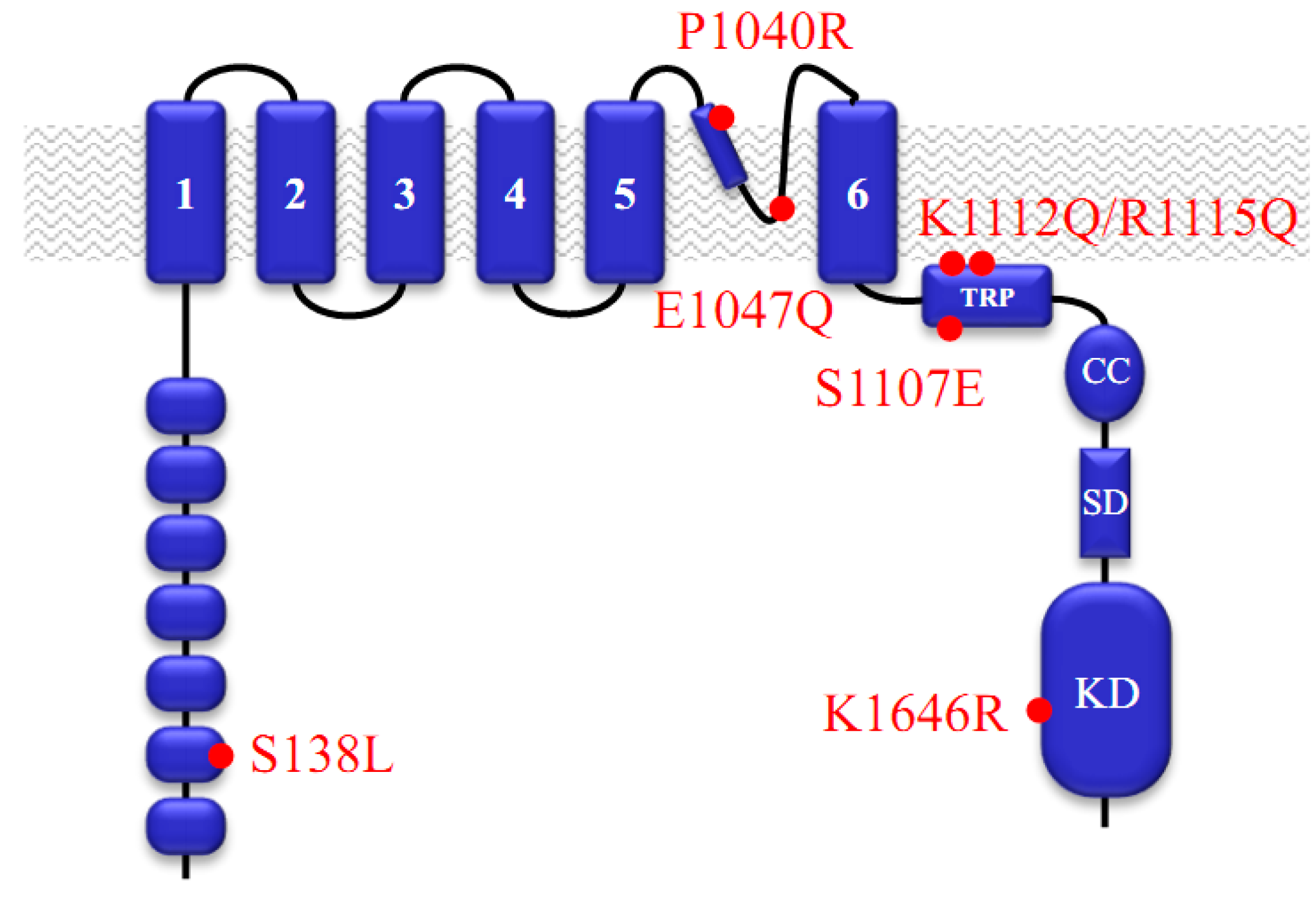
2. Pharmacological Compounds Inhibiting the TRPM7 Channel
| Compound | IC50 (μM) * | Description of the block | Reference |
|---|---|---|---|
| 2-APB | 174 | Reversible | [73,74] |
| Spermine | 2.3 † | Reversible, voltage dependent | [75] |
| SKF-96365 | n.d. | Tested only at 20 μM | [75] |
| Nafamostat | 617 | Reversible, voltage dependent | [76] |
| Carvacrol | 306 | Reversible | [77] |
| NDGA | n.d. | Tested only at 10 and 20 μM | [78] |
| AA861 | n.d. | Tested only at 10 and 40 μM | [78] |
| MK886 | n.d. | Tested only at 10 μM | [78] |
| Waixenicin A | 7.0 | Irreversible, [Mg2+]i dependent | [79] |
| NS8593 | 1.6 | Reversible, [Mg2+]i dependent | [80] |
| Quinine | n.d | Reversible, tested only at 30 μM | [80] |
| CyPPA | n.d | Tested only at 30 μM | [80] |
| Dequalinium | n.d | Tested only at 30 μM | [80] |
| SKA31 | n.d | Tested only at 30 μM | [80] |
| UCL 1684 | n.d | Tested only at 30 μM | [80] |
| Sphingosine | 0.6 | Reversible | [81] |
| FTY720 | 0.7 | Reversible | [81] |
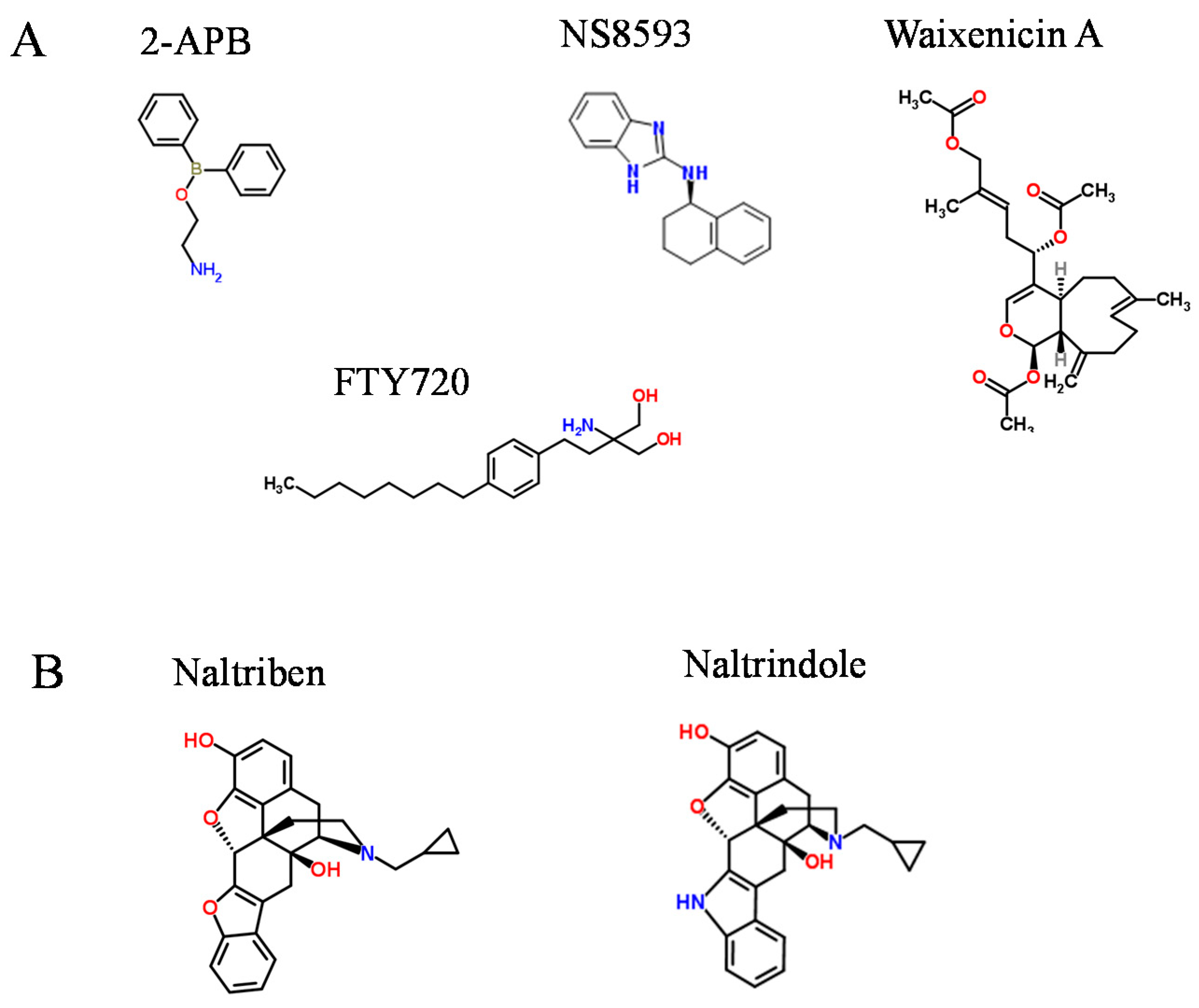
3. Drug-Like Compounds Acting as Activators of the TRPM7 Channel
| Compound | EC50 (μM) | Description of the Effect |
|---|---|---|
| Naltriben | 20.7 | Reversible, [Mg2+]i independent |
| Clozapine | n.d | Tested only at 30–50 μM |
| Proadifen | n.d | Tested only at 30–50 μM |
| Doxepin | n.d | Tested only at 30–50 μM |
| A3 hydrochloride | n.d | Tested only at 30–50 μM |
| Mibefradil | n.d | Tested only at 30–50 μM |
| U-73343 | n.d | Tested only at 30–50 μM |
| CGP-74514A | n.d | Tested only at 30–50 μM |
| Metergoline | n.d | Tested only at 30–50 μM |
| L-733,060 | n.d | Tested only at 30–50 μM |
| A-77636 | n.d | Tested only at 30–50 μM |
| ST-148 | n.d | Tested only at 30–50 μM |
| Clemastine | n.d | Tested only at 30–50 μM |
| Desipramine | n.d | Tested only at 30–50 μM |
| Sertraline | n.d | Tested only at 30–50 μM |
| Methiothepin | n.d | Tested only at 30–50 μM |
| NNC 55–0396 | n.d | Tested only at 30–50 μM |
| Prochlorperazine | n.d | Tested only at 30–50 μM |
| Nortriptyline | n.d | Tested only at 30–50 μM |
| Loperamide | n.d | Tested only at 30–50 μM |
4. Conclusions/Outlook
Acknowledgments
Author contributions
Conflicts of Interest
References
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Ryazanov, A.G.; Pavur, K.S.; Dorovkov, M.V. Alpha-kinases: A new class of protein kinases with a novel catalytic domain. Curr. Biol. 1999, 9, R43–R45. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Matsushita, M.; Nairn, A.C.; Kuriyan, J. Crystal structure of the atypical protein kinase domain of a trp channel with phosphotransferase activity. Mol. Cell 2001, 7, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Fleig, A.; Chubanov, V. TRPM7. Handb. Exp. Pharmacol. 2014, 222, 521–546. [Google Scholar] [PubMed]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7—gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Nejsum, L.N.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef]
- Walder, R.Y.; Landau, D.; Meyer, P.; Shalev, H.; Tsolia, M.; Borochowitz, Z.; Boettger, M.B.; Beck, G.E.; Englehardt, R.K.; Carmi, R.; et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002, 31, 171–174. [Google Scholar] [CrossRef]
- Chubanov, V.; Waldegger, S.; y Schnitzler, M.M.; Vitzthum, H.; Sassen, M.C.; Seyberth, H.W.; Konrad, M.; Gudermann, T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 2004, 101, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Ryazanov, A.G. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 2002, 514, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Gudermann, T. TRPM6. Handb. Exp. Pharmacol. 2014, 222, 503–520. [Google Scholar] [PubMed]
- Penner, R.; Fleig, A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb. Exp. Pharmacol. 2007, 313–328. [Google Scholar]
- Paravicini, T.M.; Chubanov, V.; Gudermann, T. TRPM7: A unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 2012, 44, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W. TRPM6 and TRPM7: A Mul-TRP-PLIK-cation of channel functions. Curr. Pharm. Biotechnol. 2010, 12, 42–53. [Google Scholar] [CrossRef]
- Bates-Withers, C.; Sah, R.; Clapham, D.E. TRPM7, the Mg2+ inhibited channel and kinase. Adv. Exp. Med. Biol. 2011, 704, 173–183. [Google Scholar] [PubMed]
- Mederos y Schnitzler, M.; Waring, J.; Gudermann, T.; Chubanov, V. Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J. 2008, 22, 1540–1551. [Google Scholar]
- Chubanov, V.; Schlingmann, K.P.; Waring, J.; Heinzinger, J.; Kaske, S.; Waldegger, S.; Mederos y Schnitzler, M.; Gudermann, T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 2007, 282, 7656–7667. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, J.; Jiang, J.; Ratzan, W.; Su, L.T.; Runnels, L.W.; Yue, L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 2007, 282, 25817–25830. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Chubanov, V.; Gudermann, T.; Montell, C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr. Biol. 2003, 13, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Kaske, S.; Krasteva, G.; Konig, P.; Kummer, W.; Hofmann, T.; Gudermann, T.; Chubanov, V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schafer, S.; Linseisen, M.; Sytik, L.; Gudermann, T.; Chubanov, V. Activation of TRPM7 channels by small molecules under physiological conditions. Pflugers. Arch. 2014. [Google Scholar]
- Xie, J.; Sun, B.; Du, J.; Yang, W.; Chen, H.C.; Overton, J.D.; Runnels, L.W.; Yue, L. Phosphatidylinositol 4,5-bisphosphate (pip(2)) controls magnesium gatekeeper TRPM6 activity. Sci. Rep. 2011, 1, 146. [Google Scholar] [CrossRef] [PubMed]
- Kaitsuka, T.; Katagiri, C.; Beesetty, P.; Nakamura, K.; Hourani, S.; Tomizawa, K.; Kozak, J.A.; Matsushita, M. Inactivation of TRPM7 kinase activity does not impair its channel function in mice. Sci. Rep. 2014, 4, 5718. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Perraud, A.L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef]
- Sahni, J.; Scharenberg, A.M. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008, 8, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Su, L.T.; Agapito, M.A.; Li, M.; Simonson, W.T.; Huttenlocher, A.; Habas, R.; Yue, L.; Runnels, L.W. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J. Biol. Chem. 2006, 281, 11260–11270. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, X.; Chen, M.; Ouyang, K.; Song, L.S.; Cheng, H. Calcium flickers steer cell migration. Nature 2009, 457, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Langeslag, M.; van Leeuwen, B.; Ran, L.; Ryazanov, A.G.; Figdor, C.G.; Moolenaar, W.H.; Jalink, K.; van Leeuwen, F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006, 25, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cai, C.; Wu, J.; Cai, S.; Ye, C.; Chen, H.; Yang, Z.; Zeng, H.; Shen, Q.; Zou, F. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013, 333, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.A.; Lively, S.; Vincent, C.; Schlichter, L.C. Regulation of podosome formation, microglial migration and invasion by Ca2+-signaling molecules expressed in podosomes. J. Neuroinflam. 2012, 9, 250. [Google Scholar] [CrossRef]
- Kuras, Z.; Yun, Y.H.; Chimote, A.A.; Neumeier, L.; Conforti, L. KCA3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells. PLoS One 2012, 7, e43859. [Google Scholar] [CrossRef] [PubMed]
- Su, L.T.; Liu, W.; Chen, H.C.; Gonzalez-Pagan, O.; Habas, R.; Runnels, L.W. TRPM7 regulates polarized cell movements. Biochem. J. 2011, 434, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Luan, Y.; You, C.X.; Chen, X.H.; Luo, R.C.; Li, R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca2+ influx. Cell Calcium 2010, 47, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Xu, X.H.; Liu, Y.; Hu, Y.; Jin, M.W.; Li, G.R. TRPM7 channels regulate proliferation and adipogenesis in 3T3-L1 preadipocytes. J. Cell. Physiol. 2013, 229, 60–67. [Google Scholar]
- Zhang, Z.; Wang, M.; Fan, X.H.; Chen, J.H.; Guan, Y.Y.; Tang, Y.B. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ. Res. 2012, 111, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Martineau, C.; Moreau, R. Role of melastatin transient receptor potential 7 channels in the osteoblastic differentiation of murine MC3T3 cells. Calcif Tissue Int. 2011, 88, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Shimizu, T.; Okada, Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell. Physiol. Biochem. 2007, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oancea, E.; Wolfe, J.T.; Clapham, D.E. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006, 98, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Brauchi, S.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 8304–8308. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Iihara, K.; Wei, W.L.; Xiong, Z.G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: Implications in hypertension. Am J. Physiol. Heart Circ. Physiol. 2008, 294, H1103–H1118. [Google Scholar] [CrossRef] [PubMed]
- Hermosura, M.C.; Nayakanti, H.; Dorovkov, M.V.; Calderon, F.R.; Ryazanov, A.G.; Haymer, D.S.; Garruto, R.M. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 11510–11515. [Google Scholar] [CrossRef] [PubMed]
- Tseveleki, V.; Rubio, R.; Vamvakas, S.S.; White, J.; Taoufik, E.; Petit, E.; Quackenbush, J.; Probert, L. Comparative gene expression analysis in mouse models for multiple sclerosis, alzheimer’s disease and stroke for identifying commonly regulated and disease-specific gene changes. Genomics 2010, 96, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Haren, N.; Sevestre, H.; Ouadid-Ahidouch, H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell Physiol. 2009, 297, C493–C502. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, E.J.; Lee, J.H.; Jeon, J.H.; Kim, S.J.; So, I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008, 99, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, M.H.; Inoue, K.; Chu, X.P.; Seeds, J.; Xiong, Z.G. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: Role in cell proliferation. Cancer Res. 2007, 67, 10929–10938. [Google Scholar] [CrossRef] [PubMed]
- Hanano, T.; Hara, Y.; Shi, J.; Morita, H.; Umebayashi, C.; Mori, E.; Sumimoto, H.; Ito, Y.; Mori, Y.; Inoue, R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J. Pharmacol. Sci. 2004, 95, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Middelbeek, J.; Kuipers, A.J.; Henneman, L.; Visser, D.; Eidhof, I.; van Horssen, R.; Wieringa, B.; Canisius, S.V.; Zwart, W.; Wessels, L.F.; et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012, 72, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 2012, 131, E851–E861. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, Y.T.; Chiu, W.T.; Shen, M.R. Remodeling of calcium signaling in tumor progression. J. Biomed. Sci. 2013, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, X.; Du, X.; Guan, B.; Liu, Y.; Zhang, H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 2011, 50, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Arking, D.E.; Pulit, S.L.; Crotti, L.; van der Harst, P.; Munroe, P.B.; Koopmann, T.T.; Sotoodehnia, N.; Rossin, E.J.; Morley, M.; Wang, X.; et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat. Genet. 2014, 46, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of TRPM7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, M.R.; Arduini, B.L.; Paulsen, J.; MacDonald, E.L.; Sabel, J.L.; Henion, P.D.; Cornell, R.A.; Parichy, D.M. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for TRPM7. Curr. Biol. 2005, 15, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wu, L.J.; Jun, J.; Cheng, X.; Xu, H.; Andrews, N.C.; Clapham, D.E. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA 2012, 109, E225–E233. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Mesirca, P.; Van den Boogert, M.; Rosen, J.; Mably, J.; Mangoni, M.E.; Clapham, D.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. USA 2013, 110, E3037–E3046. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Mesirca, P.; Mason, X.; Gibson, W.; Bates-Withers, C.; Van den Boogert, M.; Chaudhuri, D.; Pu, W.T.; Mangoni, M.E.; Clapham, D.E. Timing of myocardial TRPM7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 2013, 128, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Dorovkov, M.V.; Ryazanov, A.G. Phosphorylation of annexin I by TRPM7 channel-kinase. J. Biol. Chem. 2004, 279, 50643–50646. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Middelbeek, J.; Lasonder, E.; Dulyaninova, N.G.; Morrice, N.A.; Ryazanov, A.G.; Bresnick, A.R.; Figdor, C.G.; van Leeuwen, F.N. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 2008, 378, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Perraud, A.L.; Zhao, X.; Ryazanov, A.G.; Schmitz, C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor EEF2 via EEF2-K. Cell Signal 2011, 23, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Deason-Towne, F.; Perraud, A.L.; Schmitz, C. Identification of ser/thr phosphorylation sites in the C2-domain of phospholipase c gamma2 (plcgamma2) using TRPM7-kinase. Cell Signal 2012, 24, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.G.; Lasonder, E.; van Leeuwen, F.N. Massive autophosphorylation of the ser/thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One 2008, 3, e1876. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Kozak, J.A.; Shimizu, Y.; McLachlin, D.T.; Yamaguchi, H.; Wei, F.Y.; Tomizawa, K.; Matsui, H.; Chait, B.T.; Cahalan, M.D.; et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/CHAK1. J. Biol. Chem. 2005, 280, 20793–20803. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.N.; Krapivinsky, G.; Navarro, B.; Krapivinsky, L.; Carter, B.C.; Febvay, S.; Delling, M.; Penumaka, A.; Ramsey, I.S.; Manasian, Y.; et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in fas-induced apoptosis. Dev. Cell 2012, 22, 1149–1162. [Google Scholar]
- Krapivinsky, G.; Krapivinsky, L.; Manasian, Y.; Clapham, D.E. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 2014, 157, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Demeuse, P.; Penner, R.; Fleig, A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 2006, 127, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Deason, F.; Perraud, A.L. Molecular components of vertebrate mg2+-homeostasis regulation. Magnes. Res. 2007, 20, 6–18. [Google Scholar] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by pip(2) hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [PubMed]
- Kozak, J.A.; Matsushita, M.; Nairn, A.C.; Cahalan, M.D. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J. Gen. Physiol. 2005, 126, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Separation and characterization of currents through store-operated crac channels and Mg2+-Inhibited Cation (MIC) channels. J. Gen. Physiol. 2002, 119, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, J.; Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.A.; Kerschbaum, H.H.; Cahalan, M.D. Distinct properties of crac and mic channels in RBL cells. J. Gen. Physiol. 2002, 120, 221–235. [Google Scholar] [PubMed]
- Chen, X.; Numata, T.; Li, M.; Mori, Y.; Orser, B.A.; Jackson, M.F.; Xiong, Z.G.; MacDonald, J.F. The modulation of TRPM7 currents by nafamostat mesilate depends directly upon extracellular concentrations of divalent cations. Mol. Brain 2010, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Parnas, M.; Peters, M.; Dadon, D.; Lev, S.; Vertkin, I.; Slutsky, I.; Minke, B. Carvacrol is a novel inhibitor of drosophila trpl and mammalian TRPM7 channels. Cell Calcium 2009, 45, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Xie, J.; Zhang, Z.; Su, L.T.; Yue, L.; Runnels, L.W. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One 2010, 5, e11161. [Google Scholar]
- Zierler, S.; Yao, G.; Zhang, Z.; Kuo, W.C.; Porzgen, P.; Penner, R.; Horgen, F.D.; Fleig, A. Waixenicin a inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J. Biol. Chem. 2011, 286, 39328–39335. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; y Schnitzler, M.M.; Meissner, M.; Schafer, S.; Abstiens, K.; Hofmann, T.; Gudermann, T. Natural and synthetic modulators of SK (k(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 2012, 166, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yue, Z.; Sun, B.; Yang, W.; Xie, J.; Ni, E.; Feng, Y.; Mahmood, R.; Zhang, Y.; Yue, L. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br. J. Pharmacol. 2013, 168, 1294–1312. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.; Fruasaha, P.; Kozak, J.A. 2-aminoethyl diphenyl borinate (2-apb) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels (Austin) 2012, 6, 362–369. [Google Scholar] [CrossRef]
- Davis, F.M.; Azimi, I.; Faville, R.A.; Peters, A.A.; Jalink, K.; Putney, J.W., Jr.; Goodhill, G.J.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 2014, 33, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.; Lively, S.; Ferreira, R.; Wong, R.; Schlichter, L.C. Expression and contributions of TRPM7 and KCA2.3/SK3 channels to the increased migration and invasion of microglia in anti-inflammatory activation states. PLoS One 2014, 9, e106087. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Miralles, F.; Eder, C. TRPM7 channels regulate proliferation and polarisation of macrophages. J. Cell Sci. 2014. [Google Scholar]
- Kim, B.J.; Nam, J.H.; Kwon, Y.K.; So, I.; Kim, S.J. The role of waixenicin a as transient receptor potential melastatin 7 blocker. Basic Clin. Pharmacol. Toxicol. 2013, 112, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Visser, D.; Langeslag, M.; Kedziora, K.M.; Klarenbeek, J.; Kamermans, A.; Horgen, F.D.; Fleig, A.; van Leeuwen, F.N.; Jalink, K. TRPM7 triggers Ca2+ sparks and invadosome formation in neuroblastoma cells. Cell Calcium 2013, 54, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Kim, W.K.; Kim, B.J. Sphingosine and FTY720 modulate pacemaking activity in interstitial cells of cajal from mouse small intestine. Mol. Cells 2013, 36, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sofuoglu, M.; Portoghese, P.S.; Takemori, A.E. Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: Evidence for delta opioid receptor subtypes. J. Pharmacol. Exp. Ther. 1991, 257, 676–680. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chubanov, V.; Schäfer, S.; Ferioli, S.; Gudermann, T. Natural and Synthetic Modulators of the TRPM7 Channel. Cells 2014, 3, 1089-1101. https://doi.org/10.3390/cells3041089
Chubanov V, Schäfer S, Ferioli S, Gudermann T. Natural and Synthetic Modulators of the TRPM7 Channel. Cells. 2014; 3(4):1089-1101. https://doi.org/10.3390/cells3041089
Chicago/Turabian StyleChubanov, Vladimir, Sebastian Schäfer, Silvia Ferioli, and Thomas Gudermann. 2014. "Natural and Synthetic Modulators of the TRPM7 Channel" Cells 3, no. 4: 1089-1101. https://doi.org/10.3390/cells3041089
APA StyleChubanov, V., Schäfer, S., Ferioli, S., & Gudermann, T. (2014). Natural and Synthetic Modulators of the TRPM7 Channel. Cells, 3(4), 1089-1101. https://doi.org/10.3390/cells3041089






