Flotillins in Receptor Tyrosine Kinase Signaling and Cancer
Abstract
:1. Introduction
1.1. Receptor Tyrosine Kinase Signaling
1.2. The Flotillin Protein Family
2. Flotillins in Receptor Tyrosine Kinase and MAP Kinase Signaling
2.1. Cell Culture Studies
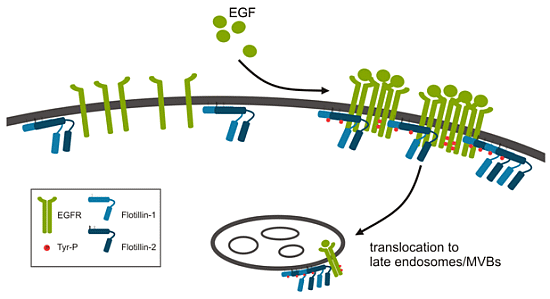
2.1.1. Role of Flotillins in EGFR Signaling

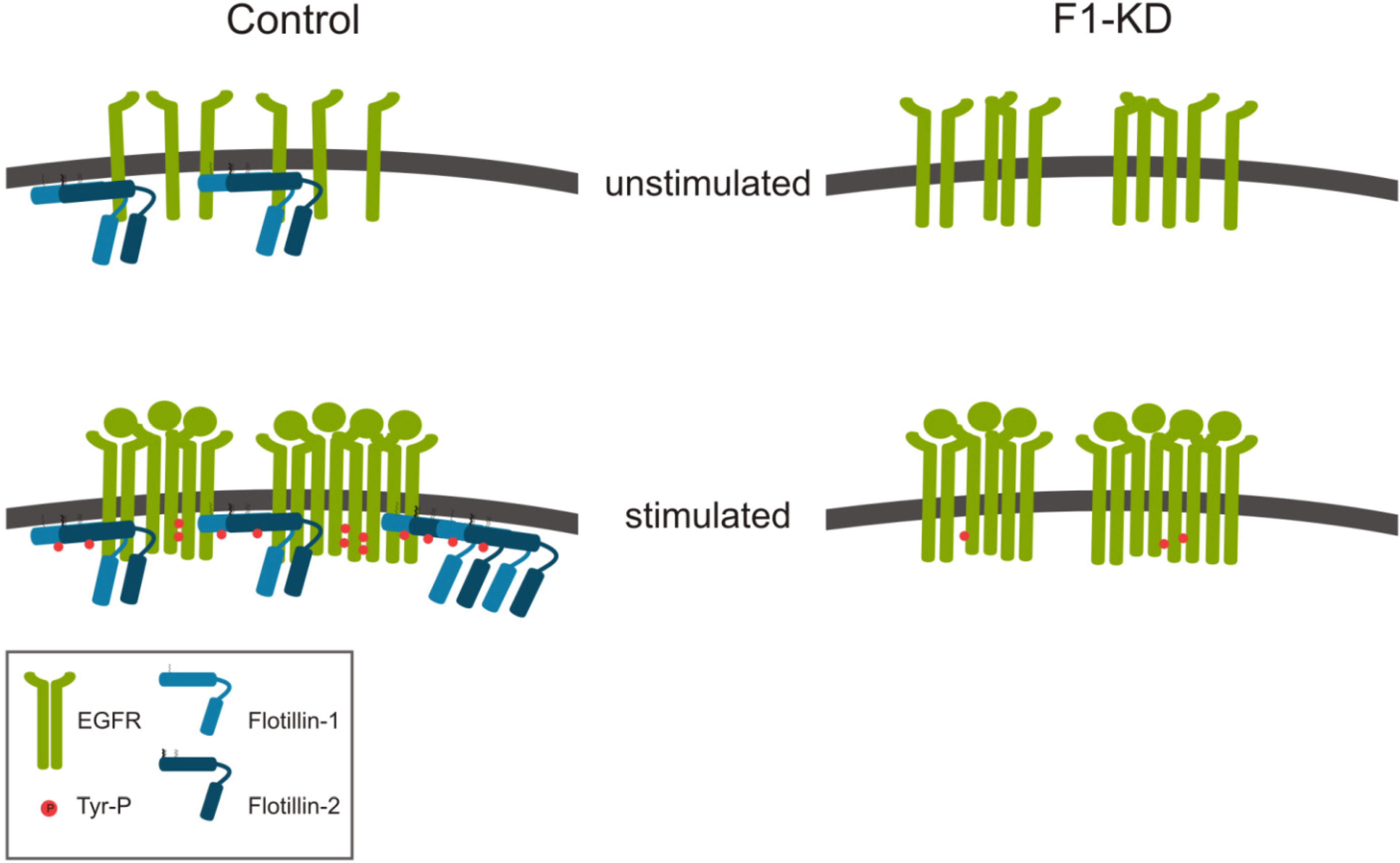
2.1.2. Flotillins and FGFR Signaling
2.1.3. Flotillins and Neurotrophin Receptor Signaling
2.1.4. Function of Flotillins in Insulin Signaling
2.1.5. Flotillins and Other Signaling Pathways
2.2. Animal Models
2.2.1. Flotillin-1 Knockout Mice
2.2.2. Flotillin-2 Knockout Mice
3. Flotillins in Cancer and Metastasis Formation
| Cancer Type | Patient Phenotype |
|---|---|
| Lung adeno-carcinoma | Expression correlates with advanced clinical stage, lymph node metastasis; decreased overall survival time |
| Esophageal squamous cell carcinoma | Expression correlates with clinical stage and NF-κB activity; decreased overall survival time |
| Breast cancer | Expression correlates with clinical stage and poor patient survival |
| Hepatocellular carcinoma | Expression correlates with tumor size, clinical stage, CLIP stage, vascular invasion, relapse and serum AFP; decreased overall survival time |
| Gastric cancer | Expression correlates with histological type, Lauren grade, ErbB2 expression, lymphovascular invasion, lymph node metastasis and T-stage; decreased overall survival time |
| Breast cancer | Expression correlates with clinical stage, T classification, M classification, histological differentiation, ErbB2 expression and poor patient survival |
| Melanoma | Expression correlates with melanoma progression, lymph node metastases and Breslow depth |
3.1. Flotillins in Breast Cancer
3.2. Flotillins in Melanoma and Metastasis Formation
4. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Banning, A.; Tomasovic, A.; Tikkanen, R. Functional aspects of membrane association of reggie/flotillin proteins. Curr. Protein Pept. Sci. 2011, 12, 725–735. [Google Scholar] [CrossRef]
- Kurrle, N.; John, B.; Meister, M.; Tikkanen, R. Function of Flotillins in Receptor Tyrosine Kinase Signaling and Endocytosis: Role of Tyrosine Phosphorylation and Oligomerization. In Protein Phosphorylation in Human Health; Huang, C., Ed.; InTech Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Ramos, J.W. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol. 2008, 40, 2707–2719. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Schubbert, S.; Shannon, K.; Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 2007, 7, 295–308. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Schulte, T.; Paschke, K.A.; Laessing, U.; Lottspeich, F.; Stuermer, C.A. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 1997, 124, 577–587. [Google Scholar]
- Bickel, P.E.; Scherer, P.E.; Schnitzer, J.E.; Oh, P.; Lisanti, M.P.; Lodish, H.F. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 1997, 272, 13793–13802. [Google Scholar]
- Edgar, A.J.; Polak, J.M. Flotillin-1: gene structure: cDNA cloning from human lung and the identification of alternative polyadenylation signals. Int. J. Biochem. Cell Biol. 2001, 33, 53–64. [Google Scholar] [CrossRef]
- Rivera-Milla, E.; Stuermer, C.A.; Malaga-Trillo, E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell. Mol. Life Sci 2006, 63, 343–357. [Google Scholar] [CrossRef]
- Malaga-Trillo, E.; Laessing, U.; Lang, D.M.; Meyer, A.; Stuermer, C.A. Evolution of duplicated reggie genes in zebrafish and goldfish. J. Mol. Evol. 2002, 54, 235–245. [Google Scholar] [CrossRef]
- Browman, D.T.; Hoegg, M.B.; Robbins, S.M. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007, 17, 394–402. [Google Scholar] [CrossRef]
- Tavernarakis, N.; Driscoll, M.; Kyrpides, N.C. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem. Sci. 1999, 24, 425–427. [Google Scholar] [CrossRef]
- Salzer, U.; Prohaska, R. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 2001, 97, 1141–1143. [Google Scholar] [CrossRef]
- Snyers, L.; Umlauf, E.; Prohaska, R. Oligomeric nature of the integral membrane protein stomatin. J. Biol. Chem. 1998, 273, 17221–17226. [Google Scholar] [CrossRef]
- Huber, T.B.; Simons, M.; Hartleben, B.; Sernetz, L.; Schmidts, M.; Gundlach, E.; Saleem, M.A.; Walz, G.; Benzing, T. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 2003, 12, 3397–3405. [Google Scholar] [CrossRef]
- Tatsuta, T.; Model, K.; Langer, T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol. Biol. Cell 2005, 16, 248–259. [Google Scholar] [CrossRef]
- Babuke, T.; Ruonala, M.; Meister, M.; Amaddii, M.; Genzler, C.; Esposito, A.; Tikkanen, R. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell. Signalling 2009, 21, 1287–1297. [Google Scholar] [CrossRef]
- Solis, G.P.; Hoegg, M.; Munderloh, C.; Schrock, Y.; Malaga-Trillo, E.; Rivera-Milla, E.; Stuermer, C.A. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem. J. 2007, 403, 313–322. [Google Scholar] [CrossRef]
- Neumann-Giesen, C.; Falkenbach, B.; Beicht, P.; Claasen, S.; Luers, G.; Stuermer, C.A.; Herzog, V.; Tikkanen, R. Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 2004, 378, 509–518. [Google Scholar] [CrossRef]
- Amaddii, M.; Meister, M.; Banning, A.; Tomasovic, A.; Mooz, J.; Rajalingam, K.; Tikkanen, R. Flotillin-1/reggie-2 plays a dual role in the activation of receptor tyrosine kinase/map kinase signaling. J. Biol. Chem. 2012, 287, 7265–7278. [Google Scholar]
- Banning, A.; Regenbrecht, C.R.; Tikkanen, R. Increased activity of mitogen activated protein kinase pathway in flotillin-2 knockout mouse model. Cell. Signalling 2014, 26, 198–207. [Google Scholar]
- Volonte, D.; Galbiati, F.; Li, S.; Nishiyama, K.; Okamoto, T.; Lisanti, M.P. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J. Biol. Chem. 1999, 274, 12702–12709. [Google Scholar]
- Fernow, I.; Icking, A.; Tikkanen, R. Reggie-1 and reggie-2 localize in non-caveolar rafts in epithelial cells: cellular localization is not dependent on the expression of caveolin proteins. Eur. J. Cell Biol. 2007, 86, 345–352. [Google Scholar] [CrossRef]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54. [Google Scholar] [CrossRef]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Morrow, I.C.; Rea, S.; Martin, S.; Prior, I.A.; Prohaska, R.; Hancock, J.F.; James, D.E.; Parton, R.G. Flotillin-1/reggie-2 traffics to surface raft domains via a novel golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J. Biol. Chem. 2002, 277, 48834–48841. [Google Scholar] [CrossRef]
- Liu, J.; Deyoung, S.M.; Zhang, M.; Dold, L.H.; Saltiel, A.R. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 2005, 280, 16125–16134. [Google Scholar] [CrossRef]
- Neumann-Giesen, C.; Fernow, I.; Amaddii, M.; Tikkanen, R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J. Cell Sci. 2007, 120, 395–406. [Google Scholar] [CrossRef]
- Riento, K.; Frick, M.; Schafer, I.; Nichols, B.J. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J. Cell Sci. 2009, 122, 912–918. [Google Scholar] [CrossRef]
- Banning, A.; Ockenga, W.; Finger, F.; Siebrasse, P.; Tikkanen, R. Transcriptional regulation of flotillins by the extracellularly regulated kinases and retinoid X receptor complexes. PLoS one 2012, 7, e45514. [Google Scholar]
- Stuermer, C.A.; Lang, D.M.; Kirsch, F.; Wiechers, M.; Deininger, S.O.; Plattner, H. Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol. Biol. Cell 2001, 12, 3031–3045. [Google Scholar] [CrossRef]
- de Gassart, A.; Geminard, C.; Fevrier, B.; Raposo, G.; Vidal, M. Lipid raft-associated protein sorting in exosomes. Blood 2003, 102, 4336–4344. [Google Scholar] [CrossRef]
- Strauss, K.; Goebel, C.; Runz, H.; Mobius, W.; Weiss, S.; Feussner, I.; Simons, M.; Schneider, A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 2010, 285, 26279–26288. [Google Scholar]
- Gkantiragas, I.; Brugger, B.; Stuven, E.; Kaloyanova, D.; Li, X.Y.; Lohr, K.; Lottspeich, F.; Wieland, F.T.; Helms, J.B. Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell 2001, 12, 1819–1833. [Google Scholar] [CrossRef]
- Santamaria, A.; Fernandez, P.L.; Farre, X.; Benedit, P.; Reventos, J.; Morote, J.; Paciucci, R.; Thomson, T.M. PTOV-1, a novel protein overexpressed in prostate cancer, shuttles between the cytoplasm and the nucleus and promotes entry into the S phase of the cell division cycle. Am. J. Pathol. 2003, 162, 897–905. [Google Scholar] [CrossRef]
- Ha, H.; Kwak, H.B.; Lee, S.K.; Na, D.S.; Rudd, C.E.; Lee, Z.H.; Kim, H.H. Membrane rafts play a crucial role in receptor activator of nuclear factor kappaB signaling and osteoclast function. J. Biol. Chem. 2003, 278, 18573–18580. [Google Scholar]
- Berger, T.; Ueda, T.; Arpaia, E.; Chio, C., II; Shirdel, E.A.; Jurisica, I.; Hamada, K.; You-Ten, A.; Haight, J.; Wakeham, A.; et al. Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene 2013, 32, 4989–4994. [Google Scholar] [CrossRef]
- Ludwig, A.; Otto, G.P.; Riento, K.; Hams, E.; Fallon, P.G.; Nichols, B.J. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J. Cell. Biol. 2010, 191, 771–781. [Google Scholar] [CrossRef]
- Kato, N.; Nakanishi, M.; Hirashima, N. Flotillin-1 regulates IgE receptor-mediated signaling in rat basophilic leukemia (RBL-2H3) cells. J. Immunol. 2006, 177, 147–154. [Google Scholar]
- Solis, G.P.; Schrock, Y.; Hulsbusch, N.; Wiechers, M.; Plattner, H.; Stuermer, C.A. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol. Biol. Cell. 2012, 23, 1812–1825. [Google Scholar] [CrossRef]
- Meister, M.; Tomasovic, A.; Banning, A.; Tikkanen, R. Mitogen-activated protein (MAP) kinase scaffolding proteins: a recount. Int. J. Mol. Sci. 2013, 14, 4854–4884. [Google Scholar] [CrossRef]
- Kurrle, N.; Ockenga, W.; Meister, M.; Vollner, F.; Kuhne, S.; John, B.A.; Banning, A.; Tikkanen, R. Phosphatidylinositol 3-Kinase dependent up-regulation of the epidermal growth factor receptor upon Flotillin-1 depletion in breast cancer cells. BMC Cancer 2013, 13, 575. [Google Scholar] [CrossRef]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef]
- Dixon, S.J.; MacDonald, J.I.; Robinson, K.N.; Kubu, C.J.; Meakin, S.O. Trk receptor binding and neurotrophin/fibroblast growth factor (FGF)-dependent activation of the FGF receptor substrate (FRS)-3. Biochim. Biophys. Acta 2006, 1763, 366–380. [Google Scholar] [CrossRef]
- Hadari, Y.R.; Gotoh, N.; Kouhara, H.; Lax, I.; Schlessinger, J. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 8578–8583. [Google Scholar] [CrossRef]
- Xu, H.; Lee, K.W.; Goldfarb, M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 1998, 273, 17987–17990. [Google Scholar] [CrossRef]
- Gotoh, N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci 2008, 99, 1319–1325. [Google Scholar] [CrossRef]
- Kouhara, H.; Hadari, Y.R.; Spivak-Kroizman, T.; Schilling, J.; Bar-Sagi, D.; Lax, I.; Schlessinger, J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997, 89, 693–702. [Google Scholar] [CrossRef]
- Tomasovic, A.; Traub, S.; Tikkanen, R. Molecular networks in FGF signaling: flotillin-1 and cbl-associated protein compete for the binding to fibroblast growth factor receptor substrate 2. PLoS One 2012, 7, e29739. [Google Scholar] [CrossRef]
- Ong, S.H.; Guy, G.R.; Hadari, Y.R.; Laks, S.; Gotoh, N.; Schlessinger, J.; Lax, I. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 2000, 20, 979–989. [Google Scholar] [CrossRef]
- Limpert, A.S.; Karlo, J.C.; Landreth, G.E. Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein. Mol. Cell. Biol. 2007, 27, 5686–5698. [Google Scholar] [CrossRef]
- Kimura, A.; Baumann, C.A.; Chiang, S.H.; Saltiel, A.R. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 9098–9103. [Google Scholar]
- Melillo, R.M.; Santoro, M.; Ong, S.H.; Billaud, M.; Fusco, A.; Hadari, Y.R.; Schlessinger, J.; Lax, I. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol. Cell Biol. 2001, 21, 4177–4187. [Google Scholar] [CrossRef]
- Degoutin, J.; Vigny, M.; Gouzi, J.Y. ALK activation induces Shc and FRS2 recruitment: Signaling and phenotypic outcomes in PC12 cells differentiation. FEBS Lett. 2007, 581, 727–734. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Ullrich, A. EGFR and FGFR signaling through FRS2 is subject to negative feedback control by ERK1/2. Biol. Chem. 2003, 384, 1215–1226. [Google Scholar]
- Kurokawa, K.; Iwashita, T.; Murakami, H.; Hayashi, H.; Kawai, K.; Takahashi, M. Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction. Oncogene 2001, 20, 1929–1938. [Google Scholar] [CrossRef]
- Stoletov, K.V.; Ratcliffe, K.E.; Terman, B.I. Fibroblast growth factor receptor substrate 2 participates in vascular endothelial growth factor-induced signaling. Faseb J. 2002, 16, 1283–1285. [Google Scholar]
- Baumann, C.A.; Ribon, V.; Kanzaki, M.; Thurmond, D.C.; Mora, S.; Shigematsu, S.; Bickel, P.E.; Pessin, J.E.; Saltiel, A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 2000, 407, 202–207. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Katsanakis, K.D.; Bheda, F.; Pillay, T.S. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J. Biol. Chem. 2004, 279, 21526–21532. [Google Scholar]
- Ribon, V.; Saltiel, A.R. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem. J. 1997, 324, 839–845. [Google Scholar]
- Chiang, S.H.; Baumann, C.A.; Kanzaki, M.; Thurmond, D.C.; Watson, R.T.; Neudauer, C.L.; Macara, I.G.; Pessin, J.E.; Saltiel, A.R. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 2001, 410, 944–948. [Google Scholar] [CrossRef]
- Fecchi, K.; Volonte, D.; Hezel, M.P.; Schmeck, K.; Galbiati, F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J. 2006, 20, 705–707. [Google Scholar]
- Delahaye, L.; Rocchi, S.; Van Obberghen, E. Potential involvement of FRS2 in insulin signaling. Endocrinology 2000, 141, 621–628. [Google Scholar]
- Sugawara, Y.; Nishii, H.; Takahashi, T.; Yamauchi, J.; Mizuno, N.; Tago, K.; Itoh, H. The lipid raft proteins flotillins/reggies interact with Galphaq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell. Signalling 2007, 19, 1301–1308. [Google Scholar] [CrossRef]
- Yamauchi, J.; Nagao, M.; Kaziro, Y.; Itoh, H. Activation of p38 mitogen-activated protein kinase by signaling through G protein-coupled receptors. Involvement of Gbetagamma and Galphaq/11 subunits. J. Biol. Chem. 1997, 272, 27771–27777. [Google Scholar] [CrossRef]
- Rajendran, L.; Beckmann, J.; Magenau, A.; Boneberg, E.M.; Gaus, K.; Viola, A.; Giebel, B.; Illges, H. Flotillins are involved in the polarization of primitive and mature hematopoietic cells. PLoS One 2009, 4, e8290. [Google Scholar] [CrossRef]
- Rossy, J.; Schlicht, D.; Engelhardt, B.; Niggli, V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS One 2009, 4, e5403. [Google Scholar] [CrossRef]
- Affentranger, S.; Martinelli, S.; Hahn, J.; Rossy, J.; Niggli, V. Dynamic reorganization of flotillins in chemokine-stimulated human T-lymphocytes. BMC Cell Biol. 2011, 12, 28. [Google Scholar] [CrossRef]
- Fais, S.; Malorni, W. Leukocyte uropod formation and membrane/cytoskeleton linkage in immune interactions. J. Leukoc. Biol. 2003, 73, 556–563. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, C.J.; Shi, L.; Li, X.H.; Zhou, J.; Song, L.B.; Liao, W.T. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. PLoS One 2013, 8, e64709. [Google Scholar]
- Song, L.; Gong, H.; Lin, C.; Wang, C.; Liu, L.; Wu, J.; Li, M.; Li, J. Flotillin-1 promotes tumor necrosis factor-alpha receptor signaling and activation of NF-kappaB in esophageal squamous cell carcinoma cells. Gastroenterology 2012, 143, 995–1005.e12. [Google Scholar] [CrossRef]
- Lin, C.; Wu, Z.; Lin, X.; Yu, C.; Shi, T.; Zeng, Y.; Wang, X.; Li, J.; Song, L. Knockdown of FLOT1 impairs cell proliferation and tumorigenicity in breast cancer through up-regulation of FOXO3a. Clin. Cancer Res. 2011, 17, 3089–3099. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Sun, Z.; Sun, X.; Wang, Z.; Xu, H. Flotillin2 expression correlates with HER2 levels and poor prognosis in gastric cancer. PLoS One 2013, 8, e62365. [Google Scholar]
- Pust, S.; Klokk, T.I.; Musa, N.; Jenstad, M.; Risberg, B.; Erikstein, B.; Tcatchoff, L.; Liestol, K.; Danielsen, H.E.; van Deurs, B.; Sandvig, K. Flotillins as regulators of ErbB2 levels in breast cancer. Oncogene 2013, 32, 3443–3451. [Google Scholar]
- Wang, X.; Yang, Q.; Guo, L.; Li, X.H.; Zhao, X.H.; Song, L.B.; Lin, H.X. Flotillin-2 is associated with breast cancer progression and poor survival outcomes. J. Transl. Med. 2013, 11, 190. [Google Scholar] [CrossRef]
- Hazarika, P.; McCarty, M.F.; Prieto, V.G.; George, S.; Babu, D.; Koul, D.; Bar-Eli, M.; Duvic, M. Up-regulation of Flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res. 2004, 64, 7361–7369. [Google Scholar] [CrossRef]
- Doherty, S.D.; Prieto, V.G.; George, S.; Hazarika, P.; Duvic, M. High flotillin-2 expression is associated with lymph node metastasis and Breslow depth in melanoma. Melanoma Res. 2006, 16, 461–463. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Citri, A.; Kochupurakkal, B.S.; Yarden, Y. The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle 2004, 3, 51–60. [Google Scholar]
- Citri, A.; Gan, J.; Mosesson, Y.; Vereb, G.; Szollosi, J.; Yarden, Y. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep. 2004, 5, 1165–1170. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; Tang, P. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer 2010, 4, 35–41. [Google Scholar]
- COSMIC catalogue of somatic mutations in cancer. Available online: http://cancer.sanger.ac.uk/cosmic (accessed on 12 December 2013).
- Verschraegen, C.F.; Giovanella, B.C.; Mendoza, J.T.; Kozielski, A.J.; Stehlin, J.S., Jr. Specific organ metastases of human melanoma cells injected into the arterial circulation of nude mice. Anticancer Res. 1991, 11, 529–535. [Google Scholar]
- Chay, C.H.; Cooper, C.R.; Gendernalik, J.D.; Dhanasekaran, S.M.; Chinnaiyan, A.M.; Rubin, M.A.; Schmaier, A.H.; Pienta, K.J. A functional thrombin receptor (PAR1) is expressed on bone-derived prostate cancer cell lines. Urology 2002, 60, 760–765. [Google Scholar] [CrossRef]
- Shi, X.; Gangadharan, B.; Brass, L.F.; Ruf, W.; Mueller, B.M. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol. Cancer Res. 2004, 2, 395–402. [Google Scholar]
- Martinelli, S.; Chen, E.J.; Clarke, F.; Lyck, R.; Affentranger, S.; Burkhardt, J.K.; Niggli, V. Ezrin/Radixin/Moesin proteins and flotillins cooperate to promote uropod formation in T cells. Front. Immunol. 2013, 4, 84. [Google Scholar]
- Li, L.; Luo, J.; Wang, B.; Wang, D.; Xie, X.; Yuan, L.; Guo, J.; Xi, S.; Gao, J.; Lin, X.; et al. Microrna-124 targets flotillin-1 to regulate proliferation and migration in breast cancer. Mol. Cancer 2013, 12, 163. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Banning, A.; Kurrle, N.; Meister, M.; Tikkanen, R. Flotillins in Receptor Tyrosine Kinase Signaling and Cancer. Cells 2014, 3, 129-149. https://doi.org/10.3390/cells3010129
Banning A, Kurrle N, Meister M, Tikkanen R. Flotillins in Receptor Tyrosine Kinase Signaling and Cancer. Cells. 2014; 3(1):129-149. https://doi.org/10.3390/cells3010129
Chicago/Turabian StyleBanning, Antje, Nina Kurrle, Melanie Meister, and Ritva Tikkanen. 2014. "Flotillins in Receptor Tyrosine Kinase Signaling and Cancer" Cells 3, no. 1: 129-149. https://doi.org/10.3390/cells3010129
APA StyleBanning, A., Kurrle, N., Meister, M., & Tikkanen, R. (2014). Flotillins in Receptor Tyrosine Kinase Signaling and Cancer. Cells, 3(1), 129-149. https://doi.org/10.3390/cells3010129