The WISP1/Src/MIF Axis Promotes the Malignant Phenotype of Non-Invasive MCF7 Breast Cancer Cells
Highlights
- WISP1 drives metastatic plasticity in ER+ breast cancer through Src-dependent induction of MIF.
- The WISP1/Src/MIF axis promotes EMT, extracellular matrix remodeling, and invasiveness of breast cancer cells.
- WISP1 regulates hyaluronan metabolism and proteolytic activity reshaping the tumor microenviroment.
- The WISP1/Src/MIF axis may serve as a therapeutic target in ER-positive breast cancer.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Determination of Secreted Hyaluronan Concentration
2.3. Enzyme-Linked Immunosorbent Assays (ELISA)
2.4. Detection of Phosphorylated Src Family Kinases by Capture ELISA
2.5. Immunofluorescence Microscopy
2.6. Western Blotting
2.7. Quantitative Real-Time PCR
2.8. Wound Healing Assay
2.9. MTT Assay
2.10. Transwell Invasion Assay
2.11. Statistical Analysis
3. Results
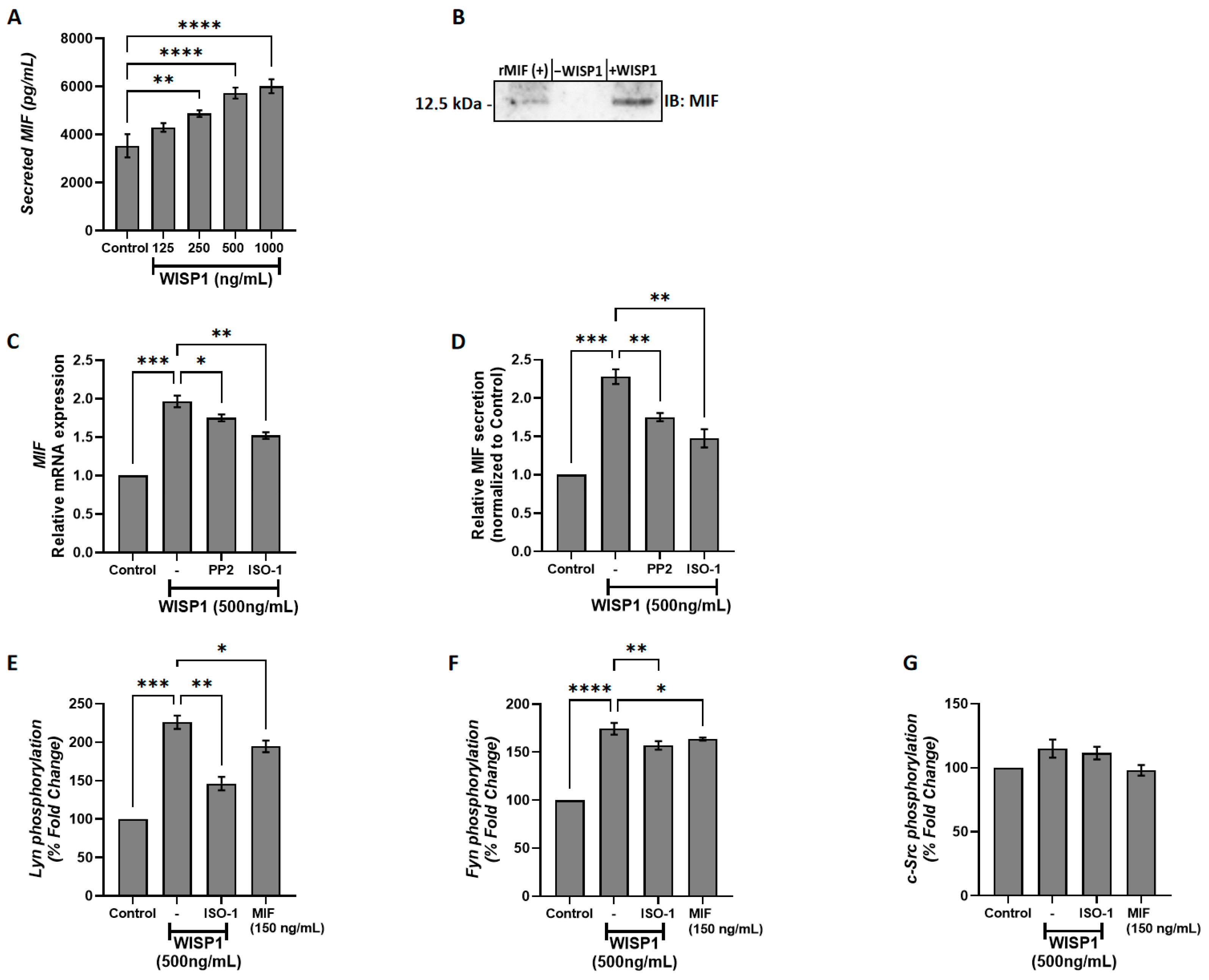
3.1. WISP1 Induces MIF Secretion in MCF7 Breast Cancer Cells Through Src Kinase-Dependent Mechanisms
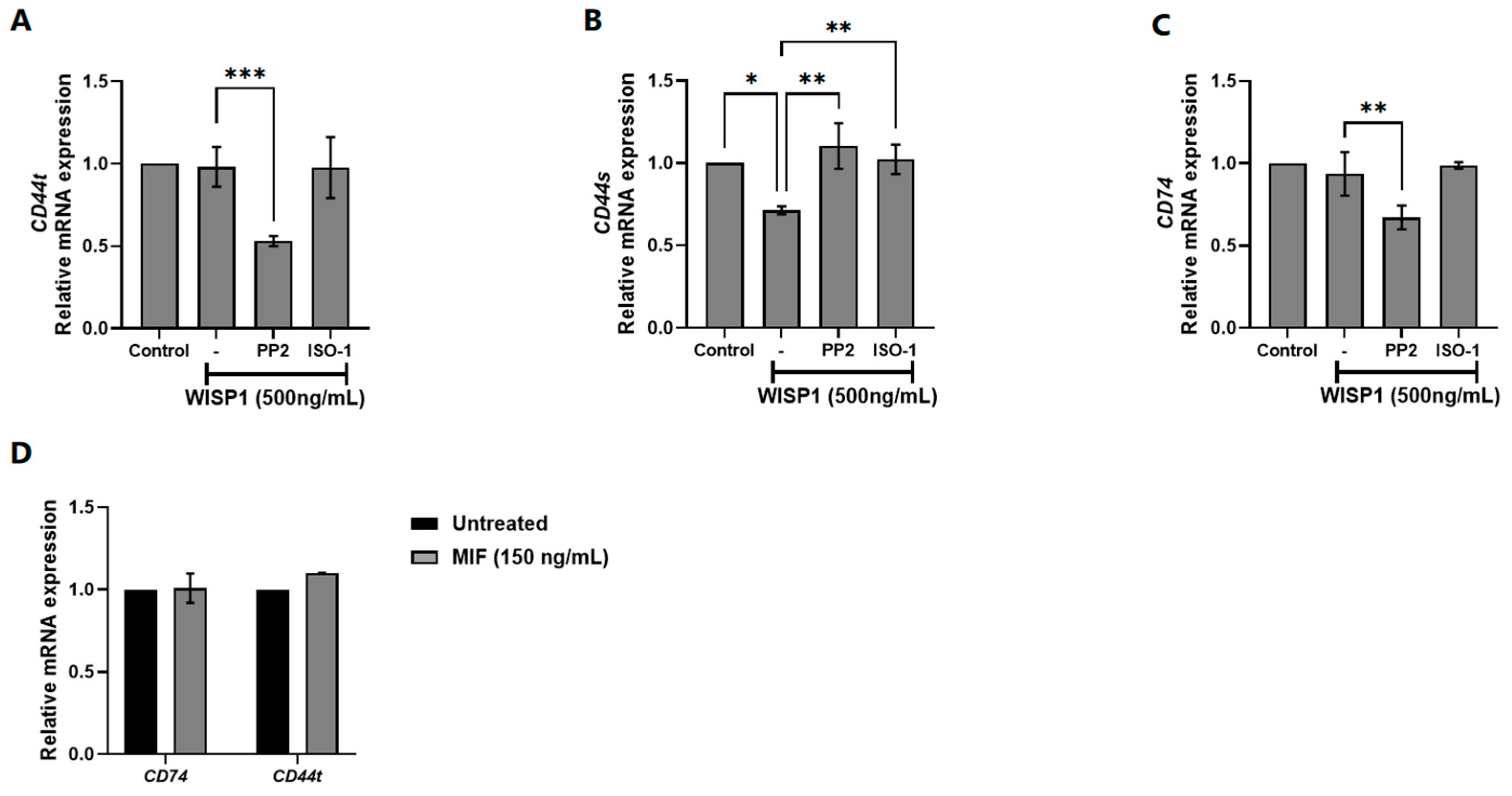
3.2. Implication of Src Kinases and MIF in WISP1-Mediated Changes in CD44/CD74 Receptors Expression
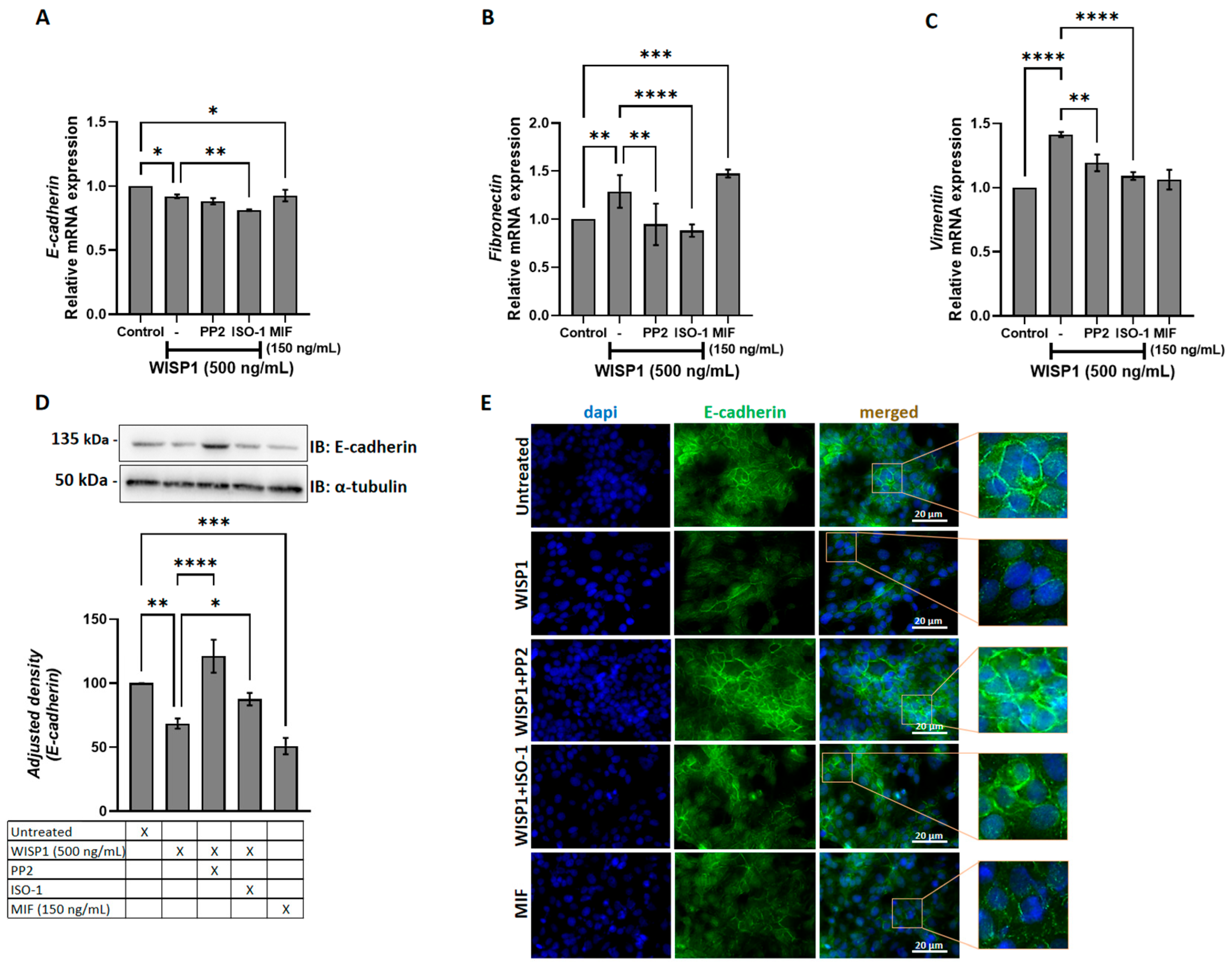
3.3. WISP1 Promotes EMT Features in Breast Cancer Cells Through Src and MIF Activities
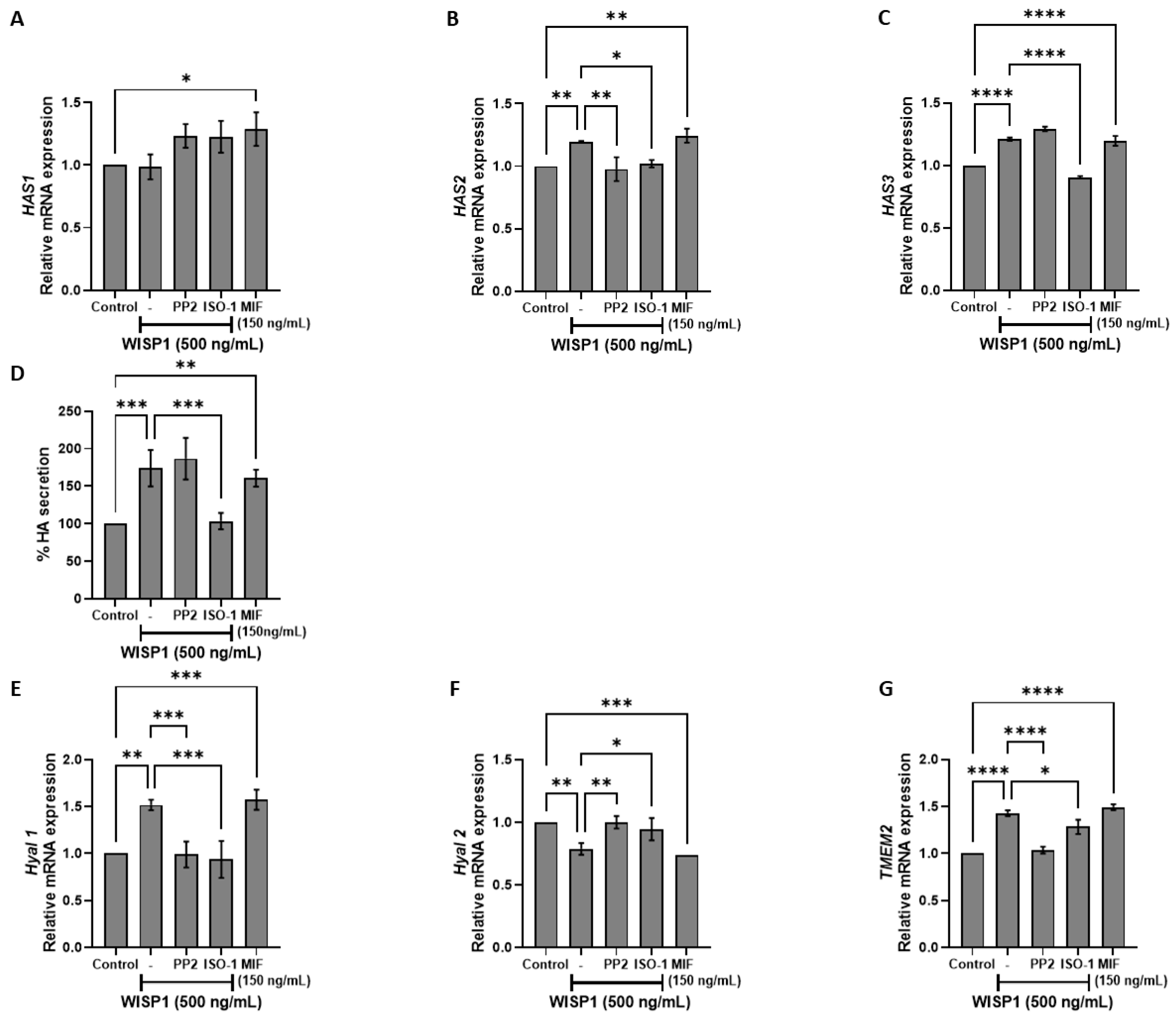
3.4. WISP1/MIF Axis Affects the Oncogenic Hyaluronan Network
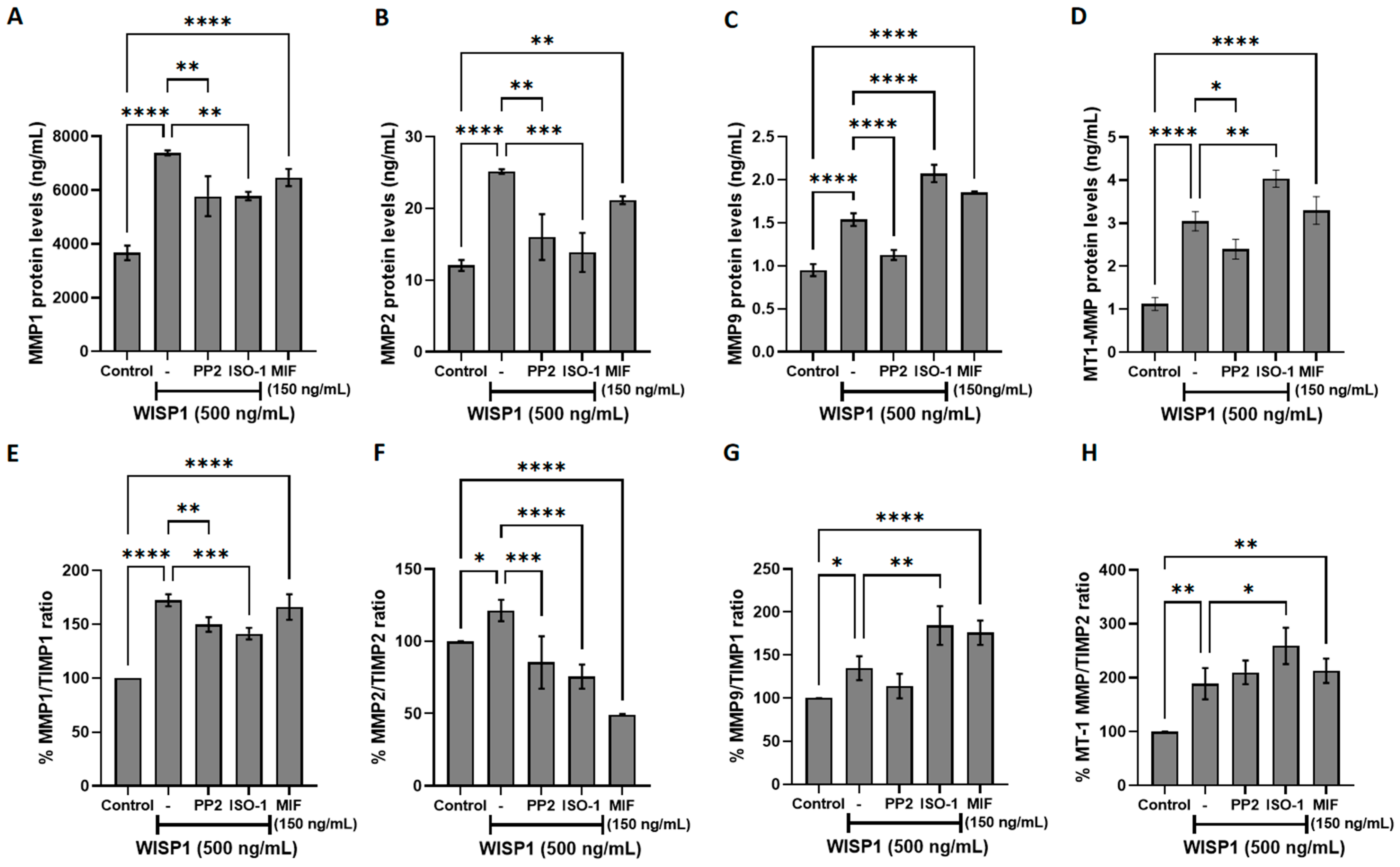
3.5. WISP1/MIF Axis Modulates MMPs/TIMPs and ECM Remodeling
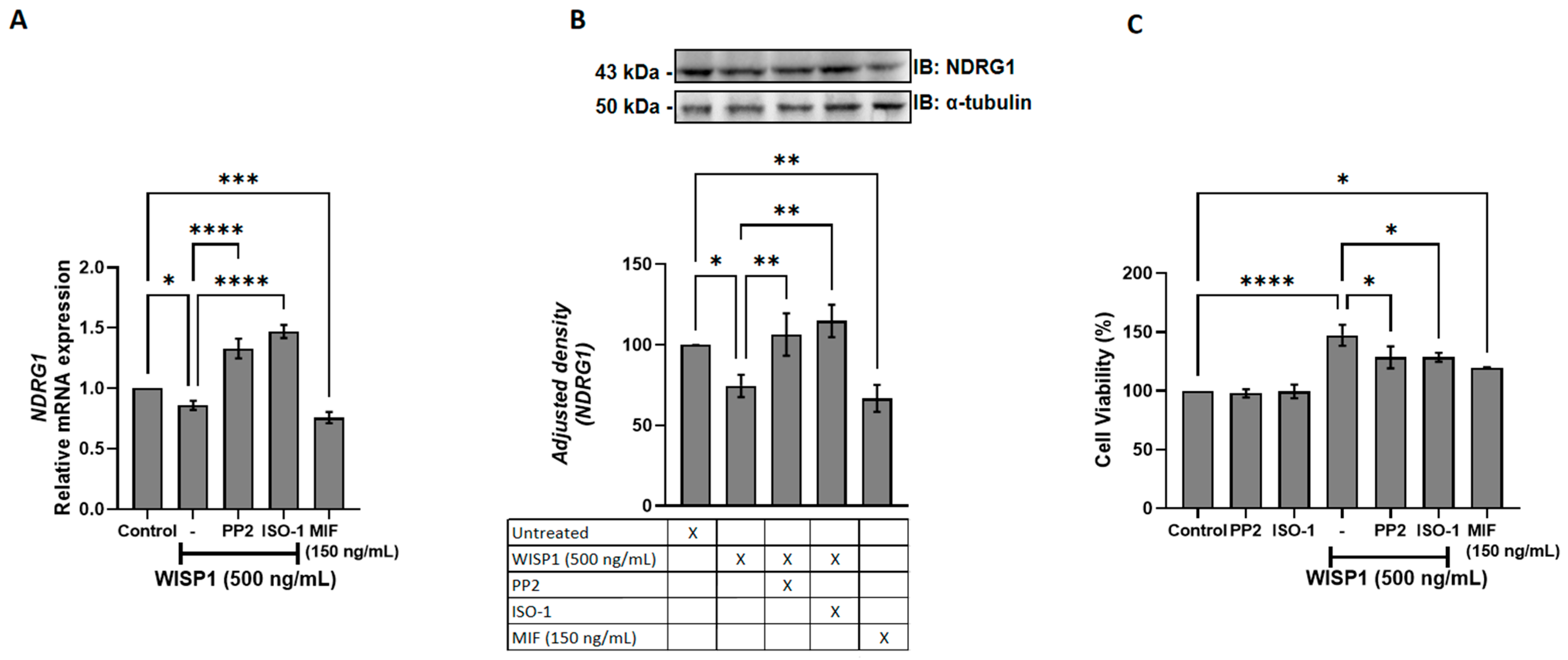
3.6. WISP1 Promotes Breast Cancer Cell Viability via Src Kinases and MIF
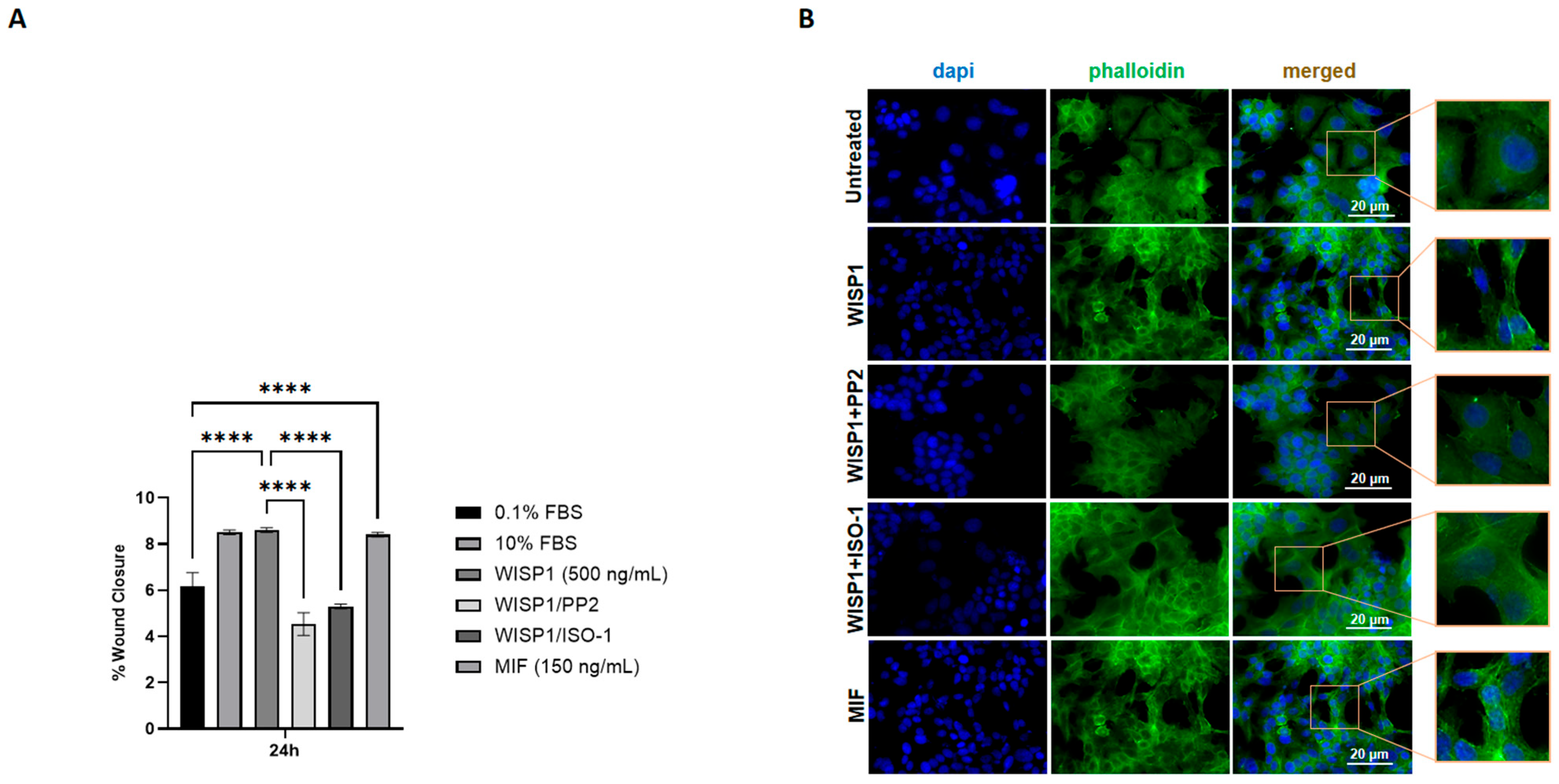
3.7. WISP1 Promotes Breast Cancer Cell Migration Through Src Kinases and MIF
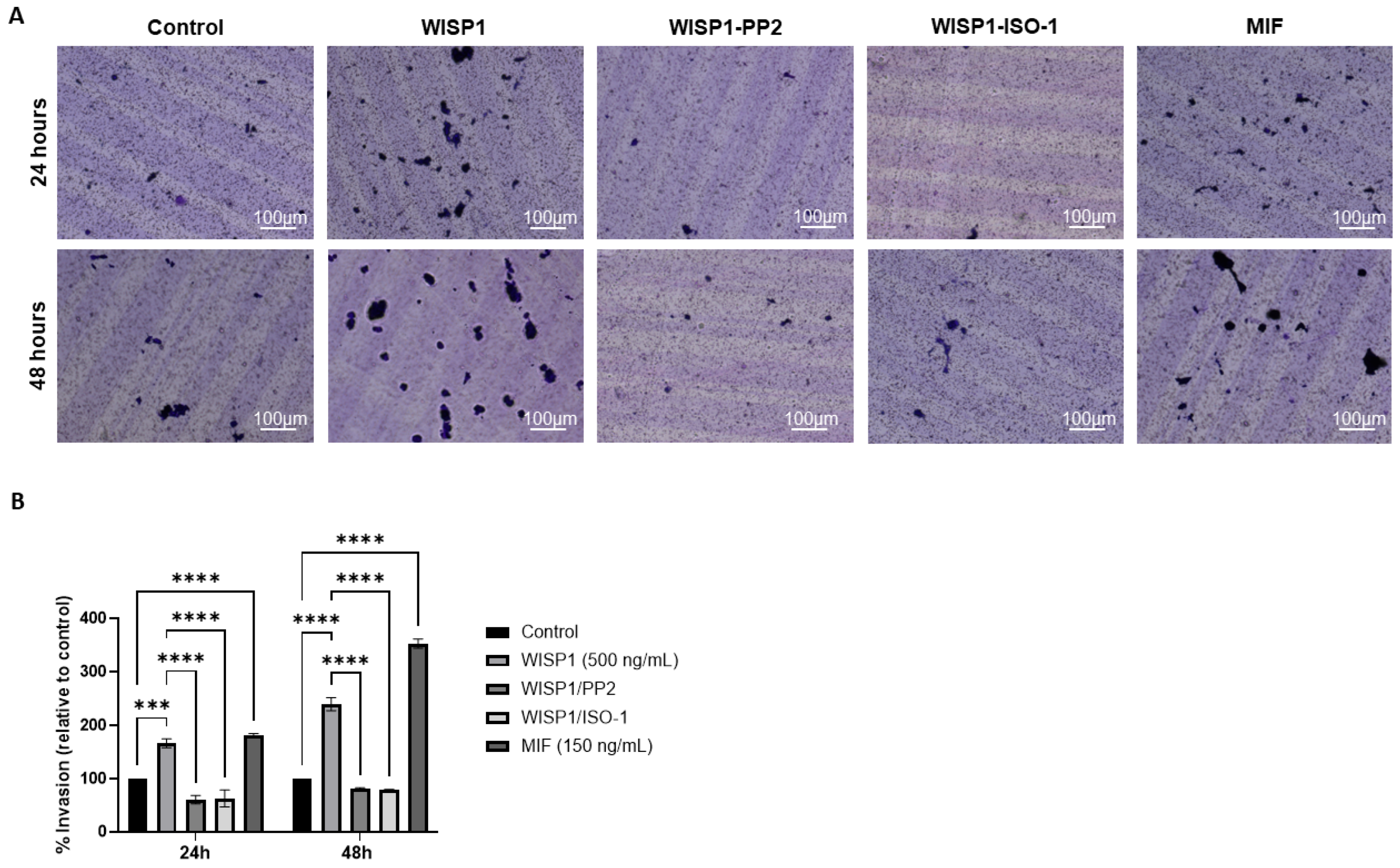
3.8. WISP1 Enhances the Invasive Capacity of MCF7 Cells via Src Kinases and MIF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fumagalli, C.; Barberis, M. Breast Cancer Heterogeneity. Diagnostics 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Luond, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-c. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Sflomos, G.; Dormoy, V.; Metsalu, T.; Jeitziner, R.; Battista, L.; Scabia, V.; Raffoul, W.; Delaloye, J.F.; Treboux, A.; Fiche, M.; et al. A Preclinical Model for ERα-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell 2016, 29, 407–422. [Google Scholar] [CrossRef]
- Li, J.J.; Tsang, J.Y.; Tse, G.M. Tumor Microenvironment in Breast Cancer-Updates on Therapeutic Implications and Pathologic Assessment. Cancers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Fico, F.; Santamaria-Martinez, A. The Tumor Microenvironment as a Driving Force of Breast Cancer Stem Cell Plasticity. Cancers 2020, 12, 3863. [Google Scholar] [CrossRef]
- Skandalis, S.S. CD44 Intracellular Domain: A Long Tale of a Short Tail. Cancers 2023, 15, 5041. [Google Scholar] [CrossRef]
- Asleh, K.; Riaz, N.; Nielsen, T.O. Heterogeneity of triple negative breast cancer: Current advances in subtyping and treatment implications. J. Exp. Clin. Cancer Res. 2022, 41, 265. [Google Scholar] [CrossRef]
- Wang, Y.; Minden, A. Current Molecular Combination Therapies Used for the Treatment of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 11046. [Google Scholar] [CrossRef]
- Christopoulou, M.E.; Aletras, A.J.; Papakonstantinou, E.; Stolz, D.; Skandalis, S.S. WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD. Int. J. Mol. Sci. 2024, 25, 10049. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, R.; García-Alamán, A.; Esteban, Y.; Mir-Coll, J.; Serra-Navarro, B.; Fontcuberta-PiSunyer, M.; Broca, C.; Armanet, M.; Wojtusciszyn, A.; Kram, V.; et al. Wisp1 is a circulating factor that stimulates proliferation of adult mouse and human beta cells. Nat. Commun. 2020, 11, 5982. [Google Scholar] [CrossRef]
- Tao, W.; Chu, C.; Zhou, W.; Huang, Z.; Zhai, K.; Fang, X.; Huang, Q.; Zhang, A.; Wang, X.; Yu, X.; et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 2020, 11, 3015. [Google Scholar] [CrossRef]
- Maeda, A.; Ono, M.; Holmbeck, K.; Li, L.; Kilts, T.M.; Kram, V.; Noonan, M.L.; Yoshioka, Y.; McNerny, E.M.B.; Tantillo, M.A.; et al. WNT1-induced Secreted Protein-1 (WISP1), a Novel Regulator of Bone Turnover and Wnt Signaling. J. Biol. Chem. 2015, 290, 14004–14018. [Google Scholar] [CrossRef]
- Wu, J.; Long, Z.; Cai, H.; Du, C.; Liu, X.; Yu, S.; Wang, Y. High expression of WISP1 in colon cancer is associated with apoptosis, invasion and poor prognosis. Oncotarget 2016, 7, 49834–49847. [Google Scholar] [CrossRef][Green Version]
- Chiang, K.C.; Hsu, S.Y.; Lin, S.J.; Yeh, C.N.; Pang, J.H.; Wang, S.Y.; Hsu, J.T.; Yeh, T.S.; Chen, L.W.; Kuo, S.F.; et al. PTEN Insufficiency Increases Breast Cancer Cell Metastasis In Vitro and In Vivo in a Xenograft Zebrafish Model. Anticancer Res. 2016, 36, 3997–4005. [Google Scholar]
- Liu, Y.; Song, Y.; Ye, M.; Hu, X.; Wang, Z.P.; Zhu, X. The emerging role of WISP proteins in tumorigenesis and cancer therapy. J. Transl. Med. 2019, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Nakachi, K.; Wang, H.; Elashoff, R.; Koeffler, H.P. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001, 61, 8917–8923. [Google Scholar] [PubMed]
- Deng, W.; Fernandez, A.; McLaughlin, S.L.; Klinke, D.J., 2nd. WNT1-inducible signaling pathway protein 1 (WISP1/CCN4) stimulates melanoma invasion and metastasis by promoting the epithelial-mesenchymal transition. J. Biol. Chem. 2019, 294, 5261–5280. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Lien, M.Y.; Tsai, M.H.; Hua, C.H.; Tang, C.H. WISP-1 Promotes Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Cells Via the miR-153-3p/Snail Axis. Cancers 2019, 11, 1903. [Google Scholar]
- Dong, T.; Liu, L.; You, Y.; Liu, J.; Wang, F.; Li, S.; Yu, Z. WISP1 inhibition of YAP phosphorylation drives breast cancer growth and chemoresistance via TEAD4 activation. Anticancer Drugs 2025, 36, 157–176. [Google Scholar]
- Richard, V.; Kindt, N.; Saussez, S. Macrophage migration inhibitory factor involvement in breast cancer (Review). Int. J. Oncol. 2015, 47, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Richard, V.; Kindt, N.; Decaestecker, C.; Gabius, H.J.; Laurent, G.; Noel, J.C.; Saussez, S. Involvement of macrophage migration inhibitory factor and its receptor (CD74) in human breast cancer. Oncol. Rep. 2014, 32, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Charan, M.; Das, S.; Mishra, S.; Chatterjee, N.; Varikuti, S.; Kaul, K.; Misri, S.; Ahirwar, D.K.; Satoskar, A.R.; Ganju, R.K. Macrophage migration inhibitory factor inhibition as a novel therapeutic approach against triple-negative breast cancer. Cell Death Dis. 2020, 11, 774. [Google Scholar] [CrossRef]
- Verjans, E.; Noetzel, E.; Bektas, N.; Schutz, A.K.; Lue, H.; Lennartz, B.; Hartmann, A.; Dahl, E.; Bernhagen, J. Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer 2009, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Mora Barthelmess, R.; Stijlemans, B.; Van Ginderachter, J.A. Hallmarks of Cancer Affected by the MIF Cytokine Family. Cancers 2023, 15, 395. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ye, C.; Yao, C.; Lin, Y.; Huang, X.; Zhang, Y.; Wang, S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008, 261, 147–157. [Google Scholar] [CrossRef]
- Conroy, H.; Mawhinney, L.; Donnelly, S.C. Inflammation and cancer: Macrophage migration inhibitory factor (MIF)—The potential missing link. QJM 2010, 103, 831–836. [Google Scholar] [CrossRef]
- Mitchell, R.A. Mechanisms and effectors of MIF-dependent promotion of tumourigenesis. Cell. Signal. 2004, 16, 13–19. [Google Scholar] [CrossRef]
- Wang, S.S.; Cen, X.; Liang, X.H.; Tang, Y.L. Macrophage migration inhibitory factor: A potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget 2017, 8, 10650–10661. [Google Scholar] [CrossRef]
- Valdez, C.N.; Sánchez-Zuno, G.A.; Bucala, R.; Tran, T.T. Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (DDT): Pathways to Tumorigenesis and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 4849. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, M.E.; Skandalis, S.S.; Papakonstantinou, E.; Stolz, D.; Aletras, A.J. WISP1 induces the expression of macrophage migration inhibitory factor in human lung fibroblasts through Src kinases and EGFR-activated signaling pathways. Am. J. Physiol. Cell Physiol. 2024, 326, C850–C865. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, P.O.; Clairefond, S.; Class, C.A.; Boulay, P.L.; Chrobak, P.; Allard, B.; Azzi, F.; Pommey, S.; Do, K.A.; Saad, F.; et al. WISP1 is associated to advanced disease, EMT and an inflamed tumor microenvironment in multiple solid tumors. Oncoimmunology 2019, 8, e1581545. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, C.N.; Chung, L.C.; Feng, T.H.; Sun, C.C.; Chen, M.F.; Jan, Y.Y.; Yeh, T.S.; Chen, S.C.; Juang, H.H. WNT-1 inducible signaling pathway protein-1 enhances growth and tumorigenesis in human breast cancer. Sci. Rep. 2015, 5, 8686. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Tsoukala, E.; Sarantopoulou, T.D.; Christopoulou, M.E. Matrix: A complex amalgam of structures and functions in tumor microenvironment. FEBS Open Bio 2025, 15, 1552–1569. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef]
- Nivison, M.P.; Meier, K.E. The role of CCN4/WISP-1 in the cancerous phenotype. Cancer Manag. Res. 2018, 10, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.K.; Kim, S.A.; Yoon, T.M.; Lee, K.H.; Kim, H.K.; Lee, D.H.; Lee, J.K.; Chung, I.J.; Joo, Y.E.; Lim, S.C. WNT1-inducible signaling pathway protein-1 contributes to tumor progression and treatment failure in oral squamous cell carcinoma. Oncol. Lett. 2017, 14, 1719–1724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.Y.; Feng, Y.J.; Ji, R. High expression of WISP1 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10445–10451. [Google Scholar]
- Fernandez, A.; Deng, W.; McLaughlin, S.L.; Pirkey, A.C.; Rellick, S.L.; Razazan, A.; Klinke, D.J., 2nd. Cell Communication Network factor 4 promotes tumor-induced immunosuppression in melanoma. EMBO Rep. 2022, 23, e54127. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.R.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann. Surg. Oncol. 2007, 14, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Chen, N.; Lin, Y.; Ma, H.; Ruan, Y.; Li, Z.; Li, X.; Pan, X.; Tian, X. Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axis. Cancer Lett. 2016, 375, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Balogh, K.N.; Templeton, D.J.; Cross, J.V. Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses. PLoS ONE 2018, 13, e0197702. [Google Scholar] [CrossRef]
- Simpson, K.D.; Templeton, D.J.; Cross, J.V. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J. Immunol. 2012, 189, 5533–5540. [Google Scholar] [CrossRef]
- Koh, H.M.; Kim, D.C. Prognostic significance of macrophage migration inhibitory factor expression in cancer patients: A systematic review and meta-analysis. Medicine 2020, 99, e21575. [Google Scholar] [CrossRef]
- Patel, A.; Sabbineni, H.; Clarke, A.; Somanath, P.R. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016, 157, 52–61. [Google Scholar] [CrossRef]
- Durst, B.; Sorg, R.V.; Röder, G.; Betz, B.; Beckmann, M.W.; Niederacher, D.; Bender, H.G.; Dall, P. The influence of hormones on CD44 expression in endometrial and breast carcinomas. Oncol. Rep. 2001, 8, 987–993. [Google Scholar] [CrossRef]
- Friedrichs, K.; Franke, F.; Lisboa, B.W.; Kügler, G.; Gille, I.; Terpe, H.J.; Hölzel, F.; Maass, H.; Günthert, U. CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res. 1995, 55, 5424–5433. [Google Scholar]
- Liu, Z.; Chu, S.; Yao, S.; Li, Y.; Fan, S.; Sun, X.; Su, L.; Liu, X. CD74 interacts with CD44 and enhances tumorigenesis and metastasis via RHOA-mediated cofilin phosphorylation in human breast cancer cells. Oncotarget 2016, 7, 68303–68313. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’ev, A. Cell-cell adhesion: Linking Wnt/beta-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, 1488. [Google Scholar] [CrossRef]
- Santarosa, M.; Maestro, R. The Autophagic Route of E-Cadherin and Cell Adhesion Molecules in Cancer Progression. Cancers 2021, 13, 6328. [Google Scholar] [CrossRef]
- Shi, X.; Leng, L.; Wang, T.; Wang, W.; Du, X.; Li, J.; McDonald, C.; Chen, Z.; Murphy, J.W.; Lolis, E.; et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006, 25, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell. Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef]
- Juarez-Cruz, J.C.; Zuniga-Eulogio, M.D.; Olea-Flores, M.; Castaneda-Saucedo, E.; Mendoza-Catalan, M.A.; Ortuno-Pineda, C.; Moreno-Godinez, M.E.; Villegas-Comonfort, S.; Padilla-Benavides, T.; Navarro-Tito, N. Leptin induces cell migration and invasion in a FAK-Src-dependent manner in breast cancer cells. Endocr. Connect. 2019, 8, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Janjanam, J.; Wu, S.C.; Wang, R.; Pano, G.; Celestine, M.; Martinot, O.; Breeze-Jones, H.; Clayton, G.; Garcin, C.; et al. The tumor cell-secreted matricellular protein WISP1 drives pro-metastatic collagen linearization. EMBO J. 2019, 38, e101302. [Google Scholar] [CrossRef]
- Vitale, D.L.; Parnigoni, A.; Viola, M.; Karousou, E.; Sevic, I.; Moretto, P.; Passi, A.; Alaniz, L.; Vigetti, D. Deciphering Drug Resistance: Investigating the Emerging Role of Hyaluronan Metabolism and Signaling and Tumor Extracellular Matrix in Cancer Chemotherapy. Int. J. Mol. Sci. 2024, 25, 7607. [Google Scholar] [CrossRef]
- Singh, K.; Oladipupo, S.S. An overview of CCN4 (WISP1) role in human diseases. J. Transl. Med. 2024, 22, 601. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Karousou, E.; Kamiryo, M.; Skandalis, S.S.; Ruusala, A.; Asteriou, T.; Passi, A.; Yamashita, H.; Hellman, U.; Heldin, C.H.; Heldin, P. The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J. Biol. Chem. 2010, 285, 23647–23654. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tobisawa, Y.; Inubushi, T.; Irie, F.; Ohyama, C.; Yamaguchi, Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J. Biol. Chem. 2017, 292, 7304–7313. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Piperigkou, Z.; Barbera, C.; Beninatto, R.; Masola, V.; Caon, I.; Onisto, M.; Franchi, M.; Galesso, D.; Karamanos, N.K. Molecular size-dependent specificity of hyaluronan on functional properties, morphology and matrix composition of mammary cancer cells. Matrix Biol. Plus 2019, 3, 100008. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Wan, X.; Liu, J.; Lu, J.F.; Tzelepi, V.; Yang, J.; Starbuck, M.W.; Diao, L.; Wang, J.; Efstathiou, E.; Vazquez, E.S.; et al. Activation of beta-catenin signaling in androgen receptor-negative prostate cancer cells. Clin. Cancer Res. 2012, 18, 726–736. [Google Scholar] [CrossRef]
- Luo, J.; Zou, H.; Guo, Y.; Tong, T.; Ye, L.; Zhu, C.; Deng, L.; Wang, B.; Pan, Y.; Li, P. SRC kinase-mediated signaling pathways and targeted therapies in breast cancer. Breast Cancer Res. 2022, 24, 99. [Google Scholar] [CrossRef]
- Gautam, J.; Banskota, S.; Lee, H.; Lee, Y.-J.; Jeon, Y.H.; Kim, J.-A.; Jeong, B.-S. Down-regulation of cathepsin S and matrix metalloproteinase-9 via Src, a non-receptor tyrosine kinase, suppresses triple-negative breast cancer growth and metastasis. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. Biomed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef]
- Tan, X.; Yan, Y.; Song, B.; Zhu, S.; Mei, Q.; Wu, K. Focal adhesion kinase: From biological functions to therapeutic strategies. Exp. Hematol. Oncol. 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Reynosa, P.; Robledo, T.; Macias-Silva, M.; Wu, S.V.; Salazar, E.P. Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008, 27, 220–231. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Menezes, S.V.; Sahni, S.; Kalinowski, D.S.; Bae, D.H.; Lane, D.J.; Richardson, D.R. The Metastasis Suppressor, N-MYC Downstream-regulated Gene-1 (NDRG1), Down-regulates the ErbB Family of Receptors to Inhibit Downstream Oncogenic Signaling Pathways. J. Biol. Chem. 2016, 291, 1029–1052. [Google Scholar] [CrossRef]
- Menezes, S.V.; Fouani, L.; Huang, M.L.H.; Geleta, B.; Maleki, S.; Richardson, A.; Richardson, D.R.; Kovacevic, Z. The metastasis suppressor, NDRG1, attenuates oncogenic TGF-beta and NF-kappaB signaling to enhance membrane E-cadherin expression in pancreatic cancer cells. Carcinogenesis 2019, 40, 805–818. [Google Scholar] [CrossRef]
- Kotepui, K.; Kotepui, M.; Majima, H.J.; Tangpong, J. Association between NDRG1 protein expression and aggressive features of breast cancer: A systematic review and meta-analysis. BMC Cancer 2023, 23, 1003. [Google Scholar] [CrossRef]
- Paul, D.; Vukelja, S.J.; Ann Holmes, F.; Blum, J.L.; McIntyre, K.J.; Lindquist, D.L.; Osborne, C.R.; Sanchez, I.J.; Goldschmidt, J.H.; Wang, Y.; et al. Randomized phase-II evaluation of letrozole plus dasatinib in hormone receptor positive metastatic breast cancer patients. npj Breast Cancer 2019, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Vale, N. Dual Drug Repurposing: The Example of Saracatinib. Int. J. Mol. Sci. 2024, 25, 4565. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence | Tannealing (°C) |
|---|---|---|
| CD44s | F: 5′-ATA ATA AAG GAG CAG CAC TTC AGG A-3′ R: 5′-ATA ATT TGT GTC TTG GTC TCT GGT AGC-3′ | 60 |
| CD44t | F: 5′-ATA ATT GCC GCT TTG CAG GTG TAT T-3′ R: 5′-ATA ATG GCA AGG TGC TAT TGA AAG CCT-3′ | 60 |
| CD74 | F: 5′-TGC ATT CAC ATT TGT GCT GTA G-3′ R: 5′-TGT ACA GAG CTC TCC ACG GCT G-3′ | 60 |
| E-Cadherin | F: 5′-TAC GCC TGG GAC TCC ACC TA-3′ R: 5′-CCA GAA ACG GAG GCC TGA T-3′ | 57 |
| Fibronectin | F: 5′-CAT CGA GCG GAT CTG GCC C-3′ R: 5′-GCA GCT GAC TCC GTT GCC CA-3′ | 57 |
| GAPDH | F: 5′-AGG CTG TTG TCA TAC TTC TCA T-3′ R: 5′-GGA GTC CAC TGG CGT CTT-3′ | 57 |
| HAS-1 | F: 5′-GGA ATA ACC TCT TGC AGC AGT TTC-3′ R: 5′-GCC GGT CAT CCC CAA AAG-3′ | 61 |
| HAS-2 | F: 5′-TCG CAA CAC GTA ACG CAA T-3′ R: 5′-ACT TCT CTT TTT CCA CCC CAT TT-3′ | 57 |
| HAS-3 | F: 5′-AAC AAG TAC GAC TCA TGG ATT TCC T-3′ R: 5′-GCC CGC TCC ACG TTG A-3′ | 61 |
| Hyal1 | F: 5′-GAT TGC AGT GTC TTC GAT GTG GTA-3′ R: 5′-GGG AGC TAT AGA AAA TTG TCA TGT CA-3′ | 61 |
| Hyal2 | F: 5′-CTA ATG AGG GTT TTG TGA ACC AGA ATA T-3′ R: 5′-GCA GAA TCG AAG CGT GGA TAC-3′ | 61 |
| MIF | F: 5′-CCG GAC AGG GTC TAC ATC AAC TAT TAC-3′ R: 5′-TAG GCG AAG GTG GAG TTG TTC C-3′ | 60 |
| MMP-1 | F: 5′-TGT GAC CTC CAT CCC CAA CT-3′ R: 5′-AAC TCA GGT CAT CTT CTG TCC GT-3′ | 57 |
| MMP-2 | F: 5′-ACT GTT GGT GGG AAC TCA GAA G-3′ R: 5′-CAA GGT CAA TGT CAG GAG AGG-3′ | 57 |
| MMP-9 | F: 5′-TTC CAG TAC CGA GAG AAA GCC TAT-3′ R: 5′-GGT CAC GTA GCC CAC TTG GT-3′ | 57 |
| MT1-MMP | F: 5′-ACT GTT GGT GGG AAC TCA GAA G-3′ R: 5′-CAA GGT CAA TGT CAG GAG AGG-3′ | 57 |
| TIMP-1 | F: 5′-CGC TGA CAT CCG GTT CGT-3′ R: 5′-TGT GGA AGT ATC CGC AGA CAC T-3′ | 59 |
| TIMP-2 | F: 5′-GGG CAC CAG GCC AAG TT-3′ R: 5′-CGC ACA GGA GCC ATC ACT-3′ | 60 |
| TMEM2 | F: 5′-GGAATAGGACTGACCTTTGCCAG-3′ R: 5′-TTCTGACCACCCTGAAAGCCGT-3′ | 57 |
| Vimentin | F: 5′-GGC TCG TCA CCT TCG TGA AT-3′ R: 5′-GAG AAA TCC TGC TCT CCT CGC-3′ | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Christopoulou, M.-E.; Karamitsou, P.; Aletras, A.; Skandalis, S.S. The WISP1/Src/MIF Axis Promotes the Malignant Phenotype of Non-Invasive MCF7 Breast Cancer Cells. Cells 2026, 15, 160. https://doi.org/10.3390/cells15020160
Christopoulou M-E, Karamitsou P, Aletras A, Skandalis SS. The WISP1/Src/MIF Axis Promotes the Malignant Phenotype of Non-Invasive MCF7 Breast Cancer Cells. Cells. 2026; 15(2):160. https://doi.org/10.3390/cells15020160
Chicago/Turabian StyleChristopoulou, Maria-Elpida, Panagiota Karamitsou, Alexios Aletras, and Spyros S. Skandalis. 2026. "The WISP1/Src/MIF Axis Promotes the Malignant Phenotype of Non-Invasive MCF7 Breast Cancer Cells" Cells 15, no. 2: 160. https://doi.org/10.3390/cells15020160
APA StyleChristopoulou, M.-E., Karamitsou, P., Aletras, A., & Skandalis, S. S. (2026). The WISP1/Src/MIF Axis Promotes the Malignant Phenotype of Non-Invasive MCF7 Breast Cancer Cells. Cells, 15(2), 160. https://doi.org/10.3390/cells15020160



_Wang.png)





