Topical CCL3 Is Well-Tolerated and Improves Liver Function in Diabetic Mice: Evidence from a 14-Day Toxicity Study
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Animals and Grouping
2.3. Wounding and Treatment
2.4. Monitoring and Clinical Observations
2.5. Immunological, Hematological, and Biochemical Analysis
2.6. Histopathological Analysis
2.7. Statistical Analysis
3. Results
3.1. Physiological and Behavioral Monitoring Following CCL3 Treatment
3.2. CCL3 Therapy Immunological Assessment in Diabetic Mice
3.3. CCL3 Therapy Biochemical Safety Assessment in Diabetic Mice
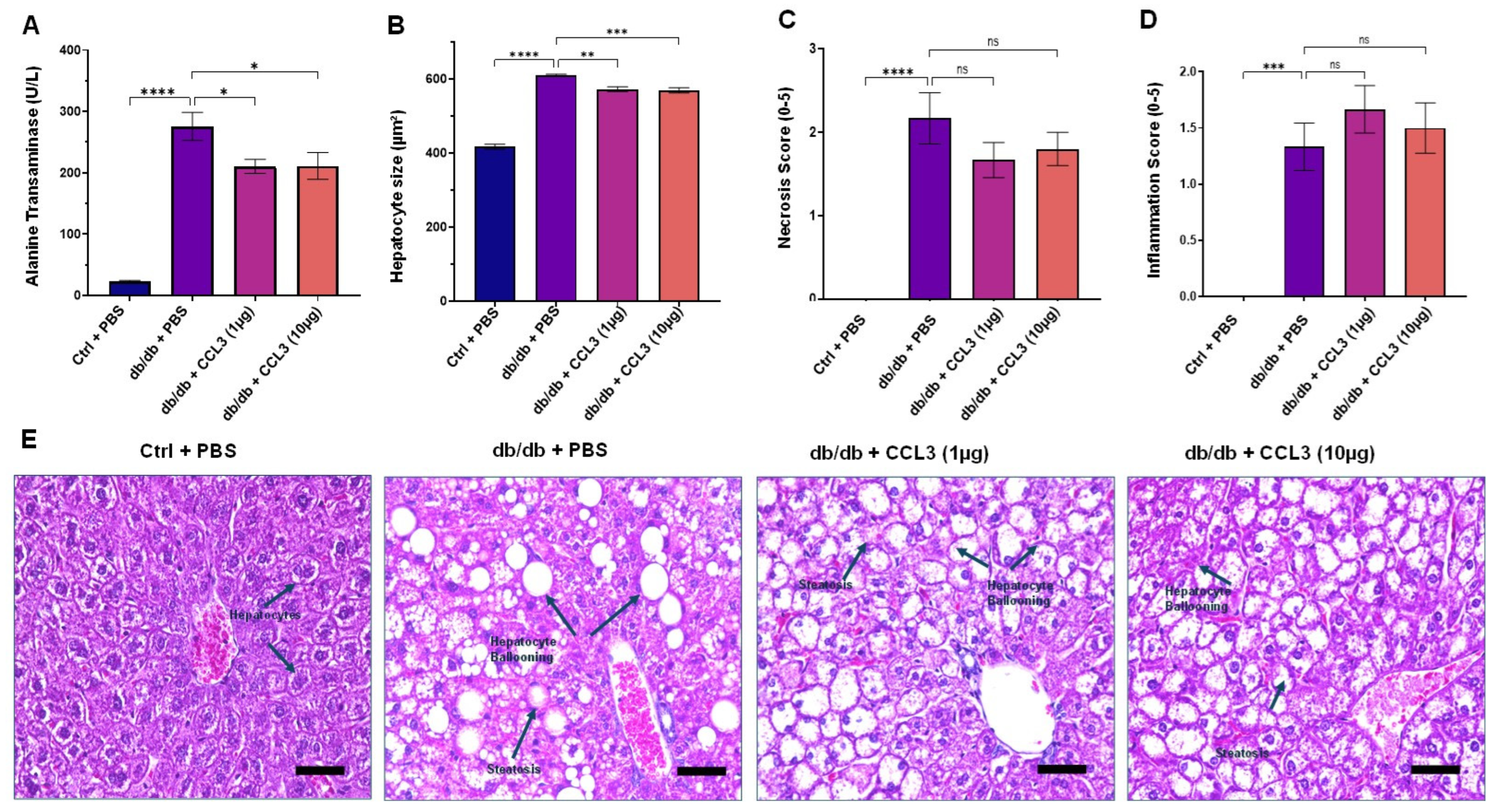
3.4. CCL3 Does Not Cause Organ Toxicity: Histopathological Evidence
3.5. CCL3 Therapy Hematological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, R.; Singh, R.; Shafikhani, S.H. Infection in Diabetes: Epidemiology, Immune Dysfunctions, and Therapeutics. In The Diabetic Foot: Medical and Surgical Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2024; pp. 299–326. [Google Scholar]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Zayas, J.; Singh, S.K.; Delgado, K.; Wood, S.J.; Mohamed, M.F.; Frausto, D.M.; Albalawi, Y.A.; Price, T.P.; Estupinian, R.; et al. Overriding impaired FPR chemotaxis signaling in diabetic neutrophil stimulates infection control in murine diabetic wound. eLife 2022, 11, e72071. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; Dipietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef]
- Roy, R.; Zayas, J.; Mohamed, M.F.; Aboonabi, A.; Delgado, K.; Wallace, J.; Bayat, M.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. IL-10 Dysregulation Underlies Chemokine Insufficiency, Delayed Macrophage Response, and Impaired Healing in Diabetic Wounds. J. Investig. Dermatol. 2022, 142, 692–704.e614. [Google Scholar] [CrossRef]
- Goldufsky, J.; Wood, S.J.; Jayaraman, V.; Majdobeh, O.; Chen, L.; Qin, S.; Zhang, C.; DiPietro, L.A.; Shafikhani, S.H. Pseudomonas aeruginosa uses T3SS to inhibit diabetic wound healing. Wound Repair Regen. 2015, 23, 557–564. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Gupta, K.; Goldufsky, J.W.; Roy, R.; Callaghan, L.T.; Wetzel, D.M.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. CrkII/Abl phosphorylation cascade is critical for NLRC4 inflammasome activity and is blocked by Pseudomonas aeruginosa ExoT. Nat. Commun. 2022, 13, 1295. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Wood, S.J.; Roy, R.; Reiser, J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT induces G1 cell cycle arrest in melanoma cells. Cell Microbiol. 2021, 23, e13339. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Bello, D.; Masood, S.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT induces mitochondrial apoptosis in target host cells in a manner that depends on its GTPase-activating protein (GAP) domain activity. J. Biol. Chem. 2015, 290, 29063–29073. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin. PLoS Pathog. 2015, 11, e1004934. [Google Scholar] [CrossRef]
- Dovi, J.V.; Szpaderska, A.M.; DiPietro, L.A. Neutrophil function in the healing wound: Adding insult to injury? Thromb. Haemost. 2004, 92, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.M.; Shafikhani, S.H.; Soulika, A.M. Macrophage and Neutrophil Dysregulation in Diabetic Wounds. Adv. Wound Care 2024, 13, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.C.; Allannic, H.; Genetet, B. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Ziyadeh, N.; Fife, D.; Walker, A.M.; Wilkinson, G.S.; Seeger, J.D. A matched cohort study of the risk of cancer in users of becaplermin. Adv. Ski. Wound Care 2011, 24, 31–39. [Google Scholar] [CrossRef]
- Blume, P.; Bowlby, M.; Schmidt, B.M.; Donegan, R. Safety and efficacy of Becaplermin gel in the treatment of diabetic foot ulcers. Chronic Wound Care Manag. Res. 2014, 1, 11–14. [Google Scholar] [CrossRef]
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D.W. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [Google Scholar] [CrossRef]
- Abbas, M.; Uckay, I.; Lipsky, B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin. Pharmacother. 2015, 16, 821–832. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C. Antimicrobial resistance: A concise update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef]
- Gu, S.; Rajendiran, G.; Forest, K.; Tran, T.C.; Denny, J.C.; Larson, E.A.; Wilke, R.A. Drug-induced liver injury with commonly used antibiotics in the all of us research program. Clin. Pharmacol. Ther. 2023, 114, 404–412. [Google Scholar] [CrossRef]
- Rivetti, S.; Romano, A.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Ruggiero, A. Aminoglycosides-related ototoxicity: Mechanisms, risk factors, and prevention in pediatric patients. Pharmaceuticals 2023, 16, 1353. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.C. Science, Medicine, and Animals: Teacher’s Guide; ERIC: Washington, DC, USA, 2005. [Google Scholar]
- Prasad, C.B. A review on drug testing in animals. Transl. Biomed. 2016, 7, 99. [Google Scholar] [CrossRef]
- Singh, R.; Gholipourmalekabadi, M.; Shafikhani, S.H. Animal models for type 1 and type 2 diabetes: Advantages and limitations. Front. Endocrinol. 2024, 15, 1359685. [Google Scholar] [CrossRef] [PubMed]
- Goldufsky, J.; Wood, S.; Hajihossainlou, B.; Rehman, T.; Majdobeh, O.; Kaufman, H.L.; Ruby, C.E.; Shafikhani, S.H. Pseudomonas aeruginosa exotoxin T induces potent cytotoxicity against a variety of murine and human cancer cell lines. J. Med. Microbiol. 2015, 64, 164–173. [Google Scholar] [CrossRef]
- Kohlhapp, F.J.; Huelsmann, E.J.; Lacek, A.T.; Schenkel, J.M.; Lusciks, J.; Broucek, J.R.; Goldufsky, J.W.; Hughes, T.; Zayas, J.P.; Dolubizno, H.; et al. Non-oncogenic Acute Viral Infections Disrupt Anti-cancer Responses and Lead to Accelerated Cancer-Specific Host Death. Cell Rep. 2016, 17, 957–965. [Google Scholar] [CrossRef]
- Gil, C.R.E.; Lund, J.; Żylicz, J.J.; Ranea-Robles, P.; Sørensen, T.I.A.; Clemmensen, C. Food insecurity promotes adiposity in mice. Obesity 2025, 33, 1087–1100. [Google Scholar] [CrossRef]
- Rathkolb, B.; Hans, W.; Prehn, C.; Fuchs, H.; Gailus-Durner, V.; Aigner, B.; Adamski, J.; Wolf, E.; Hrabě de Angelis, M. Clinical Chemistry and Other Laboratory Tests on Mouse Plasma or Serum. Curr. Protoc. Mouse Biol. 2013, 3, 69–100. [Google Scholar] [CrossRef]
- McClure, D.E. Clinical pathology and sample collection in the laboratory rodent. Vet. Clin. N. Am. Exot. Anim. Pract. 1999, 2, 565–590. [Google Scholar] [CrossRef]
- Shrimali, R.K.; Weaver, J.A.; Miller, G.F.; Starost, M.F.; Carlson, B.A.; Novoselov, S.V.; Kumaraswamy, E.; Gladyshev, V.N.; Hatfield, D.L. Selenoprotein expression is essential in endothelial cell development and cardiac muscle function. Neuromuscul. Disord. NMD 2007, 17, 135–142. [Google Scholar] [CrossRef]
- Rutgers, M.; van Pelt, M.J.; Dhert, W.J.; Creemers, L.B.; Saris, D.B. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr. Cartil. 2010, 18, 12–23. [Google Scholar] [CrossRef]
- Mahmud, F.; Roy, R.; Mohamed, M.F.; Aboonabi, A.; Moric, M.; Ghoreishi, K.; Bayat, M.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. Therapeutic evaluation of immunomodulators in reducing surgical wound infection. FASEB J. 2022, 36, e22090. [Google Scholar] [CrossRef] [PubMed]
- Ulker, E.; Caillaud, M.; Koseli, E.; Contreras, K.; Alkhlaif, Y.; Lindley, E.; Barik, M.; Ghani, S.; Bryant, C.D.; Damaj, M.I. Comparison of Pain-Like behaviors in two surgical incision animal models in C57BL/6J mice. Neurobiol. Pain 2022, 12, 100103. [Google Scholar] [CrossRef]
- Abdollahi Nejat, M.; Stiedl, O.; Smit, A.B.; van Kesteren, R.E. Continuous locomotor activity monitoring to assess animal welfare following intracranial surgery in mice. Front. Behav. Neurosci. 2024, 18, 1457894. [Google Scholar] [CrossRef] [PubMed]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.; Nasrat, H.; Bahnassy, A. Serum immunoglobulin concentrations in diabetic patients. Diabet. Med. 1994, 11, 384–387. [Google Scholar] [CrossRef]
- Tang, X.; Wan, F.; Zhu, Q.; Ye, T.; Jiang, X.; Yang, H. IgG subclass deposition in diabetic nephropathy. Eur. J. Med. Res. 2022, 27, 147. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Tawfik, N.M.; Tony, E.A.-E.; Mahmoud, A.A.; Ali, S.M.; Khairallah, M.K. Serum immunoglobulin G as a predictive marker of early renal affection in type-2 diabetic patients: A single-center study. J. Egypt. Soc. Nephrol. Transplant. 2023, 23, 17–25. [Google Scholar] [CrossRef]
- Klamt, S.; Vogel, M.; Kapellen, T.M.; Hiemisch, A.; Prenzel, F.; Zachariae, S.; Ceglarek, U.; Thiery, J.; Kiess, W. Association between IgE-mediated allergies and diabetes mellitus type 1 in children and adolescents. Pediatr. Diabetes 2015, 16, 493–503. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, J.S.; Han, K.; Baek, K.H.; Song, K.-H.; Kwon, H.-S. House dust mite and Cockroach specific Immunoglobulin E sensitization is associated with diabetes mellitus in the adult Korean population. Sci. Rep. 2018, 8, 2614. [Google Scholar] [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Liu, X.; Duan, H.; Kong, J.; Li, Z. Relationship between C-reactive protein level and diabetic retinopathy: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0144406. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Blazhev, A. Elevated igG and igM autoantibodies to advanced glycation end products of vascular elastin in hypertensive patients with type 2 diabetes: Relevance to disease initiation and progression. Pathophysiology 2022, 29, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liu, Y.; Wang, H.; Tian, S.; Sun, C. The association of alanine aminotransferase and diabetic microvascular complications: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1104963. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Islam, M.Z.; Sultana, D.; Hossain, N.; Afrin, N.; Akhter, S.; Iqram, T.; Siddique, M.A.A. Serum Alanine TransaminaseLevel in Type 2 Diabetes Mellitus and It’s Relationship with Glycemic Status. East. Med. Coll. J. 2024, 9, 68–72. [Google Scholar] [CrossRef]
- Shibabaw, T.; Dessie, G.; Molla, M.D.; Zerihun, M.F.; Ayelign, B. Assessment of liver marker enzymes and its association with type 2 diabetes mellitus in Northwest Ethiopia. BMC Res. Notes 2019, 12, 707. [Google Scholar] [CrossRef]
- Mandal, A.; Bhattarai, B.; Kafle, P.; Khalid, M.; Jonnadula, S.K.; Lamicchane, J.; Kanth, R.; Gayam, V.; Jonnadula, S. Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus 2018, 10, e3626. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in People with Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar] [CrossRef]
- Makaju, M.; Luitel, P.; Nepal, R.; Kunwar, S.; Shrestha, R.K.; Akela, G.; Jaiswal, S.; Thakur, R.K. Pattern of Serum Liver Enzymes in the Patients with Type 2 Diabetes Mellitus. J. Res. Appl. Basic Med. Sci. 2023, 9, 272–279. [Google Scholar] [CrossRef]
- Prashanth, M.; Ganesh, H.; Vima, M.; John, M.; Bandgar, T.; Joshi, S.R.; Shah, S.; Rathi, P.; Joshi, A.; Thakkar, H. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J. Assoc. Physicians India 2009, 57, 205–210. [Google Scholar]
- Dai, W.; Ye, L.; Liu, A.; Wen, S.W.; Deng, J.; Wu, X.; Lai, Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine 2017, 96, e8179. [Google Scholar] [CrossRef]
- Leite, N.C.; Villela-Nogueira, C.A.; Pannain, V.L.; Bottino, A.C.; Rezende, G.F.; Cardoso, C.R.; Salles, G.F. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: Prevalences and correlated factors. Liver Int. 2011, 31, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.L. Kidney atrophy vs hypertrophy in diabetes: Which cells are involved? Cell Cycle 2018, 17, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, G.; Wang, B.; Xiong, J.; Xu, J.; Zheng, P.; Yuan, Y.; Li, Y.; Jiang, T.; Al Mamun, A. Fibroblast growth factor 1 ameliorates diabetes-induced splenomegaly via suppressing inflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2020, 528, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.J.; Kattner, N.; Al-Selwi, Y.; Honkanen-Scott, M.; Shaw, M.F.; Brack, C.A.; Coulthard, R.; Flaxman, C.S.; Richardson, S.J.; Shaw, J.A. Quantitative analysis of human adult pancreatic histology reveals separate fatty and fibrotic phenotypes in type 2 diabetes. Diabetologia 2025, 68, 2840–2853. [Google Scholar] [CrossRef]
- Granlund, L.; Hedin, A.; Wahlhütter, M.; Seiron, P.; Korsgren, O.; Skog, O.; Lundberg, M. Histological and transcriptional characterization of the pancreatic acinar tissue in type 1 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002076. [Google Scholar] [CrossRef]
- Voulgari, C.; Papadogiannis, D.; Tentolouris, N. Diabetic cardiomyopathy: From the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc. Health Risk Manag. 2010, 6, 883–903. [Google Scholar] [CrossRef]
- Marzona, I.; Avanzini, F.; Lucisano, G.; Tettamanti, M.; Baviera, M.; Nicolucci, A.; Roncaglioni, M.C. Are all people with diabetes and cardiovascular risk factors or microvascular complications at very high risk? Findings from the Risk and Prevention Study. Acta Diabetol. 2017, 54, 123–131. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhang, W.; Niu, Y.; Li, X.; Qin, L.; Su, Q. White blood cell subtypes and risk of type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 31–37. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Bergenstal, R.; Ziemen, M.; Wardecki, M.; Muehlen-Bartmer, I.; Boelle, E.; Riddle, M.C. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: Glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014, 37, 3235–3243. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Charmoy, M.; Brunner-Agten, S.; Aebischer, D.; Auderset, F.; Launois, P.; Milon, G.; Proudfoot, A.E.; Tacchini-Cottier, F. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010, 6, e1000755. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Memarian, E.; Heijmans, R.; Slieker, R.C.; Sierra, A.; Gornik, O.; Beulens, J.W.; Hanic, M.; Elders, P.; Pascual, J.; Sijbrands, E. IgG N-glycans are associated with prevalent and incident complications of type 2 diabetes. Diabetes Metab. Res. Rev. 2023, 39, e3685. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, M.Y.; Wang, G.D.; Lv, Q.Y.; Huang, Y.Q.; Zhang, P.; Wang, W.; Zhang, Y.; Bai, Y.P.; Guo, L.Q. Metformin improves nonalcoholic fatty liver disease in db/db mice by inhibiting ferroptosis. Eur. J. Pharmacol. 2024, 966, 176341. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef]
- Filep, J.G. Targeting Neutrophils for Promoting the Resolution of Inflammation. Front. Immunol. 2022, 13, 866747. [Google Scholar] [CrossRef]
- Cruz, T.B.; Carvalho, F.A.; Matafome, P.N.; Soares, R.A.; Santos, N.C.; Travasso, R.D.; Oliveira, M.J. Mice with Type 2 Diabetes Present Significant Alterations in Their Tissue Biomechanical Properties and Histological Features. Biomedicines 2021, 10, 57. [Google Scholar] [CrossRef]
- Xu, Z.; Xiang, X.; Su, S.; Zhu, Y.; Yan, H.; Guo, S.; Guo, J.; Shang, E.X.; Qian, D.; Duan, J.A. Multi-omics analysis reveals the pathogenesis of db/db mice diabetic kidney disease and the treatment mechanisms of multi-bioactive compounds combination from Salvia miltiorrhiza. Front. Pharmacol. 2022, 13, 987668. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Roy, R.; Mahmud, F.; Zayas, J.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. Reduced Bioactive Microbial Products (Pathogen-Associated Molecular Patterns) Contribute to Dysregulated Immune Responses and Impaired Healing in Infected Wounds in Mice with Diabetes. J. Investig. Dermatol. 2024, 144, 387–397.e311. [Google Scholar] [CrossRef] [PubMed]
- Shafikhani, S. Closing Editorial: Immunopathogenesis of Bacterial Infection. Cells 2025, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Mohamed, M.F.; Witt, B.R.; Wimmer, M.A.; Shafikhani, S.H. Therapeutic assessment of N-formyl-methionyl-leucyl-phenylalanine (fMLP) in reducing periprosthetic joint infection. Eur. Cell Mater. 2021, 41, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Kroin, J.S.; Li, J.; Shafikhani, S.; Gupta, K.H.; Moric, M.; Buvanendran, A. Local vancomycin effectively reduces surgical site infection at implant site in rodents. Reg. Anesth. Pain Med. 2018, 43, 795–804. [Google Scholar] [CrossRef]
- Kroin, J.S.; Li, J.; Goldufsky, J.W.; Gupta, K.H.; Moghtaderi, M.; Buvanendran, A.; Shafikhani, S.H. Perioperative high inspired oxygen fraction therapy reduces surgical site infection with Pseudomonas aeruginosa in rats. J. Med. Microbiol. 2016, 65, 738–744. [Google Scholar] [CrossRef]
- Kroin, J.S.; Buvanendran, A.; Li, J.; Moric, M.; Im, H.-J.; Tuman, K.J.; Shafikhani, S.H. Short-term glycemic control is effective in reducing surgical site infection in diabetic rats. Anesth. Analg. 2015, 120, 1289–1296. [Google Scholar] [CrossRef]




| Parameters | Ctrl + PBS | db/db + PBS | db/db + CCL3 (1 µg) | db/db + CCL3 (10 µg) |
|---|---|---|---|---|
| IgG1 (µg/mL) | 192.45 ± 13.51 | 285.75 ± 86.27 | 137.97 ± 21.18 | 198.86 ± 7.50 |
| IgE (µg/mL) | 94.29 ± 22.03 | 207.35 ± 57.64 | 150.41 ± 17.4 | 266.25 ± 51.61 |
| CRP (ng/mL) | 1.83 ± 0.33 | 8.14 ± 1.86 | 6.97 ± 1.66 | 8.29 ± 0.88 |
| Parameters | Ctrl + PBS | db/db + PBS | db/db + CCL3 (1 µg) | db/db + CCL3 (10 µg) | Standard Range |
|---|---|---|---|---|---|
| Alanine Transaminase U/L | 23.27 ± 1.33 | 275.65 ± 22.94 | 210.35 ± 11.43 | 211.18 ± 22.02 | 0–403 |
| Aspartate Transaminase U/L | 83.39 ± 6.18 | 247.58 ± 11.97 | 264.39 ± 14.76 | 209.20 ± 14.21 | 0–552 |
| Alkaline Phosphatase U/L | 97.93 ± 16.37 | 217.00 ± 6.32 | 191.98 ± 21.03 | 212.70 ± 27.02 | 49–172 |
| Blood Urea Nitrogen mg/dL | 26.97 ± 0.83 | 30.40 ± 1.10 | 31.05 ± 0.99 | 29.60 ± 1.39 | 15.2–34.7 |
| Calcium mg/dL | 10.17 ± 0.07 | 10.89 ± 0.18 | 10.52 ± 0.38 | 10.05 ± 0.40 | 9.6–11.5 |
| Chloride mmol/L | 114.92 ± 0.76 | 109.42 ± 0.61 | 109.13 ± 1.53 | 110.28 ± 2.03 | 105–118 |
| Creatinine mg/dL | 0.03 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.0–0.3 |
| Glucose mg/dL | 231.68 ± 24.41 | 402.48 ± 34.02 | 464.52 ± 27.46 | 477.40 ± 87.53 | 130–254 |
| Potassium mmol/L | 4.36 ± 0.10 | 5.06 ± 0.23 | 6.84 ± 1.05 | 6.11 ± 1.04 | 6.9–10.0 |
| Sodium mol/L | 155.33 ± 1.02 | 154.33 ± 0.92 | 155.50 ± 1.28 | 156.80 ± 3.32 | 150–160 |
| Phosphorus mg/dL | 9.98 ± 0.82 | 11.10 ± 0.62 | 14.03 ± 0.85 | 14.39 ± 1.35 | 7.5–10.7 |
| Total Bilirubin mg/dL | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.0–0.2 |
| Total Protein g/dL | 4.71 ± 0.18 | 5.80 ± 0.11 | 5.01 ± 0.87 | 5.88 ± 0.31 | 4.7–6.1 |
| Parameters | Ctrl + PBS | db/db + PBS | db/db + CCL3 (1 µg) | db/db + CCL3 (10 µg) | Standard Range |
|---|---|---|---|---|---|
| WBC (K/µl) | 9.89 ± 1.55 | 15.10 ± 0.79 | 9.46 ± 1.60 | 9.08 ± 1.28 | 5.1–14.7 |
| Hemoglobin (g/dL) | 10.77 ± 0.29 | 12.03 ± 0.54 | 12.33 ± 0.62 | 11.78 ± 0.37 | 11.7–16.2 |
| Neutrophil % | 17.48 ± 2.60 | 20.75 ± 1.34 | 16.79 ± 2.02 | 17.44 ± 0.91 | 12.5–31.2 |
| Lymphocyte % | 72.07 ± 3.84 | 68.00 ± 1.95 | 72.94 ± 2.81 | 73.16 ± 1.23 | 62.9–82.7 |
| Monocyte % | 6.32 ± 0.47 | 6.66 ± 0.27 | 5.55 ± 0.36 | 5.69 ± 0.37 | 2.5–7.5 |
| Eosinophil % | 3.33 ± 0.68 | 3.77 ± 0.46 | 3.56 ± 0.67 | 3.11 ± 0.13 | 1–3% |
| Basophil % | 0.89 ± 0.16 | 0.89 ± 0.10 | 0.92 ± 0.11 | 0.58 ± 0.10 | <1% |
| Hemoglobin (g/dL) | 10.77 ± 0.29 | 12.03 ± 0.54 | 12.33 ± 0.62 | 11.78 ± 0.37 | 11.0–16.2 |
| MCV (fL) | 55.67 ± 2.90 | 58.33 ± 0.41 | 58.65 ± 1.06 | 58.78 ± 0.96 | 45.0–55.0 |
| Platelets (K/µL) | 757.67 ± 139.47 | 746.33 ± 67.57 | 813.67 ± 108.18 | 925.20 ± 95.57 | 574–1079 |
| MPV (fL) | 6.00 ± 0.15 | 6.10 ± 0.08 | 6.17 ± 0.12 | 6.00 ± 0.10 | 5.0–20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dehari, D.; Padmakumari, R.; Tesfaw, G.; Fierro, F.A.; Ameer, G.A.; Shafikhani, S.H. Topical CCL3 Is Well-Tolerated and Improves Liver Function in Diabetic Mice: Evidence from a 14-Day Toxicity Study. Cells 2026, 15, 120. https://doi.org/10.3390/cells15020120
Dehari D, Padmakumari R, Tesfaw G, Fierro FA, Ameer GA, Shafikhani SH. Topical CCL3 Is Well-Tolerated and Improves Liver Function in Diabetic Mice: Evidence from a 14-Day Toxicity Study. Cells. 2026; 15(2):120. https://doi.org/10.3390/cells15020120
Chicago/Turabian StyleDehari, Deepa, Rajalekshmy Padmakumari, Getnet Tesfaw, Fernando A. Fierro, Guillermo A. Ameer, and Sasha H. Shafikhani. 2026. "Topical CCL3 Is Well-Tolerated and Improves Liver Function in Diabetic Mice: Evidence from a 14-Day Toxicity Study" Cells 15, no. 2: 120. https://doi.org/10.3390/cells15020120
APA StyleDehari, D., Padmakumari, R., Tesfaw, G., Fierro, F. A., Ameer, G. A., & Shafikhani, S. H. (2026). Topical CCL3 Is Well-Tolerated and Improves Liver Function in Diabetic Mice: Evidence from a 14-Day Toxicity Study. Cells, 15(2), 120. https://doi.org/10.3390/cells15020120







