Separate BNST Microcircuits Targeted by Direct Versus Amygdala-Relayed Prefrontal Inputs Mediate Dissociable Phenotypes After Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Social Isolation
2.2. Behavioral Assessment
2.3. Virus Injection
2.4. Chemogenetic Manipulation
2.5. Immunohistochemistry
2.6. Quantification of c-Fos Immunostaining
2.7. Statistical Analysis
3. Results
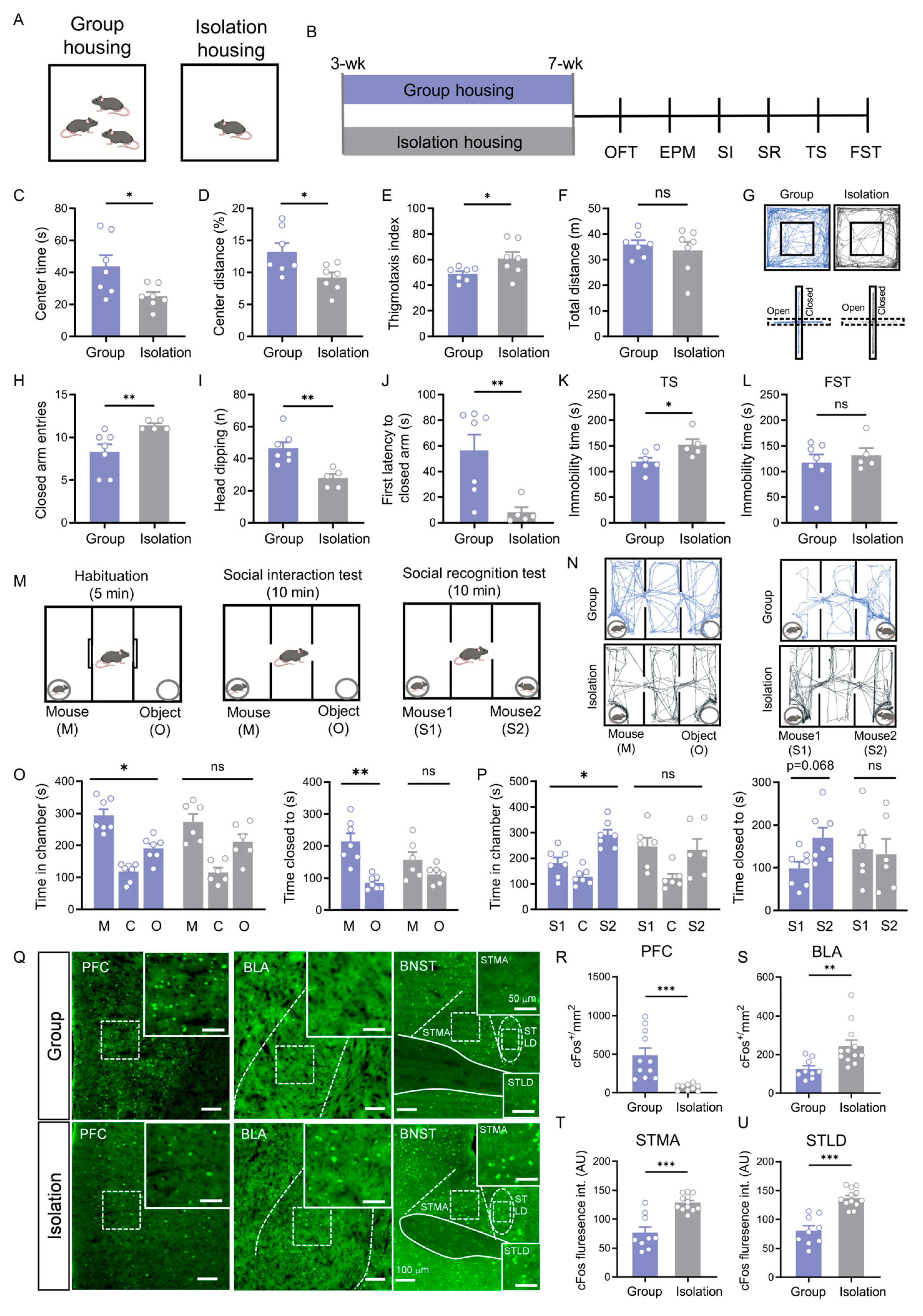
3.1. Social Isolation Induces a Complex Behavioral Syndrome and Co-Activation of Specific Brain Regions
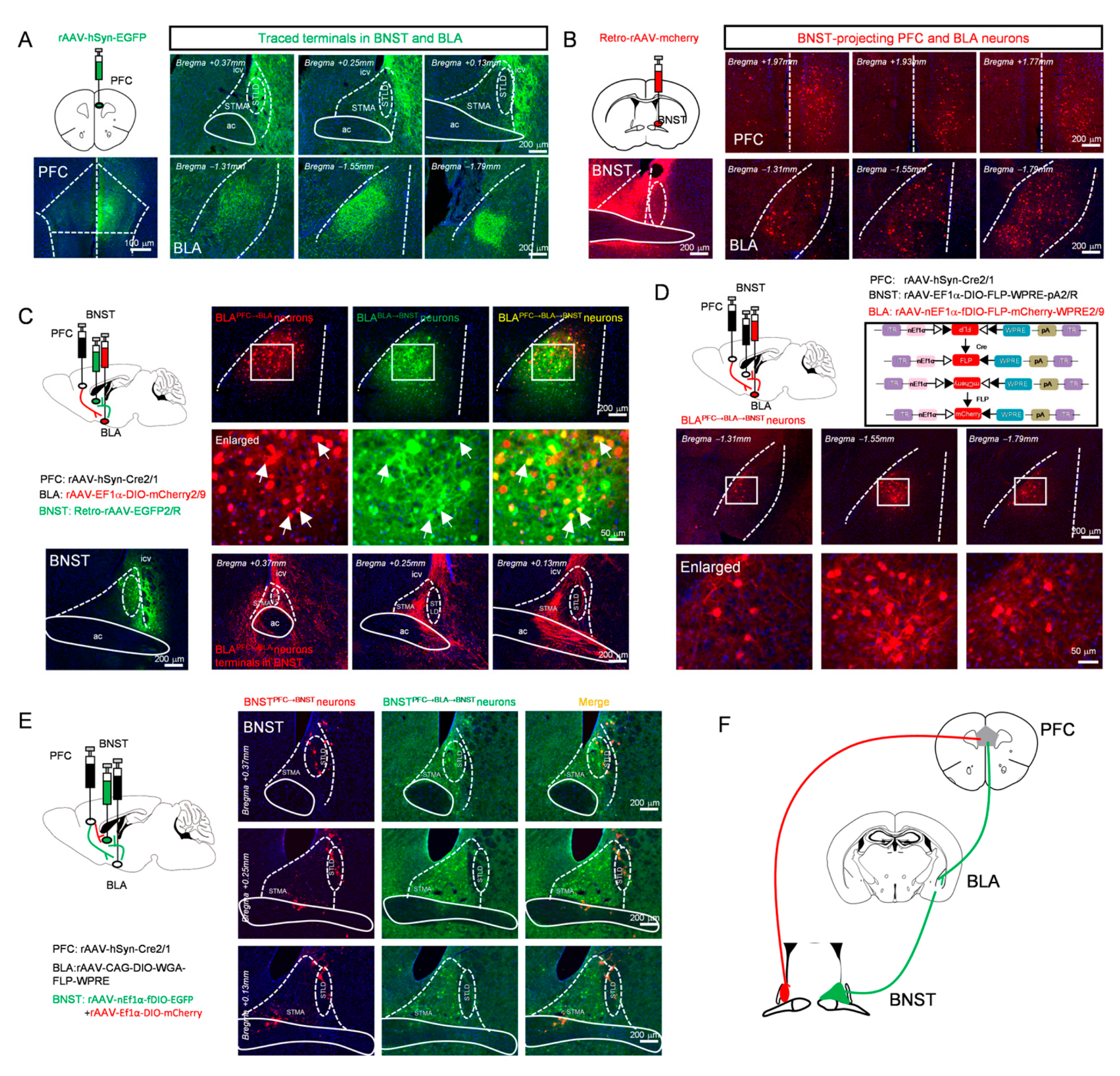
3.2. Anatomical Dissection of Parallel Prefrontal Pathways to the BNST
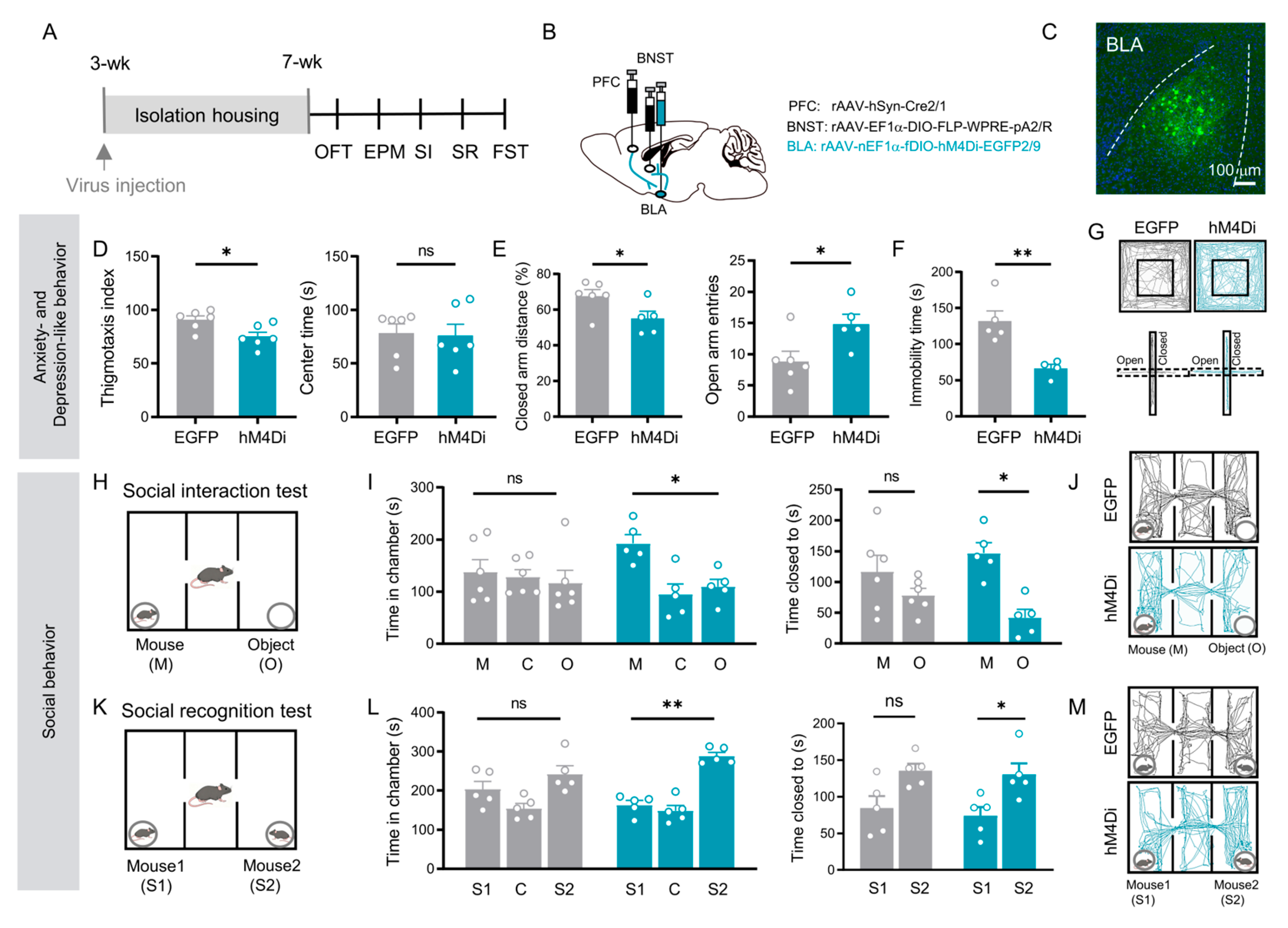
3.3. BLA Relay Neurons in the PFC → BLA → BNST Pathway Integrate Affective and Social Behaviors
3.4. BNST Neurons in the PFC → BLA → BNST Pathway Selectively Mediate Affective Deficits
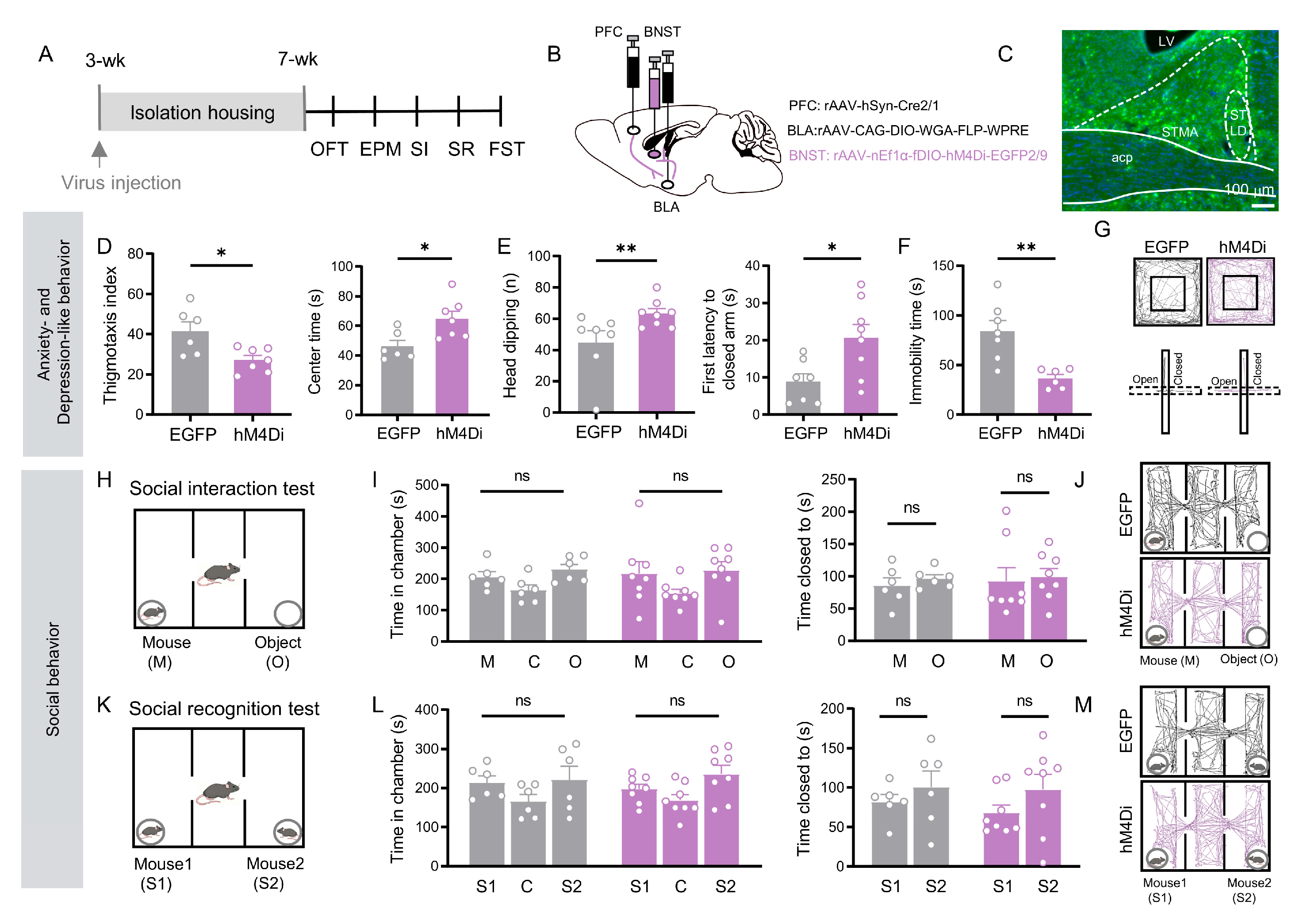
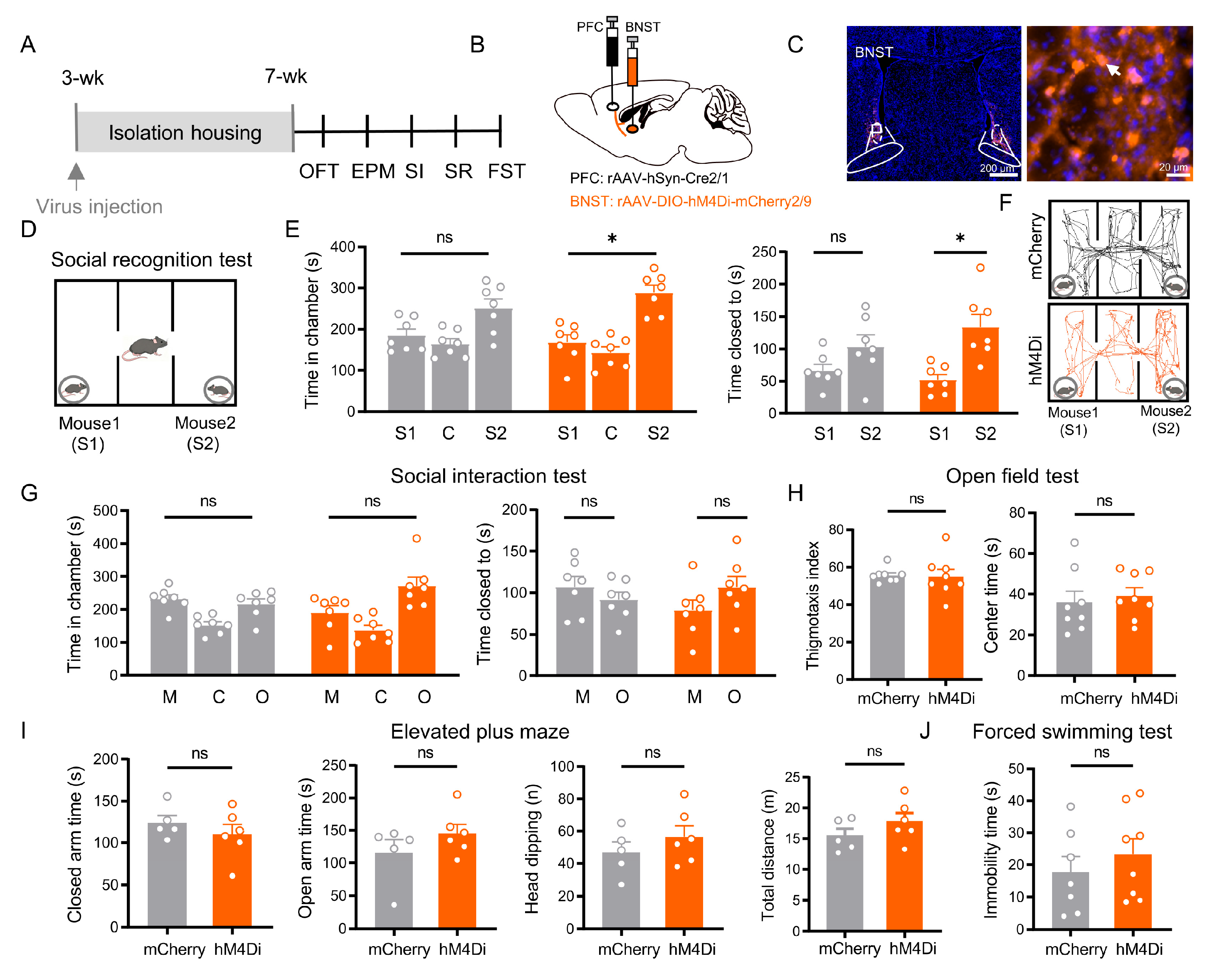
3.5. BNST Neurons in the PFC → BNST Pathway Specifically Mediate Social Recognition
4. Discussion
4.1. BLA: A Hub for Integrating and Amplifying Stress Signals
4.2. BNST: A Parallel Processing Center for Affective and Social Information
4.2.1. BNST Neurons in the PFC → BLA → BNST Pathway Mediate Affective Behaviors
4.2.2. BNST Neurons in the Direct PFC → BNST Pathway Independently Regulates Social Recognition
4.3. Limitations and Conceptual Framework
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFC | Prefrontal cortex |
| BLA | basolateral amygdala |
| BNST | bed nucleus of the stria terminalis |
| STMA | bed nucleus of the stria terminalis, medial division, anterior part |
| STLD | bed nucleus of the stria terminalis, lateral division, dorsal part |
| GH | Group-housing |
| IH | Isolation-housing |
| OFT | Open field test |
| EPM | Elevated plus maze |
| SI | Social interaction test |
| SR | Social recognition test |
| TS | Tail suspension |
| FST | Forced swimming test |
Appendix A
| Reagent | Source | Identifier | Concentration/Volume | Storage Temperature |
|---|---|---|---|---|
| Antibodies | ||||
| Anti-cFos polyclonal antibody | Abcam, Cambridge, Cambridgeshire, UK | ab190289 | 1:1000 | −20 °C |
| Alexa Flour 488 donkey anti-rabbit IgG (H + L) | Invitrogen, Carlsbad, CA, USA | A21206 | 1:500 | 4 °C |
| Virus | ||||
| rAAV2/1-hSyn-Cre-WPRE-hGH pA | Brain VTA, Wuhan, Hubei, China | PT-0136 | 200 nL | −80 °C |
| rAAV2/9-CAG-DIO-WGA-FLP-WPRE-hGH pA | Brain VTA, Wuhan, Hubei, China | PT-0557 | 200 nL | −80 °C |
| rAAV2/9-nEf1α-fDIO-hM4Di-EGFP-WPRE-hGH pA | Brain VTA, Wuhan, Hubei, China | PT-0159 | 200 nL | −80 °C |
| rAAV2/R-Ef1α-DIO-FLP-WPRE pA | Brain VTA, Wuhan, Hubei, China | PT-0075 | 200 nL | −80 °C |
| rAAV2/9-nEf1α-fDIO-mcherry-WPRE pA | Brain VTA, Wuhan, Hubei, China | PT-0339 | 200 nL | −80 °C |
| rAAV2/9-hSyn-DIO-hM4Di-mcherry | OBiO, Shanghai, China | H2481 | 200 nL | −80 °C |
| rAAV2/9-hSyn-EGFP-WPRE-hGH polyA | Brain VTA, Wuhan, Hubei, China | PT-0241 | 200 nL | −80 °C |
| pAAV2/R-hSyn-MCS-mCherry-3FLAG | OBiO, Shanghai, China | AOV063 | 200 nL | −80 °C |
| pAAV2/R-hSyn-MCS-EGFP-3FLAG | OBiO, Shanghai, China | AOV062 | 200 nL | −80 °C |
| Chemical reagent | ||||
| Clozapine-N-oxide | MedChemExpress, Monmouth Junction, NJ, USA | HY-17366 | 1 mg/kg 0.1 μg | −20 °C |
References
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ned, H.; Kalin, M.D. The Critical Relationship Between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Kupferberg, A.; Bicks, L.; Hasler, G. Social functioning in major depressive disorder. Neurosci. Biobehav. Rev. 2016, 69, 313–332. [Google Scholar] [CrossRef]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef]
- Avery, S.N.; Clauss, J.A.; Blackford, J.U. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology 2016, 41, 126–141. [Google Scholar] [CrossRef]
- Jin, S.; Liu, W.; Hu, Y.; Liu, Z.; Xia, Y.; Zhang, X.; Ding, Y.; Zhang, L.; Xie, S.; Ma, C.; et al. Aberrant functional connectivity of the bed nucleus of the stria terminalis and its age dependence in children and adolescents with social anxiety disorder. Asian J. Psychiatr. 2023, 82, 103498. [Google Scholar] [CrossRef]
- Pang, M.; Zhong, Y.; Hao, Z.; Xu, H.; Wu, Y.; Teng, C.; Li, J.; Xiao, C.; Fox, P.T.; Zhang, N.; et al. Resting-state causal connectivity of the bed nucleus of the stria terminalis in panic disorder. Brain Imaging Behav. 2021, 15, 25–35. [Google Scholar] [CrossRef]
- Williams, L.M. Precision psychiatry: A neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016, 3, 472–480. [Google Scholar] [CrossRef]
- Calhoon, G.G.; Tye, K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Morrison, J.H. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Liston, C.; Miller, M.M.; Goldwater, D.S.; Radley, J.J.; Rocher, A.B.; Hof, P.R.; Morrison, J.H.; McEwen, B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006, 26, 7870–7874. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Wellman, C.L. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009, 33, 773–783. [Google Scholar] [CrossRef]
- Yamamuro, K.; Bicks, L.K.; Leventhal, M.B.; Kato, D.; Im, S.; Flanigan, M.E.; Garkun, Y.; Norman, K.J.; Caro, K.; Sadahiro, M.; et al. A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat. Neurosci. 2020, 23, 1240–1252. [Google Scholar] [CrossRef]
- Britt, J.P.; Benaliouad, F.; McDevitt, R.A.; Stuber, G.D.; Wise, R.A.; Bonci, A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 2012, 76, 790–803. [Google Scholar] [CrossRef]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015, 527, 179–185. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Burgos-Robles, A.; Bhagat, N.D.; Leppla, C.A.; Tye, K.M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 2016, 321, 197–209. [Google Scholar] [CrossRef]
- Jhang, J.; Lee, H.; Kang, M.S.; Lee, H.S.; Park, H.; Han, J.H. Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response. Nat. Commun. 2018, 9, 2744. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- McGarry, L.M.; Carter, A.G. Prefrontal Cortex Drives Distinct Projection Neurons in the Basolateral Amygdala. Cell Rep. 2017, 21, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lowery-Gionta, E.G.; Crowley, N.A.; Bukalo, O.; Silverstein, S.; Holmes, A.; Kash, T.L. Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology 2018, 139, 68–75. [Google Scholar] [CrossRef]
- Wei, J.; Zhong, P.; Qin, L.; Tan, T.; Yan, Z. Chemicogenetic Restoration of the Prefrontal Cortex to Amygdala Pathway Ameliorates Stress-Induced Deficits. Cereb. Cortex 2018, 28, 1980–1990. [Google Scholar] [CrossRef]
- Kietzman, H.W.; Trinoskey-Rice, G.; Seo, E.H.; Guo, J.; Gourley, S.L. Neuronal Ensembles in the Amygdala Allow Social Information to Motivate Later Decisions. J. Neurosci. 2024, 44, e1848232024. [Google Scholar] [CrossRef]
- Felix-Ortiz, A.C.; Tye, K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014, 34, 586–595. [Google Scholar] [CrossRef]
- Ciocchi, S.; Herry, C.; Grenier, F.; Wolff, S.B.; Letzkus, J.J.; Vlachos, I.; Ehrlich, I.; Sprengel, R.; Deisseroth, K.; Stadler, M.B.; et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010, 468, 277–282. [Google Scholar] [CrossRef]
- Tye, K.M.; Prakash, R.; Kim, S.Y.; Fenno, L.E.; Grosenick, L.; Zarabi, H.; Thompson, K.R.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011, 471, 358–362. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, J.Y.; Holmes, A.; Pan, B.X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef]
- Duvarci, S.; Pare, D. Amygdala microcircuits controlling learned fear. Neuron 2014, 82, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.L.; Toufexis, D.J.; Davis, M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003, 463, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Gungor, N.Z.; Pare, D. Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J. Neurosci. 2016, 36, 8038–8049. [Google Scholar] [CrossRef]
- Dong, H.W.; Swanson, L.W. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: Implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J. Comp. Neurol. 2006, 494, 75–107. [Google Scholar] [CrossRef]
- Daniel, S.E.; Rainnie, D.G. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology 2016, 41, 103–125. [Google Scholar] [CrossRef]
- Marcinkiewcz, C.A.; Mazzone, C.M.; D’Agostino, G.; Halladay, L.R.; Hardaway, J.A.; DiBerto, J.F.; Navarro, M.; Burnham, N.; Cristiano, C.; Dorrier, C.E.; et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016, 537, 97–101. [Google Scholar] [CrossRef]
- Johnson, S.B.; Emmons, E.B.; Lingg, R.T.; Anderson, R.M.; Romig-Martin, S.A.; LaLumiere, R.T.; Narayanan, N.S.; Viau, V.; Radley, J.J. Prefrontal-Bed Nucleus Circuit Modulation of a Passive Coping Response Set. J. Neurosci. 2019, 39, 1405–1419. [Google Scholar] [CrossRef]
- Han, R.W.; Zhang, Z.Y.; Jiao, C.; Hu, Z.Y.; Pan, B.X. Synergism between two BLA-to-BNST pathways for appropriate expression of anxiety-like behaviors in male mice. Nat. Commun. 2024, 15, 3455. [Google Scholar] [CrossRef]
- Vantrease, J.E.; Avonts, B.; Padival, M.; DeJoseph, M.R.; Urban, J.H.; Rosenkranz, J.A. Sex Differences in the Activity of Basolateral Amygdalar Neurons That Project to the Bed Nucleus of the Stria Terminalis and Their Role in Anticipatory Anxiety. J. Neurosci. 2022, 42, 4488–4504. [Google Scholar] [CrossRef]
- Kim, S.Y.; Adhikari, A.; Lee, S.Y.; Marshel, J.H.; Kim, C.K.; Mallory, C.S.; Lo, M.; Pak, S.; Mattis, J.; Lim, B.K.; et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 2013, 496, 219–223. [Google Scholar] [CrossRef]
- Wang, J.; Jin, S.; Fu, W.; Liang, Y.; Yang, Y.; Xu, X. Pubertal exposure to bisphenol-A affects social recognition and arginine vasopressin in the brain of male mice. Ecotoxicol. Environ. Saf. 2021, 226, 112843. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, S.; Morishita, M. Sexually Dimorphic Formation of the Preoptic Area and the Bed Nucleus of the Stria Terminalis by Neuroestrogens. Front. Neurosci. 2020, 14, 797. [Google Scholar] [CrossRef] [PubMed]
- Rigney, N.; Campos-Lira, E.; Kirchner, M.K.; Wei, W.; Belkasim, S.; Beaumont, R.; Singh, S.; Suarez, S.G.; Hartswick, D.; Stern, J.E.; et al. A vasopressin circuit that modulates mouse social investigation and anxiety-like behavior in a sex-specific manner. Proc. Natl. Acad. Sci. USA 2024, 121, e2319641121. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.W.; Neuner, S.; Polepalli, J.S.; Beier, K.T.; Wright, M.; Walsh, J.J.; Lewis, E.M.; Luo, L.; Deisseroth, K.; Dolen, G.; et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 2017, 357, 1406–1411. [Google Scholar] [CrossRef]
- Grippo, A.J.; Wu, K.D.; Hassan, I.; Carter, C.S. Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depress. Anxiety 2008, 25, E17–E26. [Google Scholar] [CrossRef]
- Mamedova, D.I.; Nedogreeva, O.A.; Manolova, A.O.; Ovchinnikova, V.O.; Kostryukov, P.A.; Lazareva, N.A.; Moiseeva, Y.V.; Tret’yakova, L.V.; Kvichansky, A.A.; Onufriev, M.V.; et al. The impact of long-term isolation on anxiety, depressive-like and social behavior in aging Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) male rats. Sci. Rep. 2024, 14, 28135. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016, 2016, 6212983. [Google Scholar] [CrossRef]
- Sakurai, K.; Itou, T.; Morita, M.; Kasahara, E.; Moriyama, T.; Macpherson, T.; Ozawa, T.; Miyamoto, Y.; Yoneda, Y.; Sekiyama, A.; et al. Effects of Importin alpha1/KPNA1 deletion and adolescent social isolation stress on psychiatric disorder-associated behaviors in mice. PLoS ONE 2021, 16, e0258364. [Google Scholar] [CrossRef]
- Burrows, E.L.; Eastwood, A.F.; May, C.; Kolbe, S.C.; Hill, T.; McLachlan, N.M.; Churilov, L.; Hannan, A.J. Social Isolation Alters Social and Mating Behavior in the R451C Neuroligin Mouse Model of Autism. Neural Plast. 2017, 2017, 8361290. [Google Scholar] [CrossRef]
- Kercmar, J.; Budefeld, T.; Grgurevic, N.; Tobet, S.A.; Majdic, G. Adolescent social isolation changes social recognition in adult mice. Behav. Brain Res. 2011, 216, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Gu, Q.; Petralia, R.S.; Wang, Y.X.; Panja, D.; Liu, X.; Lehmann, M.L.; Zhu, H.; Zhu, J.; Li, Z. Mitophagy in the basolateral amygdala mediates increased anxiety induced by aversive social experience. Neuron 2021, 109, 3793–3809 e3798. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, P.; Qi, G.; Zhang, L.; Li, T.; Li, M.; Lv, X.; Lei, J.; Ming, J.; Tian, B. Systematic Input-Output Mapping Reveals Structural Plasticity of VTA Dopamine Neurons-Zona Incerta Loop Underlying the Social Buffering Effects in Learned Helplessness. Mol. Neurobiol. 2022, 59, 856–871. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.T.T.; Kim, P.J.; Kim, H.M.; Je, H.S. Neural circuit analysis using a novel intersectional split intein-mediated split-Cre recombinase system. Mol. Brain 2020, 13, 101. [Google Scholar] [CrossRef]
- Cai, Y.; Ge, J.; Pan, Z.Z. The projection from dorsal medial prefrontal cortex to basolateral amygdala promotes behaviors of negative emotion in rats. Front. Neurosci. 2024, 18, 1331864. [Google Scholar] [CrossRef]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef]
- Wang, Z.J.; Shwani, T.; Liu, J.; Zhong, P.; Yang, F.; Schatz, K.; Zhang, F.; Pralle, A.; Yan, Z. Molecular and cellular mechanisms for differential effects of chronic social isolation stress in males and females. Mol. Psychiatry 2022, 27, 3056–3068. [Google Scholar] [CrossRef]
- Allsop, S.A.; Wichmann, R.; Mills, F.; Burgos-Robles, A.; Chang, C.J.; Felix-Ortiz, A.C.; Vienne, A.; Beyeler, A.; Izadmehr, E.M.; Glober, G.; et al. Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 2018, 173, 1329–1342.e18. [Google Scholar] [CrossRef]
- Vyas, A.; Mitra, R.; Shankaranarayana Rao, B.S.; Chattarji, S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [CrossRef]
- McGaugh, J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004, 27, 1–28. [Google Scholar] [CrossRef]
- Goode, T.D.; Maren, S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn. Mem. 2017, 24, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, J.; Hazra, R.; Ahern, T.H.; Guo, J.D.; McDonald, A.J.; Mascagni, F.; Muller, J.F.; Young, L.J.; Rainnie, D.G. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 2011, 36, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Giardino, W.J.; Eban-Rothschild, A.; Christoffel, D.J.; Li, S.B.; Malenka, R.C.; de Lecea, L. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 2018, 21, 1084–1095. [Google Scholar] [CrossRef]
- Jennings, J.H.; Sparta, D.R.; Stamatakis, A.M.; Ung, R.L.; Pleil, K.E.; Kash, T.L.; Stuber, G.D. Distinct extended amygdala circuits for divergent motivational states. Nature 2013, 496, 224–228. [Google Scholar] [CrossRef]
- Rigney, N.; Zbib, A.; de Vries, G.J.; Petrulis, A. Knockdown of sexually differentiated vasopressin expression in the bed nucleus of the stria terminalis reduces social and sexual behaviour in male, but not female, mice. J. Neuroendocr. 2022, 34, e13083. [Google Scholar] [CrossRef]
- Duque-Wilckens, N.; Steinman, M.Q.; Busnelli, M.; Chini, B.; Yokoyama, S.; Pham, M.; Laredo, S.A.; Hao, R.; Perkeybile, A.M.; Minie, V.A.; et al. Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol. Psychiatry 2018, 83, 203–213. [Google Scholar] [CrossRef]
- Duque-Wilckens, N.; Torres, L.Y.; Yokoyama, S.; Minie, V.A.; Tran, A.M.; Petkova, S.P.; Hao, R.; Ramos-Maciel, S.; Rios, R.A.; Jackson, K.; et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA 2020, 117, 26406–26413. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Stein, D.J.; Albrechet-Souza, L.; Miczek, K.A.; de Almeida, R.M.M. Recovery of stress-impaired social behavior by an antagonist of the CRF binding protein, CRF(6-33,) in the bed nucleus of the stria terminalis of male rats. Behav. Brain Res. 2019, 357–358, 104–110. [Google Scholar] [CrossRef]
- Luo, P.X.; Zakharenkov, H.C.; Torres, L.Y.; Rios, R.A.; Gegenhuber, B.; Black, A.M.; Xu, C.K.; Minie, V.A.; Tran, A.M.; Tollkuhn, J.; et al. Oxytocin receptor behavioral effects and cell types in the bed nucleus of the stria terminalis. Horm. Behav. 2022, 143, 105203. [Google Scholar] [CrossRef]
- Schwabe, K.; Alam, M.; Saryyeva, A.; Lutjens, G.; Heissler, H.E.; Winter, L.; Heitland, I.; Krauss, J.K.; Kahl, K.G. Oscillatory activity in the BNST/ALIC and the frontal cortex in OCD: Acute effects of DBS. J. Neural Transm. 2021, 128, 215–224. [Google Scholar] [CrossRef]
- Hammack, S.E.; Braas, K.M.; May, V. Chemoarchitecture of the bed nucleus of the stria terminalis: Neurophenotypic diversity and function. Handb. Clin. Neurol. 2021, 179, 385–402. [Google Scholar] [CrossRef]
| Purpose | Brain Area | ||
|---|---|---|---|
| PFC | BLA | BNST | |
| label the PFC → BLA → BNST relay neurons | rAAV 2/1-Cre | rAAV 2/9-nEfIα-fDIO-mCherry-WPRE pA | rAAV 2/R-EfIα-DIO-FLP-WPRE pA |
| label the BNST neurons in the PFC → BLA → BNST circuit | rAAV 2/1-Cre | rAAV 2/9-CAG-DIO-WGA-FLP-WPRE-hGH pA | rAAV 2/9-nEfIα-fDIO-EGFP-WPRE-hGH pA |
| chemogenetic inhibits the PFC → BLA → BNST relay neurons | rAAV 2/1-Cre | rAAV 2/9-nEfIα-fDIO-hM4Di-EGFP-WPRE-hGH pA | rAAV 2/R-EfIα-DIO-FLP-WPRE pA |
| chemogenetic inhibits the BNST neurons in the PFC → BLA → BNST circuit | rAAV 2/1-Cre | rAAV 2/9-CAG-DIO-WGA-FLP-WPRE-hGH pA | rAAV 2/9-nEfIα-fDIO-hM4Di-EGFP-WPRE-hGH pA |
| chemogenetic inhibits the PFC-innervated BNST neurons | rAAV 2/1-Cre | / | rAAV 2/9-EfIα-DIO-hM4Di-mCherry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yuan, H.; Zhong, Y.; Zhang, X. Separate BNST Microcircuits Targeted by Direct Versus Amygdala-Relayed Prefrontal Inputs Mediate Dissociable Phenotypes After Isolation. Cells 2026, 15, 116. https://doi.org/10.3390/cells15020116
Yuan H, Zhong Y, Zhang X. Separate BNST Microcircuits Targeted by Direct Versus Amygdala-Relayed Prefrontal Inputs Mediate Dissociable Phenotypes After Isolation. Cells. 2026; 15(2):116. https://doi.org/10.3390/cells15020116
Chicago/Turabian StyleYuan, Hongxia, Yongmei Zhong, and Xuehan Zhang. 2026. "Separate BNST Microcircuits Targeted by Direct Versus Amygdala-Relayed Prefrontal Inputs Mediate Dissociable Phenotypes After Isolation" Cells 15, no. 2: 116. https://doi.org/10.3390/cells15020116
APA StyleYuan, H., Zhong, Y., & Zhang, X. (2026). Separate BNST Microcircuits Targeted by Direct Versus Amygdala-Relayed Prefrontal Inputs Mediate Dissociable Phenotypes After Isolation. Cells, 15(2), 116. https://doi.org/10.3390/cells15020116







