Integrin-Mediated TIMP1 Signaling Reprograms Liver Macrophages and Accelerates Colorectal Cancer Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Vector and the Establishment of Overexpression and Knockdown Cell Lines
2.3. GEO Data Processing
2.4. TCGA and GTEx Data Processing
2.5. CCLE Data Processing
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Western Blot Analysis
2.8. Co-Immunoprecipitation (Co-IP)
2.9. Hematoxylin and Eosin (H&E) Staining
2.10. Tissue Microarray (TMA) Construction and Immunohistochemistry (IHC)
2.11. Immunofluorescence Staining of Mouse Liver
2.12. Cellular Immunofluorescence
2.13. Preparation of Tumor-Conditioned Medium (CM)
2.14. Flow Cytometry Analysis
2.15. ELISA
2.16. Murine Tumor Metastasis Models
2.17. Drug and Immune Cell Depletion Treatments
2.18. Statistical Analysis
3. Results
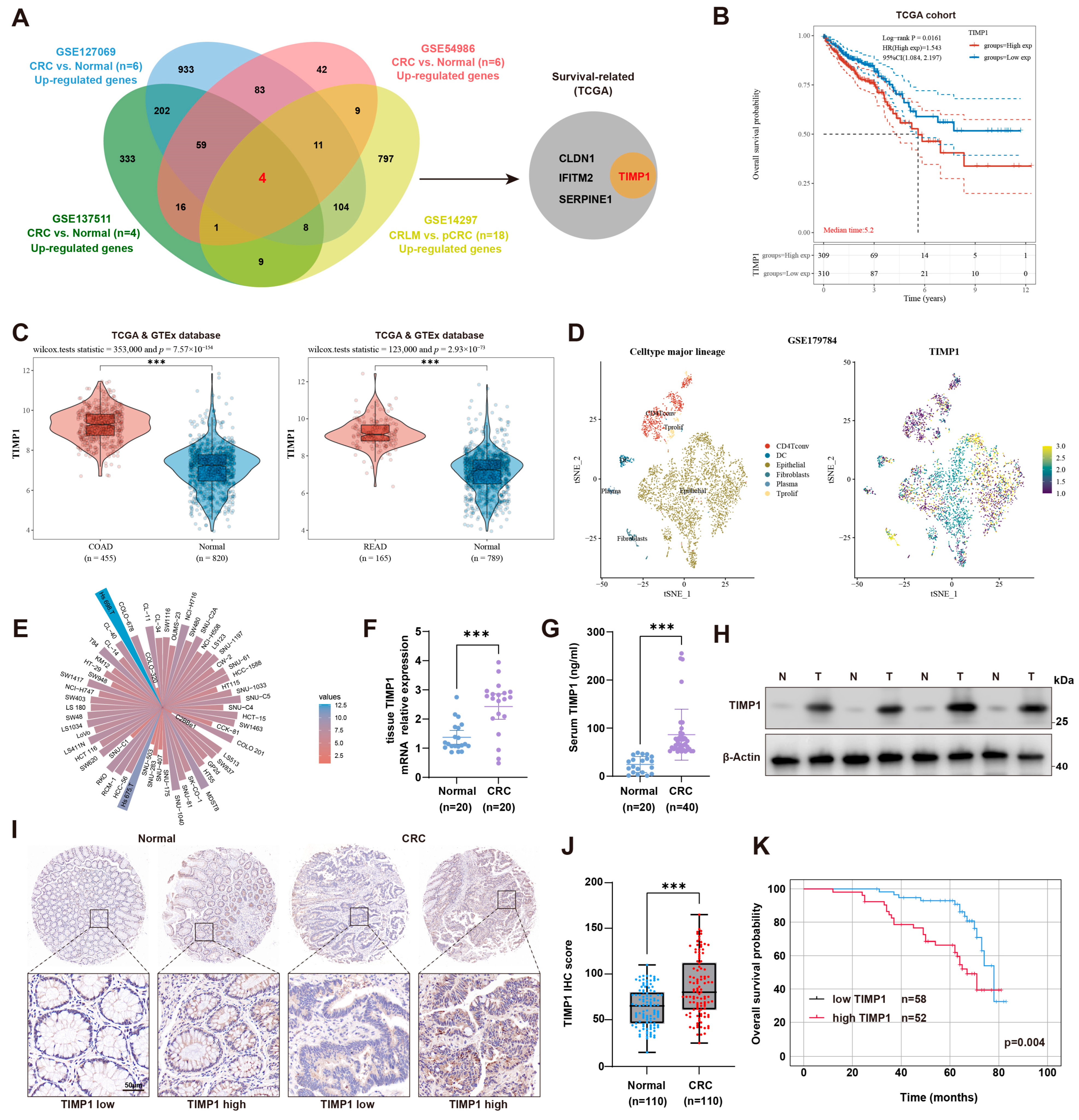
3.1. TIMP1 Is Overexpressed in CRC and Correlates with Poor Prognosis
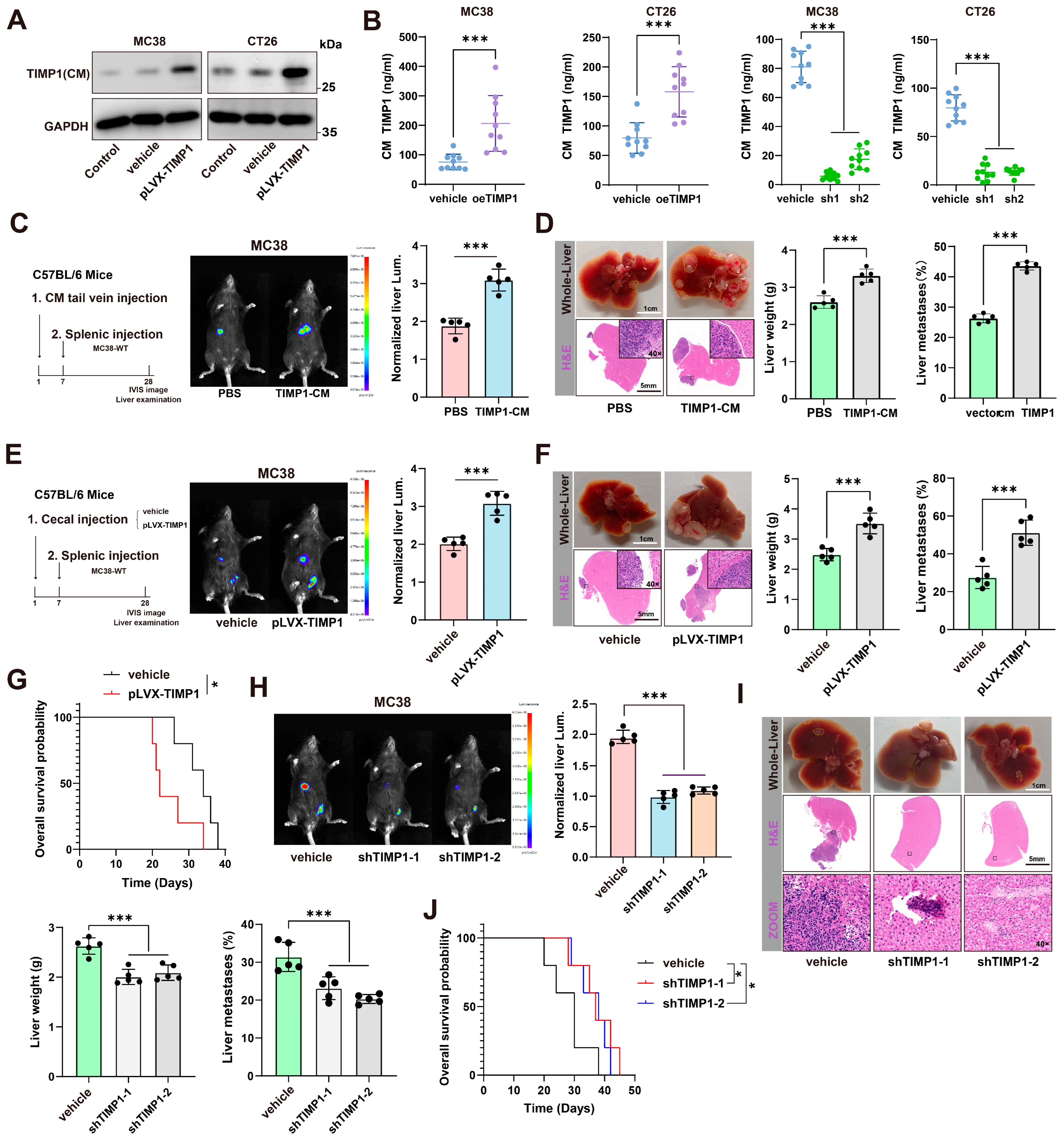
3.2. CRC Cells Can Secrete TIMP1 and Promote CRLM
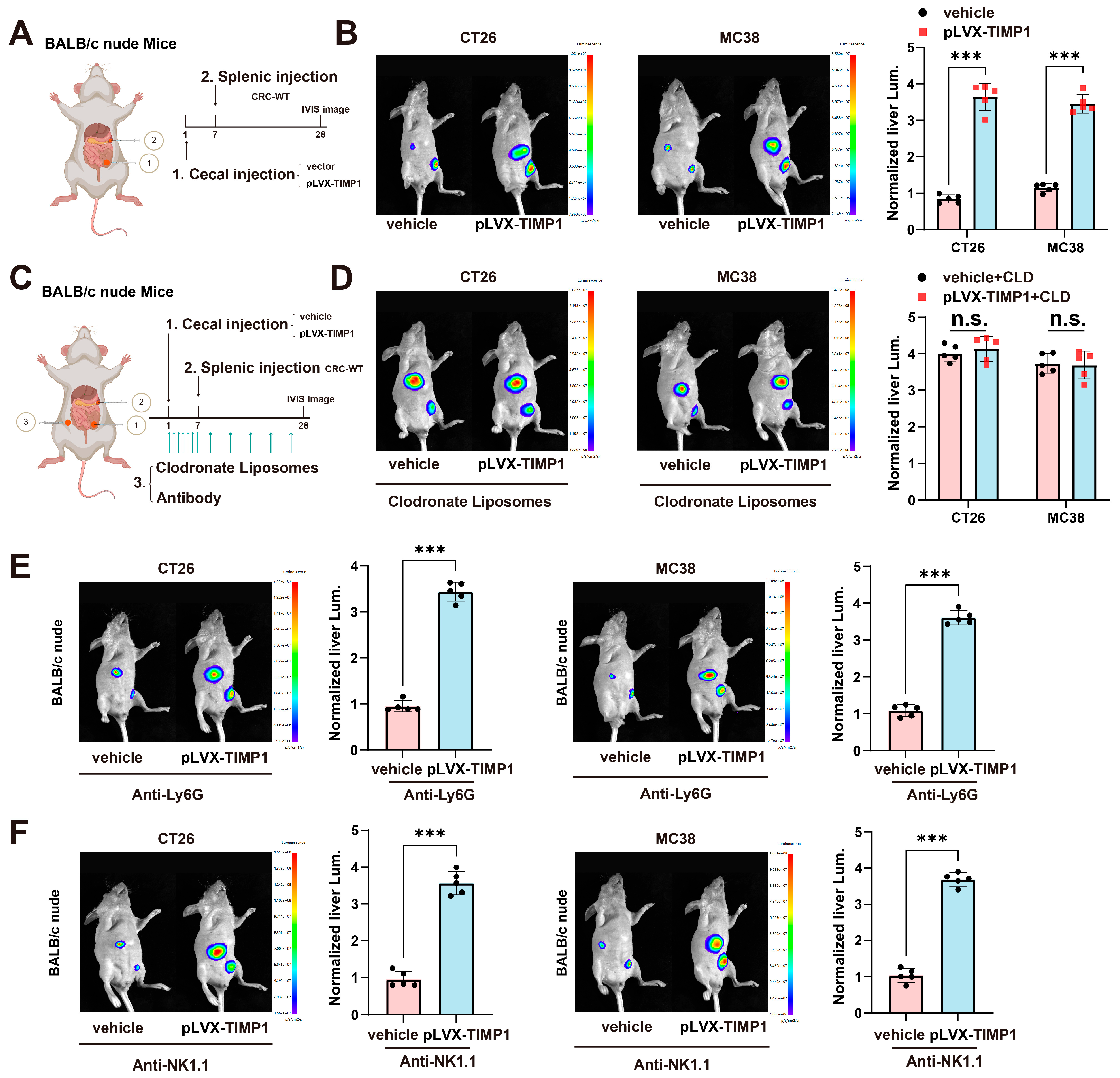
3.3. TIMP1 Promotes CRLM in a Macrophage-Dependent Manner
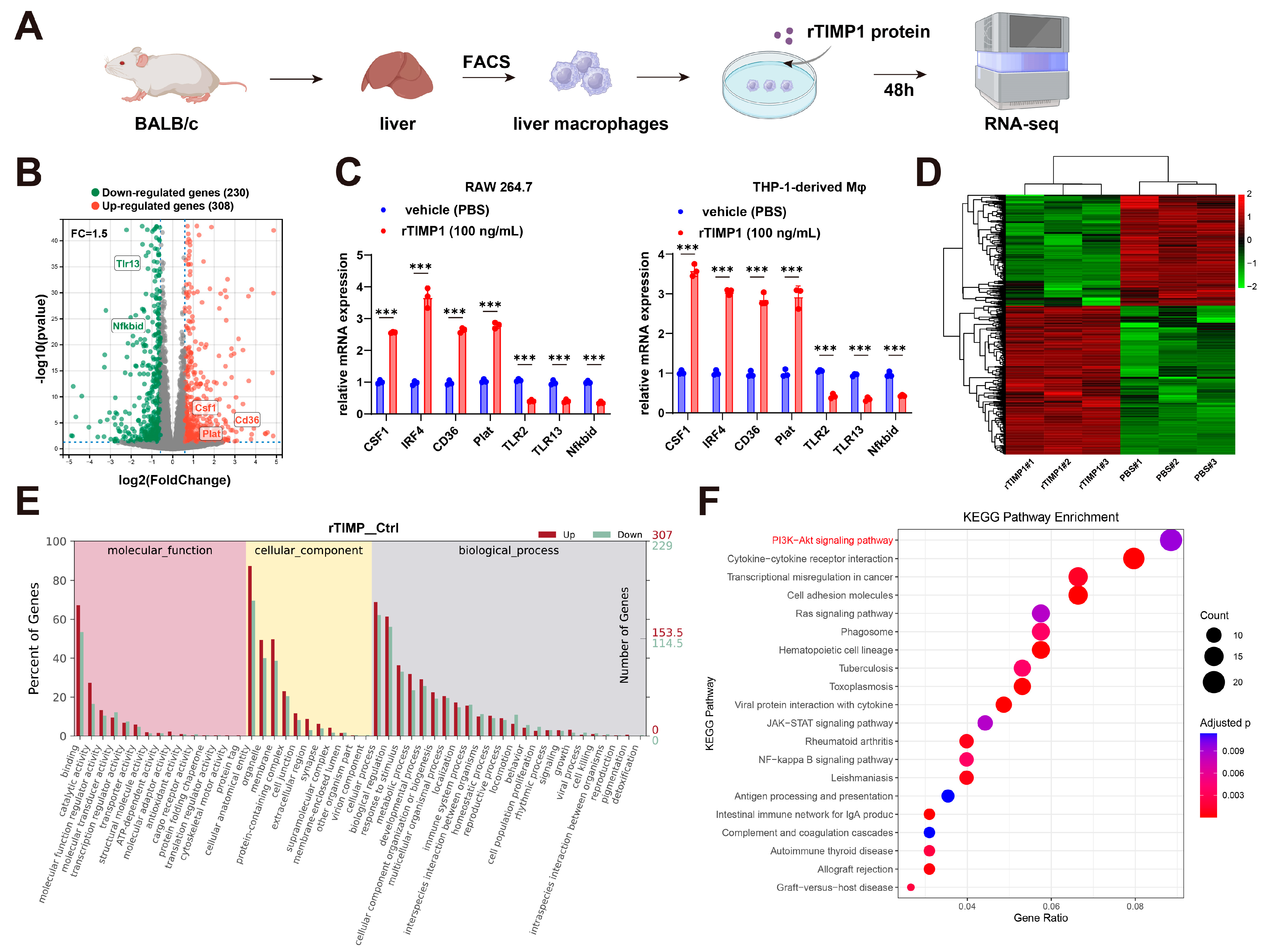
3.4. TIMP1 Stimulation Reprograms Liver Macrophage Gene Expression
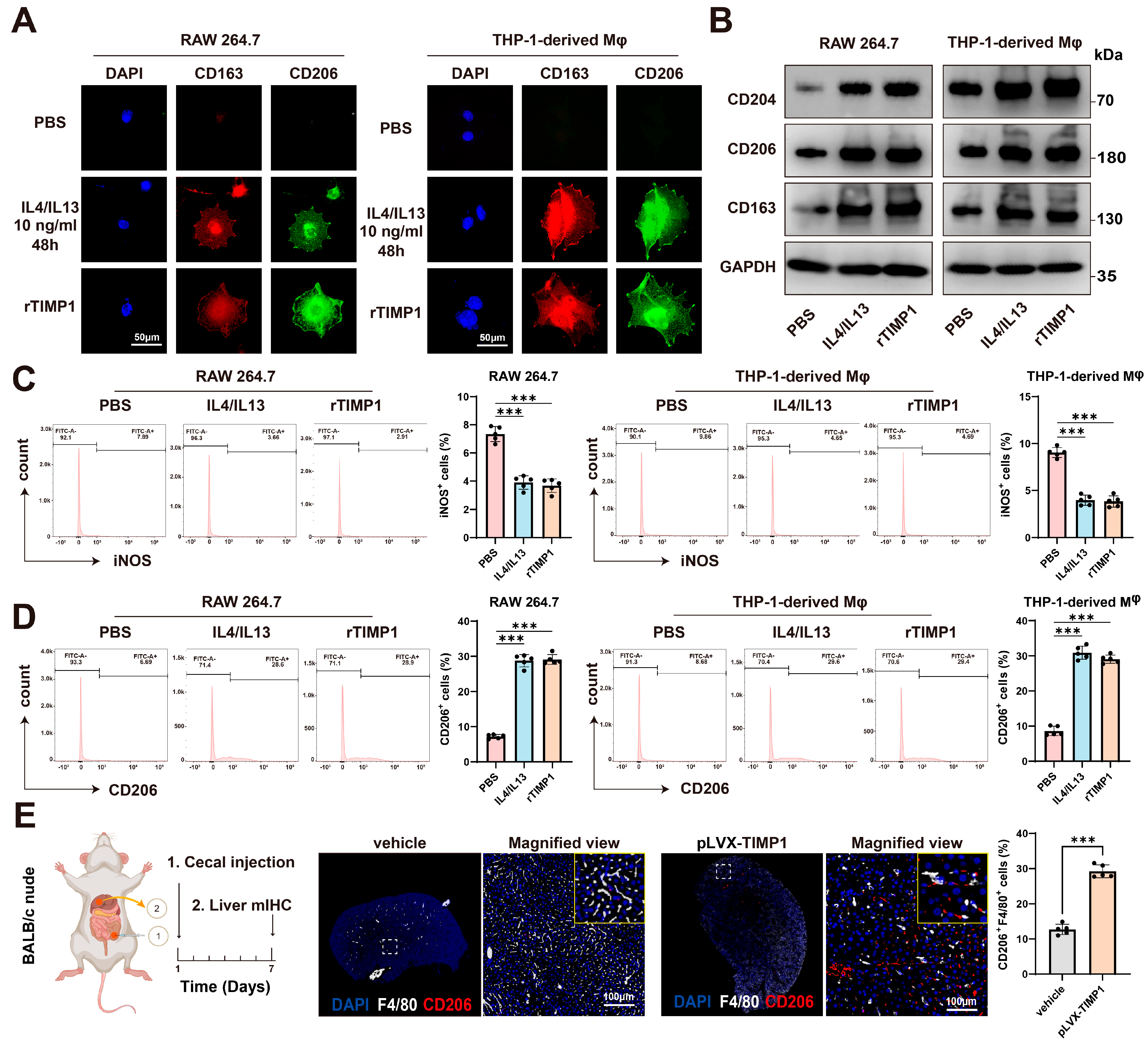
3.5. TIMP1 Drives Macrophage M2 Polarization and Pre-Metastatic Niche Formation
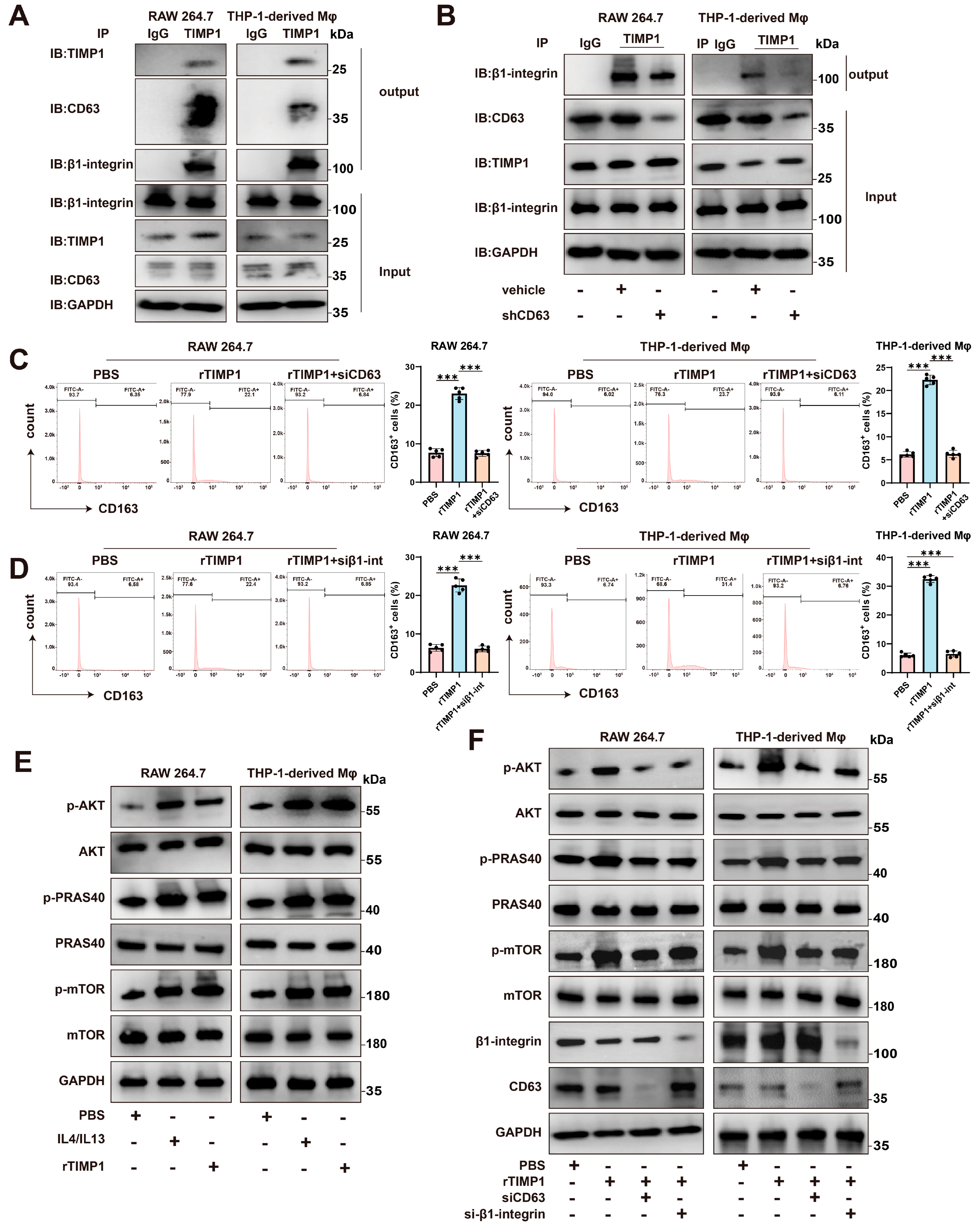
3.6. TIMP1 Interacts with CD63 and β1-Integrin to Activate AKT/mTOR Signaling and Promote M2 Macrophage Polarization
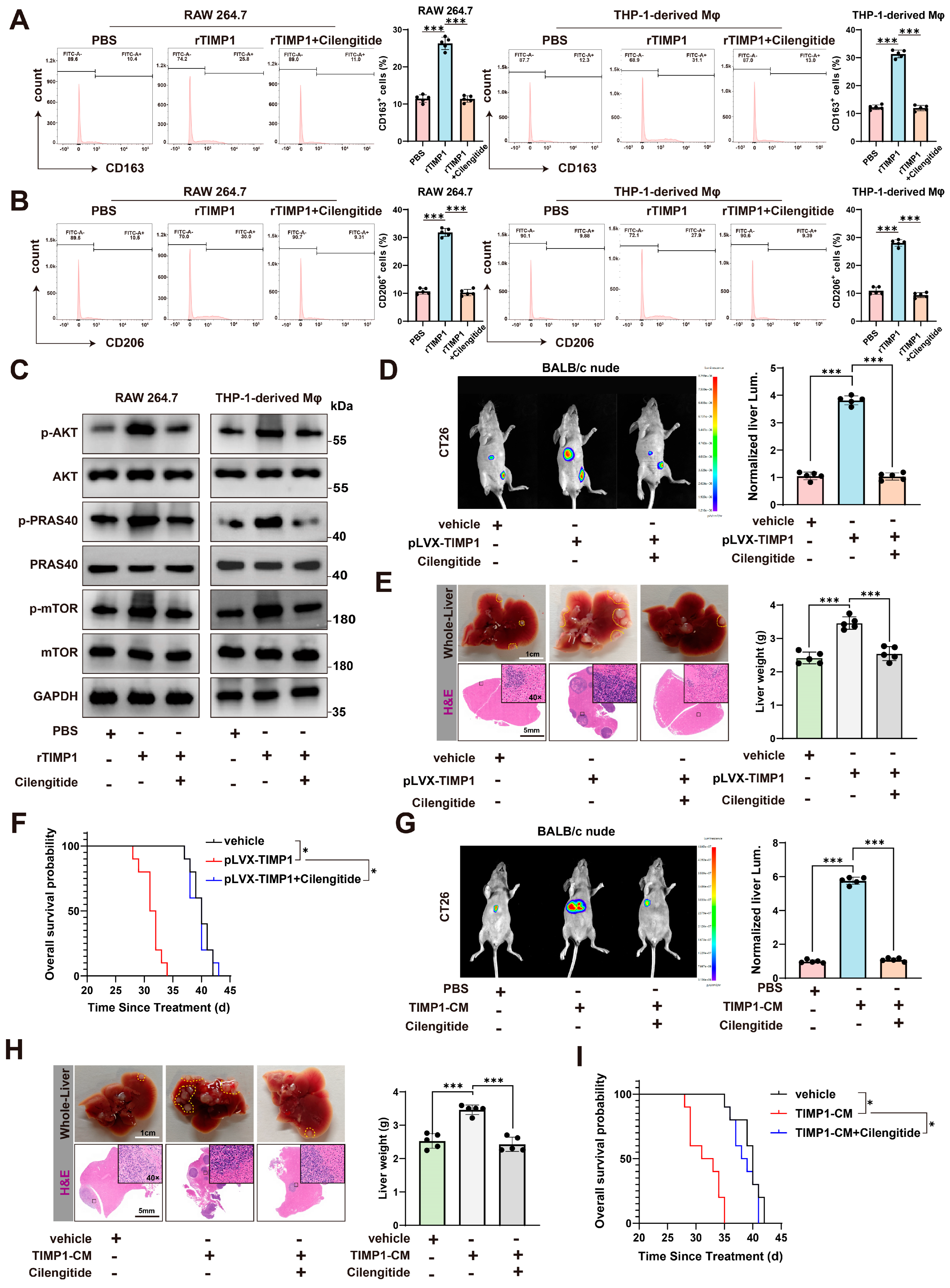
3.7. Cilengitide Blocks TIMP1-Induced M2 Polarization and Reduces Liver Metastasis In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, X.; He, X.; Hu, Z.; Huang, H.; Chen, J.; Chen, K.; Zhao, S.; Wei, P.; Li, D. Liver metastasis from colorectal cancer: Pathogenetic development, immune landscape of the tumour microenvironment and therapeutic approaches. J. Exp. Clin. Cancer Res. 2023, 42, 177. [Google Scholar] [CrossRef] [PubMed]
- van der Pool, A.E.; Damhuis, R.A.; Ijzermans, J.N.; de Wilt, J.H.; Eggermont, A.M.; Kranse, R.; Verhoef, C. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: A population-based series. Color Dis. 2012, 14, 56–61. [Google Scholar] [CrossRef]
- Zeineddine, F.A.; Zeineddine, M.A.; Yousef, A.; Gu, Y.; Chowdhury, S.; Dasari, A.; Huey, R.W.; Johnson, B.; Kee, B.; Lee, M.S.; et al. Survival improvement for patients with metastatic colorectal cancer over twenty years. NPJ Precis. Oncol. 2023, 7, 16. [Google Scholar] [CrossRef]
- Su, Y.M.; Liu, W.; Yan, X.L.; Wang, L.J.; Liu, M.; Wang, H.W.; Jin, K.M.; Bao, Q.; Wang, K.; Li, J.; et al. Five-year survival post hepatectomy for colorectal liver metastases in a real-world Chinese cohort: Recurrence patterns and prediction for potential cure. Cancer Med. 2023, 12, 9559–9569. [Google Scholar] [CrossRef]
- Chin, A.R.; Wang, S.E. Cancer Tills the Premetastatic Field: Mechanistic Basis and Clinical Implications. Clin. Cancer Res. 2016, 22, 3725–3733. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Li, T.; Li, T.; Liang, Y.; Yuan, Y.; Liu, Y.; Yao, Y.; Lei, X. Colorectal cancer cells-derived exosomal miR-188-3p promotes liver metastasis by creating a pre-metastatic niche via activation of hepatic stellate cells. J. Transl. Med. 2025, 23, 369. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, D.; Zhai, X.; Chen, F.; Niu, J.; Zhu, H. Macrophages in the premetastatic and metastatic niche: Key functions and therapeutic directions. J. Transl. Med. 2025, 23, 602. [Google Scholar] [CrossRef]
- Hou, S.; Zhao, Y.; Chen, J.; Lin, Y.; Qi, X. Tumor-associated macrophages in colorectal cancer metastasis: Molecular insights and translational perspectives. J. Transl. Med. 2024, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and β1-Integrin and the Functional Impact of Their Interaction in Cancer. Int. J. Mol. Sci. 2021, 22, 9319. [Google Scholar] [CrossRef] [PubMed]
- Kehusmaa, A.; Tuomisto, A.; Sirniö, P.; Karjalainen, H.; Kastinen, M.; Tapiainen, V.V.; Äijälä, V.K.; Tervahartiala, T.; Sorsa, T.; Rintala, J.; et al. Associations of serum and tissue TIMP1 with host response and survival in colorectal cancer. Sci. Rep. 2025, 15, 1440. [Google Scholar] [CrossRef]
- D’Angelo, R.C.; Liu, X.W.; Najy, A.J.; Jung, Y.S.; Won, J.; Chai, K.X.; Fridman, R.; Kim, H.R. TIMP-1 via TWIST1 induces EMT phenotypes in human breast epithelial cells. Mol. Cancer Res. 2014, 12, 1324–1333. [Google Scholar] [CrossRef]
- Kollet, O.; Das, A.; Karamanos, N.; Auf dem Keller, U.; Sagi, I. Redefining metalloproteases specificity through network proteolysis. Trends Mol. Med. 2024, 30, 147–163. [Google Scholar] [CrossRef]
- Nowak-Wąs, M.; Wąs, P.; Czuba, Z.; Wojnicz, R.; Wyględowska-Promieńska, D. Expression of Tissue Inhibitors of Metalloproteinases (TIMP-1, TIMP-2, TIMP-3, TIMP-4) in Blood Serum of Patients with Keratoconus. J. Clin. Med. 2024, 13, 1168. [Google Scholar] [CrossRef]
- Rao, V.S.; Gu, Q.; Tzschentke, S.; Lin, K.; Ganig, N.; Thepkaysone, M.L.; Wong, F.C.; Polster, H.; Seifert, L.; Seifert, A.M.; et al. Extravesicular TIMP-1 is a non-invasive independent prognostic marker and potential therapeutic target in colorectal liver metastases. Oncogene 2022, 41, 1809–1820. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Pan, X.; Yi, M.; Liu, C.; Jin, Y.; Liu, B.; Hu, G.; Yuan, X. Cilengitide, an αvβ3-integrin inhibitor, enhances the efficacy of anti-programmed cell death-1 therapy in a murine melanoma model. Bioengineered 2022, 13, 4557–4572. [Google Scholar] [CrossRef]
- Patras, L.; Shaashua, L.; Matei, I.; Lyden, D. Immune determinants of the pre-metastatic niche. Cancer Cell 2023, 41, 546–572. [Google Scholar] [CrossRef]
- Seubert, B.; Grünwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, B.; Harant, V.; Schaten, S.; Frühschütz, M.; Spallek, R.; Höchst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology 2016, 151, 1011–1024.e1017. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Liu, Y.; Xu, J.; Liu, J.; Xu, T.; Tong, J.; Liu, L.; Hou, Y.; Liu, D.; Yang, H.; et al. TIMP1 Indicates Poor Prognosis of Renal Cell Carcinoma and Accelerates Tumorigenesis via EMT Signaling Pathway. Front. Genet. 2022, 13, 648134. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Liu, X.W.; Chirco, R.; Warner, R.B.; Fridman, R.; Kim, H.R. TIMP-1 induces an EMT-like phenotypic conversion in MDCK cells independent of its MMP-inhibitory domain. PLoS ONE 2012, 7, e38773. [Google Scholar] [CrossRef]
- Dittmer, A.; Dittmer, J. A CAF-Fueled TIMP-1/CD63/ITGB1/STAT3 Feedback Loop Promotes Migration and Growth of Breast Cancer Cells. Cancers 2022, 14, 4983. [Google Scholar] [CrossRef]
- Duch, P.; Díaz-Valdivia, N.; Ikemori, R.; Gabasa, M.; Radisky, E.S.; Arshakyan, M.; Gea-Sorlí, S.; Mateu-Bosch, A.; Bragado, P.; Carrasco, J.L.; et al. Aberrant TIMP-1 overexpression in tumor-associated fibroblasts drives tumor progression through CD63 in lung adenocarcinoma. Matrix Biol. 2022, 111, 207–225. [Google Scholar] [CrossRef]
- Hermann, C.D.; Schoeps, B.; Eckfeld, C.; Munkhbaatar, E.; Kniep, L.; Prokopchuk, O.; Wirges, N.; Steiger, K.; Häußler, D.; Knolle, P.; et al. TIMP1 expression underlies sex disparity in liver metastasis and survival in pancreatic cancer. J. Exp. Med. 2021, 218, e20210911. [Google Scholar] [CrossRef]
- Negrin, L.L.; Carlin, G.L.; Ristl, R.; Hajdu, S. Serum levels of matrix metalloproteinases 1, 2, and 7, and their tissue inhibitors 1, 2, 3, and 4 in polytraumatized patients: Time trajectories, correlations, and their ability to predict mortality. PLoS ONE 2024, 19, e0300258. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Wang, Y.; Shen, S.; Chen, W.; Liu, T.; Wang, P.; Fan, X.; Liu, L.; Jia, J.; et al. TIMP-1 Promotes Expression of MCP-1 and Macrophage Migration by Inducing Fli-1 in Experimental Liver Fibrosis. J. Clin. Transl. Hepatol. 2024, 12, 634–645. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Opzoomer, J.W.; Anstee, J.E.; Dean, I.; Hill, E.J.; Bouybayoune, I.; Caron, J.; Muliaditan, T.; Gordon, P.; Sosnowska, D.; Nuamah, R.; et al. Macrophages orchestrate the expansion of a proangiogenic perivascular niche during cancer progression. Sci. Adv. 2021, 7, eabg9518. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Nabors, L.B.; Fink, K.L.; Mikkelsen, T.; Grujicic, D.; Tarnawski, R.; Nam, D.H.; Mazurkiewicz, M.; Salacz, M.; Ashby, L.; Zagonel, V.; et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: Results of the open-label, controlled, randomized phase II CORE study. Neuro-Oncology 2015, 17, 708–717. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Dong, Z.; Liu, K. Integrins in cancer: Emerging mechanisms and therapeutic opportunities. Pharmacol. Ther. 2023, 247, 108458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Liu, J.; Zhao, L.; Wang, L.; Sheng, G.; Cheng, P.; Han, M.; Li, G.; Zheng, Z. Integrin-Mediated TIMP1 Signaling Reprograms Liver Macrophages and Accelerates Colorectal Cancer Metastasis. Cells 2026, 15, 29. https://doi.org/10.3390/cells15010029
Liu J, Zhao L, Wang L, Sheng G, Cheng P, Han M, Li G, Zheng Z. Integrin-Mediated TIMP1 Signaling Reprograms Liver Macrophages and Accelerates Colorectal Cancer Metastasis. Cells. 2026; 15(1):29. https://doi.org/10.3390/cells15010029
Chicago/Turabian StyleLiu, Jialiang, Liming Zhao, Lin Wang, Guoli Sheng, Pu Cheng, Mingyu Han, Guoxin Li, and Zhaoxu Zheng. 2026. "Integrin-Mediated TIMP1 Signaling Reprograms Liver Macrophages and Accelerates Colorectal Cancer Metastasis" Cells 15, no. 1: 29. https://doi.org/10.3390/cells15010029
APA StyleLiu, J., Zhao, L., Wang, L., Sheng, G., Cheng, P., Han, M., Li, G., & Zheng, Z. (2026). Integrin-Mediated TIMP1 Signaling Reprograms Liver Macrophages and Accelerates Colorectal Cancer Metastasis. Cells, 15(1), 29. https://doi.org/10.3390/cells15010029




