Lymphatic Endothelial Cells and Organ-Associated Lymphangiogenesis in Tumor Microenvironment
Highlights
- LECs have heterogeneous and plastic features.
- Lymphatic vessels have a dual role in cancer progression and metastasis.
- Organ-associated lymphangiogenesis is regulated by various signaling pathways in TME.
- Functional diversity of LECs will contribute to elucidating the pathogenic mechanism of lymphatic-related diseases.
- Lymphatic modulation is focused on inhibiting pro-metastatic functions and enhancing protective anti-tumor immunity.
- Intervention of organ-specific lymphangiogenesis may be promising for molecular target and gene therapy.
Abstract
1. Introduction
2. The Heterogeneity and Functional Plasticity of Tumor-Associated Lymphatic Vessels
2.1. Endothelial Cells (ECs)
2.2. ScRNA-Seq of LECs
2.3. Lymphatic Heterogeneity and Plasticity
2.4. Tumor Microenvironment (TME) and Lymphatic Remodeling
3. Tumor Lymphangiogenesis and Immunotherapy
3.1. Dual Role of Lymphatic Vessels in Cancer Progression and Metastasis
3.2. Lymphangiogenesis and Immunotherapy
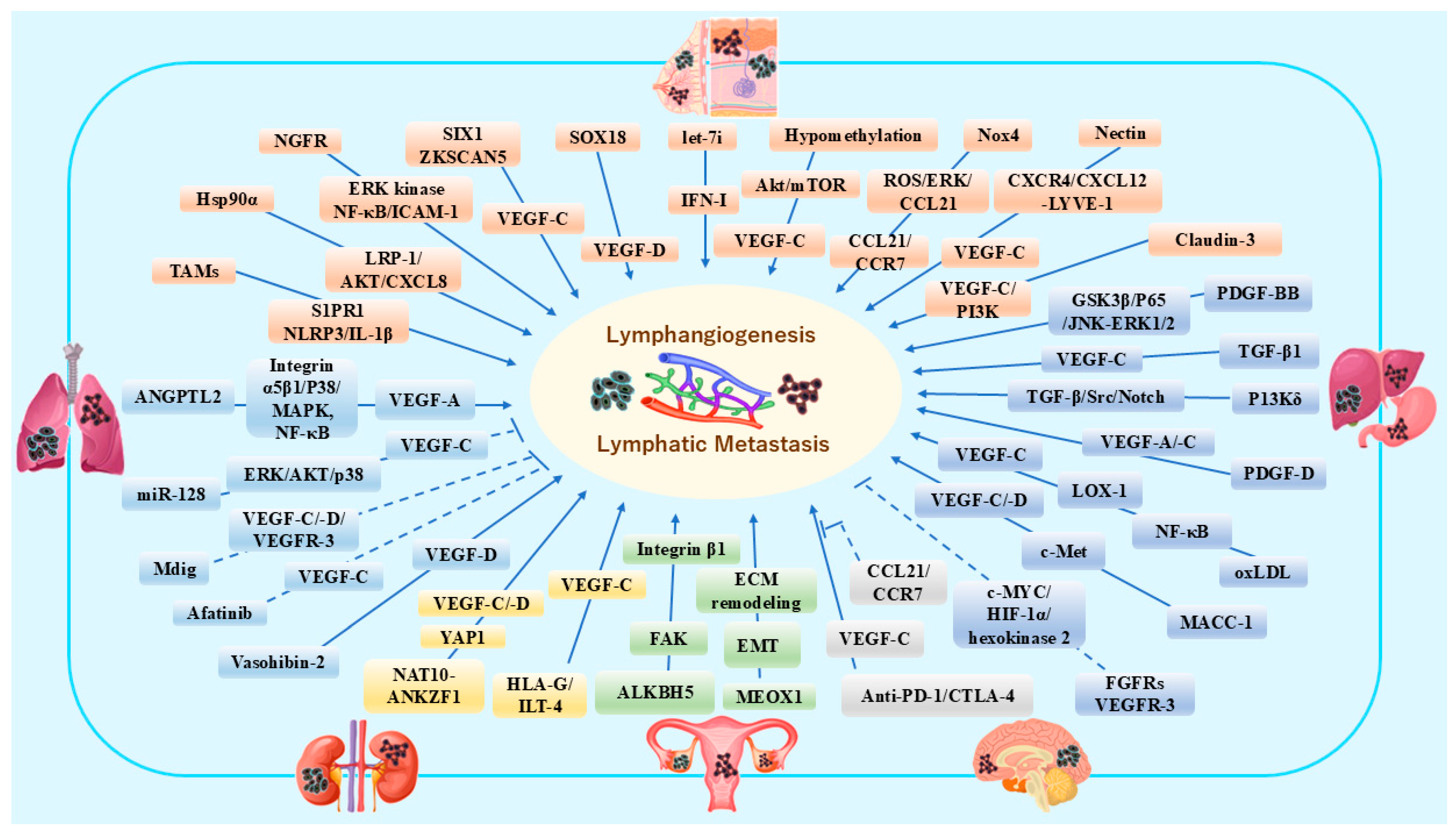
4. Organ-Associated Lymphatic Vessels and Lymphangiogenesis in Cancers
4.1. Breast Cancer and Melanoma
4.2. Lung Cancer
4.3. Gastric Cancer and Hepatic Cancer
4.4. Renal Cancer
4.5. Ovarian Cancer
4.6. Brain Malignant Tumors
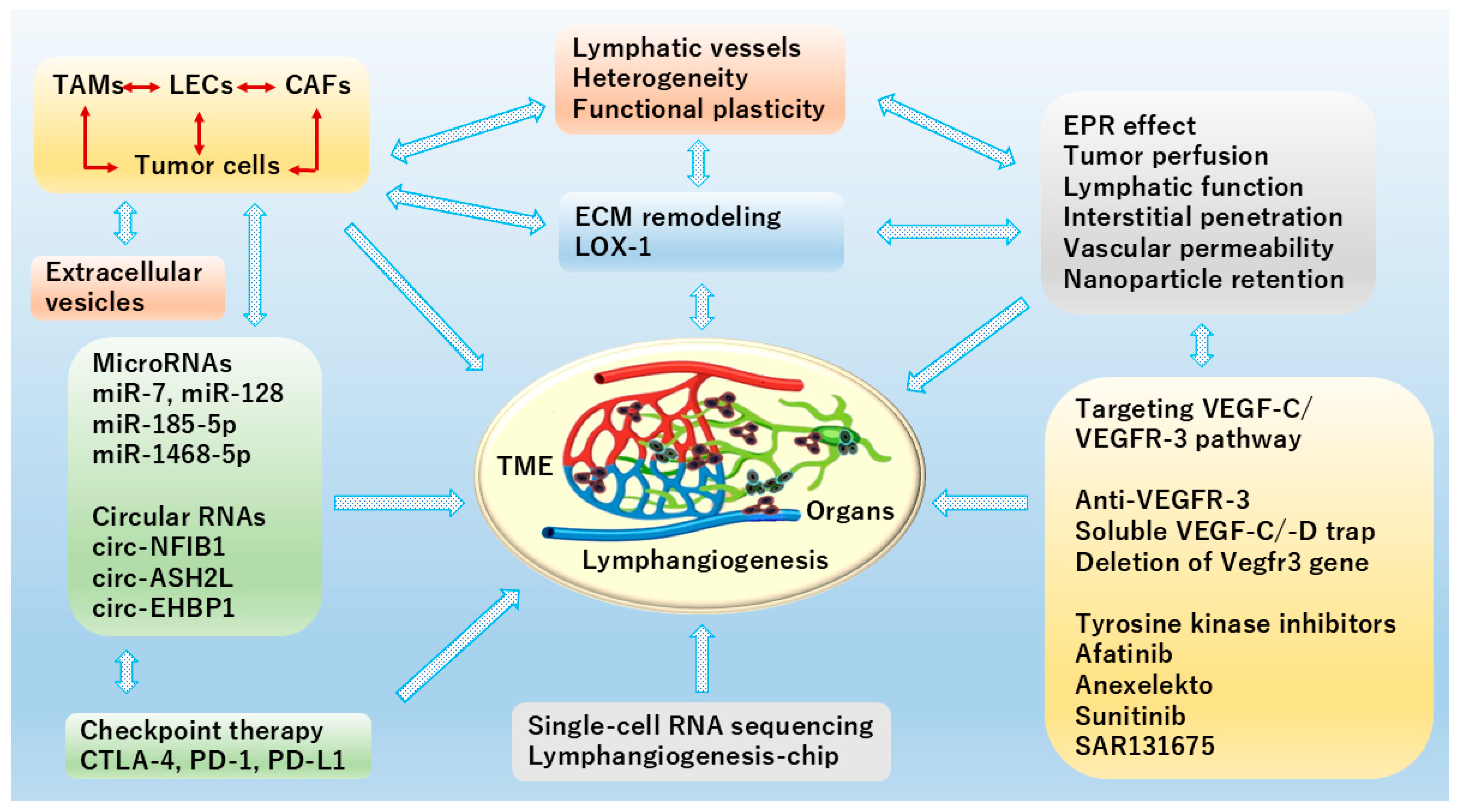
5. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ji, R.C.; Eshita, Y. Rapamycin inhibition of CFA-induced lymphangiogenesis in PLN is independent of mast cells. Mol. Biol. Rep. 2014, 41, 2217–2228. [Google Scholar] [CrossRef]
- Ji, R.C.; Eshita, Y.; Kobayashi, T.; Hidano, S.; Kamiyama, N.; Onishi, Y. Role of simvastatin in tumor lymphangiogenesis and lymph node metastasis. Clin. Exp. Metastasis 2018, 35, 785–796. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Jones, D. Parallels of Resistance between Angiogenesis and Lymphangiogenesis Inhibition in Cancer Therapy. Cells 2020, 9, 762. [Google Scholar] [CrossRef]
- Dejana, E.; Hirschi, K.K.; Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Tombor, L.S.; John, D.; Glaser, S.F.; Luxán, G.; Forte, E.; Furtado, M.; Rosenthal, N.; Baumgarten, N.; Schulz, M.H.; Wittig, J.; et al. Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction. Nat. Commun. 2021, 12, 681. [Google Scholar] [CrossRef]
- Ma, W.; Oliver, G. Lymphatic Endothelial Cell Plasticity in Development and Disease. Physiology 2017, 32, 444–452. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Breiteneder-Geleff, S.; Soleiman, A.; Kowalski, H.; Horvat, R.; Amann, G.; Kriehuber, E.; Diem, K.; Weninger, W.; Tschachler, E.; Alitalo, K.; et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999, 154, 385–394. [Google Scholar] [CrossRef]
- Morris, V.A.; Punjabi, A.S.; Lagunoff, M. Activation of Akt through gp130 receptor signaling is required for Kaposi’s sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J. Virol. 2008, 82, 8771–8779. [Google Scholar] [CrossRef]
- Oliver, G.; Srinivasan, R.S. Endothelial cell plasticity: How to become and remain a lymphatic endothelial cell. Development 2010, 137, 363–372. [Google Scholar] [CrossRef]
- Wang, H.W.; Trotter, M.W.; Lagos, D.; Bourboulia, D.; Henderson, S.; Mäkinen, T.; Elliman, S.; Flanagan, A.M.; Alitalo, K.; Boshoff, C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 2004, 36, 687–693. [Google Scholar] [CrossRef]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Augusto, M.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; Manganelli, V. Role of Lipid Rafts on LRP8 Signaling Triggered by Anti-β2-GPI Antibodies in Endothelial Cells. Biomedicines 2023, 11, 3135. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Shen, C.; Jeong, S.; Xu, N.; Chen, C.; Hang, H.L.; Tong, Y.; Cao, J. Novel discovery of PDPN-positive CAFs contributing to tumor-associated lymphangiogenesis through mesenchymal to lymphatic endothelial transition in intrahepatic cholangiocarcinoma. Genes Dis. 2023, 10, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, W.; Cui, X.; Huang, Z.; Wen, D.; Yang, Y.; Yu, W.; Cui, L.; Liu, C.Y. CCBE1 promotes tumor lymphangiogenesis and is negatively regulated by TGFbeta signaling in colorectal cancer. Theranostics 2020, 10, 2327–2341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, T.; Zhang, Y.; Wu, Y.; Zuo, A.; Liu, S.; Ba, Y.; Liu, B.; Yang, S.; Chen, Y.; et al. Cancer-associated fibroblasts enhance colorectal cancer lymphatic metastasis via CLEC11A/LGR5-mediated WNT pathway activation. J. Clin. Investig. 2025, 135, e194243. [Google Scholar] [CrossRef]
- Oo, M.W.; Hikita, T.; Mashima, T.; Torigata, K.; Thu, Y.M.; Habu, T.; Kawai, H.; Ohara, T.; Tomida, S.; Ito, S.; et al. Cancer-associated fibroblast-derived SOD3 enhances lymphangiogenesis to drive metastasis in lung adenocarcinoma. Angiogenesis 2025, 28, 51. [Google Scholar] [CrossRef]
- Xie, J.; Lin, X.; Deng, X.; Tang, H.; Zou, Y.; Chen, W.; Xie, X. Cancer-associated fibroblast-derived extracellular vesicles: Regulators and therapeutic targets in the tumor microenvironment. Cancer Drug Resist. 2025, 8, 2. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, H.; Jia, L.; Luo, Y.; Zhang, D.; An, M.; Pang, M.; Diao, X.; Li, W.; Chen, J.; et al. Integrin α6-containing extracellular vesicles promote lymphatic remodelling for pre-metastatic niche formation in lymph nodes via interplay with CD151. J. Extracell. Vesicles 2024, 13, e12518. [Google Scholar] [CrossRef]
- den Braanker, H.; van Stigt, A.C.; Kok, M.R.; Lubberts, E.; Bisoendial, R.J. Single-Cell RNA Sequencing Reveals Heterogeneity and Functional Diversity of Lymphatic Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11976. [Google Scholar] [CrossRef]
- Hedlund, E.; Deng, Q. Single-cell RNA sequencing: Technical advancements and biological applications. Mol. Aspects Med. 2018, 59, 36–46. [Google Scholar] [CrossRef]
- Wiggins, B.G.; Wang, Y.F.; Burke, A.; Grunberg, N.; Vlachaki Walker, J.M.; Dore, M.; Chahrour, C.; Pennycook, B.R.; Sanchez-Garrido, J.; Vernia, S.; et al. Endothelial sensing of AHR ligands regulates intestinal homeostasis. Nature 2023, 621, 821–829. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.P.M.H.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Arasa, J.; Collado-Diaz, V.; Kritikos, I.; Medina-Sanchez, J.D.; Friess, M.C.; Sigmund, E.C.; Schineis, P.; Hunter, M.C.; Tacconi, C.; Paterson, N.; et al. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J. Exp. Med. 2021, 218, e20201413. [Google Scholar] [CrossRef]
- Tai-Nagara, I.; Hasumi, Y.; Kusumoto, D.; Hasumi, H.; Okabe, K.; Ando, T.; Matsuzaki, F.; Itoh, F.; Saya, H.; Liu, C.; et al. Blood and lymphatic systems are segregated by the FLCN tumor suppressor. Nat. Commun. 2020, 11, 6314. [Google Scholar] [CrossRef]
- Goveia, J.; Rohlenova, K.; Taverna, F.; Treps, L.; Conradi, L.C.; Pircher, A.; Geldhof, V.; de Rooij, L.P.M.H.; Kalucka, J.; Sokol, L.; et al. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell 2020, 37, 21–36. [Google Scholar] [CrossRef]
- Takeda, A.; Hollmén, M.; Dermadi, D.; Pan, J.; Brulois, K.F.; Kaukonen, R.; Lönnberg, T.; Boström, P.; Koskivuo, I.; Irjala, H.; et al. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 2019, 51, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Houbaert, D.; Nikolakopoulos, A.P.; Jacobs, K.A.; Meçe, O.; Roels, J.; Shankar, G.; Agrawal, M.; More, S.; Ganne, M.; Rillaerts, K.; et al. An autophagy program that promotes T cell egress from the lymph node controls responses to immune checkpoint blockade. Cell Rep. 2024, 43, 114020. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, Y.; Luo, Y.; Zhu, J.; Zheng, H.; Gao, B.; Guo, X.; Li, Z.; Chen, R.; Chen, C. circNFIB1 inhibits lymphangiogenesis and lymphatic metastasis via the miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol. Cancer 2020, 19, 82. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Yang, L.; Li, M.; Zhang, Y.; Zhang, J. CircASH2L Promotes Ovarian Cancer Tumorigenesis, Angiogenesis, and Lymphangiogenesis by Regulating the miR-665/VEGFA Axis as a Competing Endogenous RNA. Front. Cell Dev. Biol. 2020, 8, 595585. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, Y.; Zhao, Y.; Kong, Y.; Zheng, H.; Li, Y.; Gao, B.; Ai, L.; Huang, H.; Huang, J.; et al. circEHBP1 promotes lymphangiogenesis and lymphatic metastasis of bladder cancer via miR-130a-3p/TGFβR1/VEGF-D signaling. Mol. Ther. 2021, 29, 1838–1852. [Google Scholar] [CrossRef]
- Aurrand-Lions, M.; Johnson-Leger, C.; Wong, C.; Du Pasquier, L.; Imhof, B.A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 2001, 98, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Dillard, M.E.; Baluk, P.; McDonald, D.M.; Harvey, N.L.; Frase, S.L.; Oliver, G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008, 22, 3282–3291. [Google Scholar] [CrossRef]
- Petrova, T.V.; Koh, G.Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 2018, 215, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ulvmar, M.H.; Stanczuk, L.; Martinez-Corral, I.; Frye, M.; Alitalo, K.; Mäkinen, T. Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms. Nat. Commun. 2018, 9, 1296. [Google Scholar] [CrossRef]
- González-Loyola, A.; Bovay, E.; Kim, J.; Lozano, T.W.; Sabine, A.; Renevey, F.; Arroz-Madeira, S.; Rapin, A.; Wypych, T.P.; Rota, G.; et al. FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure. Sci. Adv. 2021, 7, eabf4335. [Google Scholar] [CrossRef]
- Muley, A.; Kim Uh, M.; Salazar-De Simone, G.; Swaminathan, B.; James, J.M.; Murtomaki, A.; Youn, S.W.; McCarron, J.D.; Kitajewski, C.; Gnarra Buethe, M.; et al. Unique functions for Notch4 in murine embryonic lymphangiogenesis. Angiogenesis 2022, 25, 205–224. [Google Scholar] [CrossRef]
- Ulvmar, M.H.; Mäkinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 2016, 111, 310–321. [Google Scholar] [CrossRef]
- Cimini, M.; Cannatá, A.; Pasquinelli, G.; Rota, M.; Goichberg, P. Phenotypically heterogeneous podoplanin-expressing cell populations are associated with the lymphatic vessel growth and fibrogenic responses in the acutely and chronically infarcted myocardium. PLoS ONE 2017, 12, e0173927. [Google Scholar] [CrossRef] [PubMed]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; She, Y.; He, X.; Feng, H.; Li, X.; Wei, Y.; Fan, Y.; Zhao, W.E.; Yin, M.; et al. Long-term exercise enhances meningeal lymphatic vessel plasticity and drainage in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 2025, 14, 37. [Google Scholar] [CrossRef]
- Hitpass Romero, K.; Stevenson, T.J.; Smyth, L.C.D.; Watkin, B.; McCullough, S.J.C.; Vinnell, L.; Smith, A.M.; Schweder, P.; Correia, J.A.; Kipnis, J.; et al. Age-related meningeal extracellular matrix remodeling compromises CNS lymphatic function. J. Neuroinflamm. 2025, 22, 109. [Google Scholar] [CrossRef]
- Reiterer, M.; Branco, C.M. Endothelial cells and organ function: Applications and implications of understanding unique and reciprocal remodelling. FEBS J. 2020, 287, 1088–1100. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Thommen, D.S. Tertiary lymphoid structures in cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef]
- Yuan, H.; Mao, X.; Yan, Y.; Huang, R.; Zhang, Q.; Zeng, Y.; Bao, M.; Dai, Y.; Fang, B.; Mi, J.; et al. Single-cell sequencing reveals the heterogeneity of B cells and tertiary lymphoid structures in muscle-invasive bladder cancer. J. Transl. Med. 2024, 22, 48. [Google Scholar]
- Luo, H.; Xia, X.; Huang, L.B.; An, H.; Cao, M.; Kim, G.D.; Chen, H.N.; Zhang, W.H.; Shu, Y.; Kong, X.; et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022, 13, 6619. [Google Scholar] [CrossRef]
- Alve, S.; Gramolelli, S.; Jukonen, J.; Juteau, S.; Pink, A.; Manninen, A.A.; Hänninen, S.; Monto, E.; Lackman, M.H.; Carpén, O.; et al. DLL4/Notch3/WNT5B axis mediates bidirectional prometastatic crosstalk between melanoma and lymphatic endothelial cells. JCI Insight 2024, 9, e171821. [Google Scholar] [PubMed]
- Cousin, N.; Cap, S.; Dihr, M.; Tacconi, C.; Detmar, M.; Dieterich, L.C. Lymphatic PD-L1 Expression Restricts Tumor-Specific CD8+ T-cell Responses. Cancer Res. 2021, 81, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Pirson, S.; Gautier-Isola, M.; Baudin, L.; Rouaud, L.; Vanwynsberghe, A.; Deroye, J.; Bekisz, S.; Gucciardo, F.; Lebeau, A.; Buntinx, F.; et al. AXL promotes lymphangiogenesis by amplifying VEGF-C-mediated AKT pathway. Cell Mol. Life Sci. 2025, 82, 95. [Google Scholar] [PubMed]
- Karnezis, T.; Shayan, R.; Caesar, C.; Roufail, S.; Harris, N.C.; Ardipradja, K.; Zhang, Y.F.; Williams, S.P.; Farnsworth, R.H.; Chai, M.G.; et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012, 21, 181–195. [Google Scholar] [CrossRef]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Characteristics of lymphatic endothelial cells in physiological and pathological conditions. Histol. Histopathol. 2005, 20, 155–175. [Google Scholar]
- Pereira, E.R.; Jones, D.; Jung, K.; Padera, T.P. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin. Cell Dev. Biol. 2015, 38, 98–105. [Google Scholar] [CrossRef]
- Viúdez-Pareja, C.; Kreft, E.; García-Caballero, M. Immunomodulatory properties of the lymphatic endothelium in the tumor microenvironment. Front. Immunol. 2023, 14, 1235812. [Google Scholar] [CrossRef]
- Lund, A.W.; Wagner, M.; Fankhauser, M.; Steinskog, E.S.; Broggi, M.A.; Spranger, S.; Gajewski, T.F.; Alitalo, K.; Eikesdal, H.P.; Wiig, H.; et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Investig. 2016, 126, 3389–3402. [Google Scholar] [CrossRef]
- Bordry, N.; Broggi, M.A.S.; de Jonge, K.; Schaeuble, K.; Gannon, P.O.; Foukas, P.G.; Danenberg, E.; Romano, E.; Baumgaertner, P.; Fankhauser, M.; et al. Lymphatic vessel density is associated with CD8+ T cell infiltration and immunosuppressive factors in human melanoma. Oncoimmunology 2018, 7, e1462878. [Google Scholar] [CrossRef]
- Garnier, L.; Pick, R.; Montorfani, J.; Sun, M.; Brighouse, D.; Liaudet, N.; Kammertoens, T.; Blankenstein, T.; Page, N.; Bernier-Latamani, J.; et al. IFN-γ-dependent tumor-antigen cross-presentation by lymphatic endothelial cells promotes their killing by T cells and inhibits metastasis. Sci. Adv. 2022, 8, eabl5162. [Google Scholar]
- Lukacs-Kornek, V.; Malhotra, D.; Fletcher, A.L.; Acton, S.E.; Elpek, K.G.; Tayalia, P.; Collier, A.R.; Turley, S.J. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 2011, 12, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Ikenberg, K.; Cetintas, T.; Kapaklikaya, K.; Hutmacher, C.; Detmar, M. Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation. Front. Immunol. 2017, 8, 66. [Google Scholar] [CrossRef]
- Lane, R.S.; Femel, J.; Breazeale, A.P.; Loo, C.P.; Thibault, G.; Kaempf, A.; Mori, M.; Tsujikawa, T.; Chang, Y.H.; Lund, A.W. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018, 215, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, L.; Yang, S.H.; Huang, C.; Zhuang, W.; Huang, S.; Xia, X.; Tang, Y.; Li, Z.; Zhao, Z.B.; et al. Lymphatic endothelial cell-mediated accumulation of CD177+Treg cells suppresses antitumor immunity in human esophageal squamous cell carcinoma. Oncoimmunology 2024, 13, 2327692. [Google Scholar] [CrossRef]
- You, S.; Li, S.; Zeng, L.; Song, J.; Li, Z.; Li, W.; Ni, H.; Xiao, X.; Deng, W.; Li, H.; et al. Lymphatic-localized Treg-mregDC crosstalk limits antigen trafficking and restrains anti-tumor immunity. Cancer Cell 2024, 42, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.M.; Jaiswal, A.; Delclaux, I.; Dryg, I.D.; Murugan, D.; Femel, J.; Son, S.; du Bois, H.; Hill, C.; Leachman, S.A.; et al. T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat. Immunol. 2023, 24, 664–675. [Google Scholar] [CrossRef]
- Sasso, M.S.; Mitrousis, N.; Wang, Y.; Briquez, P.S.; Hauert, S.; Ishihara, J.; Hubbell, J.A.; Swartz, M.A. Lymphangiogenesis-inducing vaccines elicit potent and long-lasting T cell immunity against melanomas. Sci. Adv. 2021, 7, eabe4362. [Google Scholar] [CrossRef]
- Malhotra, D.; Fletcher, A.L.; Astarita, J.; Lukacs-Kornek, V.; Tayalia, P.; Gonzalez, S.F.; Elpek, K.G.; Chang, S.K.; Knoblich, K.; Hemler, M.E.; et al. Immunological Genome Project Consortium. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 2012, 13, 499–510. [Google Scholar] [CrossRef]
- Pan, X.; Li, X.; Dong, L.; Liu, T.; Zhang, M.; Zhang, L.; Zhang, X.; Huang, L.; Shi, W.; Sun, H.; et al. Tumour vasculature at single-cell resolution. Nature 2024, 632, 429–436. [Google Scholar] [CrossRef]
- Gkountidi, A.O.; Garnier, L.; Dubrot, J.; Angelillo, J.; Harlé, G.; Brighouse, D.; Wrobel, L.J.; Pick, R.; Scheiermann, C.; Swartz, M.A.; et al. MHC Class II Antigen Presentation by Lymphatic Endothelial Cells in Tumors Promotes Intratumoral Regulatory T cell-Suppressive Functions. Cancer Immunol. Res. 2021, 9, 748–764. [Google Scholar] [CrossRef]
- Ji, R.C. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014, 346, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol. Life Sci. 2012, 69, 897–914. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Wang, Y.; Chen, Y.; Zhang, W.; Pan, X.; Su, C.; Li, Z.; Wang, L.; Gu, J. IgG4-mediated M2 macrophage polarization in tertiary lymphoid structures of esophageal cancer: Implications for immunosuppression. Front. Immunol. 2025, 15, 1497783. [Google Scholar] [CrossRef]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limón, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, Y.; Fang, Y.; Li, Z.; Gu, X.; Xiang, J. Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int. J. Cancer 2019, 145, 1648–1659. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, W.; Ma, J.; Yang, Y.; Liang, L.; Zhang, Y.; Wang, Z.; Chen, X.; Huang, L.; Wang, W.; et al. Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Mol. Ther. 2021, 29, 1512–1528. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, W.; Chen, Z.; Xiang, C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp. Cell Res. 2017, 359, 449–457. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, M.; Broggi, M.A.S.; Potin, L.; Bordry, N.; Jeanbart, L.; Lund, A.W.; Da Costa, E.; Hauert, S.; Rincon-Restrepo, M.; Tremblay, C.; et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 2017, 9, eaal4712. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Cheng, X.; Sun, G.; Li, F.; Nie, G.; Zhang, Y. Anti-lymphangiogenesis for boosting drug accumulation in tumors. Signal Transduct. Target Ther. 2024, 9, 89. [Google Scholar] [CrossRef]
- Sun, M.; Garnier, L.; Chevalier, R.; Roumain, M.; Wang, C.; Angelillo, J.; Montorfani, J.; Pick, R.; Brighouse, D.; Fournier, N.; et al. Lymphatic-derived oxysterols promote anti-tumor immunity and response to immunotherapy in melanoma. Nat. Commun. 2025, 16, 1217. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.; Yeom, J.H.; Liu, Z.; Kim, K.M.; Kang, K.P. Inhibition of VEGFR-3 by SAR131675 decreases renal inflammation and lymphangiogenesis in the murine lupus nephritis model. Cell Death Discov. 2025, 11, 320. [Google Scholar] [CrossRef]
- Kuonqui, K.; Campbell, A.C.; Sarker, A.; Roberts, A.; Pollack, B.L.; Park, H.J.; Shin, J.; Brown, S.; Mehrara, B.J.; Kataru, R.P. Dysregulation of Lymphatic Endothelial VEGFR3 Signaling in Disease. Cells 2023, 13, 68. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Pula, B.; Olbromski, M.; Wojnar, A.; Gomulkiewicz, A.; Witkiewicz, W.; Ugorski, M.; Dziegiel, P.; Podhorska-Okolow, M. Impact of SOX18 expression in cancer cells and vessels on the outcome of invasive ductal breast carcinoma. Cell Oncol. 2013, 36, 469–483. [Google Scholar] [CrossRef]
- Weichand, B.; Popp, R.; Dziumbla, S.; Mora, J.; Strack, E.; Elwakeel, E.; Frank, A.C.; Scholich, K.; Pierre, S.; Syed, S.N.; et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 2017, 214, 2695–2713. [Google Scholar] [CrossRef]
- Wang, C.A.; Jedlicka, P.; Patrick, A.N.; Micalizzi, D.S.; Lemmer, K.C.; Deitsch, E.; Casás-Selves, M.; Harrell, J.C.; Ford, H.L. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J. Clin. Investig. 2012, 122, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Goutam, K.; Das, B.; Dash, S.R.; Kundu, C.N. Nectin-4 promotes lymphangiogenesis and lymphatic metastasis in breast cancer by regulating CXCR4-LYVE-1 axis. Vascul. Pharmacol. 2021, 140, 106865. [Google Scholar] [CrossRef]
- Hou, Q.; Chen, S.; An, Q.; Li, B.; Fu, Y.; Luo, Y. Extracellular Hsp90α Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Sun, J.; Song, X.; Bian, M.; Wang, F.; Yan, F.; Yu, Z. Inhibition of NADPH oxidase 4 attenuates lymphangiogenesis and tumor metastasis in breast cancer. FASEB J. 2021, 35, e21531. [Google Scholar] [CrossRef]
- García-Silva, S.; Benito-Martín, A.; Nogués, L.; Hernández-Barranco, A.; Mazariegos, M.S.; Santos, V.; Hergueta-Redondo, M.; Ximénez-Embún, P.; Kataru, R.P.; Lopez, A.A.; et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat. Cancer 2021, 2, 1387–1405. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Cheng, Y.; Wan, J.; Blasig, R.; Li, A.; Bai, Y.; Haseloff, R.F.; Blasig, I.E.; Zhu, L.; Qin, Z. Claudin-3 inhibits tumor-induced lymphangiogenesis via regulating the PI3K signaling pathway in lymphatic endothelial cells. Sci. Rep. 2022, 12, 17440. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, L.; Zou, Y.; Liang, J.Y.; Liu, P.; Gao, G.; Yang, A.; Tang, H.; Xie, X. Long non-coding RNA HUMT hypomethylation promotes lymphangiogenesis and metastasis via activating FOXK1 transcription in triple-negative breast cancer. J. Hematol. Oncol. 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Leichner, G.S.; Schweitzer, I.; Dror, S.; Levin, L.; Geva, P.; Golan, T.; Zaremba, L.; Shapira, G.; Parikh, R.; Shomron, N.; et al. Primary Melanoma miRNA Trafficking Induces Lymphangiogenesis. J. Investig. Dermatol. 2023, 143, 1788–1798. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, R.; Han, L.; Zhang, X.; Ye, Y.; Yu, W.; Ren, X.; Zhang, W.; Yu, J. Vasohibin 2 promotes lymphangiogenesis of lung squamous cell carcinoma through snail-dependent vascular endothelial growth factor-D (VEGF-D) signaling pathway. Ann. Transl. Med. 2022, 10, 39. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, Y.; Li, Y.; Jin, Z.; Pan, Y.; Liu, G.; Fu, S.; Zhang, Y.; Feng, K.; Feng, Y. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur. J. Cancer 2014, 50, 2336–2350. [Google Scholar] [CrossRef]
- Liu, P.I.; Jiang, Y.J.; Chang, A.C.; Huang, C.L.; Fong, Y.C.; Guo, J.H.; Liu, C.L.; Wang, S.W.; Liu, J.F.; Chang, S.L.; et al. ANGPTL2 promotes VEGF-A synthesis in human lung cancer and facilitates lymphangiogenesis. Aging 2023, 15, 1652–1667. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liu, H.; Cai, M.; Shentu, Y. Inhibition of Tumor Lymphangiogenesis is an Important Part that EGFR-TKIs Play in the Treatment of NSCLC. J. Cancer 2020, 11, 241–250. [Google Scholar] [CrossRef]
- Zhou, H.; Geng, F.; Chen, Y.; Du, J.; Zhang, X.; Liu, B.; Song, D.; Hou, H.; Zhao, H. The mineral dust-induced gene, mdig, regulates angiogenesis and lymphangiogenesis in lung adenocarcinoma by modulating the expression of VEGF-A/C/D via EGFR and HIF-1α signaling. Oncol. Rep. 2021, 45, 60. [Google Scholar] [CrossRef]
- Sun, L.; Duan, J.; Jiang, Y.; Wang, L.; Huang, N.; Lin, L.; Liao, Y.; Liao, W. Metastasis-associated in colon cancer-1 upregulates vascular endothelial growth factor-C/D to promote lymphangiogenesis in human gastric cancer. Cancer Lett. 2015, 357, 242–253. [Google Scholar] [CrossRef]
- Pak, K.H.; Park, K.C.; Cheong, J.H. VEGF-C induced by TGF- β1 signaling in gastric cancer enhances tumor-induced lymphangiogenesis. BMC Cancer 2019, 19, 799. [Google Scholar] [CrossRef]
- Ma, C.; Xie, J.; Luo, C.; Yin, H.; Li, R.; Wang, X.; Xiong, W.; Zhang, T.; Jiang, P.; Qi, W.; et al. OxLDL promotes lymphangiogenesis and lymphatic metastasis in gastric cancer by upregulating VEGF-C expression and secretion. Int. J. Oncol. 2019, 54, 572–584. [Google Scholar] [CrossRef]
- Bou Malham, V.; Benzoubir, N.; Vaquero, J.; Desterke, C.; Agnetti, J.; Song, P.X.; Gonzalez-Sanchez, E.; Arbelaiz, A.; Jacques, S.; Di Valentin, E.; et al. Intrinsic cancer cell phosphoinositide 3-kinase delta regulates fibrosis and vascular development in cholangiocarcinoma. Liver Int. 2023, 43, 2776–2793. [Google Scholar] [CrossRef]
- Cadamuro, M.; Brivio, S.; Mertens, J.; Vismara, M.; Moncsek, A.; Milani, C.; Fingas, C.; Cristina Malerba, M.; Nardo, G.; Dall’Olmo, L.; et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol. 2019, 70, 700–709. [Google Scholar] [CrossRef]
- Peng, M.; Li, H.; Cao, H.; Huang, Y.; Yu, W.; Shen, C.; Gu, J. Dual FGFR and VEGFR inhibition synergistically restrain hexokinase 2-dependent lymphangiogenesis and immune escape in intrahepatic cholangiocarcinoma. J. Gastroenterol. 2023, 58, 908–924. [Google Scholar] [CrossRef]
- Yan, J.; Xiao, G.; Yang, C.; Liu, Q.; Lv, C.; Yu, X.; Zhou, Z.; Lin, S.; Bai, Z.; Lin, H.; et al. Cancer-Associated Fibroblasts Promote Lymphatic Metastasis in Cholangiocarcinoma via the PDGF-BB/PDGFR-β Mediated Paracrine Signaling Network. Aging Dis. 2024, 15, 369–389. [Google Scholar] [CrossRef]
- Miao, D.; Shi, J.; Lv, Q.; Tan, D.; Zhao, C.; Xiong, Z.; Zhang, X. NAT10-mediated ac4C-modified ANKZF1 promotes tumor progression and lymphangiogenesis in clear-cell renal cell carcinoma by attenuating YWHAE-driven cytoplasmic retention of YAP1. Cancer Commun. 2024, 44, 361–383. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Palma, M.B.; Verine, J.; Miriuka, S.; Inda, A.M.; Errecalde, A.L.; Desgrandchamps, F.; Carosella, E.D.; Tronik-Le Roux, D. The immune-checkpoint HLA-G/ILT4 is involved in the regulation of VEGF expression in clear cell renal cell carcinoma. BMC Cancer 2020, 20, 624. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yuan, L.; Jiang, Y.; Wan, Y.; Ma, X.; Yang, J.; Sun, G.; Zhou, S.; Wang, H.; Qiu, J.; et al. ALKBH5 activates FAK signaling through m6A demethylation in ITGB1 mRNA and enhances tumor-associated lymphangiogenesis and lymph node metastasis in ovarian cancer. Theranostics 2023, 13, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Y.; Zhi, X.; Sun, Y.; Abudousalamu, Z.; Lin, Q.; Li, B.; Yao, L.; Chen, M. Unraveling the molecular mechanisms of lymph node metastasis in ovarian cancer: Focus on MEOX1. J. Ovarian Res. 2024, 17, 61. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.C. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006, 25, 677–694. [Google Scholar] [CrossRef]
- Miao, K.; Zhang, A.; Yang, X.; Zhang, Y.; Lin, A.; Wang, L.; Zhang, X.; Sun, H.; Xu, J.; Zhang, J.; et al. Lymphatic system is the mainstream for breast cancer dissemination and metastasis revealed by single-cell lineage tracing. Mol. Cancer 2025, 24, 75. [Google Scholar] [CrossRef]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Esparza, S.; Azimi, M.S.; Cornelison, R.; Azar, F.N.; Llaneza, D.C.; Belanger, M.; Mathew, A.; Tkachenko, S.; Perez, M.J.; et al. Platinum Chemotherapy Induces Lymphangiogenesis in Cancerous and Healthy Tissues That Can be Prevented With Adjuvant Anti-VEGFR3 Therapy. Front. Oncol. 2022, 12, 801764. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, Z.; Ma, J.; Chu, Z.; Li, H.; Guo, J.; Zhang, Q.; Zhao, H.; Li, Y.; Wang, T. ZKSCAN5 Activates VEGFC Expression by Recruiting SETD7 to Promote the Lymphangiogenesis, Tumour Growth, and Metastasis of Breast Cancer. Front. Oncol. 2022, 12, 875033. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Kapaklikaya, K.; Cetintas, T.; Proulx, S.T.; Commerford, C.D.; Ikenberg, K.; Bachmann, S.B.; Scholl, J.; Detmar, M. Transcriptional profiling of breast cancer-associated lymphatic vessels reveals VCAM-1 as regulator of lymphatic invasion and permeability. Int. J. Cancer 2019, 145, 2804–2815. [Google Scholar] [CrossRef]
- Kataru, R.P.; Ly, C.L.; Shin, J.; Park, H.J.; Baik, J.E.; Rehal, S.; Ortega, S.; Lyden, D.; Mehrara, B.J. Tumor Lymphatic Function Regulates Tumor Inflammatory and Immunosuppressive Microenvironments. Cancer Immunol. Res. 2019, 7, 1345–1358. [Google Scholar] [CrossRef]
- Wouters, J.; Kalender-Atak, Z.; Minnoye, L.; Spanier, K.I.; De Waegeneer, M.; Bravo González-Blas, C.; Mauduit, D.; Davie, K.; Hulselmans, G.; Najem, A.; et al. Robust gene expression programs underlie recurrent cell states and phenotype switching in melanoma. Nat. Cell Biol. 2020, 22, 986–998. [Google Scholar] [CrossRef]
- Reticker-Flynn, N.E.; Zhang, W.; Belk, J.A.; Basto, P.A.; Escalante, N.K.; Pilarowski, G.O.W.; Bejnood, A.; Martins, M.M.; Kenkel, J.A.; Linde, I.L.; et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 2022, 185, 1924–1942. [Google Scholar] [CrossRef]
- O’Melia, M.J.; Manspeaker, M.P.; Thomas, S.N. Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer Immunol. Immunother. 2021, 70, 2179–2195. [Google Scholar] [CrossRef]
- Leary, N.; Walser, S.; He, Y.; Cousin, N.; Pereira, P.; Gallo, A.; Collado-Diaz, V.; Halin, C.; Garcia-Silva, S.; Peinado, H.; et al. Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. J. Extracell. Vesicles 2022, 11, e12197. [Google Scholar] [CrossRef]
- Reger de Moura, C.; Landras, A.; Khayati, F.; Maskos, U.; Maouche, K.; Battistella, M.; Menashi, S.; Lebbé, C.; Mourah, S. CD147 Promotes Tumor Lymphangiogenesis in Melanoma via PROX-1. Cancers 2021, 13, 4859. [Google Scholar] [CrossRef]
- Berta, J.; Török, S.; Tárnoki-Zách, J.; Drozdovszky, O.; Tóvári, J.; Paku, S.; Kovács, I.; Czirók, A.; Masri, B.; Megyesfalvi, Z.; et al. Apelin promotes blood and lymph vessel formation and the growth of melanoma lung metastasis. Sci. Rep. 2021, 11, 5798. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Wen, G.Z.; Wang, Q.; Yuan, S.M. Rapamycin suppresses angiogenesis and lymphangiogenesis in melanoma by downregulating VEGF-A/VEGFR-2 and VEGF-C/VEGFR-3 expression. Onco. Targets Ther. 2019, 12, 4643–4654. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, J.I.; Oh, N.; Cho, H.J.; Park, J.H.; Park, K.S. ELK3 expressed in lymphatic endothelial cells promotes breast cancer progression and metastasis through exosomal miRNAs. Sci. Rep. 2019, 9, 8418. [Google Scholar] [CrossRef]
- Renyi-Vamos, F.; Tovari, J.; Fillinger, J.; Timar, J.; Paku, S.; Kenessey, I.; Ostoros, G.; Agocs, L.; Soltesz, I.; Dome, B. Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 7344–7353. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.Y.; Hewitt, S.M. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J. Transl. Med. 2020, 18, 443. [Google Scholar] [CrossRef]
- Watari, K.; Shibata, T.; Kawahara, A.; Sata, K.; Nabeshima, H.; Shinoda, A.; Abe, H.; Azuma, K.; Murakami, Y.; Izumi, H.; et al. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS ONE 2014, 9, e99568. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, J.; Xu, A.; Bao, J.; Cho, W.C.; Zhu, J.; Shen, J. Integrin α6 overexpression promotes lymphangiogenesis and lymphatic metastasis via activating the NF-κB signaling pathway in lung adenocarcinoma. Cell Oncol. 2022, 45, 57–67. [Google Scholar] [CrossRef]
- Fukasawa, K.; Hanada, K.; Ichikawa, K.; Hirashima, M.; Takagi, T.; Itoh, S.; Watabe, T.; Itoh, F. Endothelial-specific depletion of TGF-β signaling affects lymphatic function. Inflamm. Regen. 2021, 41, 35. [Google Scholar] [CrossRef]
- Cleary, S.J.; Qiu, L.; Seo, Y.; Baluk, P.; Liu, D.; Serwas, N.K.; Taylor, C.A.; Zhang, D.; Cyster, J.G.; McDonald, D.M.; et al. Intravital imaging of pulmonary lymphatics in inflammation and metastatic cancer. J. Exp. Med. 2025, 222, e20241359. [Google Scholar] [CrossRef]
- Ye, T.; Yang, M.; Huang, D.; Wang, X.; Xue, B.; Tian, N.; Xu, X.; Bao, L.; Hu, H.; Lv, T.; et al. MicroRNA-7 as a potential therapeutic target for aberrant NF-kappaB-driven distant metastasis of gastric cancer. J. Exp. Clin. Cancer Res. 2019, 38, 55. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, B.; Yuan, M.; Liu, J.; Zhou, C.; Sun, M.; Guo, Z.; Zhang, Y.; Song, Y.; Wang, Z. CRIP1 Reshapes the Gastric Cancer Microenvironment to Facilitate Development of Lymphatic Metastasis. Adv. Sci. 2023, 10, e2303246. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, Z.; Zou, T.; Xu, L.; Zhang, S.; Chen, Y.; Chen, C.; Zhang, W.; Wang, S.; Ding, Q.; et al. SOAT1 Promotes Gastric Cancer Lymph Node Metastasis Through Lipid Synthesis. Front. Pharmacol. 2021, 12, 769647. [Google Scholar] [CrossRef]
- Shen, X.; Kong, S.; Ma, S.; Shen, L.; Zheng, M.; Qin, S.; Qi, J.; Wang, Q.; Cui, X.; Ju, S. Hsa_circ_0000437 promotes pathogenesis of gastric cancer and lymph node metastasis. Oncogene 2022, 41, 4724–4735. [Google Scholar] [CrossRef]
- Wen, J.Y.; Li, X.; Chen, J.N.; Chen, J.; Zhang, J.Y.; Du, Y.; Zhu, W.H.; Chen, Y.J.; Yang, R.H.; Shao, C.K. CD45- erythroid progenitor cells promote lymph node metastasis in gastric cancer by inducing a hybrid epithelial/mesenchymal state in lymphatic endothelial cells. Gastric. Cancer 2023, 26, 918–933. [Google Scholar] [CrossRef]
- Thelen, A.; Scholz, A.; Benckert, C.; von Marschall, Z.; Schröder, M.; Wiedenmann, B.; Neuhaus, P.; Rosewicz, S.; Jonas, S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int. J. Cancer 2008, 122, 2471–2481. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Di Giamberardino, A.; Overi, D.; Donsante, S.; Colasanti, T.; Amato, G.; Mennini, G.; Franchitto, M.; Conti, F.; et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J. Hepatol. 2021, 75, 1377–1386. [Google Scholar] [CrossRef]
- Dufies, M.; Giuliano, S.; Ambrosetti, D.; Claren, A.; Ndiaye, P.D.; Mastri, M.; Moghrabi, W.; Cooley, L.S.; Ettaiche, M.; Chamorey, E.; et al. Sunitinib Stimulates Expression of VEGFC by Tumor Cells and Promotes Lymphangiogenesis in Clear Cell Renal Cell Carcinomas. Cancer Res. 2017, 77, 1212–1226. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, Y.; Ma, X.; Gao, Y.; Li, X.; Niu, Y.; Zhang, X.; Chang, C. Androgen receptor increases hematogenous metastasis yet decreases lymphatic metastasis of renal cell carcinoma. Nat. Commun. 2017, 8, 918. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Z.; Pang, S.; Wu, J.; Liang, J.; Sun, L. Predicative value of IFITM2 in renal clear cell carcinoma: IFITM2 is associated with lymphatic metastasis and poor clinical outcome. Biochem. Biophys. Res. Commun. 2021, 534, 157–164. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, L.; Liang, X.; Cheng, P.; Li, Q.; Chang, X.; Wang, K.; Huang, S.; Li, Y.; et al. Lymphangiogenesis in renal fibrosis arises from macrophages via VEGF-C/VEGFR3-dependent autophagy and polarization. Cell Death Dis. 2021, 12, 109. [Google Scholar] [CrossRef]
- Pal, S.; Bhowmick, S.; Sharma, A.; Sierra-Fonseca, J.A.; Mondal, S.; Afolabi, F.; Roy, D. Lymphatic vasculature in ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188950. [Google Scholar] [CrossRef]
- Sapoznik, S.; Cohen, B.; Tzuman, Y.; Meir, G.; Ben-Dor, S.; Harmelin, A.; Neeman, M. Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by LEDGF-induced expression of VEGF-C. Cancer Res. 2009, 69, 9306–9314. [Google Scholar] [CrossRef]
- Wei, R.; Lv, M.; Li, F.; Cheng, T.; Zhang, Z.; Jiang, G.; Zhou, Y.; Gao, R.; Wei, X.; Lou, J.; et al. Human CAFs promote lymphangiogenesis in ovarian cancer via the Hh-VEGF-C signaling axis. Oncotarget 2017, 8, 67315–67328. [Google Scholar] [CrossRef]
- Olbrecht, S.; Busschaert, P.; Qian, J.; Vanderstichele, A.; Loverix, L.; Van Gorp, T.; Van Nieuwenhuysen, E.; Han, S.; Van den Broeck, A.; Coosemans, A.; et al. High-grade serous tubo-ovarian cancer refined with single-cell RNA sequencing: Specific cell subtypes influence survival and determine molecular subtype classification. Genome Med. 2021, 13, 111. [Google Scholar] [CrossRef]
- Ji, R.C. The role of lymphangiogenesis in cardiovascular diseases and heart transplantation. Heart Fail Rev. 2022, 27, 1837–1856. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Yanev, P.; Poinsatte, K.; Hominick, D.; Khurana, N.; Zuurbier, K.R.; Berndt, M.; Plautz, E.J.; Dellinger, M.T.; Stowe, A.M. Impaired meningeal lymphatic vessel development worsens stroke outcome. J. Cereb. Blood Flow Metab. 2020, 40, 263–275. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef]
- Antila, S.; Chilov, D.; Nurmi, H.; Li, Z.; Näsi, A.; Gotkiewicz, M.; Sitnikova, V.; Jäntti, H.; Acosta, N.; Koivisto, H.; et al. Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer’s disease-related amyloid pathology. Nat. Cardiovasc. Res 2024, 3, 474–491. [Google Scholar] [CrossRef]
- Li, Z.; Antila, S.; Nurmi, H.; Chilov, D.; Korhonen, E.A.; Fang, S.; Karaman, S.; Engelhardt, B.; Alitalo, K. Blockade of VEGFR3 signaling leads to functional impairment of dural lymphatic vessels without affecting autoimmune neuroinflammation. Sci. Immunol. 2023, 8, eabq0375. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Tronolone, J.J.; Mohamed, N.; Jain, A. Engineering Lymphangiogenesis-On-Chip: The Independent and Cooperative Regulation by Biochemical Factors, Gradients, and Interstitial Fluid Flow. Adv. Biol. 2024, 8, e2400031. [Google Scholar] [CrossRef] [PubMed]
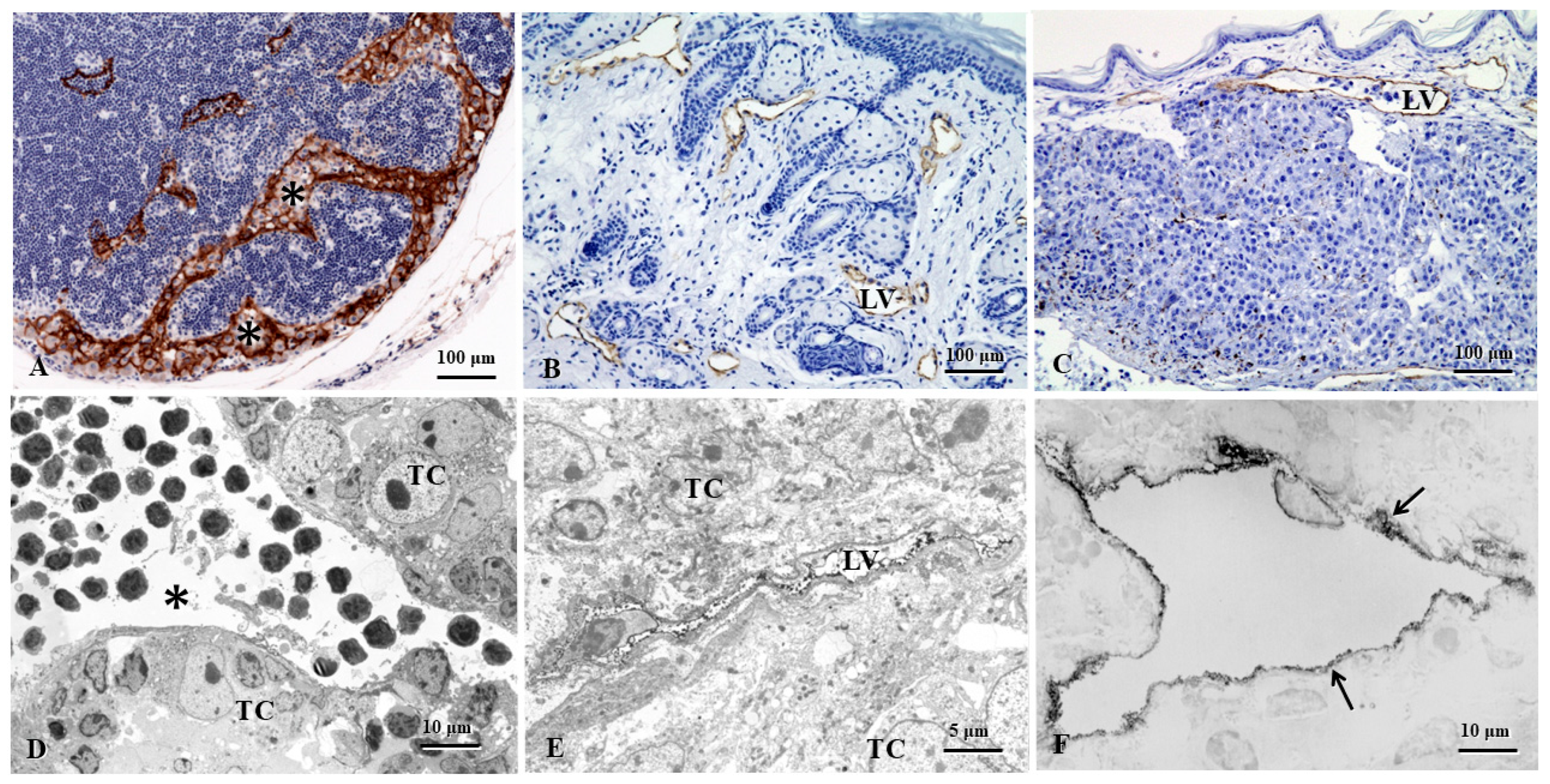
| Cancers | Animal/ Human | Molecules | Biofunctional Characteristics | References |
|---|---|---|---|---|
| Breast cancer | Mouse Human | Galectin 8 Integrin β1 Podoplanin | Peri-lymphatic TAM localization is mediated by galectin 8 expression in LECs Podoplanin-expressing macrophages promote lymphangiogenesis and tumor invasion, and their migration and adhesion depend on galectin 8-mediated integrin β1 activation | [115] |
| Breast cancer | Mouse | VCAM-1 | VCAM-1 and integrin α4 are upregulated by LECs VCAM-1 regulates lymphatic invasion and permeability through junction remodeling | [118] |
| Breast cancer | Mouse | ELK3 | ELK3 expressed in LECs promotes cancer progression and metastasis through exosomal miRNAs | [127] |
| Breast cancer | Mouse | VEGFR-3 inhibitor (SAR131675) Anlotinib | Anti-lymphangiogenesis therapy mediated by anlotinib and SAR131675 and improves anti-tumor efficacy through increasing intratumoral accumulation of nanoparticles, macromolecules, and small molecular drugs | [81] |
| Breast cancer Melanomas | Mouse | TNF-α, IFN-γ IL-1β TGF-β1, IL10 PD-L1 | Lymphatic ablation increases inflammatory cell accumulation and tumor growth Excess inflammatory cytokines (TNF-α, IFN-γ, IL-1β), and immunosuppressive molecule PD-L1 and cytokines (TGF-β, IL10) are noted in peritumoral edematous fluid | [119] |
| Melanoma | Mouse Human | NGFR | Melanoma sEV-secreted NGFR promotes tumor cell adhesion and lymphangiogenesis sEVs taken up by LECs and macrophages, reinforce LN pre-metastatic niche formation and metastasis | [92] |
| Melanomas | Mouse | VEGF-C | VEGF-C overexpression in irradiated tumor cell vaccines promotes T cell priming in the injection site and draining LNs Lymphangiogenic vaccines elicit a strong melanoma-specific T cell immunity and provide effective tumor control and long-term immunological memory | [66] |
| Melanomas | Mouse | VCAM-1 | Uptake of B16F10-derived EVs by LN LECs is mediated by VCAM-1 EVs induce lymphatic remodeling and tumor antigen cross-presentation by LECs in draining LNs | [123] |
| NSCLC | Human | VEGF-A VEGF-C | High expression of M2 ratio is an indicator of poor prognosis, positively correlated with VEGF-A and VEGF-C Increased M2 TAMs contribute to tumor lymphangiogenesis and progression by promoting VEGF-C expression | [129] |
| Lung adenocarcinoma | Mouse Human | Integrin α6 | Integrin α6 overexpression promotes lymphangiogenesis and lymphatic metastasis via activating NF-κB signaling | [131] |
| Gastric cancer | Mouse Human | MicroRNA-7 NF-κB RelA/p65 | miR-7 inhibits gastric metastasis by suppressing lymphangiogenesis via reducing VEGF-C secretion Loss of miR-7 in gastric cancer promotes RelA/p65-mediated aberrant NF-κB activation, facilitating tumor metastasis | [134] |
| Gastric cancer | Human | SOAT1 SREBP1/2 VEGF-C | The expression of SREBP1 and SREBP2 regulated by SOAT1 induces lymphangiogenesis via increasing VEGF-C expression Knockdown or inhibition of SOAT1 suppresses cancer proliferation and LN metastasis | [136] |
| Gastric cancer | Mouse Human | S100A8/A9 | CD45-erythroid progenitor cells increase lymphangiogenesis and promote LN metastasis through S100A8/A9 heterodimer-induced hybrid epithelial/mesenchymal state in LECs | [138] |
| Gastric cancer | Mouse Human | CRIP1 CCL5 VEGF-C | CRIP1 promotes VEGF-C secretion, lymphangiogenesis and lymphatic metastasis CRIP1 increases LVD and lymphatic permeability via CCL5-mediated recruitment of TAMs and TNF-α secretion | [135] |
| CCA | Rat Mouse Human | PDGF-D VEGF-A/-C | PDGF-D stimulates fibroblasts to secret VEGF-A/-C PDGF-D enables liver myofibroblasts to promote tumor lymphangiogenesis | [105] |
| CCA | Mouse Human | PDGF-BB/ PDGFR-β | CAFs promote lymphangiogenesis and lymphatic metastasis via PDGF-BB/PDGFR-β-mediated signaling | [107] |
| Intrahepatic CCA | Mouse Human | THBS1 THBS2 PEDF | Overexpression of THBS1, THBS2 and PEDF promotes tumor-associated lymphangiogenesis Blocking THBS1, THBS2, and PEDF reduces tumor growth and LN infiltration | [140] |
| Intrahepatic CCA | Mouse Human | PI3Kδ TGF-β | PI3Kδ promotes tumor growth, aggressiveness and ECM remodeling, as well as lymphangiogenesis via TGF-β/Src/Notch signaling | [104] |
| Intrahepatic CCA | Mouse Human | FGFRs VEGFs | FGFR-1 and VEGFR-3 expression induce lymphangiogenesis and lymphatic metastasis Dual FGFR and VEGFR inhibition inhibits lymphangiogenesis by suppression of c-MYC-dependent and HIF-1α-mediated hexokinase-2 expression, and improves antitumor immunity by downregulating PD-L1 expression in LECs | [106] |
| ccRCC | Mouse Human | miR-185-5p VEGF-C | Androgen receptor decreases lymphangiogenesis and lymphatic metastasis through suppressing VEGF-C expression by upregulating miR-185-5p | [142] |
| ccRCC | Human | IFITM2 VEGF-C | IFITM2-VEGF-C signaling contributes to enhanced lymphangiogenesis and lymphatic metastasis | [143] |
| ccRCC | Mouse Human | ANKZF1 NAT10 VEGF-C/-D | NAT10-ANKZF1 axis promotes tumor progression and lymphangiogenesis, by enhancing YAP1 nuclear import and VEGF-C/-D expression | [108] |
| Ovarian cancer | Mouse Human | SHH VEGF-C | Paracrine signaling of tumor-initiated SHH promotes lymphangiogenesis via CAF-derived VEGF-C | [148] |
| Ovarian cancer | Mouse Human | CircASH2L miR-665 VEGF-A | CircASH2L promotes ovarian cancer tumorigenesis and lymphangiogenesis by regulating the miR-665/VEGF-A axis | [32] |
| Ovarian cancer | Mouse Human | ALKBH5 FAK | ALKBH5 activates FAK signaling, and enhances lymphangiogenesis and LN metastasis | [110] |
| Striatal tumors (Intracranial gliomas) | Mouse | anti-PD-1/ CTLA-4 CCL21/CCR7 VEGF-C | Intracranial tumors induce remodeling of dorsal meningeal lymphatic vessels VEGF-C-induced lymphangiogenesis is required for dendritic cell trafficking to deep cervical LNs Immunotherapy enhancement by VEGF-C is dependent on CCL21/CCR7 signaling Lymphatic ablation impairs anti-PD-1/CTLA-4 efficacy against tumors | [112] |
| Glioblastoma (Brain tumor) | Mouse Human | VEGF-C anti-PD-1 | VEGF-C/anti-PD-1 immunotherapy suppresses tumor growth and prolongs survival Therapeutic delivery of VEGF-C potentiates checkpoint inhibitor therapy by increasing T cell priming Glioblastoma microenvironment is devoid of lymphangiogenic signals | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ji, R.-C. Lymphatic Endothelial Cells and Organ-Associated Lymphangiogenesis in Tumor Microenvironment. Cells 2026, 15, 28. https://doi.org/10.3390/cells15010028
Ji R-C. Lymphatic Endothelial Cells and Organ-Associated Lymphangiogenesis in Tumor Microenvironment. Cells. 2026; 15(1):28. https://doi.org/10.3390/cells15010028
Chicago/Turabian StyleJi, Rui-Cheng. 2026. "Lymphatic Endothelial Cells and Organ-Associated Lymphangiogenesis in Tumor Microenvironment" Cells 15, no. 1: 28. https://doi.org/10.3390/cells15010028
APA StyleJi, R.-C. (2026). Lymphatic Endothelial Cells and Organ-Associated Lymphangiogenesis in Tumor Microenvironment. Cells, 15(1), 28. https://doi.org/10.3390/cells15010028






