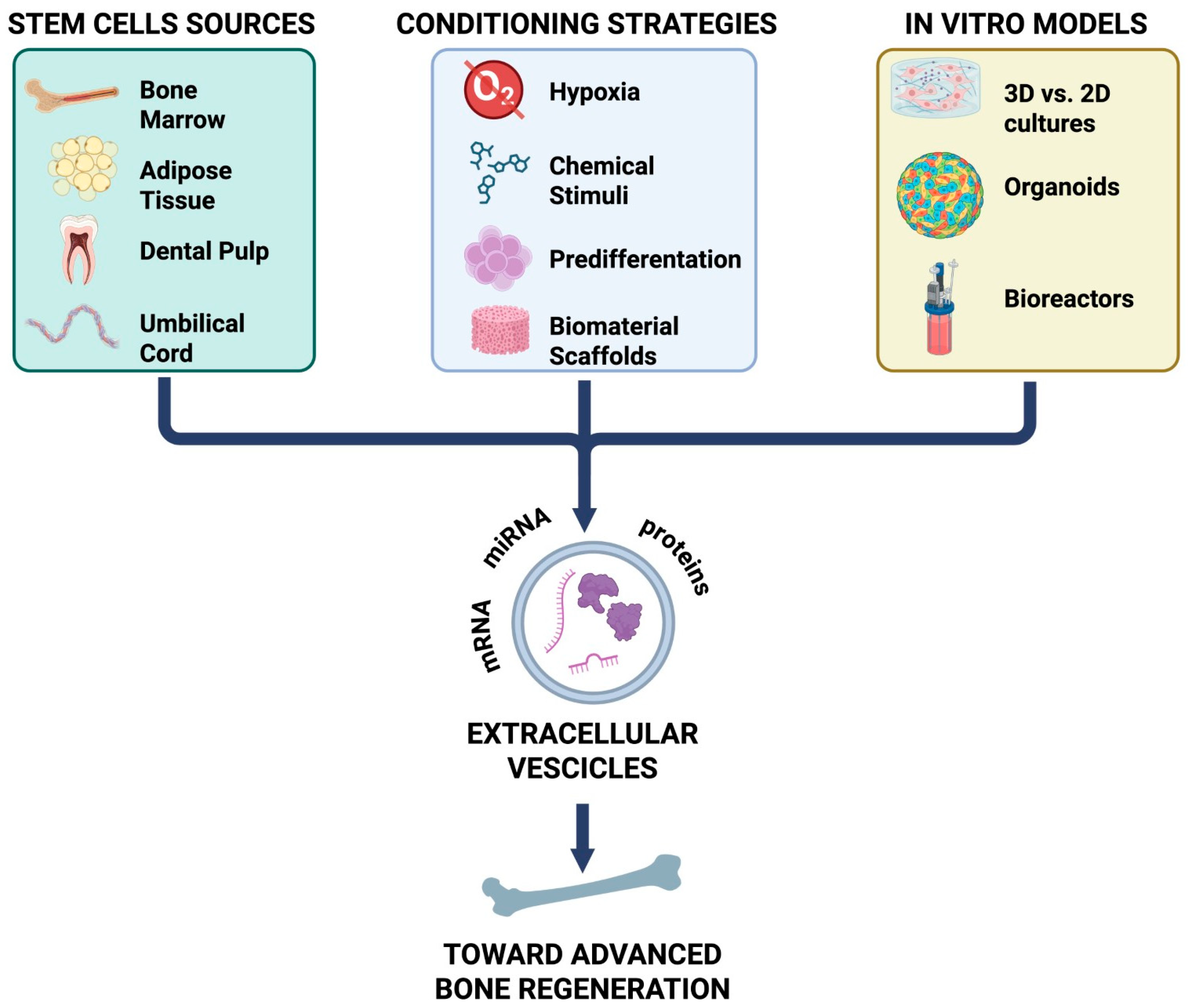
Extracellular Vesicles in Osteogenesis: Comparative Analysis of Stem Cell Sources, Conditioning Strategies, and In Vitro Models Toward Advanced Bone Regeneration
Abstract
1. Introduction
2. Osteogenesis
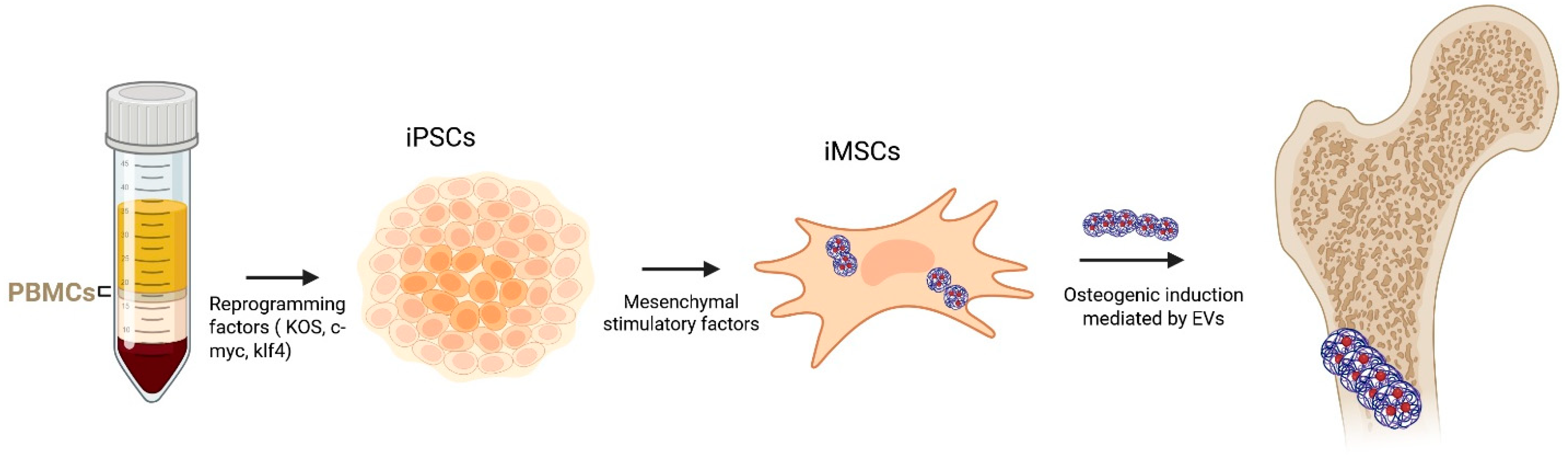
3. Stem Cell-Derived EVs in Osteogenesis
4. Strategies to Enhance the Osteogenic Potential of MSC-Derived Extracellular Vesicles
| Strategy | Stem Cell Type/Condition | Main Modifications in EV Cargo | Functional Effects on Osteogenesis/Regeneration | Biomaterial Delivery (If Present) | References |
|---|---|---|---|---|---|
| Osteogenic/odontogenic induction | Dental pulp stem cells (DPSCs), SHEDs, PDLSCs under osteogenic or odontogenic induction | ↑ Pro-osteogenic miRNAs (e.g., miR-27a-5p), changes in AMPK–mTOR, Wnt/β-catenin, BMP/Smad, TGFβ1/Smads signaling | ↑ Mineralization; ↑ RUNX2, ALP, BMP2, OCN; enhanced osteoinduction vs. naïve EVs; effects depend on induction duration (7–14 days) | [64,65,66] | |
| Bidirectional EV subsets | Dental stem cell EV subpopulations | circ_0000722 enrichment NF-κB/AKT regulation | Dual action: promotes osteogenesis and osteoclastogenesis; implications for bone remodeling | [74] | |
| Mechanical stimulation | Mechanically stimulated BMSCs | Modulation of Wnt/β-catenin pathway | ↑ Osteoblast proliferation and differentiation; promising for GIOP | [77] | |
| Magnesium preconditioning (Mg2+ activation) | Mg2+-activated DPSCs → Mg2+-EVs | ↑ miR-451a AKT/eNOS activation | ↑ Endothelial migration, angiogenesis, BMSC proliferation and osteogenesis; enhanced vascularized bone regeneration | β-TCP-modified GelMA scaffold (sustained release) | [70] |
| Magnesium-preconditioned BMSCs | BMSCs treated with Mg2+ | Modulation of angiogenic and osteogenic cargo | Rescue of Dex-induced impairment in HUVEC angiogenesis and BMSC osteogenesis | [78] | |
| MICA (Magnetic Ion Channel Activation) | MC3T3 pre-osteoblasts exposed to MICA + TREK1-functionalized nanoparticles | Increased EV output; preserved EV markers and morphology | ↑ Osteogenic differentiation and mineralization in BMSCs vs. controls | [79] | |
| Donor-age–dependent effects | EVs from young donors | ↑ miR-142-5p | ↑ Osteogenesis and bone homeostasis | [72] | |
| Hypoxia preconditioning | Dental stem cells under low O2 (1–5%) | Enrichment in angiogenic/immunomodulatory factors | ↑ Angiogenesis and immune modulation; enhanced therapeutic profile | [80] | |
| Hypoxia + biomaterial delivery | Hypoxia-preconditioned SHEDs | Cargo unchanged in morphology but ↑ osteogenic and angiogenic potential | Significantly improved cranial bone regeneration | Injectable porous PLGA microspheres with polydopamine coating | [81] |
| Genetic modification: HIF-1α overexpression | DPSCs overexpressing HIF-1α | ↑ Jagged1 | Markedly enhanced angiogenesis; potential for ischemic disorders | [83] | |
| Genetic modification: HIF-1α + telomerase + inflammatory priming | Engineered MSCs (HIF-1α + TERT + cytokine stimulation) | ↑ EV yield and uniformity; enhanced immunomodulatory profile | Stronger regenerative and immune-modulating functions | [84] | |
| General note on hypoxia sensitivity | Multiple MSC sources | Hypoxia modifies EV cargo through regulation of oxygen-sensitive pathways | Improved performance in avascular or load-bearing defects | [76,85] | |
| General genetic engineering approaches | MSCs engineered for osteoinductive genes/miRNAs | Customized EV cargo | Targeted functional enhancement for bone repair | [86] |
5. In Vitro and in Vivo Models of EV-Mediated Bone Regeneration
6. Clinical Perspectives on Extracellular Vesicles for Bone Regeneration
The Regulatory Potential of Extracellular Vesicles in Osteonecrosis
7. Extracellular Vesicles in Osteoporosis: Possible Therapeutic Targets
8. Bone Defect Repair and Fracture Healing: The Importance of Scaffolds and Microenvironment
9. Diabetic Bone-Specific Physiopathology May Affect Therapeutical Results
10. Extracellular Vesicles in Enthesis Acute and Chronic Pathology
11. Osteoarthritis and Related Clinical Complications—The Role of Subchondral Bone
12. Maxillo-Facial Defects, Periodontal Pathologies, and Dental Implant Issues
13. Opportunities and Limitations of AI/ML in EV-Mediated Bone Repair
14. Challenges for Clinical Translation of EV-Based Bone Regeneration
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Khan, K.; Kim, J.-H. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef]
- Wang, B.Z.; Luo, L.J.; Vunjak-Novakovic, G. RNA and Protein Delivery By Cell-Secreted and Bioengineered Extracellular Vesicles. Adv. Healthc. Mater. 2022, 11, 2101557. [Google Scholar]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Tamkovich, S.N.; Tutanov, O.S.; Laktionov, P.P. Exosomes: Generation, structure, transport, biological activity, and diagnostic application. Biochem. Suppl. Ser. A Membr. Cell Biol. 2016, 10, 163–173. [Google Scholar]
- Krylova, S.V.; Feng, D. The machinery of exosomes: Biogenesis, release, and uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Poon, I.K. Apoptotic cell-derived extracellular vesicles: More than just debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, J.; Lu, T.; Han, W.; Jiao, K.; Li, H. Mesenchymal stem cell-derived extracellular vesicles in bone-related diseases: Intercellular communication messengers and therapeutic engineering protagonists. Int. J. Nanomed. 2024, 19, 3233–3257. [Google Scholar]
- Hertel, F.C.; Silva, A.S.d.; Sabino, A.d.P.; Valente, F.L.; Reis, E.C.C. Preconditioning methods to improve mesenchymal stromal cell-derived extracellular vesicles in bone regeneration—A systematic review. Biology 2022, 11, 733. [Google Scholar]
- Hong, Y.; Li, R.; Sheng, S.; Zhou, F.; Bai, L.; Su, J. Bone organoid construction and evolution. J. Orthop. Transl. 2025, 53, 260–273. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Marie, P.J.; Kassem, M. Osteoblasts in osteoporosis: Past, emerging, and future anabolic targets. Eur. J. Endocrinol. 2011, 165, 1–10. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H.; Guo, J.; Yuan, Y.; Ni, G. Effects of BMSC-derived EVs on bone metabolism. Pharmaceutics 2022, 14, 1012. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Innamorati, G.; Valenti, M.T. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev. Rep. 2012, 8, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas-Baena, B.; Pulido-Escribano, V.; Dorado, G.; Gálvez-Moreno, M.Á.; Camacho-Cardenosa, M.; Casado-Díaz, A. Clinical potential of mesenchymal stem cell-derived exosomes in bone regeneration. J. Clin. Med. 2023, 12, 4385. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Confalonieri, D.; Schwab, A.; Walles, H.; Ehlicke, F. Advanced therapy medicinal products: A guide for bone marrow-derived MSC application in bone and cartilage tissue engineering. Tissue Eng. Part B Rev. 2018, 24, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, X.; Zhang, J.-T.; Liu, A.-F.; Zhang, C.; Han, J.-C.; Zhang, X.-Q.; Wu, S.; Zhang, X.-Y.; Lv, F.-Q. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J. Orthop. Surg. Res. 2021, 16, 23. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Z.; Xu, S.; Li, R.; Qian, H. Biological Nanotherapeutics Derived From Human Umbilical Cord Mesenchymal Stem Cells: Mechanisms and Translational Potential in Multisystem Therapies for Regeneration and Oncology. Int. J. Nanomed. 2025, 20, 12117–12175. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakamura, M.; Okubo, C.; Kliesmete, Z.; Ohnuki, M.; Narita, M.; Watanabe, A.; Ueda, M.; Takashima, Y.; Hellmann, I. The pluripotent stem cell-specific transcript ESRG is dispensable for human pluripotency. PLoS Genet. 2021, 17, e1009587. [Google Scholar] [CrossRef]
- Thanaskody, K.; Jusop, A.S.; Tye, G.J.; Zaman, W.S.W.K.; Dass, S.A.; Nordin, F. MSCs vs. iPSCs: Potential in therapeutic applications. Front. Cell Dev. Biol. 2022, 10, 1005926. [Google Scholar] [CrossRef]
- Taheri, B.; Soleimani, M.; Fekri Aval, S.; Esmaeili, E.; Bazi, Z.; Zarghami, N. Induced pluripotent stem cell-derived extracellular vesicles: A novel approach for cell-free regenerative medicine. J. Cell. Physiol. 2019, 234, 8455–8464. [Google Scholar]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Imanishi, Y.; Hata, M.; Matsukawa, R.; Aoyagi, A.; Omi, M.; Mizutani, M.; Naruse, K.; Ozawa, S.; Honda, M.; Matsubara, T. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm. Regen. 2021, 41, 12. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Yin, Y.; He, X.-T.; An, Y.; Tian, B.-M.; Hong, Y.-L.; Wu, L.-A.; Chen, F.-M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Li, P.; Yuan, K.; Zhao, F.; Zhu, X.; Zhang, P.; Huang, Z. Extracellular vesicles derived from human dental pulp stem cells promote osteogenesis of adipose-derived stem cells via the MAPK pathway. J. Tissue Eng. 2020, 11, 2041731420975569. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, L.; Mao, J.; Liu, W.; Ma, W.; Zhao, B. Rab27a-mediated extracellular vesicle secretion contributes to osteogenesis in periodontal ligament-bone niche communication. Sci. Rep. 2023, 13, 8479. [Google Scholar]
- Lan, Q.; Cao, J.; Bi, X.; Xiao, X.; Li, D.; Ai, Y. Curcumin-primed periodontal ligament stem cells-derived extracellular vesicles improve osteogenic ability through the Wnt/β-catenin pathway. Front. Cell Dev. Biol. 2023, 11, 1225449. [Google Scholar] [CrossRef]
- Lan, Q.; Xiao, X.; Bi, X.; Gu, Y.; Ai, Y. Effects of periodontal ligament stem cell-derived exosomes on osteoblastic proliferation, migration, differentiation, apoptosis, and signaling pathways. Oral Dis. 2024, 30, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Pranskunas, M.; Šimoliūnas, E.; Alksne, M.; Martin, V.; Gomes, P.S.; Puisys, A.; Kaupinis, A.; Juodzbalys, G. Assessment of the bone healing process mediated by periosteum-derived mesenchymal stem cells’ secretome and a xenogenic bioceramic—An in vivo study in the rabbit critical size calvarial defect model. Materials 2021, 14, 3512. [Google Scholar] [CrossRef]
- Tang, D.; Tang, W.; Chen, H.; Liu, D.; Jiao, F. Synergistic Effects of Icariin and Extracellular Vesicles Derived from Rabbit Synovial Membrane-Derived Mesenchymal Stem Cells on Osteochondral Repair via the Wnt/β-Catenin Pathway. Anal. Cell. Pathol. 2024, 2024, 1083143. [Google Scholar] [CrossRef]
- Rosu, A.; Ghaemi, B.; Bulte, J.W.; Shakeri-Zadeh, A. Tumor-tropic Trojan horses: Using mesenchymal stem cells as cellular nanotheranostics. Theranostics 2024, 14, 571. [Google Scholar] [CrossRef]
- Ding, Z.; Greenberg, Z.F.; Serafim, M.F.; Ali, S.; Jamieson, J.C.; Traktuev, D.O.; March, K.; He, M. Understanding molecular characteristics of extracellular vesicles derived from different types of mesenchymal stem cells for therapeutic translation. Extracell. Vesicle 2024, 3, 100034. [Google Scholar] [CrossRef]
- Qiu, M.; Zhai, S.; Fu, Q.; Liu, D. Bone marrow mesenchymal stem cells-derived exosomal microRNA-150-3p promotes osteoblast proliferation and differentiation in osteoporosis. Hum. Gene Ther. 2021, 32, 717–729. [Google Scholar] [PubMed]
- You, L.; Pan, L.; Chen, L.; Gu, W.; Chen, J. MiR-27a is essential for the shift from osteogenic differentiation to adipogenic differentiation of mesenchymal stem cells in postmenopausal osteoporosis. Cell. Physiol. Biochem. 2016, 39, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Wang, D. Mesenchymal stem cell–derived extracellular vesicles inhibit osteoporosis via microRNA-27a-induced inhibition of DKK2-mediated Wnt/β-catenin pathway. Inflammation 2022, 45, 780–799. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, H.; Zhou, H.; Yin, H.; Yang, J.; Song, Y.; Yang, B. miR-424-5p shuttled by bone marrow stem cells-derived exosomes attenuates osteogenesis via regulating WIF1-mediated Wnt/β-catenin axis. Aging 2021, 13, 17190. [Google Scholar]
- Al-Sharabi, N.; Mohamed-Ahmed, S.; Shanbhag, S.; Kampleitner, C.; Elnour, R.; Yamada, S.; Rana, N.; Birkeland, E.; Tangl, S.; Gruber, R. Osteogenic human MSC-derived extracellular vesicles regulate MSC activity and osteogenic differentiation and promote bone regeneration in a rat calvarial defect model. Stem Cell Res. Ther. 2024, 15, 33. [Google Scholar] [CrossRef]
- Tan, K.L.; Chia, W.C.; How, C.W.; Tor, Y.S.; Show, P.L.; Looi, Q.H.D.; Foo, J.B. Benchtop isolation and characterisation of small extracellular vesicles from human mesenchymal stem cells. Mol. Biotechnol. 2021, 63, 780–791. [Google Scholar] [CrossRef]
- Batsali, A.K.; Georgopoulou, A.; Mavroudi, I.; Matheakakis, A.; Pontikoglou, C.G.; Papadaki, H.A. The role of bone marrow mesenchymal stem cell derived extracellular vesicles (MSC-EVs) in normal and abnormal hematopoiesis and their therapeutic potential. J. Clin. Med. 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Stöckl, S.; Li, S.; Herrmann, M.; Lukas, C.; Reinders, Y.; Sickmann, A.; Grässel, S. Effects of extracellular vesicles from osteogenic differentiated human BMSCs on osteogenic and adipogenic differentiation capacity of naïve human BMSCs. Cells 2022, 11, 2491. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-L.; Hsu, C.-J.; Wu, C.-W.; Chang, L.-H.; Chen, J.-W.; Chen, C.-H.; Huang, K.-C.; Chang, J.-K.; Wu, S.-C.; Shao, P.-L. Enhancement of osteoblast function through extracellular vesicles derived from adipose-derived stem cells. Biomedicines 2022, 10, 1752. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Ni, C.-Y.; Chen, C.-Y.; Rao, S.-S.; Yin, H.; Huang, J.; Tan, Y.-J.; Wang, Z.-X.; Cao, J. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics 2020, 10, 2293. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Fan, Y.; Liu, H.; Shen, Z. Human umbilical cord mesenchymal stem cells-derived extracellular vesicles for rat jawbone regeneration in periapical periodontitis. ACS Biomater. Sci. Eng. 2024, 10, 5784–5795. [Google Scholar] [CrossRef]
- Chita, A. Engineering the Protein Cargo Levels of Extracellular Vesicles (EVs) Derived from Umbilical Cord Mesenchymal Stem Cells (UC-MSCs); Library of the School of Health Sciences: Athens, Greece, 2025. [Google Scholar]
- Liu, X.; Li, Q.; Niu, X.; Hu, B.; Chen, S.; Song, W.; Ding, J.; Zhang, C.; Wang, Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017, 13, 232. [Google Scholar] [CrossRef]
- Gao, R.; Ye, T.; Zhu, Z.; Li, Q.; Zhang, J.; Yuan, J.; Zhao, B.; Xie, Z.; Wang, Y. Small extracellular vesicles from iPSC-derived mesenchymal stem cells ameliorate tendinopathy pain by inhibiting mast cell activation. Nanomedicine 2022, 17, 513–529. [Google Scholar] [CrossRef]
- Ye, T.; Chen, Z.; Zhang, J.; Luo, L.; Gao, R.; Gong, L.; Du, Y.; Xie, Z.; Zhao, B.; Li, Q. Large extracellular vesicles secreted by human iPSC-derived MSCs ameliorate tendinopathy via regulating macrophage heterogeneity. Bioact. Mater. 2023, 21, 194–208. [Google Scholar] [CrossRef]
- Tertel, T.; Dittrich, R.; Arsène, P.; Jensen, A.; Giebel, B. EV products obtained from iPSC-derived MSCs show batch-to-batch variations in their ability to modulate allogeneic immune responses in vitro. Front. Cell Dev. Biol. 2023, 11, 1282860. [Google Scholar] [CrossRef]
- Chi, Y.; Liu, T.; Jin, Q.; Liu, H. Extracellular vesicles carrying RUNX3 promote differentiation of dental pulp stem cells. Tissue Eng. Regen. Med. 2024, 21, 111–122. [Google Scholar] [CrossRef]
- Brunello, G.; Zanotti, F.; Trentini, M.; Zanolla, I.; Pishavar, E.; Favero, V.; Favero, R.; Favero, L.; Bressan, E.; Bonora, M. Exosomes derived from dental pulp stem cells show different angiogenic and osteogenic properties in relation to the age of the donor. Pharmaceutics 2022, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Asou, Y.; Matsumura, E.; Nakagawa, Y.; Miyatake, K.; Katagiri, H.; Nakamura, T.; Koga, H.; Komori, K.; Sekiya, I. Short cytoplasmic isoform of IL1R1/CD121a mediates IL1β induced proliferation of synovium-derived mesenchymal stem/stromal cells through ERK1/2 pathway. Heliyon 2022, 8, e09476. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Pei, M. Synovium-derived stem cells: A tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo-Rodríguez, C.; Crossland, R.E.; Reis, M.; Pandit, H.; Wang, X.-n.; Jones, E. Characterization and miRNA profiling of extracellular vesicles from human osteoarthritic subchondral bone multipotential stromal cells (MSCs). Stem Cells Int. 2021, 2021, 7232773. [Google Scholar] [CrossRef]
- Morito, T.; Muneta, T.; Hara, K.; Ju, Y.-J.; Mochizuki, T.; Makino, H.; Umezawa, A.; Sekiya, I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology 2008, 47, 1137–1143. [Google Scholar] [CrossRef]
- Kim, S.; Koga, T.; Isobe, M.; Kern, B.E.; Yokochi, T.; Chin, Y.E.; Karsenty, G.; Taniguchi, T.; Takayanagi, H. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003, 17, 1979–1991. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Li, P.; Fan, Y.; Zhang, L.; Ma, X.; Sun, R.; Liu, Y.; Li, W. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021, 272, 119204. [Google Scholar] [CrossRef]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT–β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, W.; Zou, X.; Xu, J.; He, S.; Chang, L.; Li, X.; Yin, Y.; Tian, M.; Li, Z. Human periodontal ligament stem cell-derived exosomes promote bone regeneration by altering MicroRNA profiles. Stem Cells Int. 2020, 2020, 8852307. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mu, Q.; Ku, W.; Zheng, Y.; Yi, P.; Lin, L.; Li, P.; Wang, B.; Wu, J.; Yu, D. Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration. J. Nanobiotechnol. 2024, 22, 265. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 2020, 111, 1–11. [Google Scholar] [CrossRef]
- Hu, X.; Zhong, Y.; Kong, Y.; Chen, Y.; Feng, J.; Zheng, J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tian, L.; Zhang, C. Bone marrow stem cells-derived exosomes extracted from osteoporosis patients inhibit osteogenesis via microRNA-21/SMAD7. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6221–6229. [Google Scholar]
- Yang, B.-c.; Kuang, M.-j.; Kang, J.-y.; Zhao, J.; Ma, J.-x.; Ma, X.-l. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun. 2020, 524, 883–889. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Ding, Y.; Wang, Y.; Du, J.; Chen, Y.; Xu, J.; Liu, Y. MiR-451a-Enriched Small Extracellular Vesicles Derived from Mg2+-Activated DPSCs Induce Vascularized Bone Regeneration through the AKT/eNOS/NO Axis. ACS Appl. Mater. Interfaces 2025, 17, 31345–31356. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wang, H.; Chen, K.; Wang, S.; Xu, J.; Ji, P. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res. Ther. 2022, 13, 149. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Cui, X.; Wang, Y.; Xu, R.; Lu, R.; Chen, J.; Zhou, X.; Zhang, C.; Li, L. Exosomes from young plasma stimulate the osteogenic differentiation and prevent osteoporosis via miR-142-5p. Bioact. Mater. 2025, 49, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, X.; He, X.; Xiao, S.; Shi, Q.; Chen, P.; Lee, J.; Guo, X.; Liu, H.; Fan, Y. Osteoinductive dental pulp stem cell-derived extracellular vesicle-loaded multifunctional hydrogel for bone regeneration. ACS Nano 2024, 18, 8777–8797. [Google Scholar] [CrossRef]
- Xie, L.; Ren, X.; Yang, Z.; Zhou, T.; Zhang, M.; An, W.; Guan, Z. Exosomal circ_0000722 derived from periodontal ligament stem cells undergoing osteogenic differentiation promotes osteoclastogenesis. Int. Immunopharmacol. 2024, 128, 111520. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Chen, J.; Cui, Y.; Lu, X.; Xiong, S.; Yue, C.; Yang, B. Human dental pulp stem cell-derived exosomes decorated titanium scaffolds for promoting bone regeneration. Colloids Surf. B Biointerfaces 2024, 235, 113775. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Fu, J.; Wang, R.; He, J.; Yao, J.; Ye, Q.; He, Y. Tailored Extracellular Vesicles from Dental Stem Cells: Advances in Specific Modifications for Enhanced Therapeutic Applications. Int. J. Nanomed. 2025, 20, 8327–8341. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Wang, D.; Zhang, H.; Zhou, L.; Qin, M.; Li, G.; Ma, T.; Li, Y.; Dong, B. Mechanical stimulation of extracellular vesicles secreted by bone marrow mesenchymal stem cells promotes osteoblast proliferation and differentiation by activating the Wnt/β-catenin signaling pathway. Connect. Tissue Res. 2025, 67, 1–18. [Google Scholar] [CrossRef]
- Li, L.; Cheng, L.; Du, Y.; Zhang, Y.; Wang, Z.; Nie, Y.; Long, J.; Li, C.; Zhang, Y.; Lai, Y. Exosomes Derived from Mg-Preconditioned Bone Mesenchymal Stem Cells Promote Angiogenesis and Osteogenesis for Osteonecrosis Treatment. Materials 2025, 18, 4687. [Google Scholar] [CrossRef] [PubMed]
- Rajan Unnithan, A.; Man, K.; Kritika; Gethings, L.A.; Hughes, C.J.; Keenan, A.; Heaney, L.; Cox, S.C.; Davies, O.G.; El Haj, A.J. Engineering Extracellular Vesicle Production through Magnetic Ion Channel Activation for Bone Regeneration. bioRxiv 2025. bioRxiv: 2025.2008.2007.669024. [Google Scholar] [CrossRef]
- Liu, D.; Shi, B.; Zhou, W.; Tao, G. Exosomes from hypoxia-conditioned apical papilla stem cells accelerate angiogenesis in vitro through Notch/JAG1/VEGF signaling. Tissue Cell 2023, 84, 102197. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, Z.; Yuan, X.; Wan, Z.; Yu, Y.; Zhan, Q.; Zhao, Y.; Han, J.; Huang, J.; Xiong, C. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact. Mater. 2022, 14, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, M.; Niu, Y.; Wang, Y. Romance of the three kingdoms in hypoxia: HIFs, epigenetic regulators, and chromatin reprogramming. Cancer Lett. 2020, 495, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Villanueva-Badenas, E.; Sánchez-Sánchez, R.; Sánchez-López, C.M.; Baquero, M.C.; Sepúlveda, P.; Dorronsoro, A. HIF-1α and pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int. J. Mol. Sci. 2021, 22, 3416. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Chen, Z.; Cai, X.; Wang, X.; Zhou, P.; Tang, Y.; Ying, T.; Zhang, X.; Shen, Y. Hypoxic bone mesenchymal stem cell-derived exosomes direct schwann cells proliferation, migration, and paracrine to accelerate facial nerve regeneration via circRNA_Nkd2/miR-214-3p/MED19 Axis. Int. J. Nanomed. 2024, 19, 1409–1429. [Google Scholar] [CrossRef]
- Biswas, S.; Gangadaran, P.; Dhara, C.; Ghosh, S.; Phadikar, S.D.; Chakraborty, A.; Mahajan, A.A.; Mondal, R.; Chattopadhyay, D.; Banerjee, T. Extracellular Vesicles in Osteogenesis: A Comprehensive Review of Mechanisms and Therapeutic Potential for Bone Regeneration. Curr. Issues Mol. Biol. 2025, 47, 675. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef]
- Sun, X.; Mao, Y.; Liu, B.; Gu, K.; Liu, H.; Du, W.; Li, R.; Zhang, J. Mesenchymal stem cell-derived exosomes enhance 3D-printed scaffold functions and promote alveolar bone defect repair by enhancing angiogenesis. J. Pers. Med. 2023, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; D’aurora, M.; Gugliandolo, A.; Merciaro, I.; Ettorre, V.; Bramanti, A.; Piattelli, A.; Gatta, V.; Mazzon, E.; Fontana, A. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018, 13, 3805–3825. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, C.; Meng, L.a.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022, 18, 26–41. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Wu, J.; Yang, R.; Sun, Q.; Xu, Y.; Wang, B.; Cai, M.; Xu, Y.; Zhuang, C. Adipose stem cells-derived exosomes modified gelatin sponge promotes bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1096390. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv. Sci. 2023, 10, 2303650. [Google Scholar] [CrossRef] [PubMed]
- Vonk, L.A.; van Dooremalen, S.F.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- de Windt, T.S.; Saris, D.B.; Slaper-Cortenbach, I.C.; van Rijen, M.H.; Gawlitta, D.; Creemers, L.B.; de Weger, R.A.; Dhert, W.J.; Vonk, L.A. Direct cell–cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Woods, I.; Riffault, M.; Johnson, G.P.; Corrigan, M.; Lowry, M.C.; Shen, N.; Labour, M.-N.; Wynne, K.; O’Driscoll, L. Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl. Med. 2020, 9, 1431–1447. [Google Scholar] [CrossRef]
- Du, Z.; Rizzo, S.A.; Sarrafian, T.L.; Bagwell, M.S.; Mahlberg, R.C.; Amontree, A.; Schiebel, P.; Tauferner, D.M.; LeBrasseur, Z.S.; Witt, T.A. Engineered BMP2/BMP7 extracellular vesicles induce autocrine BMP release driving SMAD phosphorylation to promote bone formation. NPJ Regen. Med. 2025, 10, 26. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Q.; Zhao, L.; Mao, J.; Huang, W.; Han, X.; Liu, Y. Periodontal ligament stem cell-derived small extracellular vesicles embedded in matrigel enhance bone repair through the adenosine receptor signaling pathway. Int. J. Nanomed. 2022, 17, 519–536. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016, 12, 836. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Chen, Z.; Zhang, W. Development of an angiogenesis-promoting microvesicle-alginate-polycaprolactone composite graft for bone tissue engineering applications. PeerJ 2016, 4, e2040. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Keck, M.; Weber, V.; Kasper, C.; Egger, D. Dynamic cultivation of human mesenchymal stem/stromal cells for the production of extracellular vesicles in a 3D bioreactor system. Biotechnol. Lett. 2024, 46, 279–293. [Google Scholar] [CrossRef]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Jakl, V.; Ehmele, M.; Winkelmann, M.; Ehrenberg, S.; Eiseler, T.; Friemert, B.; Rojewski, M.T.; Schrezenmeier, H. A novel approach for large-scale manufacturing of small extracellular vesicles from bone marrow-derived mesenchymal stromal cells using a hollow fiber bioreactor. Front. Bioeng. Biotechnol. 2023, 11, 1107055. [Google Scholar] [CrossRef]
- Bahmaee, H.; Owen, R.; Boyle, L.; Perrault, C.M.; Garcia-Granada, A.A.; Reilly, G.C.; Claeyssens, F. Design and evaluation of an osteogenesis-on-a-chip microfluidic device incorporating 3D cell culture. Front. Bioeng. Biotechnol. 2020, 8, 557111. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, P.; Liu, Q.; Yu, H.; Fang, F.; Liu, X. Microfluidic bone chip to study osteogenesis of porous substrate topographies in normal and osteoporotic microenvironments. Eur. Cells Mater. 2024, 47, 238–252. [Google Scholar] [CrossRef]
- Vis, M.; Zhao, F.; Bodelier, E.; Bood, C.; Bulsink, J.; Van Doeselaar, M.; Amirabadi, H.E.; Ito, K.; Hofmann, S. Osteogenesis and osteoclastogenesis on a chip: Engineering a self-assembling 3D coculture. Bone 2023, 173, 116812. [Google Scholar] [CrossRef] [PubMed]
- Maritan, S.M.; Lian, E.Y.; Mulligan, L.M. An efficient and flexible cell aggregation method for 3D spheroid production. J. Vis. Exp. JoVE 2017, 121, 55544. [Google Scholar]
- Olivares, A.L.; Marsal, È.; Planell, J.A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef]
- Zhao, D.; Saiding, Q.; Li, Y.; Tang, Y.; Cui, W. Bone organoids: Recent advances and future challenges. Adv. Healthc. Mater. 2024, 13, 2302088. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, D.; Li, G.; Liu, J.; Chen, X.; Su, J. Engineering bone/cartilage organoids: Strategy, progress, and application. Bone Res. 2024, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, J. Organoid extracellular vesicle-based therapeutic strategies for bone therapy. Biomater. Transl. 2023, 4, 199. [Google Scholar]
- Wang, L.; Wang, D.; Ye, Z.; Xu, J. Engineering extracellular vesicles as delivery systems in therapeutic applications. Adv. Sci. 2023, 10, 2300552. [Google Scholar] [CrossRef]
- Abdollahi, S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol. Bioeng. 2021, 118, 1029–1049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yao, Z.; Xue, L.; Wang, D.; Tan, Z. The role of immune cells in modulating chronic inflammation and osteonecrosis. Front. Immunol. 2022, 13, 1064245. [Google Scholar] [CrossRef] [PubMed]
- Vig, S.; Fernandes, M.H. Bone cell exosomes and emerging strategies in bone engineering. Biomedicines 2022, 10, 767. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Du, W.; Rao, S.-S.; Tan, Y.-J.; Hu, X.-K.; Luo, M.-J.; Ou, Q.-F.; Wu, P.-F.; Qing, L.-M.; Cao, Z.-M. Extracellular vesicles from human urine-derived stem cells inhibit glucocorticoid-induced osteonecrosis of the femoral head by transporting and releasing pro-angiogenic DMBT1 and anti-apoptotic TIMP1. Acta Biomater. 2020, 111, 208–220. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Wu, P.; Xiao, Y.; Duan, N.; Quan, J.; Du, W. microRNA-148a-3p in extracellular vesicles derived from bone marrow mesenchymal stem cells suppresses SMURF1 to prevent osteonecrosis of femoral head. J. Cell. Mol. Med. 2020, 24, 11512–11523. [Google Scholar] [CrossRef]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular vesicles of stem cells to prevent BRONJ. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Tian, W. Small extracellular vesicles derived from adipose tissue prevent bisphosphonate-related osteonecrosis of the jaw by promoting angiogenesis. Int. J. Nanomed. 2021, 16, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, R.; Dang, X.; Liu, J.; Jiao, H. Experimental study on improvement of osteonecrosis of femoral head with exosomes derived from miR-27a-overexpressing vascular endothelial cells. Chin. J. Reparative Reconstr. Surg. 2021, 35, 356–365. [Google Scholar]
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390. [Google Scholar] [CrossRef]
- Nan, K.; Zhang, Y.; Zhang, X.; Li, D.; Zhao, Y.; Jing, Z.; Liu, K.; Shang, D.; Geng, Z.; Fan, L. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res. Ther. 2021, 12, 331. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Dong, B.; Li, Y.; Liang, Z. Network meta-analysis of invasive treatment for early-stage osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2024, 19, 30. [Google Scholar] [CrossRef]
- Yuan, F.-L.; Wu, Q.-Y.; Miao, Z.-N.; Xu, M.-H.; Xu, R.-S.; Jiang, D.-L.; Ye, J.-X.; Chen, F.-H.; Zhao, M.-D.; Wang, H.-J.; et al. Osteoclast-derived extracellular vesicles: Novel regulators of osteoclastogenesis and osteoclast–osteoblasts communication in bone remodeling. Front. Physiol. 2018, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, X.; Xu, C.; Cheng, Q. MicroRNAs in serum exosomes as circulating biomarkers for postmenopausal osteoporosis. Front. Endocrinol. 2022, 13, 819056. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, X.; Zhang, Y.; Zhuang, Q.; Wang, S.; Fang, X.; Xu, T.; Li, X.; Chen, G. Exosomal circFAM63Bsuppresses bone regeneration of postmenopausal osteoporosis via regulating miR-578/HMGA2 axis. J. Orthop. Res. 2024, 42, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Liu, Y.-W.; Rao, S.-S.; Yin, H.; Huang, J.; Chen, C.-Y.; Hu, Y.; Zhang, Y.; Tan, Y.-J.; Yuan, L.-Q. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef]
- Huang, B.; Su, Y.; Shen, E.; Song, M.; Liu, D.; Qi, H. Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model. Life Sci. 2021, 272, 119208. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Hou, C.; Bian, Z.; Jiang, W.; Li, M.; Zhu, L. Extracellular vesicles from bone marrow mesenchymal stem cells alleviate osteoporosis in mice through USP7-mediated YAP1 protein stability and the Wnt/β-catenin pathway. Biochem. Pharmacol. 2023, 217, 115829. [Google Scholar] [CrossRef]
- Deluca, A.; Wagner, A.; Heimel, P.; Deininger, C.; Wichlas, F.; Redl, H.; Rohde, E.; Tempfer, H.; Gimona, M.; Traweger, A. Synergistic effect of umbilical cord extracellular vesicles and rhBMP-2 to enhance the regeneration of a metaphyseal femoral defect in osteoporotic rats. Stem Cell Res. Ther. 2024, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, J.; Cai, L.; Liu, T.; Wang, X.; Zhang, Y.; Zhou, Z.; Li, T.; Liu, M.; Lai, R. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int. J. Nanomed. 2020, 15, 7967–7977. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Liu, Y.; Liu, X.; Ding, Y.; Li, D.; Zhang, X.; Liu, Y. Tailored apoptotic vesicles promote bone regeneration by releasing the osteoinductive brake. Int. J. Oral Sci. 2024, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.d. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Wang, T.; Liu, K.; Wang, J.; Xiang, G.; Hu, X.; Bai, H.; Lei, W.; Tao, T.H.; Feng, Y. Spatiotemporal regulation of injectable heterogeneous silk gel scaffolds for accelerating guided vertebral repair. Adv. Healthc. Mater. 2023, 12, 2202210. [Google Scholar] [CrossRef]
- Qayoom, I.; Teotia, A.K.; Kumar, A. Nanohydroxyapatite based ceramic carrier promotes bone formation in a femoral neck canal defect in osteoporotic rats. Biomacromolecules 2019, 21, 328–337. [Google Scholar] [CrossRef]
- Negri, S.; Wang, Y.; Sono, T.; Lee, S.; Hsu, G.C.-Y.; Xu, J.; Meyers, C.A.; Qin, Q.; Broderick, K.; Witwer, K.W. Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation. Stem Cells Transl. Med. 2020, 9, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Teotia, A.K.; Qayoom, I.; Singh, P.; Mishra, A.; Jaiman, D.; Seppälä, J.; Lidgren, L.; Kumar, A. Exosome-functionalized ceramic bone substitute promotes critical-sized bone defect repair in rats. ACS Appl. Bio Mater. 2021, 4, 3716–3726. [Google Scholar] [CrossRef]
- Mizukami, Y.; Kawao, N.; Takafuji, Y.; Ohira, T.; Okada, K.; Jo, J.-I.; Tabata, Y.; Kaji, H. Matrix vesicles promote bone repair after a femoral bone defect in mice. PLoS ONE 2023, 18, e0284258. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Federici, A.S.; Hoey, D.A.; Cox, S.C. An ECM-mimetic hydrogel to promote the therapeutic efficacy of osteoblast-derived extracellular vesicles for bone regeneration. Front. Bioeng. Biotechnol. 2022, 10, 829969. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Zhang, M.; Ge, S.; Feng, T.; Chen, R.; Shen, J.; Li, R.; Wang, Z.; Xie, Y. Histone deacetylase inhibition enhances extracellular vesicles from muscle to promote osteogenesis via miR-873-3p. Signal Transduct. Target. Ther. 2024, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sakai, K.; Watanabe, J.; Dong, J.; Maruyama, H.; Li, X.; Hibi, H. Conditioned medium of human mesenchymal stem cells affects stem cell senescence in osteoporosis. Biochem. Biophys. Res. Commun. 2024, 711, 149858. [Google Scholar] [CrossRef]
- Li, X.; Chen, R.; Li, Y.; Wang, P.; Cui, Y.; Yang, L.; Zhu, X.; Zhang, R. miR-27a-5p—Abundant small extracellular vesicles derived from Epimedium-preconditioned bone mesenchymal stem cells stimulate osteogenesis by targeting Atg4B-mediated autophagy. Front. Cell Dev. Biol. 2021, 9, 642646. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Kang, M.-K.; Park, S.-H.; Kim, D.Y.; Oh, S.Y.; Oh, M.-S.; Park, S.; Kang, I.-J.; Kang, Y.-H. Aesculetin accelerates osteoblast differentiation and matrix-vesicle-mediated mineralization. Int. J. Mol. Sci. 2021, 22, 12391. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Deng, M.; Huang, X.; Xie, D.; Gao, X.; Chen, J.; Shi, Z.; Lu, J.; Lin, H.; Li, P. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhacning autophagy. J. Control. Release 2023, 364, 644–653. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Park, Y.-S.; Kim, H.-S.; Kim, D.-h.; Lee, S.-H.; Lee, C.-H.; Lee, S.-H.; Kim, J.-E.; Lee, S.; Kim, H.M. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J. Control. Release 2023, 355, 184–198. [Google Scholar] [CrossRef]
- Go, G.; Jeon, J.; Lee, G.; Lee, J.H.; Lee, S.H. Bovine milk extracellular vesicles induce the proliferation and differentiation of osteoblasts and promote osteogenesis in rats. J. Food Biochem. 2021, 45, e13705. [Google Scholar] [CrossRef]
- Dong, M.; Shi, C.; Yu, X.; Yang, Q.; Wu, S.; Liu, R.; Liu, T.; Wang, L.; Niu, W. Milk-derived small extracellular vesicles: Nanomaterials to promote bone formation. J. Nanobiotechnol. 2022, 20, 370. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Ma, H.-W.; Xiang, G.-H.; He, G.-L.; Cai, H.-C.; Dai, Z.-H.; Chen, Y.-L.; Lin, Y.; Xu, H.-Z.; Ni, W.-F. Bone-targeting delivery of platelet lysate exosomes ameliorates glucocorticoid-induced osteoporosis by enhancing bone-vessel coupling. J. Nanobiotechnol. 2022, 20, 220. [Google Scholar] [CrossRef]
- Xie, X.; Cheng, P.; Hu, L.; Zhou, W.; Zhang, D.; Knoedler, S.; Liu, G.; Xiong, Y.; Xue, H.; Hu, Y. Bone-targeting engineered small extracellular vesicles carrying anti-miR-6359-CGGGAGC prevent valproic acid-induced bone loss. Signal Transduct. Target. Ther. 2024, 9, 24. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Jia, Y.; Qiu, S.; Xu, J.; Kang, Q.; Chai, Y. Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction osteogenesis in older rats. Calcif. Tissue Int. 2020, 106, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dou, G.; Kuang, H.; Bao, L.; Liu, H.; Ye, Q.; Wang, Z.; Yang, X.; Ren, L.; Li, Z. Apoptotic extracellular vesicles induced endothelial cell-mediated autologous stem cell recruitment dominates allogeneic stem cell therapeutic mechanism for bone repair. ACS Nano 2024, 18, 8718–8732. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xu, S.; Wang, Z. Rat sinus mucosa-and periosteum-derived exosomes accelerate osteogenesis. J. Cell. Physiol. 2019, 234, 21947–21961. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Yu, L.; Zou, L.; Yang, L.; Lin, S.; Wang, J.; Yuan, Z.; Dai, J. Exosomal miR-335 derived from mature dendritic cells enhanced mesenchymal stem cell-mediated bone regeneration of bone defects in athymic rats. Mol. Med. 2021, 27, 20. [Google Scholar] [CrossRef]
- Kang, M.; Huang, C.-C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hsu, C.-Y.; Gao, Y.; Meyers, C.A.; Chang, L.; Zhang, L.; Broderick, K.; Ding, C.; Peault, B. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife 2019, 8, e48191. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Wu, J.; Yu, Y.; Fu, Y.; Yin, S.; Li, K.; Li, Y.; Cai, L.; Du, Y. Polyphenol-Mediated Electroactive Hydrogel with Armored Exosomes Delivery for Bone Regeneration. ACS Nano 2025, 19, 17796–17812. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, X.; Gu, R.; Wang, N.; Ren, H.; Song, R.; Wan, Z.; Huang, J.; Yi, K.; Xiong, C. Affinity Modifications of Porous Microscaffolds Impact Bone Regeneration by Modulating the Delivery Kinetics of Small Extracellular Vesicles. ACS Nano 2025, 19, 17813–17823. [Google Scholar] [CrossRef]
- Pan, S.; Yin, Z.; Shi, C.; Xiu, H.; Wu, G.; Heng, Y.; Zhu, Z.; Zhang, J.; Gui, J.; Yu, Z. Multifunctional Injectable Hydrogel Microparticles Loaded With miR-29a Abundant BMSCs Derived Exosomes Enhanced Bone Regeneration by Regulating Osteogenesis and Angiogenesis. Small 2024, 20, 2306721. [Google Scholar] [CrossRef]
- Shou, J.; Li, S.; Shi, W.; Zhang, S.; Zeng, Z.; Guo, Z.; Ye, Z.; Wen, Z.; Qiu, H.; Wang, J. 3WJ RNA nanoparticles-aptamer functionalized exosomes from M2 macrophages target BMSCs to promote the healing of bone fractures. Stem Cells Transl. Med. 2023, 12, 758–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Q.-Y.; Sheng, J.-G. Exosomes in the repair of bone defects: Next-generation therapeutic tools for the treatment of nonunion. BioMed Res. Int. 2019, 2019, 1983131. [Google Scholar] [CrossRef]
- Hao, Z.C.; Lu, J.; Wang, S.Z.; Wu, H.; Zhang, Y.T.; Xu, S.G. Stem cell-derived exosomes: A promising strategy for fracture healing. Cell Prolif. 2017, 50, e12359. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Xu, Z.; Wu, Y.; Wang, G.; Yang, G.; Cao, L.; Chang, H.; Zhou, M.; Jiang, X. High-Performance Hydrogel-Encapsulated Engineered Exosomes for Supporting Endoplasmic Reticulum Homeostasis and Boosting Diabetic Bone Regeneration. Adv. Sci. 2024, 11, 2309491. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Li, X.-R.; Wei, W.-J.; Wei, Z.-Y.; Zhang, C.-R.; Wang, F.; Dawes, H.; Guo, S.-C. Polymeric coating on β-TCP scaffolds provides immobilization of small extracellular vesicles with surface-functionalization and ZEB1-Loading for bone defect repair in diabetes mellitus. Biomaterials 2022, 283, 121465. [Google Scholar] [CrossRef]
- Jing, X.; Wang, S.; Tang, H.; Li, D.; Zhou, F.; Xin, L.; He, Q.; Hu, S.; Zhang, T.; Chen, T. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl. Mater. Interfaces 2022, 14, 16082–16099. [Google Scholar] [CrossRef]
- Yang, T.; Dong, Y.; Wan, J.; Liu, X.; Liu, Y.; Huang, J.; Zhou, J.; Xiao, H.; Tang, L.; Wang, Y. Sustained release of BMSC-EVs from 3D printing gel/HA/nHAP scaffolds for promoting bone regeneration in diabetic rats. Adv. Healthc. Mater. 2023, 12, 2203131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, Y.; Li, Z.; Chen, H.; Huang, S.; Jian, C.; Yu, A. MiR-144-5p, an exosomal miRNA from bone marrow-derived macrophage in type 2 diabetes, impairs bone fracture healing via targeting Smad1. J. Nanobiotechnol. 2021, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yan, J.; Wang, C.; Qin, W.; Han, X.; Qin, Z.; Wei, Y.; Xu, H.; Gao, J.; Gao, C. Interorgan communication in neurogenic heterotopic ossification: The role of brain-derived extracellular vesicles. Bone Res. 2024, 12, 11. [Google Scholar] [CrossRef]
- Song, W.; Ma, Z.; Wang, X.; Wang, Y.; Wu, D.; Wang, C.; He, D.; Kong, L.; Yu, W.; Li, J.J. Macroporous granular hydrogels functionalized with aligned architecture and small extracellular vesicles stimulate osteoporotic tendon-to-bone healing. Adv. Sci. 2023, 10, 2304090. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Wang, Y.; Jacobson, D.S.; Reisdorf, R.L.; Kuroiwa, T.; Behfar, A.; Moran, S.L.; Steinmann, S.P.; Zhao, C. Effects of purified exosome product on rotator cuff tendon-bone healing in vitro and in vivo. Biomaterials 2021, 276, 121019. [Google Scholar] [CrossRef]
- Cai, J.; Xu, J.; Ye, Z.; Wang, L.; Zheng, T.; Zhang, T.; Li, Y.; Jiang, J.; Zhao, J. Exosomes derived from kartogenin-preconditioned mesenchymal stem cells promote cartilage formation and collagen maturation for enthesis regeneration in a rat model of chronic rotator cuff tear. Am. J. Sports Med. 2023, 51, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Jenner, F.; Wagner, A.; Gerner, I.; Ludewig, E.; Trujanovic, R.; Rohde, E.; von Rechenberg, B.; Gimona, M.; Traweger, A. Evaluation of the potential of umbilical cord mesenchymal stromal cell–derived small extracellular vesicles to improve rotator cuff healing: A pilot ovine study. Am. J. Sports Med. 2023, 51, 331–342. [Google Scholar] [CrossRef]
- Xue, Y.; Riva, N.; Zhao, L.; Shieh, J.-s.; Chin, Y.-T.; Gatt, A.; Guo, J.J. Recent advances of exosomes in soft tissue injuries in sports medicine: A critical review on biological and biomaterial applications. J. Control. Release 2023, 364, 90–108. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Sankaranarayanan, J.; Kim, H.K.; Kang, J.Y.; Kuppa, S.S.; Yang, H.Y.; Seon, J.K. Comparative Efficacy of Exosomes Derived from Different Mesenchymal Stem Cell Sources in Osteoarthritis Models: An In Vitro and Ex Vivo Analysis. Int. J. Mol. Sci. 2025, 26, 5447. [Google Scholar] [CrossRef]
- Xu, C.; Mi, Z.; Dong, Z.; Chen, X.; Ji, G.; Kang, H.; Li, K.; Zhao, B.; Wang, F. Platelet-derived exosomes alleviate knee osteoarthritis by attenuating cartilage degeneration and subchondral bone loss. Am. J. Sports Med. 2023, 51, 2975–2985. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, G.; Yan, Y.; Wang, C.; Wang, Z.; Jiang, C.; Jiang, Z.; Ma, T.; Zhang, C.; Yan, Z. Exosomes derived from bone marrow mesenchymal stem cells pretreated with decellularized extracellular matrix enhance the alleviation of osteoarthritis through miR-3473b/phosphatase and tensin homolog axis. J. Gene Med. 2023, 25, e3510. [Google Scholar] [CrossRef] [PubMed]
- Trivanovic, D.; Volkmann, N.; Stoeckl, M.; Tertel, T.; Rudert, M.; Giebel, B.; Herrmann, M. Enhancement of immunosuppressive activity of mesenchymal stromal cells by platelet-derived factors is accompanied by apoptotic priming. Stem Cell Rev. Rep. 2023, 19, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, W.; Duan, P.; Lv, X.; Feng, Y.; Yin, Z.; Luo, Z.; Li, Z.; Zhang, H.; Zhou, T. HWJMSC-derived extracellular vesicles ameliorate IL-1β-induced chondrocyte injury through regulation of the BMP2/RUNX2 axis via up-regulation TFRC. Cell. Signal. 2023, 105, 110604. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.; Lai, R.; Lim, S.; Hui, J.; Toh, W. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Niedermair, T.; Lukas, C.; Li, S.; Stöckl, S.; Craiovan, B.; Brochhausen, C.; Federlin, M.; Herrmann, M.; Grässel, S. Influence of extracellular vesicles isolated from osteoblasts of patients with cox-arthrosis and/or osteoporosis on metabolism and osteogenic differentiation of BMSCs. Front. Bioeng. Biotechnol. 2020, 8, 615520. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Si, H.-B.; Tang, L.; Xie, H.-Q.; Shen, B. Exosomes derived from human urine–derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am. J. Sports Med. 2022, 50, 1088–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, X.-L.; Wang, Y.-N.; Lu, W.; Wang, H.; Liao, R.; Zeng, M.; Yang, J.-X.; Hu, Y.; Xie, J. Extracellular vesicles from human urine-derived stem cells ameliorate particulate polyethylene-induced osteolysis. Int. J. Nanomed. 2021, 16, 7479–7494. [Google Scholar] [CrossRef]
- Xu, H.; Chai, Q.; Xu, X.; Li, Z.; Bao, W.; Man, Z.; Li, W. Exosome-functionalized Ti6Al4V scaffolds promoting osseointegration by modulating endogenous osteogenesis and osteoimmunity. ACS Appl. Mater. Interfaces 2022, 14, 46161–46175. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Wen, J.; Zhang, Y.; Mou, P.; Luo, Z.; Cai, Y.; Chen, A.; Fu, X.; Meng, W.; Zhou, Z. M2 macrophage-derived exosome-functionalized topological scaffolds regulate the foreign body response and the coupling of angio/osteoclasto/osteogenesis. Acta Biomater. 2024, 177, 91–106. [Google Scholar] [CrossRef]
- Olaechea, A.; Benabdellah, K.; Vergara-Buenaventura, A.; Gómez-Melero, S.; Cafferata, E.A.; Meza-Mauricio, J.; Padial-Molina, M.; Galindo-Moreno, P. Preclinical evidence for the use of oral mesenchymal stem cell-derived extracellular vesicles in bone regenerative therapy: A systematic review. Stem Cells Transl. Med. 2023, 12, 791–800. [Google Scholar] [CrossRef]
- Guo, S.; Gu, J.; Ma, J.; Xu, R.; Wu, Q.; Meng, L.; Liu, H.; Li, L.; Xu, Y. GATA4-driven miR-206-3p signatures control orofacial bone development by regulating osteogenic and osteoclastic activity. Theranostics 2021, 11, 8379. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Zhang, S.; Ma, Y.; Yang, X.; Huo, F.; Chen, Y.; Yang, B.; Tian, W. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res. Ther. 2022, 13, 41. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, L. Exosomes from gingival mesenchymal stem cells enhance migration and osteogenic differentiation of pre-osteoblasts. Die Pharm.-Int. J. Pharm. Sci. 2020, 75, 576–580. [Google Scholar]
- Wang, W.; Qiao, S.-C.; Wu, X.-B.; Sun, B.; Yang, J.-G.; Li, X.; Zhang, X.; Qian, S.-J.; Gu, Y.-X.; Lai, H.-C. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021, 12, 628. [Google Scholar] [CrossRef]
- Maiborodin, I.; Shevela, A.; Matveeva, V.; Morozov, V.; Toder, M.; Krasil’nikov, S.; Koryakina, A.; Shevela, A.; Yanushevich, O. First experimental study of the influence of Extracellular vesicles derived from multipotent stromal cells on Osseointegration of Dental implants. Int. J. Mol. Sci. 2021, 22, 8774. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Lin, X.; Zhang, Z.; Zhang, M.; Tang, C.; Kou, X.; Deng, F. TNF-α-licensed exosome-integrated titaniumaccelerated T2D osseointegration by promoting autophagy-regulated M2 macrophage polarization. Biochem. Biophys. Res. Commun. 2024, 727, 150316. [Google Scholar] [CrossRef]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef]
- Rana, N.; Suliman, S.; Al-Sharabi, N.; Mustafa, K. Extracellular vesicles derived from primed mesenchymal stromal cells loaded on biphasic calcium phosphate biomaterial exhibit enhanced macrophage polarization. Cells 2022, 11, 470. [Google Scholar] [CrossRef]
- Song, X.; Xue, Y.; Fan, S.; Hao, J.; Deng, R. Lipopolysaccharide-activated macrophages regulate the osteogenic differentiation of bone marrow mesenchymal stem cells through exosomes. PeerJ 2022, 10, e13442. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-R.; Li, D.-W.; Man, Q.-W. Proteomic profile of tissue-derived extracellular vesicles from benign odontogenic lesions. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101921. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tang, H.; Gu, X.; Shi, Y.; Gong, P.; Yao, Y. Radiation can regulate the expression of miRNAs associated with osteogenesis and oxidation in exosomes from peripheral blood plasma. Oxidative Med. Cell. Longev. 2021, 2021, 6646323. [Google Scholar] [CrossRef]
- Lee, A.; Choi, J.; Shi, S.; He, P.; Zhang, Q.; Le, A. DPSC-derived extracellular vesicles promote rat jawbone regeneration. J. Dent. Res. 2023, 102, 313–321. [Google Scholar] [CrossRef]
- Liu, A.; Jin, S.; Fu, C.; Cui, S.; Zhang, T.; Zhu, L.; Wang, Y.; Shen, S.G.; Jiang, N.; Liu, Y. Macrophage-derived small extracellular vesicles promote biomimetic mineralized collagen-mediated endogenous bone regeneration. Int. J. Oral Sci. 2020, 12, 33. [Google Scholar] [CrossRef]
- Ming, L.; Qu, Y.; Wang, Z.; Dong, L.; Li, Y.; Liu, F.; Wang, Q.; Zhang, D.; Li, Z.; Zhou, Z. Small extracellular vesicles laden oxygen-releasing thermosensitive hydrogel for enhanced antibacterial therapy against anaerobe-induced periodontitis alveolar bone defect. ACS Biomater. Sci. Eng. 2024, 10, 932–945. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, R.; Da, N.; Wang, Y.; Yang, J.; Li, B.; He, X. Aspirin loaded extracellular vesicles inhibit inflammation of macrophages via switching metabolic phenotype in periodontitis. Biochem. Biophys. Res. Commun. 2023, 667, 25–33. [Google Scholar] [CrossRef]
- Yu, W.; Li, S.; Guan, X.; Zhang, N.; Xie, X.; Zhang, K.; Bai, Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater. Adv. 2022, 133, 112646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pei, Z.; He, W.; Feng, W.; Hao, T.; Sun, M.; Yang, X.; Wang, X.; Kong, X.; Chang, J. 3D-printed porous zinc scaffold combined with bioactive serum exosomes promotes bone defect repair in rabbit radius. Aging 2024, 16, 9625. [Google Scholar] [CrossRef]
- Zhou, H.; Qi, Y.-X.; Zhu, C.-H.; Li, A.; Pei, D.-D. Mesenchymal stem cell-derived extracellular vesicles for treatment of bone loss within periodontitis in pre-clinical animal models: A meta-analysis. BMC Oral Health 2023, 23, 701. [Google Scholar] [CrossRef] [PubMed]
- Wiest, E.F.; Zubair, A.C. Generation of Current Good Manufacturing Practices-Grade Mesenchymal Stromal Cell-Derived Extracellular Vesicles Using Automated Bioreactors. Biology 2025, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, H.; Li, N.; Liu, P.; Yang, J.; Zhao, Y. Emerging technologies towards extracellular vesicles large-scale production. Bioact. Mater. 2025, 52, 338–365. [Google Scholar] [CrossRef]
- Liu, X.-C.; Tian, J.-W.; Xu, J.-Y.; Chen, L.-G.; Ye, Z.-W.; Huang, J.; Wu, L.-Z.; Zhang, Z.-L.; Yu, Z.-L.; Chen, G. Extracellular vesicle manufacture via FACTORY: Fully automated collection technology and optimum machinery for clinical translational applications. Trends Biotechnol. 2025, 43, 2947–2969. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, P.K.; Kashyap, V.; Kumar, S. Proteomics of Extracellular Vesicles: Recent Updates, Challenges and Limitations. Proteomes 2025, 13, 12. [Google Scholar] [CrossRef]
- Silva, T.F.; Hutchins, E.; Zhao, W.; Ciani, Y.; Kim, M.; Ko, E.; Mariscal, J.; Qiu, Z.; Bedier, F.; Kittel, A. Extracellular vesicle heterogeneity through the lens of multiomics. Cell Rep. Med. 2025, 6, 102161. [Google Scholar] [CrossRef] [PubMed]
- Shaba, E.; Vantaggiato, L.; Governini, L.; Haxhiu, A.; Sebastiani, G.; Fignani, D.; Grieco, G.E.; Bergantini, L.; Bini, L.; Landi, C. Multi-omics integrative approach of extracellular vesicles: A future challenging milestone. Proteomes 2022, 10, 12. [Google Scholar] [CrossRef]
- Wang, T.; Ng, C.Y.; Ng, B.Z.J.; Toh, W.S.; Hui, J.H.P. Multi-Omics Analysis of Small Extracellular Vesicles in Osteoarthritis: Bridging the Gap between Molecular Insights and Clinical Applications. Burn. Trauma 2025, 13, tkaf023. [Google Scholar] [CrossRef]
- Gómez-de-Mariscal, E.; Maška, M.; Kotrbová, A.; Pospíchalová, V.; Matula, P.; Munoz-Barrutia, A. Deep-learning-based segmentation of small extracellular vesicles in transmission electron microscopy images. Sci. Rep. 2019, 9, 13211. [Google Scholar] [CrossRef]
- Uthamacumaran, A.; Abdouh, M.; Sengupta, K.; Gao, Z.-h.; Forte, S.; Tsering, T.; Burnier, J.V.; Arena, G. Machine intelligence-driven classification of cancer patients-derived extracellular vesicles using fluorescence correlation spectroscopy: Results from a pilot study. Neural Comput. Appl. 2023, 35, 8407–8422. [Google Scholar] [CrossRef]
- del Real Mata, C.; Jeanne, O.; Jalali, M.; Lu, Y.; Mahshid, S. Nanostructured-Based Optical Readouts Interfaced with Machine Learning for Identification of Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, 2202123. [Google Scholar] [CrossRef] [PubMed]

| Stem Cell Source | Typical EV Markers | Key Cargo | Main Signaling Pathways, Protein Modulation | Main In Vivo Models | Advantages/Limitations | References |
|---|---|---|---|---|---|---|
| BM-MSCs | CD9, CD63, CD81, TSG101 | miR-186, miR-196a, miR-27a, miR-150-3p, miR-424-5p, combined let-7a-5p, let-7c-5p, miR-328a-5p, and miR-31a-5p proteins ((Fibulin-1 (FBLN1), Prolargin (PRELP), Matrix Gla protein (MGP), Cystatin-C (CST3), SCUBE3, FGFR1, CCN5, IGFBP4, CTHRC1, IGFBP6, Decorin (DCN), Tetranectin (CLEC3B), MMP2, IGF2, IGFBP2, IGFBP3, HMGB1, COL6A1, Versican (VCAN) |
| Calvarial defect, osteoporosis | Highly osteogenic; invasive sourcing | [14,19,36,37,38,39,40,41,42,43,44] |
| ADSCs | CD9, CD63, CD81 | miR-375, autophagy regulators | MAPK pathway, Ras protein activity | Bone defect repair | High yield; variability among donors | [20,42,45,46] |
| UC-MSCs | CD9, CD63, CD81 | CLEC11A, miRNAs |
| Fracture healing | Low immunogenicity; perinatal source | [21,47,48,49] |
| iMSCs | CD9, CD63, CD81 | miR-196a, miR-206, Wnt proteins | Wnt/β-catenin pathway | Calvarial defect, angiogenesis | Scalable; requires reprogramming | [50,51,52,53] |
| DPSCs | CD9, CD63, CD81, TSG101 | miR-27a, RUNX3, Rab27a |
| Bone and periodontal defect | Minimally invasive; tooth source | [28,29,54,55] |
| SYN-MSCs | CD9, CD63, TSG101 | chondrogenic/pro-repair miRNAs and proteins (Sox9-related) |
| osteochondral repair, osteonecrosis of femoral head (ONFH) models | cartilage-specific regenerative potential; scalable production using 3D bioreactors; donor/inflammation status affects yield | [25,33,56,57,58,59] |
| miRNA | EV Source(s) | Functional Role | Key Pathways/Targets | Effect on Osteogenesis | Refs |
|---|---|---|---|---|---|
| miR-186 | BM-MSC-EVs | Pro-osteogenic | Mob1/Hippo inhibition | ↑ Osteoblast proliferation | [19] |
| miR-150-3p | BM-MSC-EVs | Pro-osteogenic | ↑ Runx2, Osterix; ↓ apoptosis | ↑ Osteogenesis | [36] |
| miR-935 | BM-MSC-EVs | Pro-osteogenic | STAT1 inhibition → ↑ Runx2 | ↑ Osteogenic differentiation | [60,61] |
| miR-196a | BM-MSC-EVs; iMSC-EVs | Pro-osteogenic | Targets Dkk1 → Wnt/β-catenin activation | ↑ ALP, OCN, Runx2 | [14,62,63] |
| miR-27a | BM-MSC-EVs; iMSC-EVs | Pro-osteogenic | Targets Dkk2 | Protects against bone loss; ↓ osteoclasts | [14,37,38,63] |
| miR-27a-5p | Dental stem cell EVs | Pro-osteogenic | AMPK–mTOR, Wnt, BMP/Smad | ↑ Mineralization | [64,65,66,67] |
| let-7a-5p/let-7c-5p/miR-328a-5p/miR-31a-5p | BM-MSC-EVs | Pro-osteogenic | BMP/Smad modulation | ↑ Smad1/5/9; ↓ Smad2/3 | [43] |
| miR-21 | OP-BM-MSC-EVs | Anti-osteogenic | Targets Smad7 | ↓ ALP, Runx2 | [68] |
| miR-424-5p | OP-BM-MSC-EVs | Anti-osteogenic | ↓ WIF1 → Wnt disruption | ↓ Osteogenic differentiation | [39] |
| miR-375 | ADSC-EVs | Pro-osteogenic | Targets IGFBP3 | ↑ Bone regeneration | [45] |
| miR-1263 | UC-MSC-EVs | Pro-osteogenic | Mob1/Hippo | ↓ Apoptosis; ↑ balance osteoblast/adipocyte | [47,69] |
| miR-451a | Mg2+-EVs; hydrogels | Pro-osteogenic | AKT/eNOS activation | ↑ Angiogenesis and osteogenesis | [70,71] |
| miR-142-5p | Young-donor EVs; PDLSC-EVs | Pro-osteogenic | Age-dependent enrichment | ↑ Osteogenesis | [64,72] |
| miR-122-5p/miR-25-3p/miR-192-5p | PDLSC-EVs | Pro-osteogenic | Osteogenic pathway regulators | ↑ Osteogenic differentiation | [64] |
| miR-206 | BM-MSC-EVs; iMSC-EVs | Pro-osteogenic-related | Enriched osteogenic miRNA | Supports osteogenic gene upregulation | [14,63] |
| EV Origin | Model and Delivery | Key Outcomes | Refs |
|---|---|---|---|
| Periodontal MSC-derived EVs (hGMSC/hPDLSC) | In vitro and rat calvarial models; 3D scaffolds/collagen membranes | Enhanced osteogenesis, signaling upregulation (TGFβ, BMP2), improved bone repair | [87,88,89,90,91] |
| ADSC-EVs (incl. engineered and 3D-bioprinted systems) | Rat femoral and osteochondral defects; PLGA/Mg-GA, polydopamine, ECM hydrogels | Improved angiogenesis, osteogenesis, reduced inflammation; cartilage–bone dual regeneration | [71,92,93,94] |
| Induced MSC-EVs and hucMSC-EVs | In vitro OA chondrocytes; rat bone defect w/hydrogel delivery | Protection of cartilage homeostasis; increased vascularization and bone formation | [88,95,96,97] |
| Osteocyte-derived mechanically activated EVs (MA-EVs) | In vivo osteoporotic models; local delivery | Promoted bone regeneration in osteoporotic conditions | [98] |
| BMP2/BMP7-primed MSC-EVs | Rat calvarial defect; local delivery | Activated BMPRI/II–SMAD pathway; improved bone repair | [99] |
| PDLSC-EVs (Matrigel) and engineered bone-targeted EVs (BT-Exo-siShn3) | Topical gel delivery; systemic/local targeted EVs | Enhanced MSC recruitment, AKT/ERK activation; Shn3 silencing → ↑ osteogenesis, ↓ RANKL | [100,101] |
| MSC-EVs + Alginate-PCL scaffolds | In vivo subcutaneous bone formation model | Improved vascularization and engineered bone formation | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dalle Carbonare, L.; Minoia, A.; Braggio, M.; Piritore, F.C.; Vareschi, A.; Cominacini, M.; Gandini, A.; Antoniazzi, F.; Cui, D.; Romanelli, M.G.; et al. Extracellular Vesicles in Osteogenesis: Comparative Analysis of Stem Cell Sources, Conditioning Strategies, and In Vitro Models Toward Advanced Bone Regeneration. Cells 2026, 15, 27. https://doi.org/10.3390/cells15010027
Dalle Carbonare L, Minoia A, Braggio M, Piritore FC, Vareschi A, Cominacini M, Gandini A, Antoniazzi F, Cui D, Romanelli MG, et al. Extracellular Vesicles in Osteogenesis: Comparative Analysis of Stem Cell Sources, Conditioning Strategies, and In Vitro Models Toward Advanced Bone Regeneration. Cells. 2026; 15(1):27. https://doi.org/10.3390/cells15010027
Chicago/Turabian StyleDalle Carbonare, Luca, Arianna Minoia, Michele Braggio, Francesca Cristiana Piritore, Anna Vareschi, Mattia Cominacini, Alberto Gandini, Franco Antoniazzi, Daping Cui, Maria Grazia Romanelli, and et al. 2026. "Extracellular Vesicles in Osteogenesis: Comparative Analysis of Stem Cell Sources, Conditioning Strategies, and In Vitro Models Toward Advanced Bone Regeneration" Cells 15, no. 1: 27. https://doi.org/10.3390/cells15010027
APA StyleDalle Carbonare, L., Minoia, A., Braggio, M., Piritore, F. C., Vareschi, A., Cominacini, M., Gandini, A., Antoniazzi, F., Cui, D., Romanelli, M. G., & Valenti, M. T. (2026). Extracellular Vesicles in Osteogenesis: Comparative Analysis of Stem Cell Sources, Conditioning Strategies, and In Vitro Models Toward Advanced Bone Regeneration. Cells, 15(1), 27. https://doi.org/10.3390/cells15010027











