Activating KRAS Mutations Expressed in 3D Endothelial Spheroids Induce Blebbing Morphologies Associated with Amoeboid-like Migration
Highlights
- Endothelial spheroids expressing KRASG12V exhibit characteristic features associated with abnormal vessel development in arteriovenous malformations as well as novel phenotypes not previously observed in 2D monolayers.
- Expression of KRASG12V can induce blebbing morphologies associated with mesenchymal-to-amoeboid transitions (MAT) and amoeboid-like migration in brain endothelial spheroids after extended culture.
- Amoeboid migration may play a role in the aberrant angiogenesis of KRAS-driven arteriovenous malformations or in resistance to inhibitors targeting mesenchymal migration alone.
- 3D brain endothelial spheroids transduced with adeno-associated viral constructs offer a novel platform to investigate the plasticity of driver mutations associated with arteriovenous malformations.
Abstract
1. Introduction
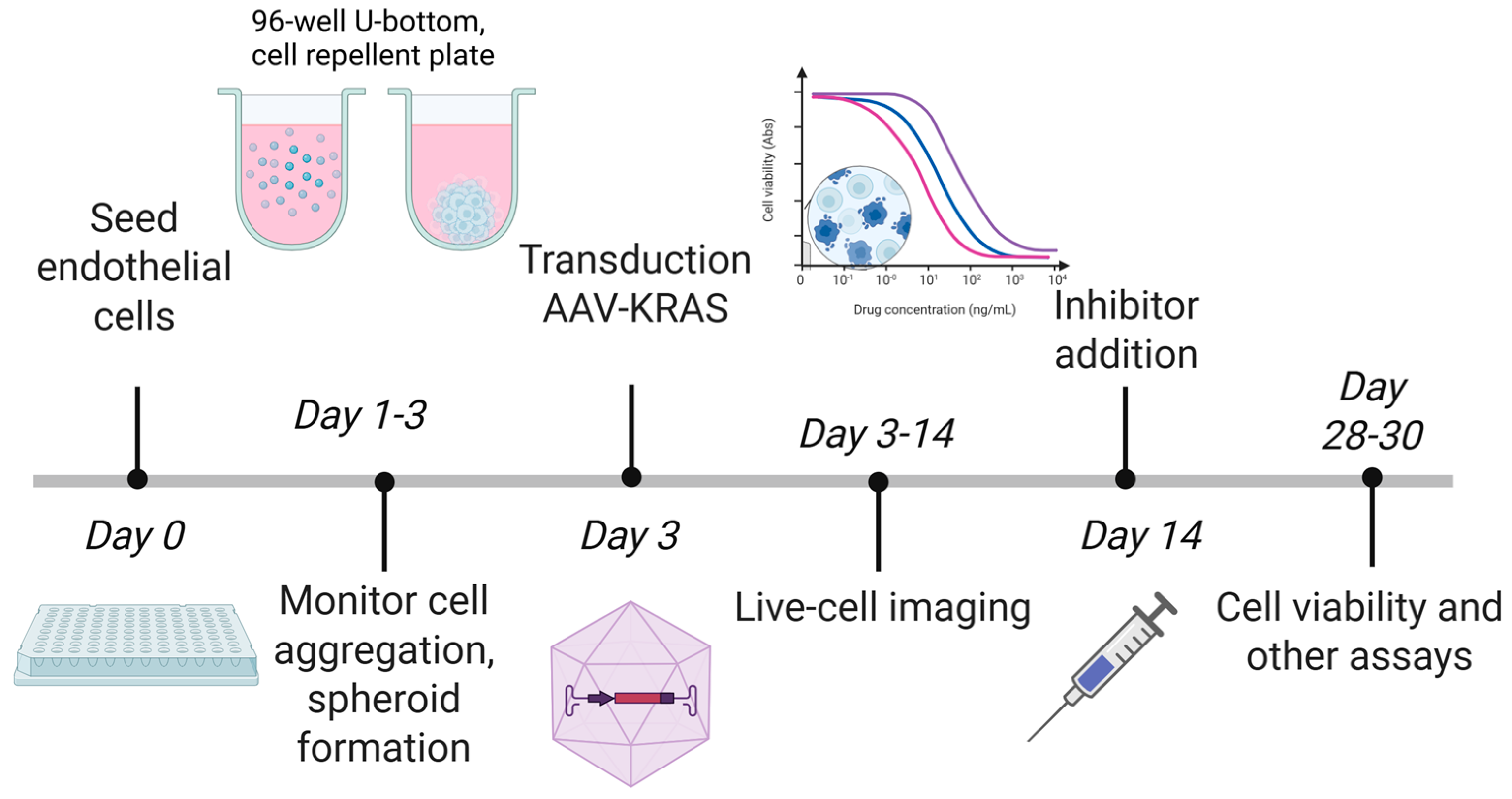
2. Materials and Methods
2.1. Cell Culture and Spheroid Formation
2.2. AAV Construction and Transduction
2.3. Western Blotting
2.4. Live-Cell Imaging
2.5. Immunocytochemistry and Microscopy
2.6. Viability and Cell Death Assays
2.7. Spheroid Sprouting Assays
2.8. Spheroid Dispersion and Cell Size Estimation
2.9. Pathway Inhibition
2.10. Statistical Analysis
3. Results
3.1. AAV2QUADYF Enables Stable Mosaic Gene Expression in hCMEC/D3 Spheroids
3.2. KRASG12V Increases Spheroid Size in the Early Culture Period
3.3. KRASG12V Stimulates Spheroid Sprouting in a Reduced Growth Factor Environment
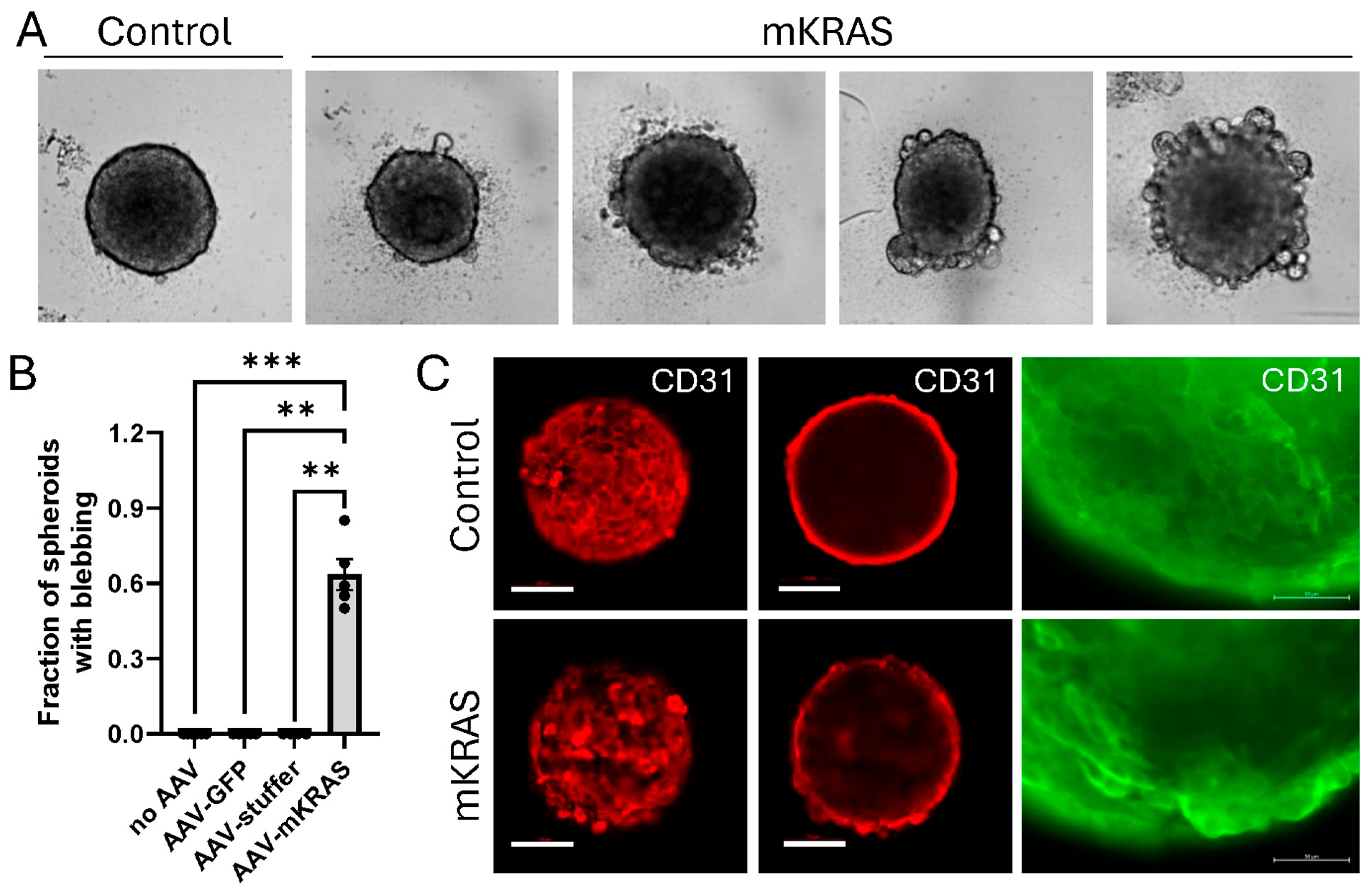
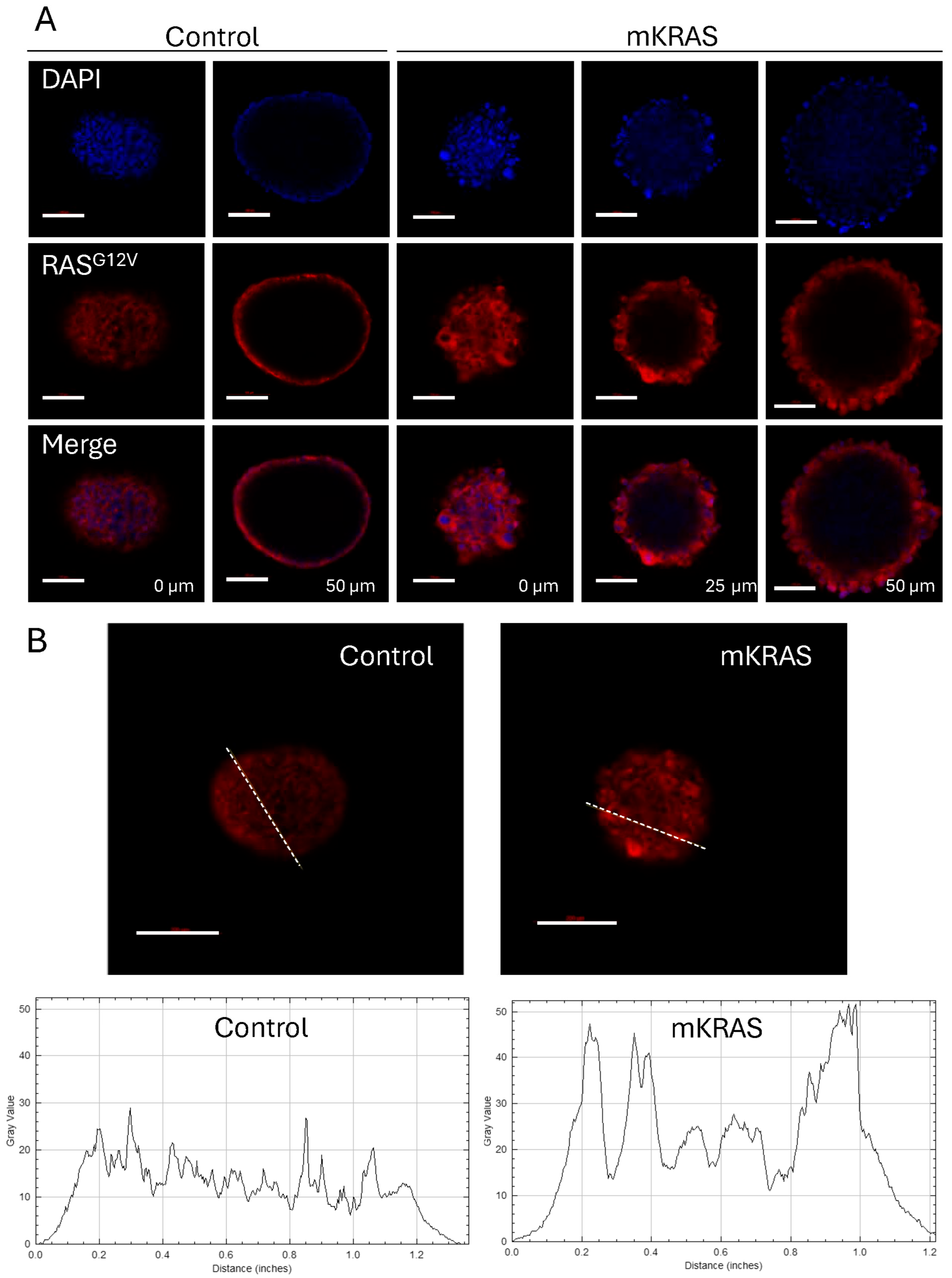
3.4. KRASG12V Alters Spheroid Morphology After Extended Culture
3.5. KRASG12V-Associated Blebs Disrupt Cell-to-Cell Adhesion
3.6. KRASG12V Leads to Cellular Hypertrophy and Amoeboid Migration
3.7. Blebbing Can Be Reversed with MEK, mTOR, and Rho/ROCK Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| AVM | Arteriovenous malformation |
| ANV-FITC | Annexin V-fluorescein isothiocynate |
| EndoMT | Endothelial–mesenchymal transition |
| GFP | Green fluorescent protein |
| hCMEC | Human cerebral microvascular endothelial cells |
| HUVEC | Human umbilical vein endothelial cell |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| MAT | Mesenchymal-amoeboid transition |
References
- Lawton, M.T.; Rutledge, W.C.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; terBrugge, K.; Kondziolka, D.; Morgan, M.K.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Primers 2015, 1, 15008. [Google Scholar] [CrossRef]
- Can, A.; Gross, B.A.; Du, R. The natural history of cerebral arteriovenous malformations. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 143, pp. 15–24. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Rezai Jahromi, B.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.R.; et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Al-Olabi, L.; Polubothu, S.; Dowsett, K.; Andrews, K.A.; Stadnik, P.; Joseph, A.P.; Knox, R.; Pittman, A.; Clark, G.; Baird, W.; et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 2018, 128, 1496–1508. [Google Scholar] [CrossRef]
- Hong, T.; Yan, Y.; Li, J.; Radovanovic, I.; Ma, X.; Shao, Y.W.; Yu, J.; Ma, Y.; Zhang, P.; Ling, F.; et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2019, 142, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Nelson, J.; Weinsheimer, S.; Winkler, E.A.; Rutledge, C.; Abla, A.A.; Gupta, N.; Shieh, J.T.; Cooke, D.L.; Hetts, S.W.; et al. Somatic mosaicism in the MAPK pathway in sporadic brain arteriovenous malformation and association with phenotype. J. Neurosurg. 2022, 136, 148–155. [Google Scholar] [CrossRef]
- Oka, M.; Kushamae, M.; Aoki, T.; Yamaguchi, T.; Kitazato, K.; Abekura, Y.; Kawamata, T.; Mizutani, T.; Miyamoto, S.; Takagi, Y. KRAS G12D or G12V Mutation in Human Brain Arteriovenous Malformations. World Neurosurg. 2019, 126, e1365–e1373. [Google Scholar] [CrossRef]
- Li, H.; Nam, Y.; Huo, R.; Fu, W.; Jiang, B.; Zhou, Q.; Song, D.; Yang, Y.; Jiao, Y.; Weng, J.; et al. De Novo Germline and Somatic Variants Convergently Promote Endothelial-to-Mesenchymal Transition in Simplex Brain Arteriovenous Malformation. Circ. Res. 2021, 129, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Flores Suarez, C.P.; Boudreau, E.; Herman, A.M.; Gutierrez, M.C.; Gustafson, D.; DiStefano, P.V.; Cui, M.; Chen, Z.; De Ruiz, K.B.; et al. Somatic gain of KRAS function in the endothelium is sufficient to cause vascular malformations that require MEK but not PI3K signaling. Circ. Res. 2020, 127, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kim, S.; Huang, S.; Yoo, J.Y.; Körbelin, J.; Lee, T.J.; Kaur, B.; Dash, P.K.; Chen, P.R.; Kim, E. Selective endothelial hyperactivation of oncogenic KRAS induces brain arteriovenous malformations in mice. Ann. Neurol. 2021, 89, 926–941. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Boon, L.M.; Vikkula, M. Trametinib as a promising therapeutic option in alleviating vascular defects in an endothelial KRAS-induced mouse model. Hum. Mol. Genet. 2023, 32, 276–289. [Google Scholar] [CrossRef]
- Saito, S.; Nakamura, Y.; Miyashita, S.; Sato, T.; Hoshina, K.; Okada, M.; Hasegawa, H.; Oishi, M.; Fujii, Y.; Körbelin, J.; et al. CRISPR/CasRx suppresses KRAS-induced brain arteriovenous malformation developed in postnatal brain endothelial cells in mice. J. Clin. Investig. 2024, 9, e179729. [Google Scholar] [CrossRef] [PubMed]
- Maynard, K.; LoPresti, M.; Iacobas, I.; Kan, P.; Lam, S. Antiangiogenic agent as a novel treatment for pediatric intracranial arteriovenous malformations: Case report. J. Neurosurg. Pediatr. 2019, 24, 673–679. [Google Scholar] [CrossRef]
- Korff, T.; Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 1998, 143, 1341–1352. [Google Scholar] [CrossRef]
- McRobb, L.S.; Lee, V.S.; Faqihi, F.; Stoodley, M.A. A Simple Model to Study Mosaic Gene Expression in 3D Endothelial Spheroids. J. Cardiovasc. Dev. Dis. 2024, 11, 305. [Google Scholar] [CrossRef]
- Subramanian, S.; Ugoya, S.O.; Zhao, Z.; McRobb, L.S.; Grau, G.E.; Combes, V.; Inglis, D.W.; Gauden, A.J.; Lee, V.S.; Moutrie, V.; et al. Stable thrombus formation on irradiated microvascular endothelial cells under pulsatile flow: Pre-testing annexin V-thrombin conjugate for treatment of brain arteriovenous malformations. Thromb. Res. 2018, 167, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Faqihi, F.; Stoodley, M.A.; McRobb, L.S. Endothelial surface translocation of mitochondrial PDCE2 involves the non-canonical secretory autophagy pathway: Putative molecular target for radiation-guided drug delivery. Exp. Cell Res. 2021, 405, 112688. [Google Scholar] [CrossRef]
- Lipinski, D.M.; Reid, C.A.; Boye, S.L.; Peterson, J.J.; Qi, X.; Boye, S.E.; Boulton, M.E.; Hauswirth, W.W. Systemic Vascular Transduction by Capsid Mutant Adeno-Associated Virus After Intravenous Injection. Hum. Gene Ther. 2015, 26, 767–776. [Google Scholar] [CrossRef] [PubMed]
- McRobb, L.S.; McKay, M.J.; Gamble, J.R.; Grace, M.; Moutrie, V.; Santos, E.D.; Lee, V.S.; Zhao, Z.; Molloy, M.P.; Stoodley, M.A. Ionizing radiation reduces ADAM10 expression in brain microvascular endothelial cells undergoing stress-induced senescence. Aging 2017, 9, 1248–1268. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef]
- Graziani, V.; Rodriguez-Hernandez, I.; Maiques, O.; Sanz-Moreno, V. The amoeboid state as part of the epithelial-to-mesenchymal transition programme. Trends Cell Biol. 2022, 32, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Zanetti, A.; Sironi, M.; Polentarutti, N.; Lanfrancone, L.; Dejana, E.; Colotta, F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J. Cell Biol. 1994, 127, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, A.B.; Shojaei, S. Mechanistic insights into mesenchymal-amoeboid transition as an intelligent cellular adaptation in cancer metastasis and resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167332. [Google Scholar] [CrossRef] [PubMed]
- Morley, S.; Hager, M.H.; Pollan, S.G.; Knudsen, B.; Di Vizio, D.; Freeman, M.R. Trading in your spindles for blebs: The amoeboid tumor cell phenotype in prostate cancer. Asian J. Androl. 2014, 16, 530–535. [Google Scholar] [CrossRef]
- Jia, W.; Czabanka, M.; Broggini, T. Cell blebbing novel therapeutic possibilities to counter metastasis. Clin. Exp. Metastasis 2024, 41, 817–828. [Google Scholar] [CrossRef]
- Weems, A.D.; Welf, E.S.; Driscoll, M.K.; Zhou, F.Y.; Mazloom-Farsibaf, H.; Chang, B.J.; Murali, V.S.; Gihana, G.M.; Weiss, B.G.; Chi, J.; et al. Blebs promote cell survival by assembling oncogenic signalling hubs. Nature 2023, 615, 517–525. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Bafor, A.E.; Freeman, B.H.; Chen, P.R.; Park, E.S.; Kim, E. Pathophysiology in Brain Arteriovenous Malformations: Focus on Endothelial Dysfunctions and Endothelial-to-Mesenchymal Transition. Biomedicines 2024, 12, 1795. [Google Scholar] [CrossRef]
- Wu, J.S.; Jiang, J.; Chen, B.J.; Wang, K.; Tang, Y.L.; Liang, X.H. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Liu, Y.J.; Le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuzé, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015, 160, 659–672. [Google Scholar] [CrossRef]
- Chillà, A.; Anceschi, C.; Frediani, E.; Scavone, F.; Del Rosso, T.; Pelagio, G.; Tufaro, A.; De Palma, G.; Del Rosso, M.; Fibbi, G.; et al. Inhibition of MMPs supports amoeboid angiogenesis hampering VEGF-targeted therapies via MLC and ERK 1/2 signaling. J. Transl. Med. 2023, 21, 102. [Google Scholar] [CrossRef]
- Chillà, A.; Margheri, F.; Biagioni, A.; Del Rosso, M.; Fibbi, G.; Laurenzana, A. Mature and progenitor endothelial cells perform angiogenesis also under protease inhibition: The amoeboid angiogenesis. J. Exp. Clin. Cancer Res. 2018, 37, 74. [Google Scholar] [CrossRef]
- Jia, Y.C.; Fu, J.Y.; Huang, P.; Zhang, Z.P.; Chao, B.; Bai, J. Characterization of Endothelial Cells Associated with Cerebral Arteriovenous Malformation. Neuropsychiatr. Dis. Treat. 2020, 16, 1015–1022. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.J.; Liu, L.; Zhao, Y.L. Ephrin B2 and EphB4 selectively mark arterial and venous vessels in cerebral arteriovenous malformation. J. Int. Med. Res. 2014, 42, 405–415. [Google Scholar] [CrossRef]
- Gabeff, R.; Boccara, O.; Soupre, V.; Lorette, G.; Bodemer, C.; Herbreteau, D.; Tavernier, E.; Maruani, A. Efficacy and Tolerance of Sirolimus (Rapamycin) for Extracranial Arteriovenous Malformations in Children and Adults. Acta Derm. Venereol. 2019, 99, 1105–1109. [Google Scholar] [CrossRef]
- Maruani, A.; Tavernier, E.; Boccara, O.; Mazereeuw-Hautier, J.; Leducq, S.; Bessis, D.; Guibaud, L.; Vabres, P.; Carmignac, V.; Mallet, S.; et al. Sirolimus (Rapamycin) for Slow-Flow Malformations in Children: The Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021, 157, 1289–1298. [Google Scholar] [CrossRef]
- Nicholson, C.L.; Flanagan, S.; Murati, M.; Boull, C.; McGough, E.; Ameduri, R.; Weigel, B.; Maguiness, S. Successful management of an arteriovenous malformation with trametinib in a patient with capillary-malformation arteriovenous malformation syndrome and cardiac compromise. Pediatr. Dermatol. 2022, 39, 316–319. [Google Scholar] [CrossRef]
- Edwards, E.A.; Phelps, A.S.; Cooke, D.; Frieden, I.J.; Zapala, M.A.; Fullerton, H.J.; Shimano, K.A. Monitoring Arteriovenous Malformation Response to Genotype-Targeted Therapy. Pediatrics 2020, 146, e20193206. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wu, Y.; Lawton, M.T.; Yang, G.Y.; Barbaro, N.M.; Young, W.L. Coexpression of angiogenic factors in brain arteriovenous malformations. Neurosurgery 2005, 56, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Emala, C.W.; Joshi, S.; Mesa-Tejada, R.; Quick, C.M.; Feng, L.; Libow, A.; Marchuk, D.A.; Young, W.L. Abnormal pattern of Tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery 2000, 47, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Baig Mirza, A.; Fayez, F.; Al-Munaer, M.; Georgiannakis, A.; Burn, L.; Ravi, K.; Vastani, A.; Syrris, C.; Patel, J.; Matloob, S. The use of Bevacizumab in the treatment of brain arteriovenous malformations: A systematic review. Neurosurg. Rev. 2025, 48, 506. [Google Scholar] [CrossRef] [PubMed]
- Makrodouli, E.; Oikonomou, E.; Koc, M.; Andera, L.; Sasazuki, T.; Shirasawa, S.; Pintzas, A. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: A comparative study. Mol. Cancer 2011, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Maldonado, C.; Zimmer, Y.; Medová, M. A comparative analysis of individual RAS mutations in cancer biology. Front. Oncol. 2019, 9, 1088. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
McRobb, L.S.; Lee, V.S.; Stoodley, M.A. Activating KRAS Mutations Expressed in 3D Endothelial Spheroids Induce Blebbing Morphologies Associated with Amoeboid-like Migration. Cells 2026, 15, 22. https://doi.org/10.3390/cells15010022
McRobb LS, Lee VS, Stoodley MA. Activating KRAS Mutations Expressed in 3D Endothelial Spheroids Induce Blebbing Morphologies Associated with Amoeboid-like Migration. Cells. 2026; 15(1):22. https://doi.org/10.3390/cells15010022
Chicago/Turabian StyleMcRobb, Lucinda S., Vivienne S. Lee, and Marcus A. Stoodley. 2026. "Activating KRAS Mutations Expressed in 3D Endothelial Spheroids Induce Blebbing Morphologies Associated with Amoeboid-like Migration" Cells 15, no. 1: 22. https://doi.org/10.3390/cells15010022
APA StyleMcRobb, L. S., Lee, V. S., & Stoodley, M. A. (2026). Activating KRAS Mutations Expressed in 3D Endothelial Spheroids Induce Blebbing Morphologies Associated with Amoeboid-like Migration. Cells, 15(1), 22. https://doi.org/10.3390/cells15010022





