Spinal Microglial TLR7 Activation Drives Hyperalgesia in a Lupus Mouse Model via Upregulation of IL-1β, IL-18, and Cav2.2 and Enhanced Glutamatergic Synaptic Activity
Highlights
- Patients with systemic lupus erythematosus (SLE) often suffer from chronic pain due to the insufficient efficacy and safety profile of currently available analgesics. In this study, we revealed that TLR7 signaling activity is elevated in the spinal dorsal horn in lupus mice with thermal hyperalgesia. TLR7 activation drives molecular, synaptic, cellular, and pain phenotype alterations in lupus mice.
- Our findings suggest that targeting TLR7 or downstream effectors may represent a promising strategy to alleviate chronic pain induced by SLE.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavior Tests
2.3. Intrathecal Injection Procedure
2.4. Topical In Vivo Drug Application
2.5. Western Blot Experiments
2.6. Immunohistochemical Experiments
2.7. Spinal Slice Preparations for Whole-Cell Patch Clamp Recording
2.8. Whole-Cell Patch Clamp Recording and Analysis of Miniature Excitatory Postsynaptic Currents (mEPSCs) from Neurons in the Spinal Dorsal Horn
2.9. Materials
2.10. Data Analysis
3. Results
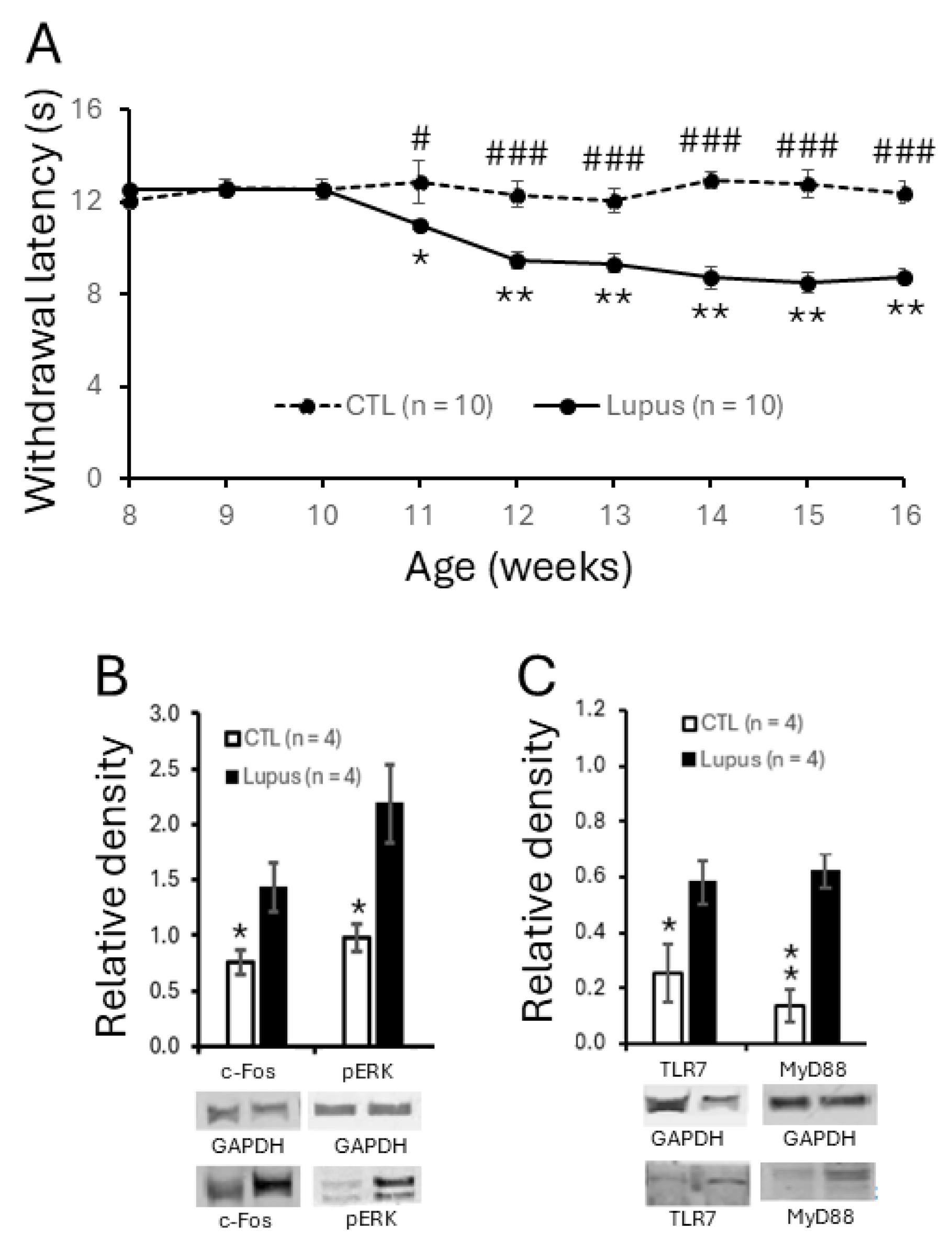
3.1. MRL/lpr Mice Spontaneously Exhibited Thermal Hypersensitivity Accompanied by Increased Neuronal Activation in the Spinal Dorsal Horn
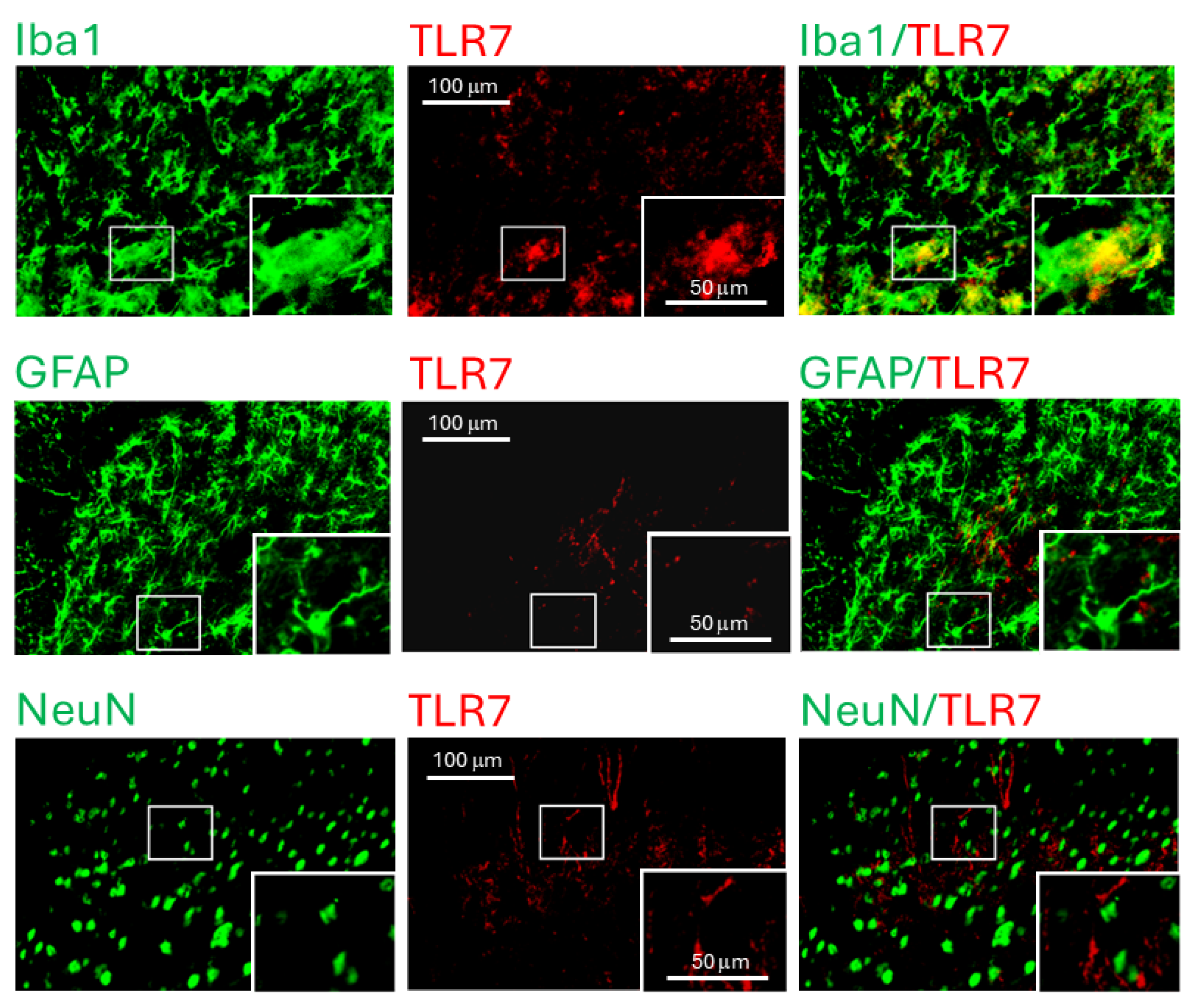
3.2. TLR7 Protein Expression Was Elevated in Spinal Dorsal Horn and Present in Microglia
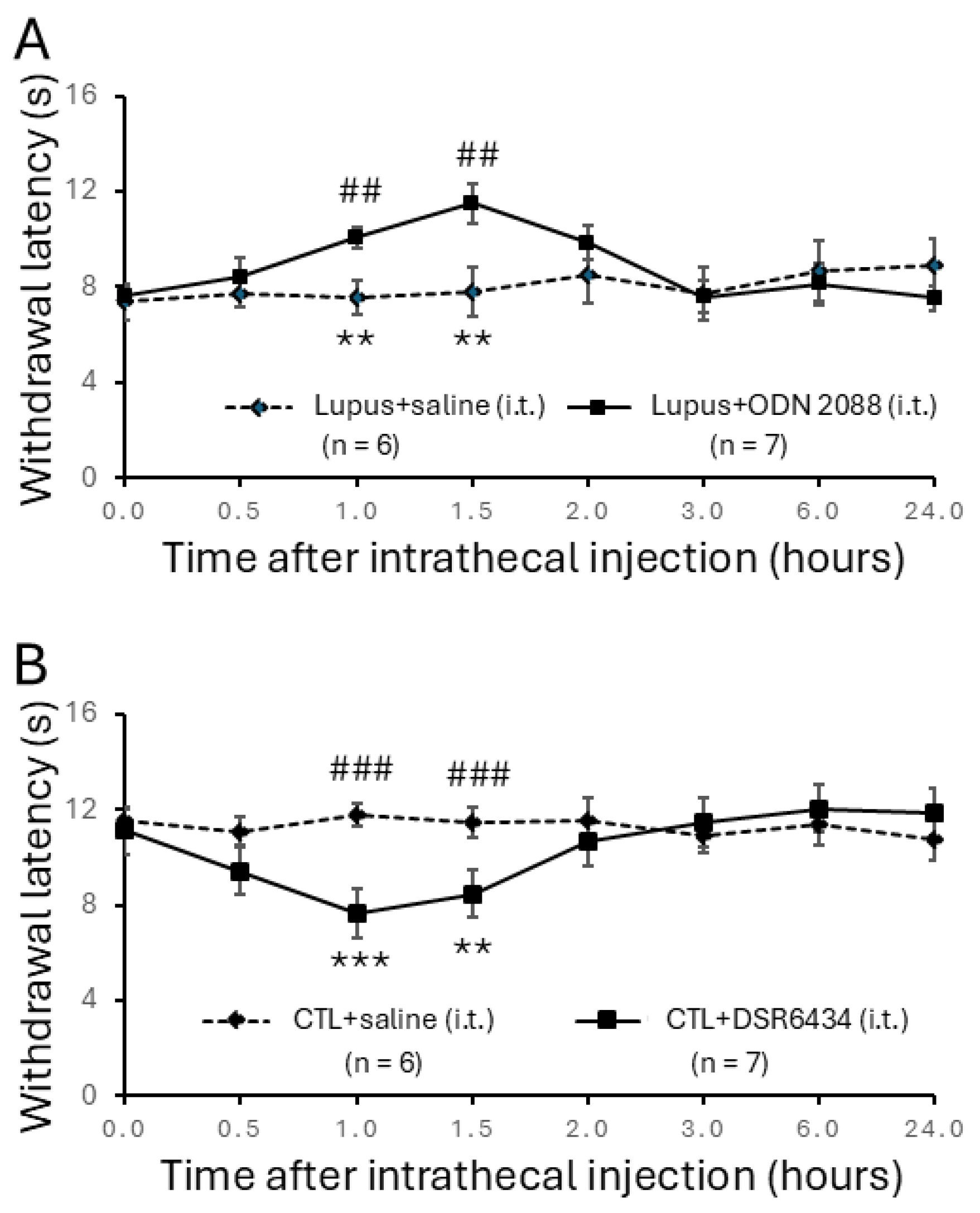
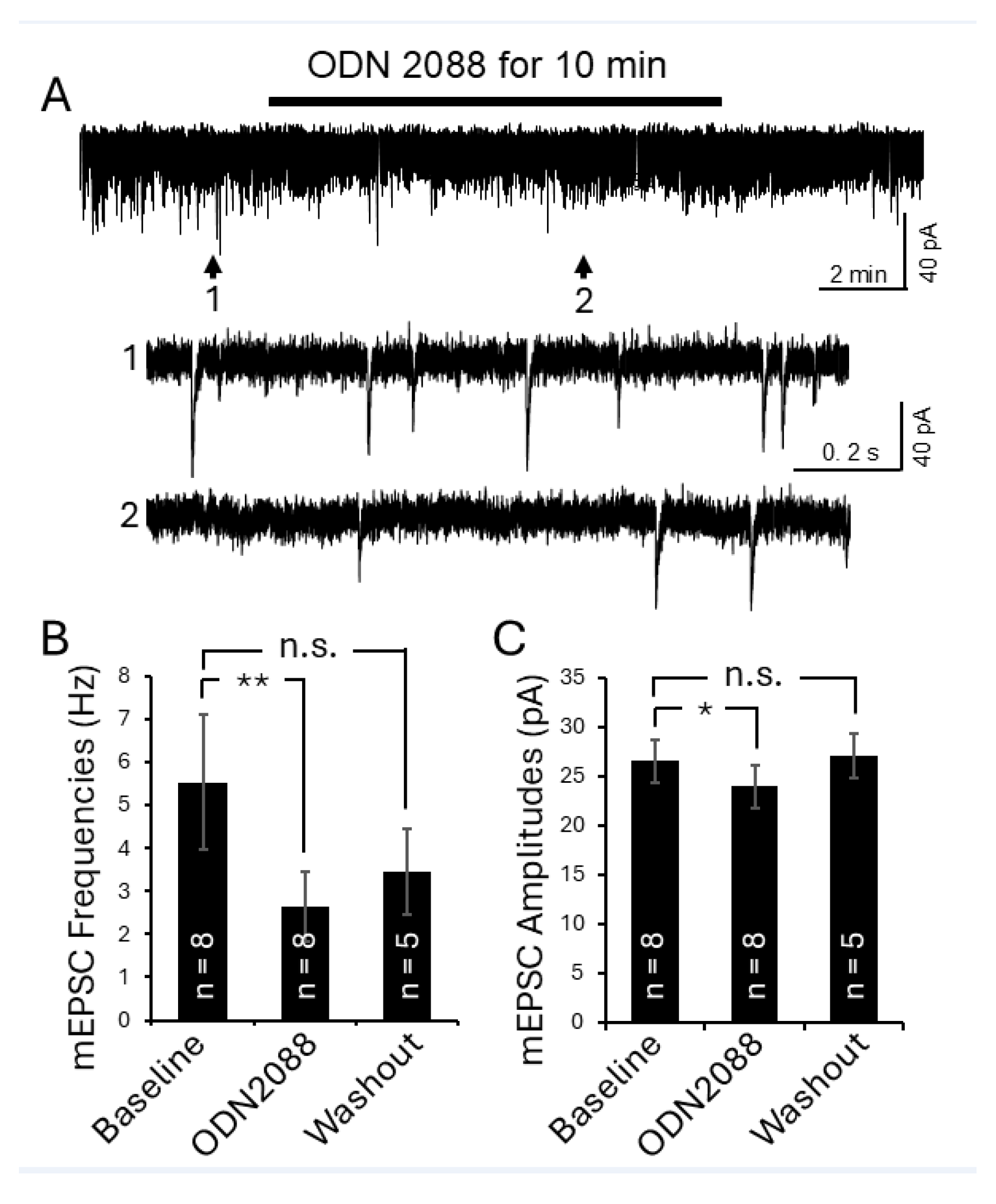
3.3. Intrathecal Administration of TLR7 Antagonists Reversed Thermal Hyperalgesia in MRL/lpr Mice
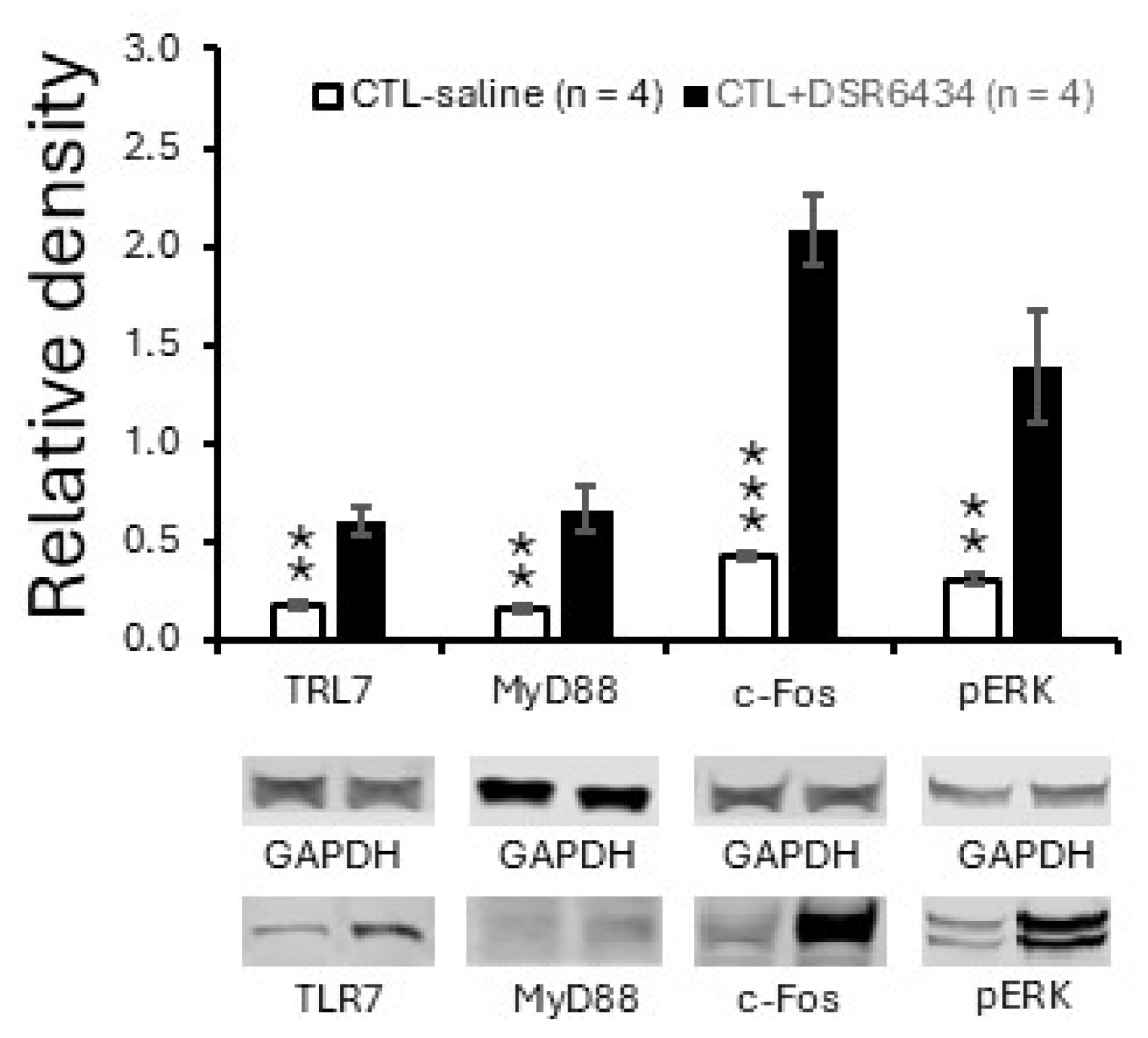
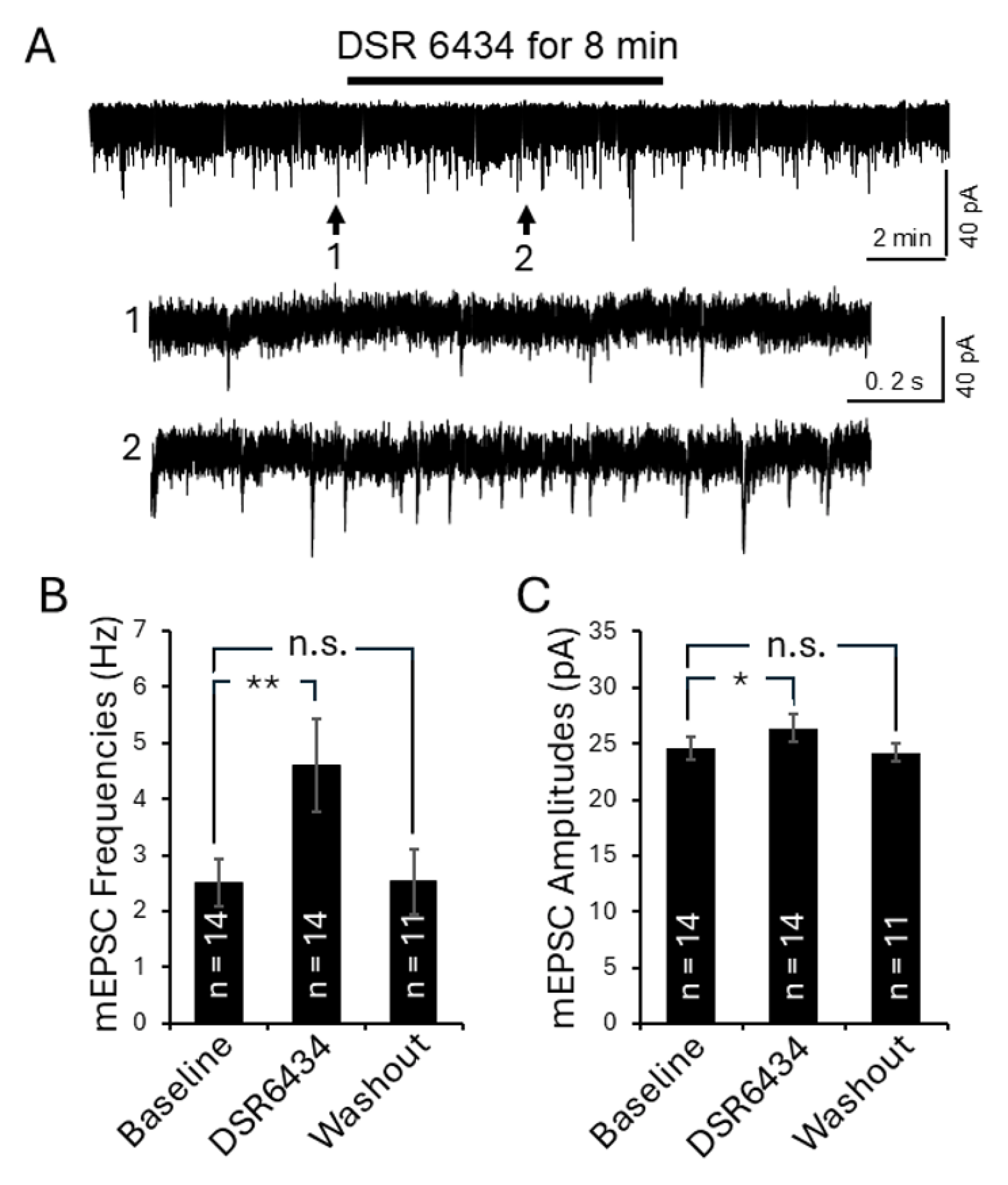
3.4. Intrathecal Administration of TLR7 Agonists Induced Thermal Hyperalgesia in Control Mice
3.5. Activation of Spinal TLR7 with the Exogenous TLR7 Agonist Increased TLR7 and MyD88 Expression and Induced Spinal Neuronal Activation
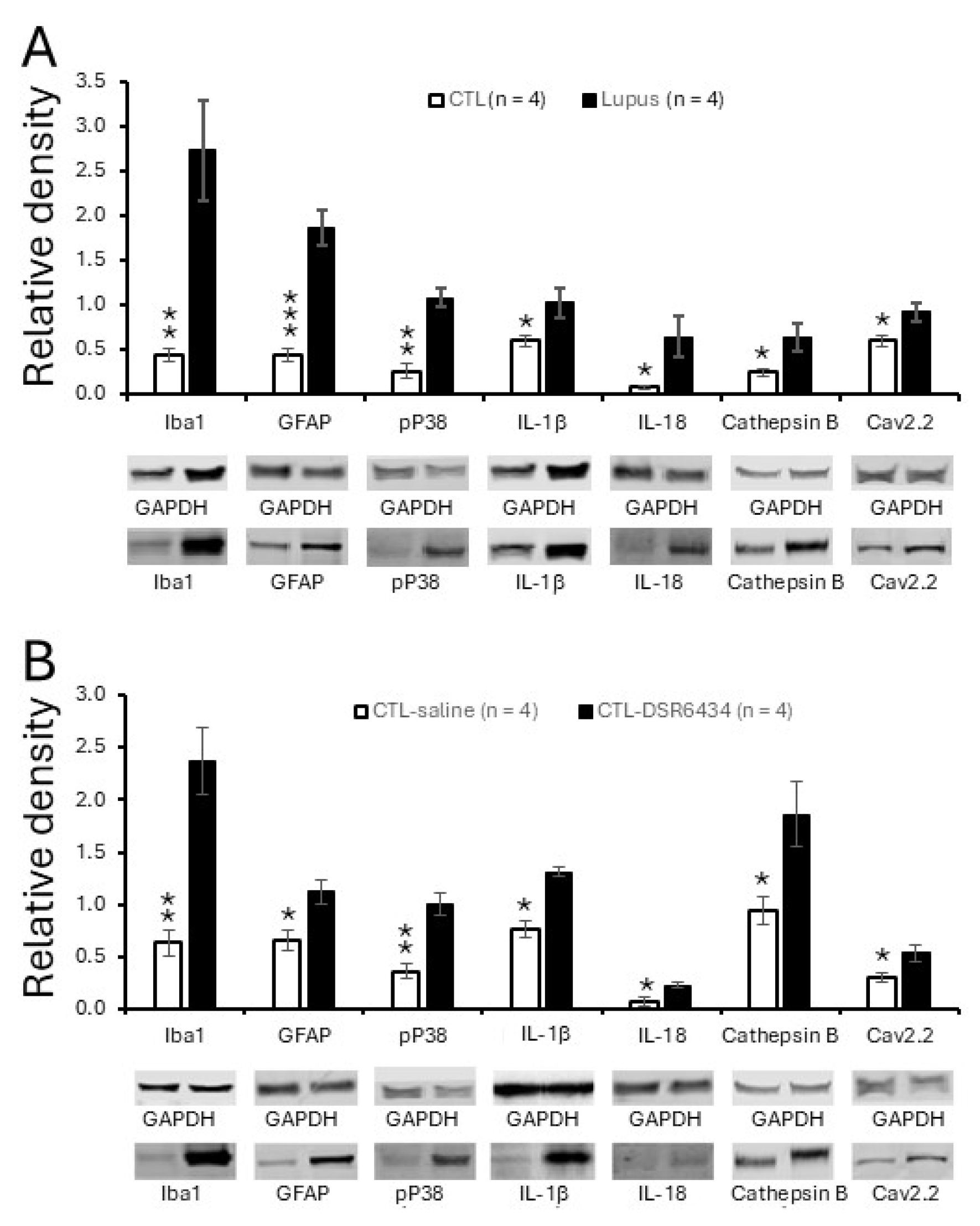
3.6. Activation of Spinal TLR7 in MRL Control Mice Enhanced Activation of Microglia and Astrocytes, P38 MAPK (P38) Phosphorylation and Production of IL-1β and IL-18
3.7. Activation of Spinal TLR7 in MRL Control Mice Enhanced Protein Expression of N-Type Voltage-Gated Calcium Channels (Cav2.2)
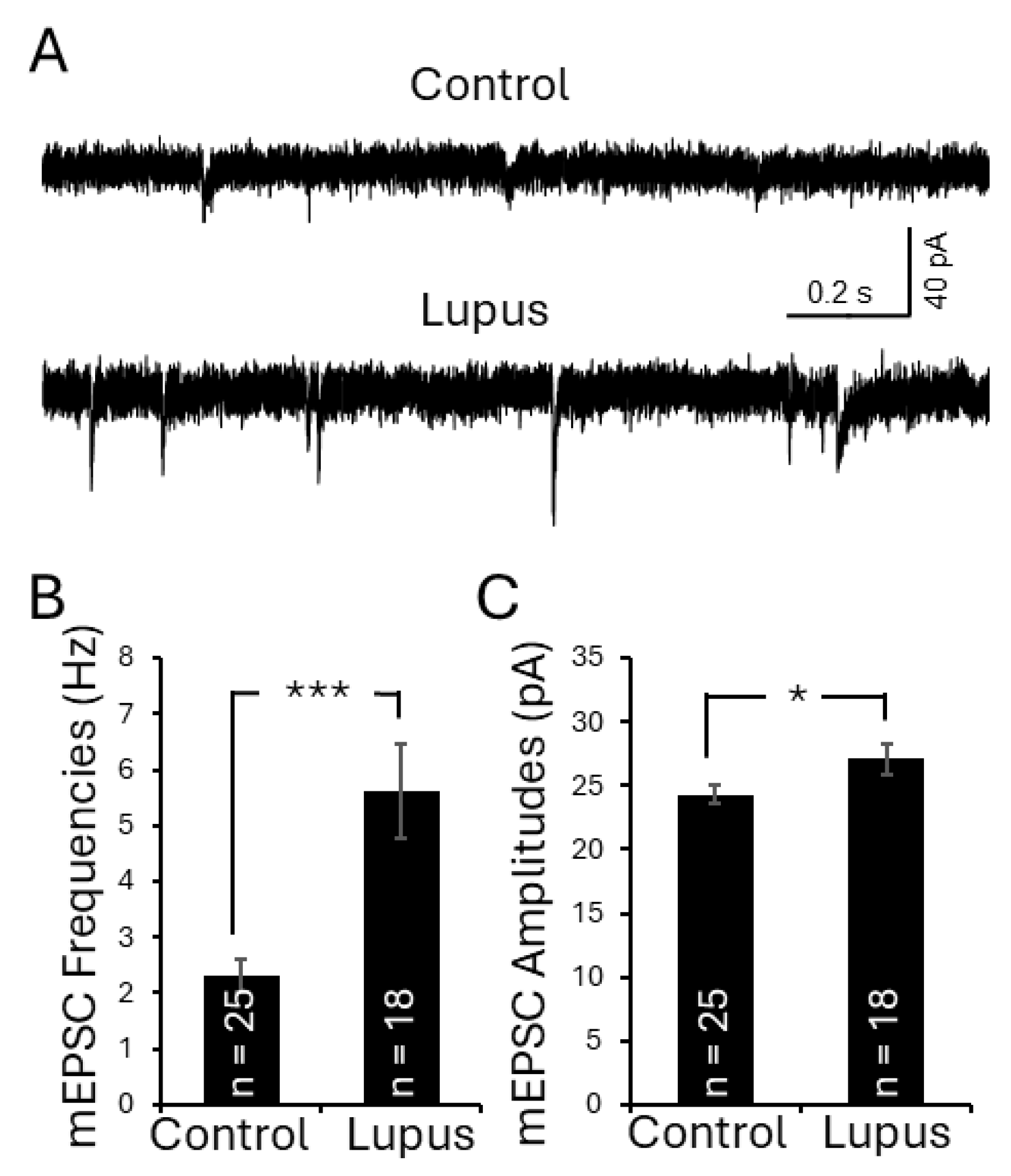
3.8. Activation of TLR7 in the Spinal Dorsal Horn in Lupus Mice Enhanced Presynaptic Glutamate Release and Postsynaptic AMPA Glutamate Receptor Activity in Superficial Dorsal Horn Neurons
4. Discussion
4.1. Role of TLR7 in the SLE Pathogenesis
4.2. Role of TLR7 in Pain
4.3. Molecular and Synaptic Mechanisms Underlying the Genesis of Chronic Pain Induced by SLE
4.4. Role of Microglia in the Pathogenesis of Chronic Pain in Females
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigor, R.; Edmonds, J.; Lewkonia, R.; Bresnihan, B.; Hughes, G.R. Systemic lupus erythematosus. A prospective analysis. Ann. Rheum. Dis. 1978, 37, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Sutton, J.D. Physical disability and psychosocial dysfunction in systemic lupus erythematosus. J. Rheumatol. 1988, 15, 959–964. [Google Scholar] [PubMed]
- Waldheim, E.; Elkan, A.C.; Bergman, S.; Frostegard, J.; van Vollenhoven, R.; Henriksson, E.W. Extent and characteristics of self-reported pain in patients with systemic lupus erythematosus. Lupus 2013, 22, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Gilkeson, G.S. Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/LPR mice. Immunol. Res. 2002, 25, 143–153. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N.; Dixon, F.J. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985, 37, 269–390. [Google Scholar]
- Perez de Lema, G.; Maier, H.; Nieto, E.; Vielhauer, V.; Luckow, B.; Mampaso, F.; Schlondorff, D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 2001, 12, 1369–1382. [Google Scholar] [CrossRef]
- Monteith, A.J.; Kang, S.; Scott, E.; Hillman, K.; Rajfur, Z.; Jacobson, K.; Costello, M.J.; Vilen, B.J. Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 2016, 113, E2142–E2151. [Google Scholar] [CrossRef]
- Yoneda, T.; Ishimaru, N.; Arakaki, R.; Kobayashi, M.; Izawa, T.; Moriyama, K.; Hayashi, Y. Estrogen deficiency accelerates murine autoimmune arthritis associated with receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenesis. Endocrinology 2004, 145, 2384–2391. [Google Scholar] [CrossRef]
- Cox, J.H.; Starr, A.E.; Kappelhoff, R.; Yan, R.; Roberts, C.R.; Overall, C.M. Matrix metalloproteinase 8 deficiency in mice exacerbates inflammatory arthritis through delayed neutrophil apoptosis and reduced caspase 11 expression. Arthritis Rheum. 2010, 62, 3645–3655. [Google Scholar] [CrossRef]
- Greco, C.M.; Rudy, T.E.; Manzi, S. Adaptation to chronic pain in systemic lupus erythematosus: Applicability of the multidimensional pain inventory. Pain Med. 2003, 4, 39–50. [Google Scholar] [CrossRef]
- Grossman, J.M. Lupus arthritis. Best Pract. Res. Clin. Rheumatol. 2009, 23, 495–506. [Google Scholar] [CrossRef]
- Moritoki, M.; Kadowaki, T.; Niki, T.; Nakano, D.; Soma, G.; Mori, H.; Kobara, H.; Masaki, T.; Kohno, M.; Hirashima, M. Galectin-9 ameliorates clinical severity of MRL/LPR lupus-prone mice by inducing plasma cell apoptosis independently of Tim-3. PLoS ONE 2013, 8, e60807. [Google Scholar] [CrossRef]
- Yen, T.H.; Yang, H.Y.; Yeh, Y.H.; Chu, P.H.; Wen, C.J.; Fu, J.F.; Wang, I.K.; Liang, C.C.; Chang, C.T.; Chen, K.H.; et al. Aliskiren attenuates proteinuria in mice with lupus nephritis by a blood pressure-independent mechanism. Lupus 2013, 22, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.C.; Cooke, A.; Moore, A.R.; Collins, C.; Hay, F.; Willoughby, D.A. Connective tissue abnormalities in MRL/1 mice. Ann. Rheum. Dis. 1986, 45, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Hack, B.; Bai, T.; Brorson, J.R.; Quigg, R.J.; Alexander, J.J. Inhibition of C5a receptor alleviates experimental CNS lupus. J. Neuroimmunol. 2010, 221, 46–52. [Google Scholar] [CrossRef]
- Yamada, A.; Miyazaki, T.; Lu, L.M.; Ono, M.; Ito, M.R.; Terada, M.; Mori, S.; Hata, K.; Nozaki, Y.; Nakatsuru, S.; et al. Genetic basis of tissue specificity of vasculitis in MRL/LPR mice. Arthritis Rheum. 2003, 48, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Viatchenko-Karpinski, V.; Kong, L.; Weng, H.R. Activation of microglial GPR109A alleviates thermal hyperalgesia in female lupus mice by suppressing IL-18 and glutamatergic synaptic activity. Glia 2022, 70, 634–649. [Google Scholar] [CrossRef]
- Weng, H.R. Emerging Molecular and Synaptic Targets for the Management of Chronic Pain Caused by Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2024, 25, 3602. [Google Scholar] [CrossRef]
- Yan, X.; Maixner, D.W.; Li, F.; Weng, H.R. Chronic pain and impaired glial glutamate transporter function in lupus-prone mice are ameliorated by blocking macrophage colony-stimulating factor-1 receptors. J. Neurochem. 2017, 140, 963–976. [Google Scholar] [CrossRef]
- Viatchenko-Karpinski, V.; Kong, L.; Weng, H.R. Deficient AMPK activity contributes to hyperexcitability in peripheral nociceptive sensory neurons and thermal hyperalgesia in lupus mice. PLoS ONE 2023, 18, e0288356. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Liu, J.; Tang, S.; Yan, J.; Li, H.; Wan, Z.; Wang, L.; Yan, X. Activation of Protease-Activated Receptor-1 Causes Chronic Pain in Lupus-Prone Mice Via Suppressing Spinal Glial Glutamate Transporter Function and Enhancing Glutamatergic Synaptic Activity. J. Pain 2023, 24, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Lauwerys, B.R.; Ducreux, J.; Houssiau, F.A. Type I interferon blockade in systemic lupus erythematosus: Where do we stand? Rheumatology 2014, 53, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Lauwerys, B.R.; Husson, S.N.; Maudoux, A.L.; Badot, V.; Houssiau, F.A. sIL7R concentrations in the serum reflect disease activity in the lupus kidney. Lupus Sci. Med. 2014, 1, e000036. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, H.A.; Gingras, S.; Kim, M.; Bastacky, S.; Tilstra, J.S.; Shlomchik, M.J. B cell-intrinsic TLR7 expression drives severe lupus in TLR9-deficient mice. JCI Insight 2023, 8, e172219. [Google Scholar] [CrossRef]
- Berland, R.; Fernandez, L.; Kari, E.; Han, J.H.; Lomakin, I.; Akira, S.; Wortis, H.H.; Kearney, J.F.; Ucci, A.A.; Imanishi-Kari, T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 2006, 25, 429–440. [Google Scholar] [CrossRef]
- Dorner, T.; Lipsky, P.E. The essential roles of memory B cells in the pathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2024, 20, 770–782. [Google Scholar] [CrossRef]
- Kielian, T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006, 83, 711–730. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shih, Y.C.; Hung, Y.F.; Hsueh, Y.P. Beyond defense: Regulation of neuronal morphogenesis and brain functions via Toll-like receptors. J. Biomed. Sci. 2019, 26, 90. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Norgard, M.A.; Levasseur, P.R.; Olson, B.; Burfeind, K.G.; Buenafe, A.C.; Zhu, X.; Jeng, S.; McWeeney, S.K.; Marks, D.L. Persistent Toll-like receptor 7 stimulation induces behavioral and molecular innate immune tolerance. Brain Behav. Immun. 2019, 82, 338–353. [Google Scholar] [CrossRef]
- Dilly, G.A.; Kittleman, C.W.; Kerr, T.M.; Messing, R.O.; Mayfield, R.D. Cell-type specific changes in PKC-delta neurons of the central amygdala during alcohol withdrawal. Transl. Psychiatry 2022, 12, 289. [Google Scholar] [CrossRef]
- Lehmann, S.M.; Kruger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Rosenberger, K.; Kruger, C.; Habbel, P.; Derkow, K.; Kaul, D.; Rybak, A.; Brandt, C.; Schott, E.; Wulczyn, F.G.; et al. Extracellularly delivered single-stranded viral RNA causes neurodegeneration dependent on TLR7. J. Immunol. 2012, 189, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Kodama, L.; Fan, L.; Zhu, D.; Zhu, J.; Wong, M.Y.; Ye, P.; Norman, K.; Foxe, N.R.; Ijaz, L.; et al. Tlr7 drives sex differences in age- and Alzheimer’s disease-related demyelination. Science 2024, 386, eadk7844. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Filippone, A.; Biondo, C.; Mancuso, G.; Casili, G.; Lanza, M.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. TLR7/8 in the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 9384. [Google Scholar] [CrossRef]
- He, L.; Han, G.; Wu, S.; Du, S.; Zhang, Y.; Liu, W.; Jiang, B.; Zhang, L.; Xia, S.; Jia, S.; et al. Toll-like receptor 7 contributes to neuropathic pain by activating NF-kappaB in primary sensory neurons. Brain Behav. Immun. 2020, 87, 840–851. [Google Scholar] [CrossRef]
- Chen, O.; Jiang, C.; Berta, T.; Powell Gray, B.; Furutani, K.; Sullenger, B.A.; Ji, R.R. MicroRNA let-7b enhances spinal cord nociceptive synaptic transmission and induces acute and persistent pain through neuronal and microglial signaling. Pain 2024, 165, 1824–1839. [Google Scholar] [CrossRef]
- Cizkova, D.; Marsala, J.; Lukacova, N.; Marsala, M.; Jergova, S.; Orendacova, J.; Yaksh, T.L. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp. Brain Res. 2002, 147, 456–463. [Google Scholar] [CrossRef]
- Nieto-Rostro, M.; Ramgoolam, K.; Pratt, W.S.; Kulik, A.; Dolphin, A.C. Ablation of α2δ-1 inhibits cell-surface trafficking of endogenous N-type calcium channels in the pain pathway in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E12043–E12052. [Google Scholar] [CrossRef]
- Sudhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef]
- Hoppanova, L.; Lacinova, L. Voltage-dependent CaV3.2 and CaV2.2 channels in nociceptive pathways. Pflügers Arch.-Eur. J. Physiol. 2022, 474, 421–434. [Google Scholar] [CrossRef]
- Vink, S.; Alewood, P.F. Targeting voltage-gated calcium channels: Developments in peptide and small-molecule inhibitors for the treatment of neuropathic pain. Br. J. Pharmacol. 2012, 167, 970–989. [Google Scholar] [CrossRef]
- Xu, J.; Chu, K.L.; Zhu, C.Z.; Niforatos, W.; Swensen, A.; Searle, X.; Lee, L.; Jarvis, M.F.; McGaraughty, S. A mixed Ca2+ channel blocker, A-1264087, utilizes peripheral and spinal mechanisms to inhibit spinal nociceptive transmission in a rat model of neuropathic pain. J. Neurophysiol. 2014, 111, 394–404. [Google Scholar] [CrossRef]
- Tang, C.; Gomez, K.; Chen, Y.; Allen, H.N.; Hestehave, S.; Rodriguez-Palma, E.J.; Loya-Lopez, S.; Calderon-Rivera, A.; Duran, P.; Nelson, T.S.; et al. C2230, a preferential use- and state-dependent CaV2.2 channel blocker, mitigates pain behaviors across multiple pain models. J. Clin. Investig. 2024, 135, e177429. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Feng, Z.P.; Zhang, L.; Pajouhesh, H.; Ding, Y.; Belardetti, F.; Pajouhesh, H.; Dolphin, D.; Mitscher, L.A.; Snutch, T.P. Scaffold-based design and synthesis of potent N-type calcium channel blockers. Bioorg. Med. Chem. Lett. 2009, 19, 6467–6472. [Google Scholar] [CrossRef]
- Hylden, J.L.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Yan, X.; Maixner, D.W.; Yadav, R.; Gao, M.; Li, P.; Bartlett, M.G.; Weng, H.R. Paclitaxel induces acute pain via directly activating toll like receptor 4. Mol. Pain 2015, 11, 10. [Google Scholar] [CrossRef]
- Yan, X.; Yadav, R.; Gao, M.; Weng, H.R. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia 2014, 62, 1093–1109. [Google Scholar] [CrossRef]
- Maixner, D.W.; Christy, D.; Kong, L.; Viatchenko-Karpinski, V.; Horner, K.A.; Hooks, S.B.; Weng, H.R. Phytohormone abscisic acid ameliorates neuropathic pain via regulating LANCL2 protein abundance and glial activation at the spinal cord. Mol. Pain 2022, 18, 17448069221107781. [Google Scholar] [CrossRef]
- Nie, H.; Weng, H.R. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. J. Neurophysiol. 2009, 101, 2041–2051. [Google Scholar] [CrossRef]
- Weng, H.R.; Chen, J.H.; Pan, Z.Z.; Nie, H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience 2007, 149, 898–907. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, E.; Gao, M.; Weng, H.R. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. J. Physiol. 2013, 591, 2001–2019. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Weng, H.R. Endogenous interleukin-1beta in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J. Biol. Chem. 2013, 288, 30544–30557. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Tsuzuki, K.; Ling, J.X.; Sonobe, H.; Gu, J.G. Distinct roles of P2X receptors in modulatiing glutamate release at different primary sensory synapses in rat spinal cord. J. Neurophysiol. 2003, 89, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, Z.; Ataei, S.; Riahi, E.; Parviz, M.; Sehati, F.; Zare, M.; Angizeh, R.; Ashabi, G.; Hosseindoost, S. Neuroprotective effects of MK-801 against cerebral ischemia reperfusion. Heliyon 2024, 10, e33821. [Google Scholar] [CrossRef]
- Rahimi, K.; Nourishirazi, A.; Delaviz, H.; Ghotbeddin, Z. Antinociceptive effects of gamma-linolenic acid in the formalin test in the rats. Ann. Med. Surg. 2024, 86, 2677–2683. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Brinson, C.W.; Lu, Z.; Li, Y.; Lopes-Virella, M.F.; Huang, Y. Lipopolysaccharide and IL-1beta coordinate a synergy on cytokine production by upregulating MyD88 expression in human gingival fibroblasts. Mol. Immunol. 2016, 79, 47–54. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, S.; Liu, L.; Liu, S.; Wang, B.; Ma, Y.; Cao, X. Promotion of TLR7-MyD88-dependent inflammation and autoimmunity in mice through stem-loop changes in Lnc-Atg16l1. Nat. Commun. 2024, 15, 10224. [Google Scholar] [CrossRef]
- Nakanishi, H. Cathepsin regulation on microglial function. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140465. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, F.; Maixner, D.W.; Yadav, R.; Gao, M.; Ali, M.W.; Hooks, S.B.; Weng, H.R. Interleukin-1beta released by microglia initiates the enhanced glutamatergic activity in the spinal dorsal horn during paclitaxel-associated acute pain syndrome. Glia 2019, 67, 482–497. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-alpha, Wnt Ligands, and Interleukin 1beta. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef]
- Chu, Y.X.; Zhang, Y.Q.; Zhao, Z.Q. Involvement of microglia and interleukin-18 in the induction of long-term potentiation of spinal nociceptive responses induced by tetanic sciatic stimulation. Neurosci. Bull. 2012, 28, 49–60. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Malcangio, M.; Sideris-Lampretsas, G. How microglia contribute to the induction and maintenance of neuropathic pain. Nat. Rev. Neurosci. 2025, 26, 263–275. [Google Scholar] [CrossRef]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef]
- Mantyh, P.W.; Rogers, S.D.; Honore, P.; Allen, B.J.; Ghilardi, J.R.; Li, J.; Daughters, R.S.; Lappi, D.A.; Wiley, R.G.; Simone, D.A. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 1997, 278, 275–279. [Google Scholar] [CrossRef]
- Noel, J.; Ralph, G.S.; Pickard, L.; Williams, J.; Molnar, E.; Uney, J.B.; Collingridge, G.L.; Henley, J.M. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 1999, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Lin, S.L.; Carroll, M.C. Neuroimmune mechanisms of neuropsychiatric systemic lupus erythematosus. Curr. Opin. Immunol. 2025, 96, 102608. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Tripathy, A.; Padhan, P.; Raghav, S.K.; Gupta, B. Toll-like receptor-7 activation in CD8+ T cells modulates inflammatory mediators in patients with rheumatoid arthritis. Rheumatol. Int. 2022, 42, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Hsu, T.C.; Hsu, G.J.; Li, S.L.; Tzang, B.S. Cystamine attenuates the expressions of NOS- and TLR-associated molecules in the brain of NZB/W F1 mice. Eur. J. Pharmacol. 2009, 607, 102–106. [Google Scholar] [CrossRef]
- Kwun, A.; Sullivan, J.K.; Shelestak, J.; Merritt, K.M.; Liu, S.S.; Mey, G.; DeSilva, T.; Jorgensen, T.N. Sustained NPSLE-like phenotype in the absence of systemic lupus-like disease in TLR7-deficient B6.Nba2 mice. Brain Behav. Immun. 2025, 128, 352–361. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, J.; Sakurai, D.; Kaufman, K.M.; Edberg, J.C.; Kimberly, R.P.; Kamen, D.L.; Gilkeson, G.S.; Jacob, C.O.; Scofield, R.H.; et al. MicroRNA-3148 modulates allelic expression of toll-like receptor 7 variant associated with systemic lupus erythematosus. PLoS Genet. 2013, 9, e1003336. [Google Scholar] [CrossRef]
- Horton, C.G.; Farris, A.D. Toll-like receptors in systemic lupus erythematosus: Potential targets for therapeutic intervention. Curr. Allergy Asthma Rep. 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Shlomchik, M.J. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr. Opin. Immunol. 2009, 21, 626–633. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.Z.; Park, C.K.; Berta, T.; Ji, R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010, 13, 1460–1462. [Google Scholar] [CrossRef]
- He, L.; Cao, J.; Jiang, B.C.; Yang, J.J.; Tao, Y.X.; Ai, Y. C/EBPbeta Participates in Nerve Trauma-Induced TLR7 Upregulation in Primary Sensory Neurons. Mol. Neurobiol. 2022, 59, 2629–2641. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Francavilla, M.; Facchetti, S.; Nativi, C.; Tassorelli, C. Insights into the Involvement of TRPA1 Channels in the Neuro-Inflammatory Machinery of Trigeminal Neuralgia. Molecules 2025, 30, 1884. [Google Scholar] [CrossRef]
- Hoshikawa, N.; Sakai, A.; Takai, S.; Suzuki, H. Targeting Extracellular miR-21-TLR7 Signaling Provides Long-Lasting Analgesia in Osteoarthritis. Mol. Ther. Nucleic Acids 2020, 19, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Xu, Z.Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.J.; Ji, R.R. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, T.; Hong, J.; Woo, J.; Min, H.; Hwang, E.; Lee, S.J.; Lee, C.J. Imiquimod enhances excitability of dorsal root ganglion neurons by inhibiting background (K2P) and voltage-gated (Kv1.1 and Kv1.2) potassium channels. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Amara, S.G. New views of glutamate transporter structure and function: Advances and challenges. Neuropharmacology 2011, 60, 172–181. [Google Scholar] [CrossRef]
- Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Honigsperger, C.; Wunderbaldinger, G.; Gassner, M.; Sandkuhler, J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J. Neurosci. 2013, 33, 6540–6551. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- Shao, P.P.; Ye, F.; Chakravarty, P.K.; Varughese, D.J.; Herrington, J.B.; Dai, G.; Bugianesi, R.M.; Haedo, R.J.; Swensen, A.M.; Warren, V.A.; et al. Aminopiperidine sulfonamide Cav2.2 channel inhibitors for the treatment of chronic pain. J. Med. Chem. 2012, 55, 9847–9855. [Google Scholar] [CrossRef]
- Kim, C.; Jun, K.; Lee, T.; Kim, S.S.; McEnery, M.W.; Chin, H.; Kim, H.L.; Park, J.M.; Kim, D.K.; Jung, S.J.; et al. Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol. Cell. Neurosci. 2001, 18, 235–245. [Google Scholar] [CrossRef]
- Saegusa, H.; Kurihara, T.; Zong, S.; Kazuno, A.; Matsuda, Y.; Nonaka, T.; Han, W.; Toriyama, H.; Tanabe, T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. Embo J. 2001, 20, 2349–2356. [Google Scholar] [CrossRef]
- Scott, D.A.; Wright, C.E.; Angus, J.A. Actions of intrathecal omega-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur. J. Pharmacol. 2002, 451, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Santiago, U.; Nelson, T.S.; Allen, H.N.; Calderon-Rivera, A.; Hestehave, S.; Rodriguez Palma, E.J.; Zhou, Y.; Duran, P.; Loya-Lopez, S.; et al. A peptidomimetic modulator of the CaV2.2 N-type calcium channel for chronic pain. Proc. Natl. Acad. Sci. USA 2023, 120, e2305215120. [Google Scholar] [CrossRef]
- Lai, C.Y.; Ho, Y.C.; Hsieh, M.C.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Peng, H.Y. Spinal Fbxo3-Dependent Fbxl2 Ubiquitination of Active Zone Protein RIM1α Mediates Neuropathic Allodynia through CaV2.2 Activation. J. Neurosci. 2016, 36, 9722–9738. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z.; Ju, J.; Chu, T.; Gao, F. Sex Differences in the Regulation of Interleukins in Chronic Pain: A Widely Recognized but Difficult-to-Tackle Factor. Int. J. Mol. Sci. 2025, 26, 3835. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef]
- Peng, J.; Gu, N.; Zhou, L.; Eyo, U.B.; Murugan, M.; Gan, W.B.; Wu, L.J. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat. Commun. 2016, 7, 12029. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.P.; Song, W.B.; Song, X.J. EphrinB-EphB receptor signaling contributes to bone cancer pain via Toll-like receptor and proinflammatory cytokines in rat spinal cord. Pain 2013, 154, 2823–2835. [Google Scholar] [CrossRef]
- Agalave, N.M.; Larsson, M.; Abdelmoaty, S.; Su, J.; Baharpoor, A.; Lundback, P.; Palmblad, K.; Andersson, U.; Harris, H.; Svensson, C.I. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 2014, 155, 1802–1813. [Google Scholar] [CrossRef]
- Nieto, F.R.; Clark, A.K.; Grist, J.; Hathway, G.J.; Chapman, V.; Malcangio, M. Neuron-immune mechanisms contribute to pain in early stages of arthritis. J. Neuroinflamm. 2016, 13, 96. [Google Scholar] [CrossRef]
- Luongo, L.; Malcangio, M.; Salvemini, D.; Starowicz, K. Chronic pain: New insights in molecular and cellular mechanisms. Biomed. Res. Int. 2015, 2015, 676725. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Y.; Wang, Z.; Zhu, J.; Liu, F.; Zhu, M.; Zheng, J.; Chen, Q.; Zhai, D.; Chen, Y.; et al. NFAT1 Signaling Contributes to Bone Cancer Pain by Regulating IL-18 Expression in Spinal Microglia. CNS Neurosci. Ther. 2025, 31, e70222. [Google Scholar] [CrossRef]
- Wang, M.J.; Jing, X.Y.; Wang, Y.Z.; Yang, B.R.; Lu, Q.; Hu, H.; Kang, L. Exercise, Spinal Microglia and Neuropathic Pain: Potential Molecular Mechanisms. Neurochem. Res. 2024, 49, 29–37. [Google Scholar] [CrossRef]
- Ruivo, J.; Tavares, I.; Pozza, D.H. Molecular targets in bone cancer pain: A systematic review of inflammatory cytokines. J. Mol. Med. 2024, 102, 1063–1088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bipin, S.; Viatchenko-Karpinski, V.; Li, C.; Lim, S.; Weng, H.-R. Spinal Microglial TLR7 Activation Drives Hyperalgesia in a Lupus Mouse Model via Upregulation of IL-1β, IL-18, and Cav2.2 and Enhanced Glutamatergic Synaptic Activity. Cells 2026, 15, 20. https://doi.org/10.3390/cells15010020
Bipin S, Viatchenko-Karpinski V, Li C, Lim S, Weng H-R. Spinal Microglial TLR7 Activation Drives Hyperalgesia in a Lupus Mouse Model via Upregulation of IL-1β, IL-18, and Cav2.2 and Enhanced Glutamatergic Synaptic Activity. Cells. 2026; 15(1):20. https://doi.org/10.3390/cells15010020
Chicago/Turabian StyleBipin, Saumya, Viacheslav Viatchenko-Karpinski, Catherine Li, Sujin Lim, and Han-Rong Weng. 2026. "Spinal Microglial TLR7 Activation Drives Hyperalgesia in a Lupus Mouse Model via Upregulation of IL-1β, IL-18, and Cav2.2 and Enhanced Glutamatergic Synaptic Activity" Cells 15, no. 1: 20. https://doi.org/10.3390/cells15010020
APA StyleBipin, S., Viatchenko-Karpinski, V., Li, C., Lim, S., & Weng, H.-R. (2026). Spinal Microglial TLR7 Activation Drives Hyperalgesia in a Lupus Mouse Model via Upregulation of IL-1β, IL-18, and Cav2.2 and Enhanced Glutamatergic Synaptic Activity. Cells, 15(1), 20. https://doi.org/10.3390/cells15010020




