Potential Role of Serum Cytokines and Chemokines as Biomarkers of Injury Severity and Functional Outcomes Following Pediatric Traumatic Brain Injury
Highlights
- Cytokines may be beneficial as biomarkers of pediatric TBI severity and prognosis
- Additional studies may be beneficial in delineating the role of cytokines in assessing pediatric TBI.
Abstract
1. Introduction
2. Materials and Methods
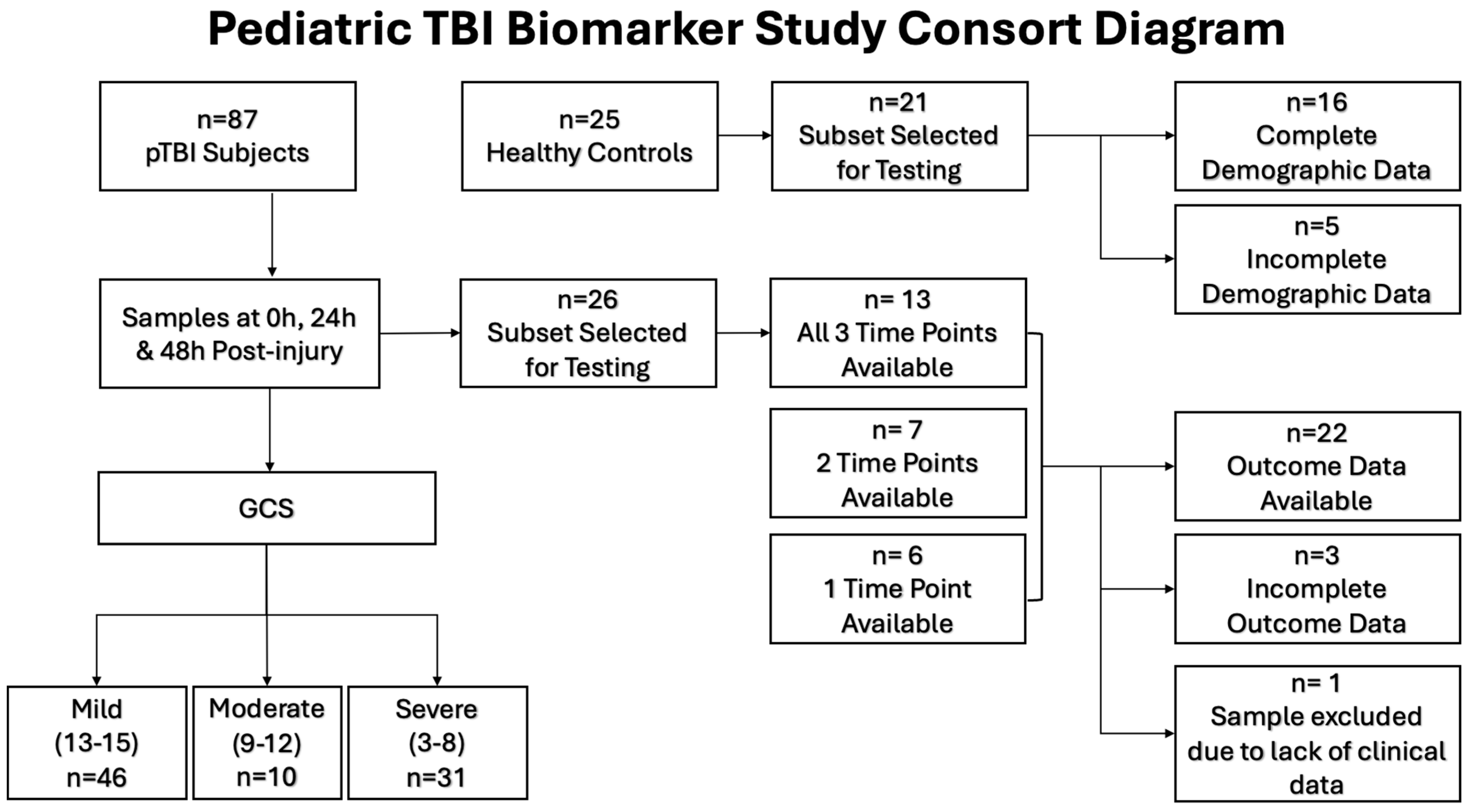
2.1. Study Design and Setting
2.2. Participants
2.3. Enhanced Chemiluminescent Immunoassays (ECLIA)
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Demographics of Pediatric TBI Patients and Controls
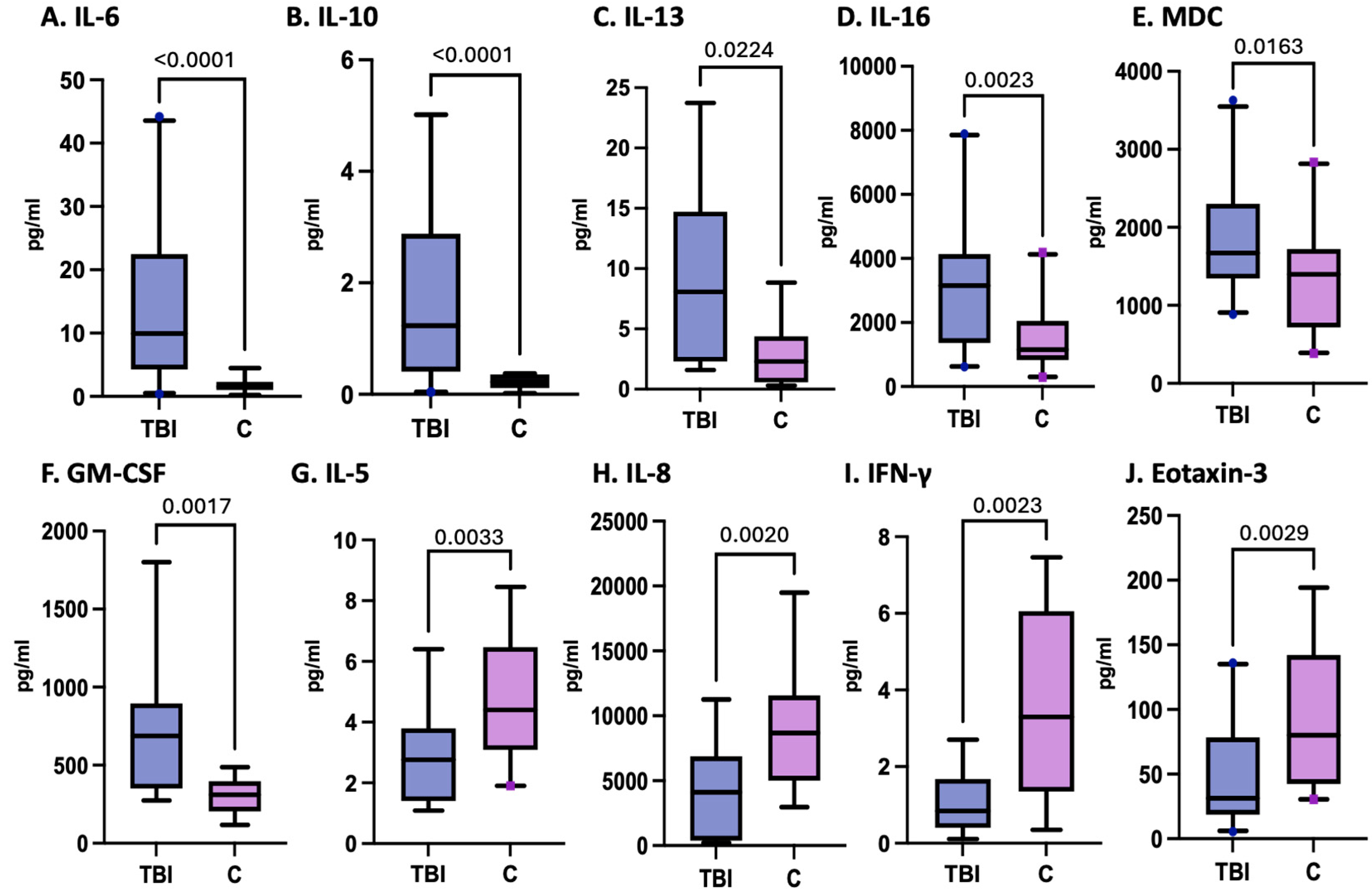
3.2. Variation in Inflammatory Cytokines and Chemokines in Pediatric TBI Patients
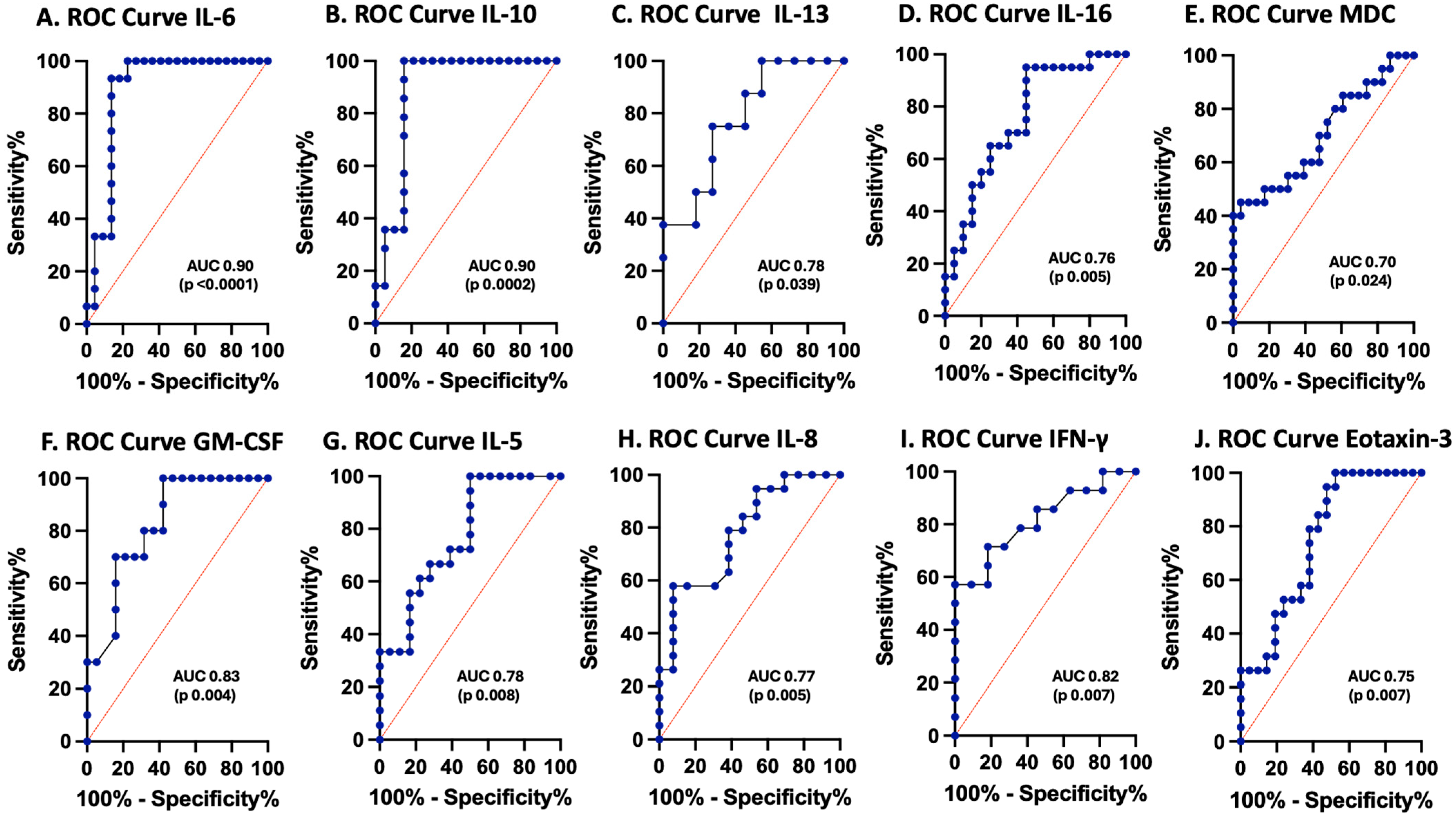
3.3. Cytokines and Chemokines as Biomarkers of Pediatric TBI
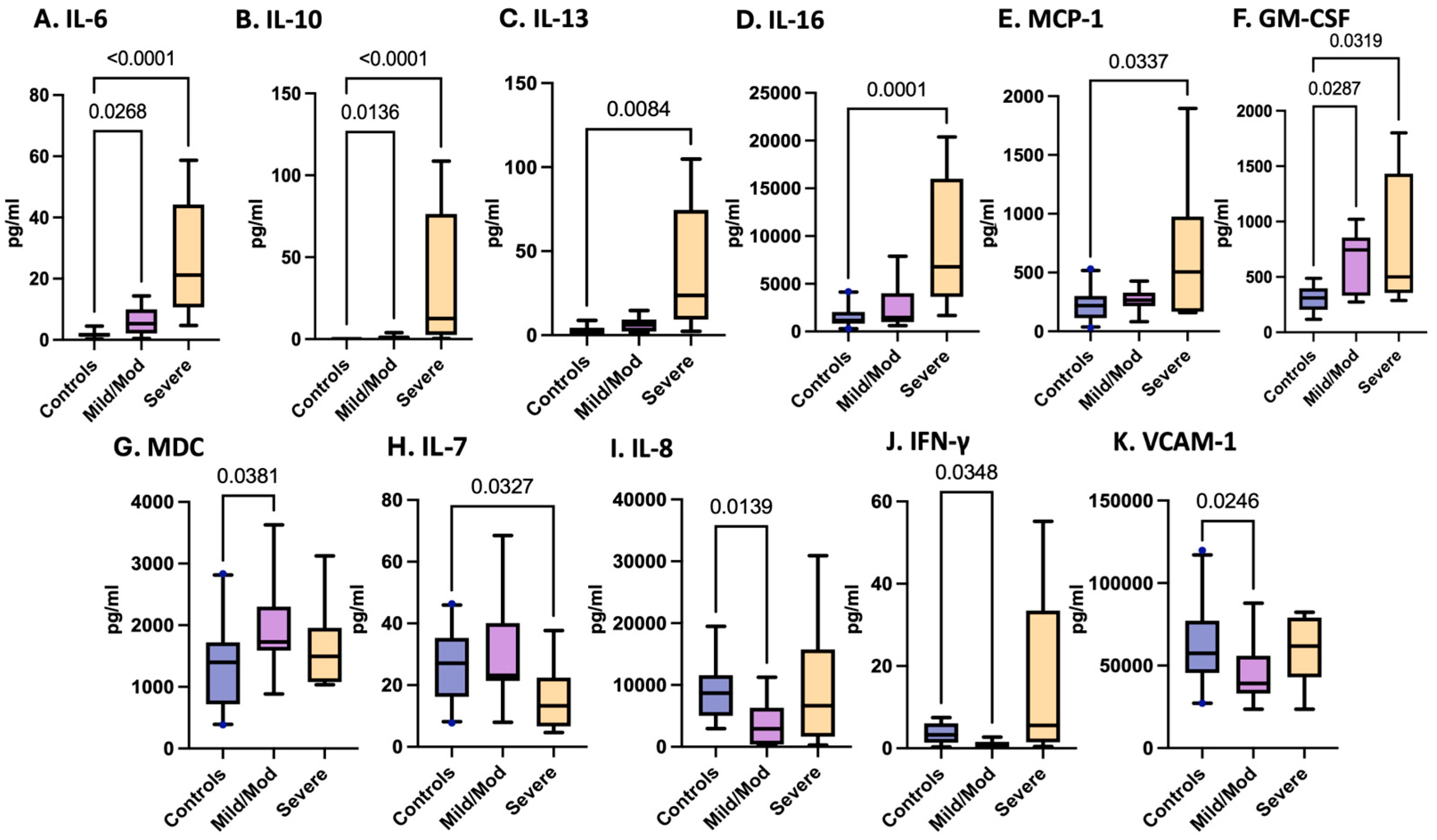
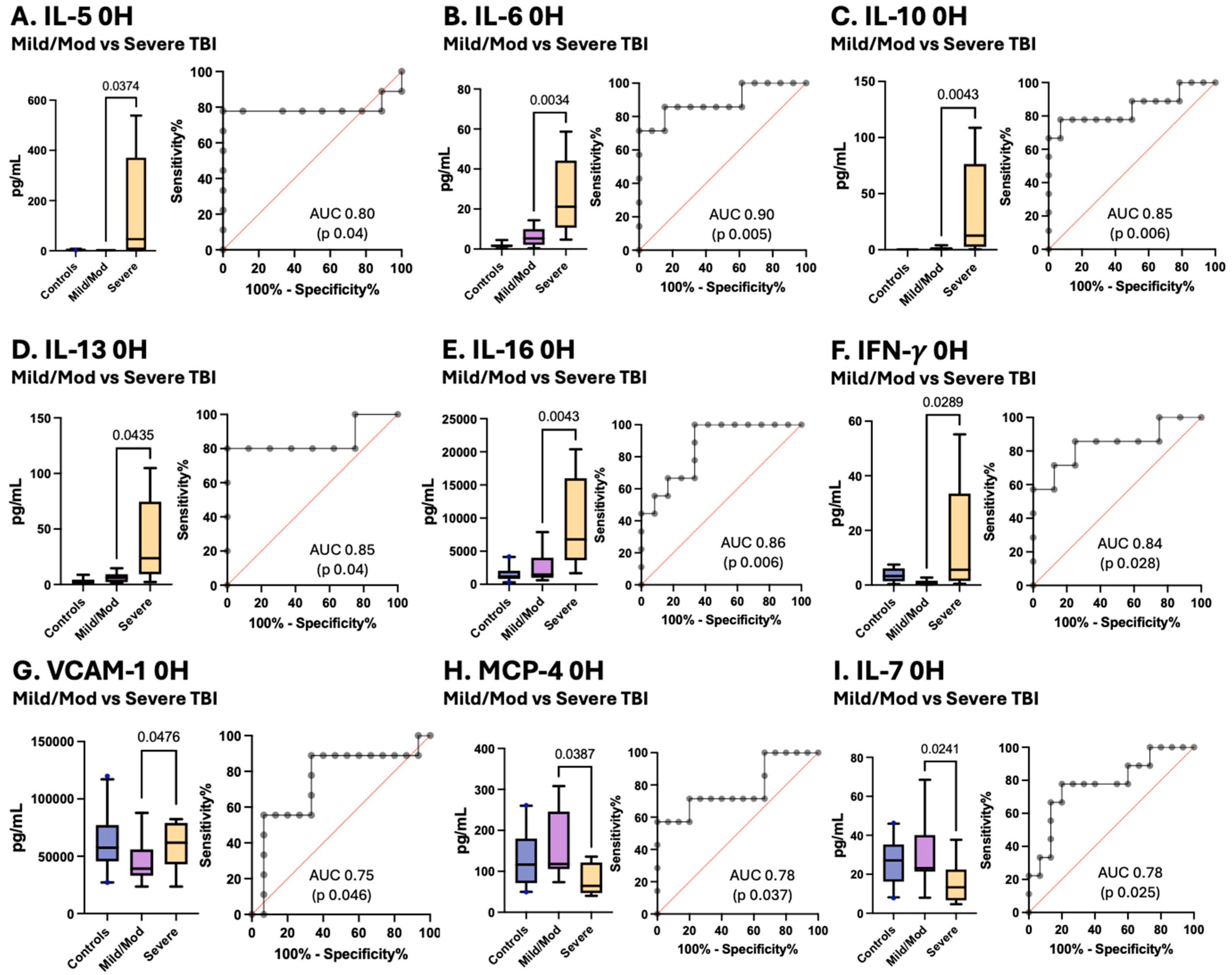
3.4. Variations in Inflammatory Cytokines and Chemokines Associated with pTBI Severity Based on the GCS
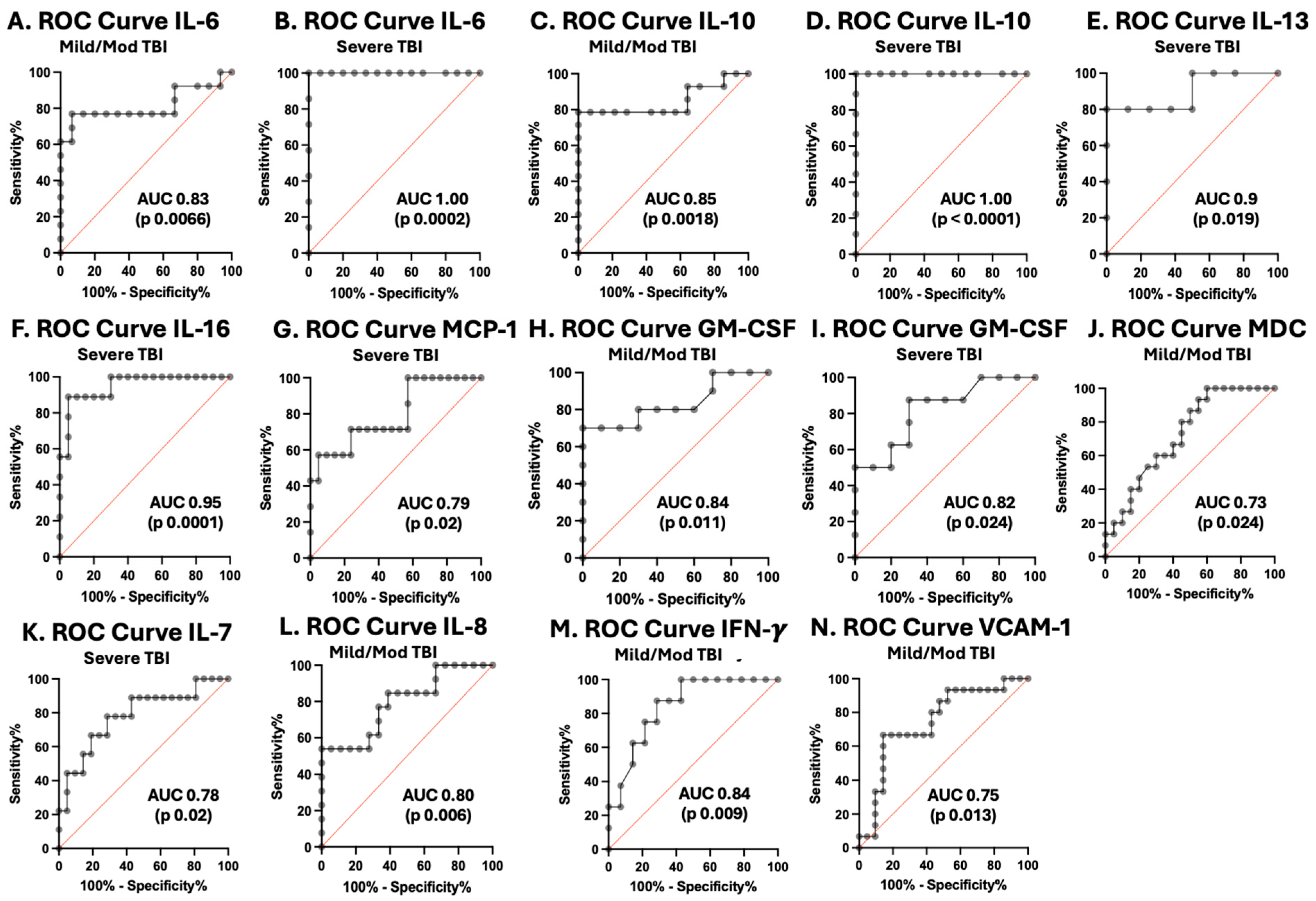
3.5. Cytokines and Chemokines as Biomarkers of Pediatric TBI Severity
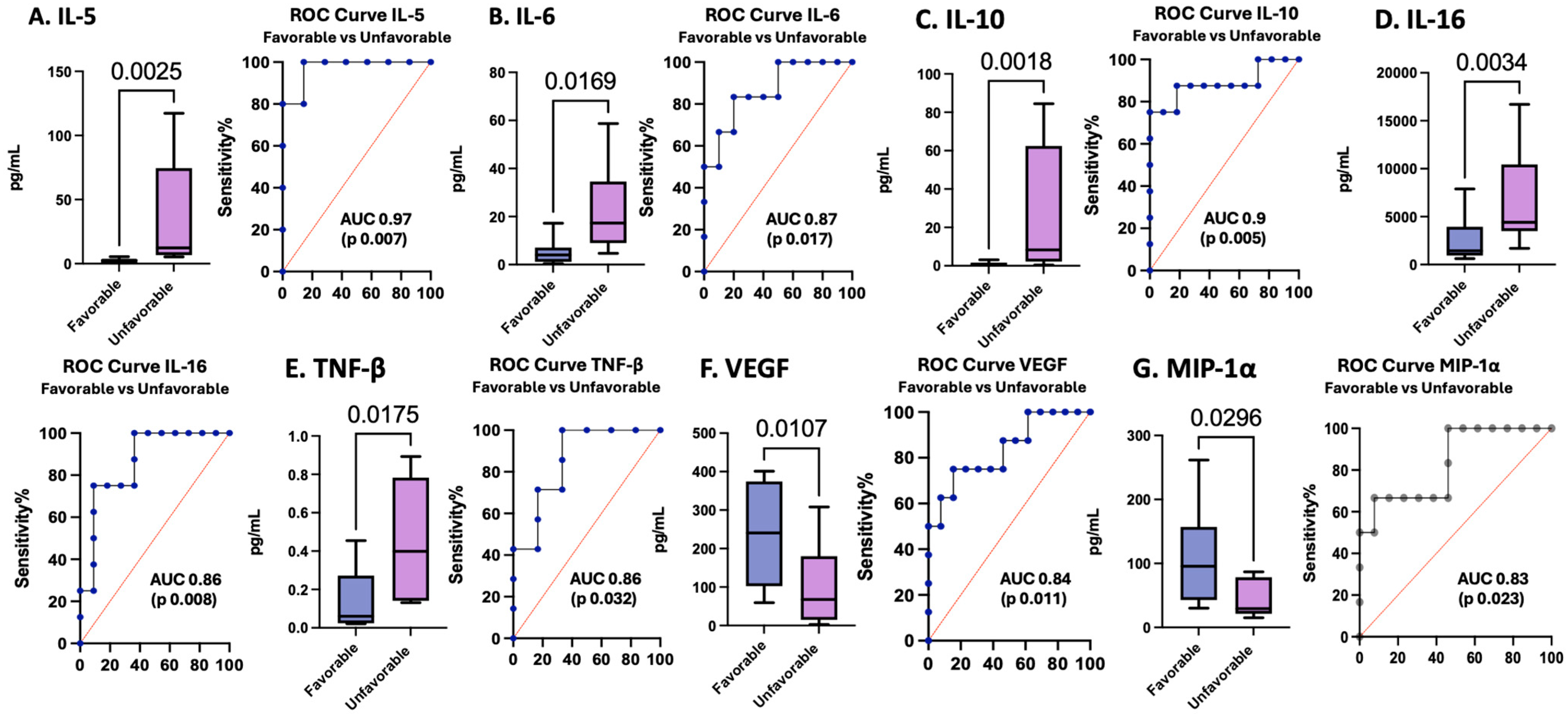
3.6. Cytokines and Chemokines as Biomarkers of Pediatric TBI Outcomes
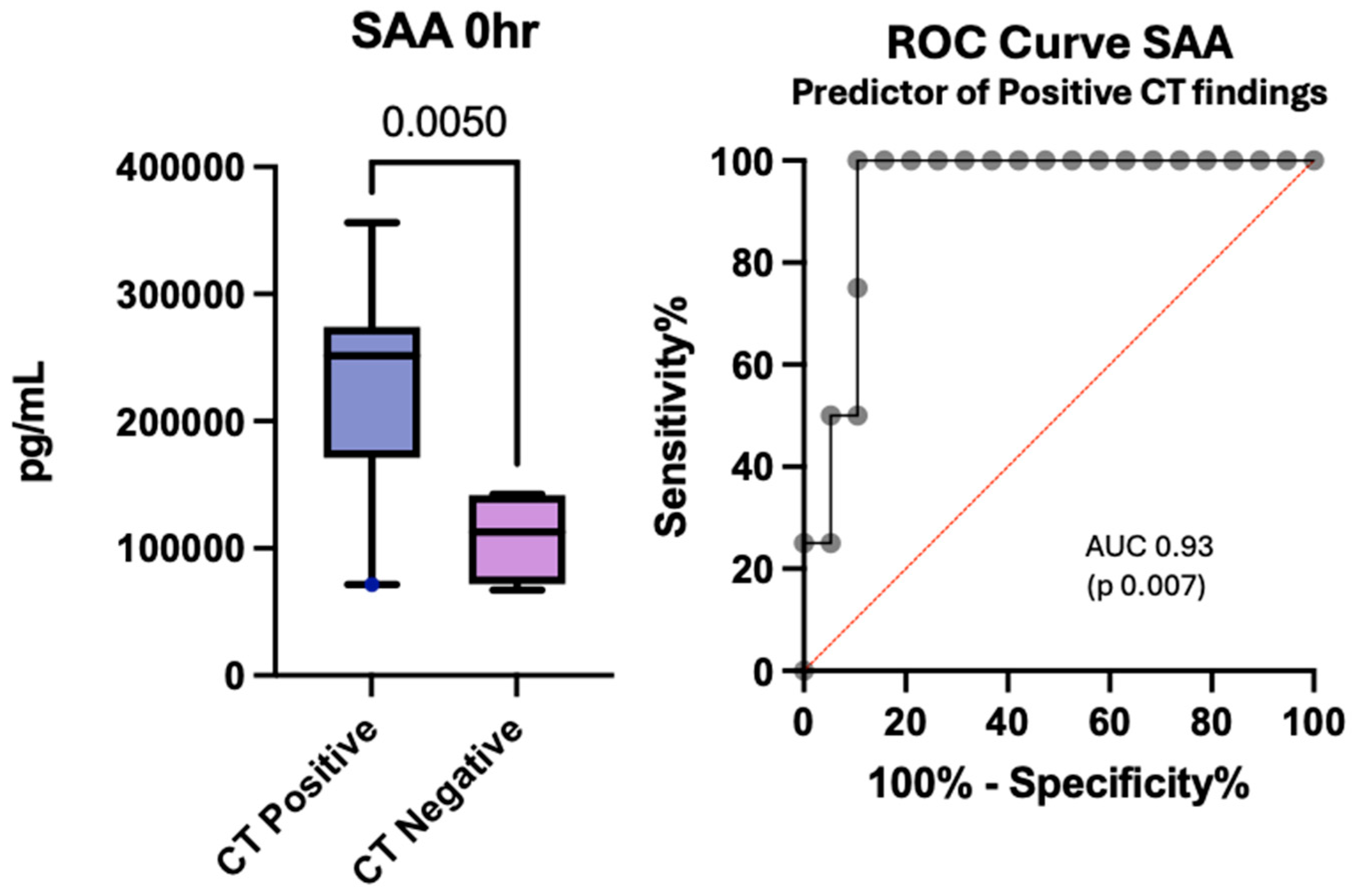
3.7. Cytokines and Chemokines as Potential Predictors of Positive Computed Tomography Findings
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet Neurology. Traumatic Brain Injury: Time to End the Silence. Lancet Neurol. 2010, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.B.P.; Thomas, K.E.M.; Zhou, H.M.M. Surveillance Report: Traumatic Brain Injury-Related Deaths by Age Group, Sex, and Mechanism of Injury—United States, 2018 and 2019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022; pp. 2–15. [Google Scholar]
- Schuchat, A.; Houry, D.; Baldwin, G. Report to Congress: The Management of Traumatic Brain Injury in Children; National Center for Injury Prevention and Control, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018; pp. 4–86. [Google Scholar]
- Humphreys, I.; Wood, R.L.; Phillips, C.J.; Macey, S. The Costs of Traumatic Brain Injury: A Literature Review. Clin. Outcomes Res. 2013, 5, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Yeates, K.O.; Swift, E.; Taylor, H.G.; Wade, S.L.; Drotar, D.; Stancin, T.; Minich, N. Short- and Long-Term Social Outcomes Following Pediatric Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2004, 10, 412–426. [Google Scholar] [CrossRef]
- Babikian, T.; Merkley, T.; Savage, R.C.; Giza, C.C.; Levin, H. Chronic Aspects of Pediatric Traumatic Brain Injury: Review of the Literature. J. Neurotrauma 2015, 32, 1849–1860. [Google Scholar] [CrossRef]
- Rashid, M.; Goez, H.R.; Mabood, N.; Damanhoury, S.; Yager, J.Y.; Joyce, A.S.; Newton, A.S. The Impact of Pediatric Traumatic Brain Injury (TBI) on Family Functioning: A Systematic Review. J. Pediatr. Rehabil. Med. 2014, 7, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.; Morganti-Kossmann, M.C.; Mondello, S.; Yong, V.W. The Role of Markers of Inflammation in Traumatic Brain Injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Waters, R.J.; Murray, G.D.; Teasdale, G.M.; Stewart, J.; Day, I.; Lee, R.J.; Nicoll, J.A.R. Cytokine Gene Polymorphisms and Outcome after Traumatic Brain Injury. J. Neurotrauma 2013, 30, 1710–1716. [Google Scholar] [CrossRef]
- Dikmen, S.; Machamer, J.; Fann, J.R.; Temkin, N.R. Rates of Symptom Reporting Following Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2010, 16, 401–411. [Google Scholar] [CrossRef]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood Biomarkers for Traumatic Brain Injury: A Quantitative Assessment of Diagnostic and Prognostic Accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Kuhle, J.; Petzold, A. What Makes a Prognostic Biomarker in CNS Diseases: Strategies for Targeted Biomarker Discovery? Part 1: Acute and Monophasic Diseases. Expert Opin. Med. Diagn. 2011, 5, 333–346. [Google Scholar] [CrossRef]
- Petron, D.J. Blood-Based Biomarkers for Traumatic Brain Injury: Evaluation of Research Approaches, Available Methods and Potential Utility from the Clinician and Clinical Laboratory Perspectives. Clin. Biochem. 2014, 47, 876–888. [Google Scholar] [CrossRef]
- Mondello, S.; Kobeissy, F.; Vestri, A.; Hayes, R.L.; Kochanek, P.M.; Berger, R.P. Serum Concentrations of Ubiquitin C-Terminal Hydrolase-L1 and Glial Fibrillary Acidic Protein after Pediatric Traumatic Brain Injury. Sci. Rep. 2016, 6, 28203. [Google Scholar] [CrossRef]
- Mehta, T.; Fayyaz, M.; Giler, G.E.; Kaur, H.; Raikwar, S.P.; Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Thangavel, R.; Zaheer, S.; et al. Current Trends in Biomarkers for Traumatic Brain Injury. Open Access J. Neurol. Neurosurg. 2020, 12, 86. [Google Scholar] [CrossRef]
- Munoz Pareja, J.C.; de Rivero Vaccari, J.P.; Chavez, M.M.; Kerrigan, M.; Pringle, C.; Guthrie, K.; Swaby, K.; Coto, J.; Kobeissy, F.; Avery, K.L.; et al. Prognostic and Diagnostic Utility of Serum Biomarkers in Pediatric Traumatic Brain Injury. J. Neurotrauma 2024, 41, 106–122. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Buonora, J.E.; Rhind, S.G.; Hutchison, M.G.; Baker, A.J.; Rizoli, S.B.; Diaz-Arrastia, R.; Mueller, G.P. Blood Biomarkers in Moderate-to-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front. Neurol. 2015, 6, 145046. [Google Scholar] [CrossRef]
- Munoz Pareja, J.C.; Li, X.; Gandham, N.; Wang, K.K.; Lautenslager, L.; Pareja, M.C.; Shanmugham, P.; Faulkinberry, S.; Ghosh, S.; Kerrigan, M.; et al. Biomarkers in Moderate to Severe Pediatric Traumatic Brain Injury: A Review of the Literature. Pediatr. Neurol. 2022, 130, 60–68. [Google Scholar] [CrossRef]
- Ondruschka, B.; Schuch, S.; Pohlers, D.; Franke, H.; Dreßler, J. Acute Phase Response after Fatal Traumatic Brain Injury. Int. J. Leg. Med. 2018, 132, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Rodney, T.; Osier, N.; Gill, J. Pro- and Anti-Inflammatory Biomarkers and Traumatic Brain Injury Outcomes: A Review. Cytokine 2018, 110, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, L.S.; Grell, R. Pediatric Traumatic Brain Injury: Impact on the Developing Brain. Pediatr. Neurol. 2023, 148, 215–222. [Google Scholar] [CrossRef]
- Wei, S.; Leng, B.; Yan, G. Targeting Autophagy Process in Center Nervous Trauma. Front. Neurosci. 2023, 17, 1128087. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Baylr, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The Far-Reaching Scope of Neuroinflammation after Traumatic Brain Injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Berger, R.P.; Beers, S.R.; Papa, L.; Bell, M. Common Data Elements for Pediatric Traumatic Brain Injury: Recommendations from the Biospecimens and Biomarkers Workgroup. J. Neurotrauma 2012, 29, 672–677. [Google Scholar] [CrossRef]
- Scott, X.O.; Chen, S.H.; Hadad, R.; Yavagal, D.; Peterson, E.C.; Starke, R.M.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Cohort Study on the Differential Expression of Inflammatory and Angiogenic Factors in Thrombi, Cerebral and Peripheral Plasma Following Acute Large Vessel Occlusion Stroke. J. Cereb. Blood Flow. Metab. 2022, 42, 1827–1839. [Google Scholar] [CrossRef]
- Newell, E.A.; Todd, B.P.; Mahoney, J.; Pieper, A.A.; Ferguson, P.J.; Bassuk, A.G. Combined Blockade of Interleukin-1α and -1β Signaling Protects Mice from Cognitive Dysfunction after Traumatic Brain Injury. eNeuro 2018, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Makwana, M.; Ahmed, A.I.; Zaben, M. Profiling Biomarkers of Traumatic Axonal Injury: From Mouse to Man. Clin. Neurol. Neurosurg. 2018, 171, 6–20. [Google Scholar] [CrossRef]
- Pu, H.; Zheng, X.; Jiang, X.; Mu, H.; Xu, F.; Zhu, W.; Ye, Q.; Jizhang, Y.; Hitchens, T.K.; Shi, Y.; et al. Interleukin-4 Improves White Matter Integrity and Functional Recovery after Murine Traumatic Brain Injury via Oligodendroglial PPARγ. J. Cereb. Blood Flow. Metab. 2021, 41, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.H.; Hadad, R.; Taylor, R.R.; Pilar, J.R.; Salazar, O.; Llompart-Pou, J.A.; Dietrich, W.D.; Keane, R.W.; Pérez-Bárcena, J.; de Rivero Vaccari, J.P. Inflammatory Biomarkers of Traumatic Brain Injury. Pharmaceuticals 2022, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, D.C.; Brichacek, A.L.; Foster, C.H.; Lucke-Wold, B.P.; Ali, A.; Colantonio, M.A.; Brown, C.M.; Qaiser, R. Pediatric Traumatic Brain Injury: An Update on Preclinical Models, Clinical Biomarkers, and the Implications of Cerebrovascular Dysfunction. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221098124. [Google Scholar] [CrossRef]
- Fraunberger, E.; Esser, M.J. Neuro-Inflammation in Pediatric Traumatic Brain Injury—From Mechanisms to Inflammatory Networks. Brain Sci. 2019, 9, 319. [Google Scholar] [CrossRef]
- Thompson, H.J.; Martha, S.R.; Wang, J.; Becker, K.J. Impact of Age on Plasma Inflammatory Biomarkers in the 6 Months Following Mild Traumatic Brain Injury. J. Head. Trauma. Rehabil. 2020, 35, 324–331. [Google Scholar] [CrossRef]
- Buttram, S.D.W.; Wisniewski, S.R.; Jackson, E.K.; Adelson, P.D.; Feldman, K.; Bayir, H.; Berger, R.P.; Clark, R.S.B.; Kochanek, P.M. Multiplex Assessment of Cytokine and Chemokine Levels in Cerebrospinal Fluid Following Severe Pediatric Traumatic Brain Injury: Effects of Moderate Hypothermia. J. Neurotrauma 2007, 24, 1707–1717. [Google Scholar] [CrossRef]
- Berger, R.P.; Ta’asan, S.; Rand, A.; Lokshin, A.; Kochanek, P. Multiplex Assessment of Serum Biomarker Concentrations in Well-Appearing Children with Inflicted Traumatic Brain Injury. Pediatr. Res. 2009, 65, 97–102. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Hutchison, M.G.; Hassan, S.; Shiu, M.Y.; Inaba, K.; Topolovec-Vranic, J.; Neto, A.C.; Rizoli, S.B.; Baker, A.J. Inflammatory Cytokine and Chemokine Profiles Are Associated with Patient Outcome and the Hyperadrenergic State Following Acute Brain Injury. J. Neuroinflamm. 2016, 13, 40. [Google Scholar] [CrossRef]
- Yue, J.K.; Kobeissy, F.H.; Jain, S.; Sun, X.; Phelps, R.R.L.; Korley, F.K.; Gardner, R.C.; Ferguson, A.R.; Huie, J.R.; Schneider, A.L.C.; et al. Neuroinflammatory Biomarkers for Traumatic Brain Injury Diagnosis and Prognosis: A TRACK-TBI Pilot Study. Neurotrauma Rep. 2023, 4, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Ley, E.J.; Clond, M.A.; Singer, M.B.; Shouhed, D.; Salim, A. IL6 Deficiency Affects Function after Traumatic Brain Injury. J. Surg. Res. 2011, 170, 253–256. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, 16295–16296. [Google Scholar] [CrossRef]
- Ryan, E.; Kelly, L.; Stacey, C.; Huggard, D.; Duff, E.; McCollum, D.; Leonard, A.; Boran, G.; Doherty, D.R.; Bolger, T.; et al. Mild-to-Severe Traumatic Brain Injury in Children: Altered Cytokines Reflect Severity. J. Neuroinflamm. 2022, 19, 36. [Google Scholar] [CrossRef]
- Park, S.H.; Hwang, S.K. Prognostic Value of Serum Levels of S100 Calcium-Binding Protein B, Neuron-Specific Enolase, and Interleukin-6 in Pediatric Patients with Traumatic Brain Injury. World Neurosurg. 2018, 118, e534–e542. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Genovese, O.; Aloe, L.; Antonelli, A.; Piastra, M.; Polidori, G.; Di Rocco, C. Interleukin 1beta and Interleukin 6 Relationship with Paediatric Head Trauma Severity and Outcome. Childs Nerv. Syst. 2005, 21, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Stillings, S.A.; Leclerc, J.L.; Phillips, H.; Edwards, N.J.; Robicsek, S.A.; Hoh, B.L.; Blackburn, S.; Doré, S. Role of Interleukin-10 in Acute Brain Injuries. Front. Neurol. 2017, 8, 244. [Google Scholar] [CrossRef]
- Negrin, L.L.; Ristl, R.; Wollner, G.; Hajdu, S. Differences in Eotaxin Serum Levels between Polytraumatized Patients with and without Concomitant Traumatic Brain Injury—A Matched Pair Analysis. J. Clin. Med. 2024, 13, 4218. [Google Scholar] [CrossRef]
- Lo, T.Y.M.; Jones, P.A.; Minns, R.A. Pediatric Brain Trauma Outcome Prediction Using Paired Serum Levels of Inflammatory Mediators and Brain-Specific Proteins. J. Neurotrauma 2009, 26, 1479–1487. [Google Scholar] [CrossRef]
- Rowland, B.; Savarraj, J.P.J.; Karri, J.; Zhang, X.; Cardenas, J.; Choi, H.A.; Holcomb, J.B.; Wade, C.E. Acute Inflammation in Traumatic Brain Injury and Polytrauma Patients Using Network Analysis. Shock 2020, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef]
- Clausen, F.; Marklund, N.; Hillered, L. Acute Inflammatory Biomarker Responses to Diffuse Traumatic Brain Injury in the Rat Monitored by a Novel Microdialysis Technique. J. Neurotrauma 2019, 36, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Tsitsipanis, C.; Miliaraki, M.; Paflioti, E.; Lazarioti, S.; Moustakis, N.; Ntotsikas, K.; Theofanopoulos, A.; Ilia, S.; Vakis, A.; Simos, P.; et al. Inflammation Biomarkers IL-6 and IL-10 May Improve the Diagnostic and Prognostic Accuracy of Currently Authorized Traumatic Brain Injury Tools. Exp. Ther. Med. 2023, 26, 364. [Google Scholar] [CrossRef]
- Knoblach, S.M.; Faden, A.I. Interleukin-10 Improves Outcome and Alters Proinflammatory Cytokine Expression after Experimental Traumatic Brain Injury. Exp. Neurol. 1998, 153, 143–151. [Google Scholar] [CrossRef]
- Crichton, A.; Ignjatovic, V.; Babl, F.E.; Oakley, E.; Greenham, M.; Hearps, S.; Delzoppo, C.; Beauchamp, M.H.; Guerguerian, A.M.; Boutis, K.; et al. Interleukin-8 Predicts Fatigue at 12 Months Post-Injury in Children with Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.J.; Carlos, T.M.; Kochanek, P.M.; Wisniewski, S.R.; Bell, M.J.; Clark, R.S.B.; DeKosky, S.T.; Marion, D.W.; Adelson, P.D. Interleukin-8 Is Increased in Cerebrospinal Fluid of Children with Severe Head Injury. Crit. Care Med. 2000, 28, 929–934. [Google Scholar] [CrossRef]
- Kossmann, T.; Stahel, P.F.; Lenzlinger, P.M.; Redl, H.; Dubs, R.W.; Trentz, O.; Schlag, G.; Morganti-Kossmann, M.C. Interleukin-8 Released into the Cerebrospinal Fluid after Brain Injury Is Associated with Blood-Brain Barrier Dysfunction and Nerve Growth Factor Production. J. Cereb. Blood Flow Metab. 1997, 17, 280–289. [Google Scholar] [CrossRef]
- Lo, T.Y.M.; Jones, P.A.; Minns, R.A. Combining Coma Score and Serum Biomarker Levels to Predict Unfavorable Outcome Following Childhood Brain Trauma. J. Neurotrauma 2010, 27, 2139–2145. [Google Scholar] [CrossRef]
- Kuppermann, N.; Holmes, J.F.; Dayan, P.S.; Hoyle, J.D.; Atabaki, S.M.; Holubkov, R.; Nadel, F.M.; Monroe, D.; Stanley, R.M.; Borgialli, D.A.; et al. Identification of Children at Very Low Risk of Clinically-Important Brain Injuries after Head Trauma: A Prospective Cohort Study. Lancet 2009, 374, 1160–1170, Erratum in Lancet 2014, 383, 308. https://doi.org/10.1016/S0140-6736(14)60105-7. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.M.; Bowman, M.J.; Ginn-Pease, M.E.; Kosnik, E.; King, D.R. Pediatric Head Injuries: Can Clinical Factors Reliably Predict an Abnormality on Computed Tomography? Ann. Emerg. Med. 1993, 22, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Olldashi, F.; Muzha, I.; Filipi, N.; Lede, R.; Copertari, P.; Traverso, C.; Copertari, A.; Vergara, E.A.; Montenegro, C.; De Huidobro, R.R.; et al. Effect of Intravenous Corticosteroids on Death within 14 Days in 10,008 Adults with Clinically Significant Head Injury (MRC CRASH Trial): Randomised Placebo-Controlled Trial. Lancet 2004, 364, 1321–1328. [Google Scholar] [CrossRef]
- Alderson, P.; Roberts, I. Corticosteroids for Acute Traumatic Brain Injury. Cochrane Database Syst. Rev. 2005, 2005, CD000196. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Kishimoto, T. Inhibition of IL-6 for the Treatment of Inflammatory Diseases. Curr. Opin. Pharmacol. 2004, 4, 386–391. [Google Scholar] [CrossRef]
- Rutgers, A.; Westerweel, P.E.; van der Holt, B.; Postma, S.; van Vonderen, M.G.A.; Piersma, D.P.; Postma, D.; van den Berge, M.; Jong, E.; de Vries, M.; et al. Timely Administration of Tocilizumab Improves Outcome of Hospitalized COVID-19 Patients. PLoS ONE 2022, 17, e0271807. [Google Scholar] [CrossRef]
- Kaye, A.G.; Siegel, R. The Efficacy of IL-6 Inhibitor Tocilizumab in Reducing Severe COVID-19 Mortality: A Systematic Review. PeerJ 2020, 8, e10322. [Google Scholar] [CrossRef]
- Yang, S.H.; Gangidine, M.; Pritts, T.A.; Goodman, M.D.; Lentsch, A.B. Interleukin 6 Mediates Neuroinflammation and Motor Coordination Deficits after Mild Traumatic Brain Injury and Brief Hypoxia in Mice. Shock 2013, 40, 471–475. [Google Scholar] [CrossRef]
- Loddick, S.A.; Turnbull, A.V.; Rothwell, N.J. Cerebral Interleukin-6 Is Neuroprotective during Permanent Focal Cerebral Ischemia in the Rat. J. Cereb. Blood Flow Metab. 1998, 18, 176–179. [Google Scholar] [CrossRef]
| Characteristic | Controls | Pediatric Traumatic Brain Injury Patients | ||||
|---|---|---|---|---|---|---|
| Favorable Outcome | Unfavorable Outcome | |||||
| (n = 16) | (GOS-E Peds ≤ 4) (n = 14) | (GOS-E Peds ≥ 5) (n = 8) | ||||
| Age in years, mean (SD) | 8.2 | 5.5 | 4.0 | 6.1 | 2.6 | 3.6 |
| Gender, n (%) | ||||||
| Female | 7 | 44% | 4 | 29% | 6 | 50% |
| Male | 8 | 50% | 10 | 71% | 2 | 17% |
| Race, n (%) | ||||||
| White | 7 | 50% | 7 | 50% | 3 | 38% |
| African American | 0 | 0% | 4 | 29% | 3 | 38% |
| Unknown | 4 | 29% | 3 | 21% | 2 | 25% |
| Payer status, n (%) | ||||||
| Medicaid | 4 | 25% | 7 | 44% | 5 | 63% |
| Other | 8 | 50% | 5 | 31% | 3 | 38% |
| BMI, mean (SD) | 19.2 | 5.7 | 21.0 | 8.1 | ||
| GCS, n (%) | ||||||
| Severe (GCS:3–8) | NA | 4 | 29% | 6 | 75% | |
| Moderate (GCS:9–12) | 3 | 21% | 2 | 25% | ||
| Mild (GCS:13–15) | 7 | 50% | 0 | 0% | ||
| ISS Score, n (%) | ||||||
| Minor (1–8) | NA | 0 | 0% | 0 | 0% | |
| Moderate (9–15) | 0 | 0% | 0 | 0% | ||
| Serious (16–24) | 3 | 21% | 0 | 0% | ||
| Severe (25–49) | 9 | 64% | 4 | 50% | ||
| Critical (50–75) | 1 | 7% | 4 | 50% | ||
| PRISM Score, n (%) | ||||||
| 5–9 | NA | 4 | 18% | 1 | 13% | |
| 10–14 | 3 | 14% | 1 | 13% | ||
| 15–19 | 3 | 14% | 1 | 13% | ||
| 20–24 | 1 | 5% | 1 | 13% | ||
| 25–29 | 0 | 0% | 1 | 13% | ||
| 30–34 | 0 | 0% | 0 | 0% | ||
| ≥35 | 0 | 0% | 1 | 13% | ||
| Neuroimaging | ||||||
| CT Positive | NA | 12 | 86% | 6 | 75% | |
| CT Negative | 2 | 14% | 1 | 13% | ||
| Marshall Score | ||||||
| 1 | NA | 2 | 14% | 1 | 13% | |
| 2 | 9 | 64% | 5 | 63% | ||
| 3 | 2 | 14% | 1 | 13% | ||
| 4 | 1 | 7% | 1 | 13% | ||
| Biomarkers | AUROC | SE | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|
| IL-5 | 0.7794 | 0.083 | 0.6167 to 0.9420 | 0.0081 | Cutoff | (pg/mL) | >3.016 |
| Sensitivity | (%) | 79 | |||||
| Specificity | (%) | 62 | |||||
| IL-6 | 0.8909 | 0.05837 | 0.7765 to 1.000 | <0.0001 | Cutoff | (pg/mL) | <4.576 |
| Sensitivity | (%) | 100 | |||||
| Specificity | (%) | 77 | |||||
| IL-10 | 0.8872 | 0.06352 | 0.7627 to 1.000 | 0.0002 | Cutoff | (pg/mL) | <0.3728 |
| Sensitivity | (%) | 100 | |||||
| Specificity | (%) | 84 | |||||
| IL-13 | 0.7841 | 0.1055 | 0.5773 to 0.9909 | 0.039 | Cutoff | (pg/mL) | <3.597 |
| Sensitivity | (%) | 75 | |||||
| Specificity | (%) | 73 | |||||
| IL-16 | 0.7575 | 0.07669 | 0.6072 to 0.9078 | 0.0053 | Cutoff | (pg/mL) | <1552 |
| Sensitivity | (%) | 70 | |||||
| Specificity | (%) | 65 | |||||
| CP-IL8 | 0.7747 | 0.07739 | 0.6230 to 0.9264 | 0.0049 | Cutoff | (pg/mL) | >2939 |
| Sensitivity | (%) | 100 | |||||
| Specificity | (%) | 50 | |||||
| IFN-γ | 0.8182 | 0.08427 | 0.6530 to 0.9833 | 0.0073 | Cutoff | (pg/mL) | >1.725 |
| Sensitivity | (%) | 71 | |||||
| Specificity | (%) | 82 | |||||
| GM-CSF | 0.8263 | 0.07616 | 0.6771 to 0.9756 | 0.0044 | Cutoff | (pg/mL) | <343.7 |
| Sensitivity | (%) | 70 | |||||
| Specificity | (%) | 84 | |||||
| MDC | 0.7011 | 0.08156 | 0.5412 to 0.8609 | 0.0243 | Cutoff | (pg/mL) | <1307 |
| Sensitivity | (%) | 80 | |||||
| Specificity | (%) | 43 | |||||
| Eotaxin-3 | 0.7519 | 0.07697 | 0.6010 to 0.9027 | 0.0065 | Cutoff | (pg/mL) | >41.43 |
| Sensitivity | (%) | 79 | |||||
| Specificity | (%) | 62 |
| Controls (n = 21) | Traumatic Brain Injury (n = 22) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarkers | Median (pg/mL) | S.D. (pg/mL) | IQR (pg/mL) | n | Median (pg/mL) | S.D. (pg/mL) | IQR (pg/mL) | Mean Rank Difference | p-Value | |
| IL-6 | 0.2275 | 1.095 | 1.003–2.301 | Severe | 7 | 21.15 | 19.12 | 10.64–44.21 | −29.91 | <0.0001 |
| IL-10 | 0.2234 | 0.1263 | 0.1113–0.3559 | Mild/Moderate | 14 | 1.022 | 1.175 | 0.348–1.871 | −15.93 | 0.0183 |
| Severe | 9 | 12.58 | 41.5 | 2.54–76.45 | −30.99 | <0.0001 | ||||
| IL-13 | 2.294 | 2.821 | 0.5675–4.376 | Severe | 5 | 23.73 | 40.14 | 9.363–74.53 | −15.05 | 0.0076 |
| IL-16 | 1150 | 953.4 | 832–2053 | Severe | 9 | 6772 | 6841 | 3632–16,008 | −25.12 | 0.0002 |
| CP-IL8 | 8668 | 4359 | 5014–11,581 | Mild/Moderate | 13 | 2917 | 3924 | 371–6314 | 14.47 | 0.0255 |
| VCAM-1 | 57,406 | 23,059 | 45,579–77,190 | Mild/Moderate | 15 | 39,218 | 16,407 | 33,099–55,924 | 14.8 | 0.0486 |
| Biomarkers | TBI Severity | AUROC | SE | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| IL-6 | Mild/Moderate | 0.8267 | 0.1046 | 0.6216 to 1.000 | 0.0066 | Cutoff | (pg/mL) | >3.050 |
| Sensitivity | (%) | 77 | ||||||
| Specificity | (%) | 93 | ||||||
| Severe | 1 | 0 | 1.000 to 1.000 | 0.0002 | Cutoff | (pg/mL) | >4.576 | |
| Sensitivity | (%) | 100 | ||||||
| Specificity | (%) | 100 | ||||||
| IL-10 | Mild/Moderate | 0.8469 | 0.08355 | 0.6832 to 1.000 | 0.0018 | Cutoff | (pg/mL) | >0.3905 |
| Sensitivity | (%) | 79 | ||||||
| Specificity | (%) | 100 | ||||||
| Severe | 1 | 0 | 1.000 to 1.000 | <0.0001 | Cutoff | (pg/mL) | >0.3728 | |
| Sensitivity | (%) | 100 | ||||||
| Specificity | (%) | 100 | ||||||
| IL-13 | Severe | 0.9 | 0.1012 | 0.7016 to 1.000 | 0.0192 | Cutoff | (pg/mL) | >12.64 |
| Sensitivity | (%) | 80 | ||||||
| Specificity | (%) | 100 | ||||||
| IL-16 | Severe | 0.95 | 0.04011 | 0.8714 to 1.000 | 0.0001 | Cutoff | (pg/mL) | >1603 |
| Sensitivity | (%) | 100 | ||||||
| Specificity | (%) | 70 | ||||||
| CP IL-8 | Mild/Moderate | 0.7949 | 0.08314 | 0.6319 to 0.9578 | 0.0057 | Cutoff | (pg/mL) | <6842 |
| Sensitivity | (%) | 85 | ||||||
| Specificity | (%) | 61 | ||||||
| IL-7 | Severe | 0.7831 | 0.09633 | 0.5943 to 0.9719 | 0.0155 | Cutoff | (pg/mL) | <26.24 |
| Sensitivity | (%) | 89 | ||||||
| Specificity | (%) | 57 | ||||||
| VCAM-1 | Mild/Moderate | 0.746 | 0.08508 | 0.5793 to 0.9128 | 0.0129 | Cutoff | (pg/mL) | <55,944 |
| Sensitivity | (%) | 80 | ||||||
| Specificity | (%) | 57 | ||||||
| GM-CSF | Mild/Moderate | 0.8350 | 0.09606 | 0.6467 to 1.000 | 0.0113 | Cutoff | (pg/mL) | >343.7 |
| Sensitivity | (%) | 80 | ||||||
| Specificity | (%) | 70 | ||||||
| Severe | 0.8188 | 0.1019 | 0.6189 to 1.000 | 0.0235 | Cutoff | (pg/mL) | >346.2 | |
| Sensitivity | (%) | 88 | ||||||
| Specificity | (%) | 70 |
| Biomarkers | Sample Time | AUROC | SE | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| IL-5 | 24 h | 0.9714 | 0.04373 | 0.8857 to 1.000 | 0.0074 | Cutoff | (pg/mL) | >4.326 |
| Sensitivity | (%) | 100 | ||||||
| Specificity | (%) | 86 | ||||||
| IL-6 | 0 h | 0.8667 | 0.09477 | 0.6809 to 1.000 | 0.017 | Cutoff | (pg/mL) | >8.135 |
| Sensitivity | (%) | 83 | ||||||
| Specificity | (%) | 80 | ||||||
| IL-10 | 0 h | 0.8864 | 0.09063 | 0.7087 to 1.000 | 0.005 | Cutoff | (pg/mL) | >2.834 |
| Sensitivity | (%) | 75 | ||||||
| Specificity | (%) | 91 | ||||||
| IL-16 | 0 h | 0.8636 | 0.086 | 0.6951 to 1.000 | 0.0082 | Cutoff | (pg/mL) | >3998 |
| Sensitivity | (%) | 75 | ||||||
| Specificity | (%) | 91 | ||||||
| TNF-β | 0 h | 0.8571 | 0.1096 | 0.6423 to 1.000 | 0.0321 | Cutoff | (pg/mL) | >0.2665 |
| Sensitivity | (%) | 71 | ||||||
| Specificity | (%) | 83 | ||||||
| MIP-1α | 0 h | 0.8333 | 0.1038 | 0.6299 to 1.000 | 0.0226 | Cutoff | (pg/mL) | <91.21 |
| Sensitivity | (%) | 100 | ||||||
| Specificity | (%) | 54 | ||||||
| VEGF | 0 h | 0.8365 | 0.0942 | 0.6519 to 1.000 | 0.0113 | Cutoff | (pg/mL) | <98.62 |
| Sensitivity | (%) | 75 | ||||||
| Specificity | (%) | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Swaby, K.; Skirvin, A.J.; Machado, N.; Mateo Chavez, M.; Bernal, J.A.; Fuentes, A.; Pringle, C.P.; Guthrie, K.; Coto, J.; Dhanashree, R.; et al. Potential Role of Serum Cytokines and Chemokines as Biomarkers of Injury Severity and Functional Outcomes Following Pediatric Traumatic Brain Injury. Cells 2026, 15, 19. https://doi.org/10.3390/cells15010019
Swaby K, Skirvin AJ, Machado N, Mateo Chavez M, Bernal JA, Fuentes A, Pringle CP, Guthrie K, Coto J, Dhanashree R, et al. Potential Role of Serum Cytokines and Chemokines as Biomarkers of Injury Severity and Functional Outcomes Following Pediatric Traumatic Brain Injury. Cells. 2026; 15(1):19. https://doi.org/10.3390/cells15010019
Chicago/Turabian StyleSwaby, Kathryn, Alexander J. Skirvin, Natalie Machado, Maria Mateo Chavez, Julia Alexis Bernal, Ana Fuentes, Charlene P. Pringle, Kourtney Guthrie, Jennifer Coto, Rajderkar Dhanashree, and et al. 2026. "Potential Role of Serum Cytokines and Chemokines as Biomarkers of Injury Severity and Functional Outcomes Following Pediatric Traumatic Brain Injury" Cells 15, no. 1: 19. https://doi.org/10.3390/cells15010019
APA StyleSwaby, K., Skirvin, A. J., Machado, N., Mateo Chavez, M., Bernal, J. A., Fuentes, A., Pringle, C. P., Guthrie, K., Coto, J., Dhanashree, R., Gober, J., Perez, P. K., Solano, J. P., McCrea, H. J., Loor-Torres, R., Kaufman, J., Alkhachroum, A., O’Phelan, K. H., Kobeissy, F., ... Munoz Pareja, J. C. (2026). Potential Role of Serum Cytokines and Chemokines as Biomarkers of Injury Severity and Functional Outcomes Following Pediatric Traumatic Brain Injury. Cells, 15(1), 19. https://doi.org/10.3390/cells15010019









