Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Recombinant AAV Vector Production and L4-SpN Injection
2.3. Peripheral Nerve Injury
2.4. Hot Plate Test
2.5. Intrathecal Injection
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
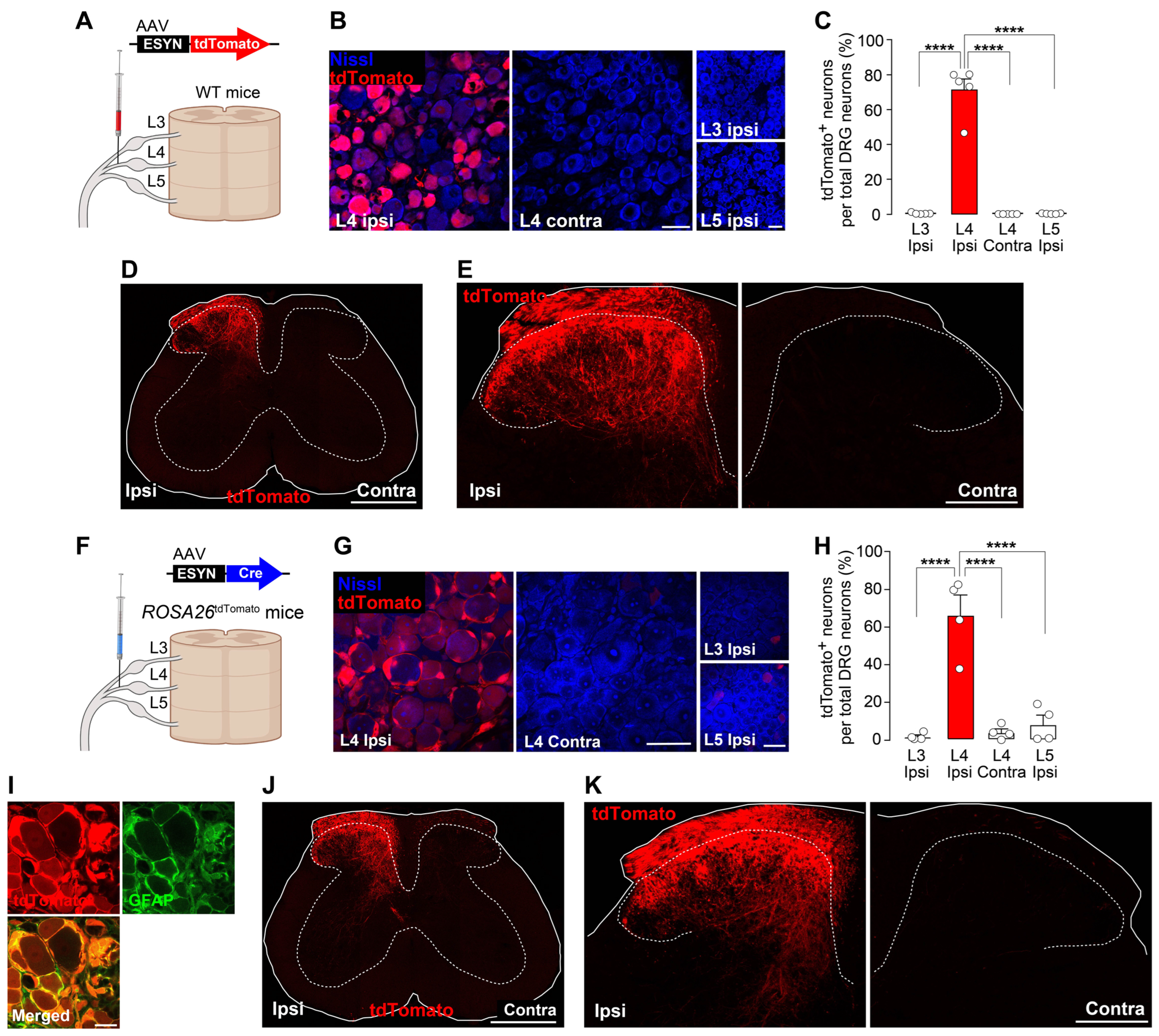
3.1. New Methods to Visualize Injured Primary Afferent Fibers in the SDH Using AAV Vectors
3.2. Spatial Correlation Between Injured Nerve Fiber Projection and Reactive Microglia
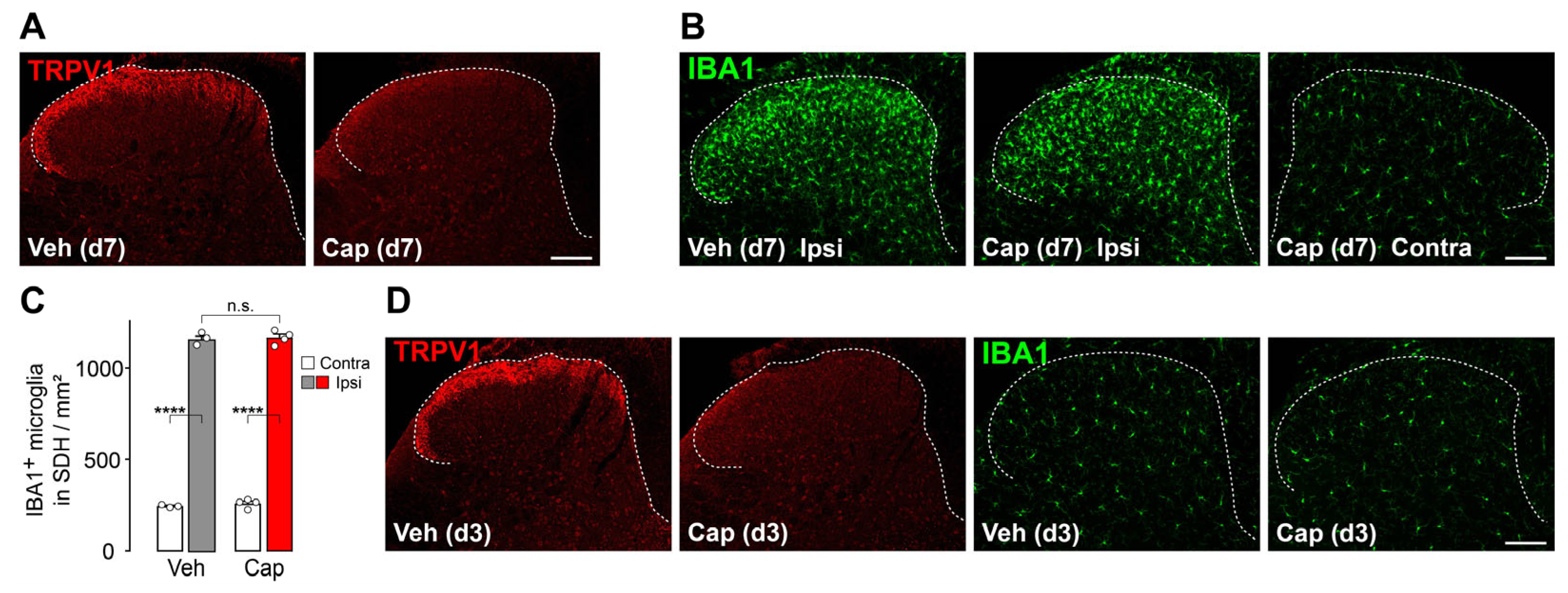
3.3. TRPV1+ Primary Afferent Fibers Are Dispensable for Microgliosis in the SDH After Peripheral Nerve Injury
3.4. Microgliosis in the SDH Involves Damage to Primary Afferent Aβ-Fibers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDH | Spinal dorsal horn |
| L4 | Fourth lumbar |
| DRG | Dorsal root ganglion |
| SpN | Spinal nerve |
| TRPV1 | Transient receptor potential vanilloid 1 |
| CNS | Central nervous system |
| IBA1 | Ionized calcium-binding adapter molecule 1 |
| AAV | Adeno-associated virus |
| ESYN | Enhanced synapsin |
| PBS | Phosphate-buffered saline |
| i.p. | Intraperitoneal |
| WT | Wild type |
| CTB | Cholera toxin B subunit |
| IB4 | Isolectin B4 |
| Ctrl | Control |
| SAP | Saporin |
| NF200 | Neurofilament 200 |
| MBP | Myelin basic protein |
| ROI | Region of interest |
| DIC | Differential interference contrast |
| n.s. | Not significant |
| GN | Gracile nucleus |
| CSF1 | Colony-stimulating factor 1 |
| LPA | Lysophosphatidic acid |
References
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the cells and circuits of touch in normal and pathological settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Tsuda, M.; Masuda, T.; Kohno, K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023, 46, 597–610. [Google Scholar] [CrossRef]
- Gilmore, S.A. Proliferation of non-neuronal cells in spinal cords of irradiated, immature rats following transection of the sciatic nerve. Anat. Rec. 1975, 181, 799–811. [Google Scholar] [CrossRef]
- Masuda, T.; Iwamoto, S.; Yoshinaga, R.; Tozaki-Saitoh, H.; Nishiyama, A.; Mak, T.W.; Tamura, T.; Tsuda, M.; Inoue, K. Transcription factor irf5 drives p2x4r+-reactive microglia gating neuropathic pain. Nat. Commun. 2014, 5, 3771. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Crow, M.; Didangelos, A.; Lopes, D.M.; McMahon, S.B. Persistent alterations in microglial enhancers in a model of chronic pain. Cell Rep. 2016, 15, 1771–1781. [Google Scholar] [CrossRef]
- Coyle, D.E. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia 1998, 23, 75–83. [Google Scholar] [CrossRef]
- Colburn, R.W.; Rickman, A.J.; DeLeo, J.A. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp. Neurol. 1999, 157, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Stuesse, S.L.; Cruce, W.L.; Lovell, J.A.; McBurney, D.L.; Crisp, T. Microglial proliferation in the spinal cord of aged rats with a sciatic nerve injury. Neurosci. Lett. 2000, 287, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2x4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.; Salter, M.W. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav. Immun. 2007, 21, 624–633. [Google Scholar] [CrossRef]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.R.; Mantyh, P.W. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuda, M.; Yoshinaga, R.; Tozaki-Saitoh, H.; Ozato, K.; Tamura, T.; Inoue, K. Irf8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012, 1, 334–340. [Google Scholar] [CrossRef]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron-derived csf1 induces microglial proliferation and dap12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef]
- Kohno, K.; Kitano, J.; Kohro, Y.; Tozaki-Saitoh, H.; Inoue, K.; Tsuda, M. Temporal kinetics of microgliosis in the spinal dorsal horn after peripheral nerve injury in rodents. Biol. Pharm. Bull. 2018, 41, 1096–1102. [Google Scholar] [CrossRef]
- Kanehisa, K.; Koga, K.; Maejima, S.; Shiraishi, Y.; Asai, K.; Shiratori-Hayashi, M.; Xiao, M.F.; Sakamoto, H.; Worley, P.F.; Tsuda, M. Neuronal pentraxin 2 is required for facilitating excitatory synaptic inputs onto spinal neurons involved in pruriceptive transmission in a model of chronic itch. Nat. Commun. 2022, 13, 2367. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J.; Martin, C.L.; Arnolda, L.F.; Minson, J.B. Tracer-toxins: Cholera toxin b-saporin as a model. J. Neurosci. Methods 2000, 103, 83–90. [Google Scholar] [CrossRef]
- Tarpley, J.W.; Kohler, M.G.; Martin, W.J. The behavioral and neuroanatomical effects of ib4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Res. 2004, 1029, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ho Kim, S.; Mo Chung, J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Kohno, K.; Shirasaka, R.; Yoshihara, K.; Mikuriya, S.; Tanaka, K.; Takanami, K.; Inoue, K.; Sakamoto, H.; Ohkawa, Y.; Masuda, T.; et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science 2022, 376, 86–90. [Google Scholar] [CrossRef]
- Kohro, Y.; Matsuda, T.; Yoshihara, K.; Kohno, K.; Koga, K.; Katsuragi, R.; Oka, T.; Tashima, R.; Muneta, S.; Yamane, T.; et al. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat. Neurosci. 2020, 23, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Hylden, J.L.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Lee, H.; Lo, L.; Shields, S.D.; Zylka, M.J.; Basbaum, A.I.; Anderson, D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 9075–9080. [Google Scholar] [CrossRef]
- Zwick, M.; Davis, B.M.; Woodbury, C.J.; Burkett, J.N.; Koerber, H.R.; Simpson, J.F.; Albers, K.M. Glial cell line-derived neurotrophic factor is a survival factor for isolectin b4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J. Neurosci. 2002, 22, 4057–4065. [Google Scholar] [CrossRef]
- Shehab, S.A.; Hughes, D.I. Simultaneous identification of unmyelinated and myelinated primary somatic afferents by co-injection of isolectin b4 and cholera toxin subunit b into the sciatic nerve of the rat. J. Neurosci. Methods 2011, 198, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Iskols, M.; Shi, D.; Reddy, P.; Walker, C.; Lezgiyeva, K.; Voisin, T.; Pawlak, M.; Kuchroo, V.K.; Chiu, I.M.; et al. A mouse drg genetic toolkit reveals morphological and physiological diversity of somatosensory neuron subtypes. Cell 2024, 187, 1508–1526.e16. [Google Scholar] [CrossRef]
- Upadhyay, A.; Gradwell, M.A.; Vajtay, T.J.; Conner, J.; Sanyal, A.A.; Azadegan, C.; Patel, K.R.; Thackray, J.K.; Bohic, M.; Imai, F.; et al. The dorsal column nuclei scale mechanical sensitivity in naive and neuropathic pain states. Cell Rep. 2025, 44, 115556. [Google Scholar] [CrossRef]
- Peirs, C.; Seal, R.P. Neural circuits for pain: Recent advances and current views. Science 2016, 354, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.A.; Skinner, R.D. Intraspinal non-neuronal cellular responses to peripheral nerve injury. Anat. Rec. 1979, 194, 369–387. [Google Scholar] [CrossRef]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef]
- Bai, L.; Lehnert, B.P.; Liu, J.; Neubarth, N.L.; Dickendesher, T.L.; Nwe, P.H.; Cassidy, C.; Woodbury, C.J.; Ginty, D.D. Genetic identification of an expansive mechanoreceptor sensitive to skin stroking. Cell 2015, 163, 1783–1795. [Google Scholar] [CrossRef]
- Hathway, G.J.; Vega-Avelaira, D.; Moss, A.; Ingram, R.; Fitzgerald, M. Brief, low frequency stimulation of rat peripheral c-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 2009, 144, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.P.; Luo, H.; Ma, Q.; Xie, Y.K.; Li, W.; Hu, H.; Xu, Z.Z. Gpr151 in nociceptors modulates neuropathic pain via regulating p2x3 function and microglial activation. Brain 2021, 144, 3405–3420. [Google Scholar] [CrossRef]
- Liu, X.J.; Liu, T.; Chen, G.; Wang, B.; Yu, X.L.; Yin, C.; Ji, R.R. Tlr signaling adaptor protein myd88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci. Rep. 2016, 6, 28188. [Google Scholar] [CrossRef] [PubMed]
- Szeredi, I.D.; Jancso, G.; Oszlacs, O.; Santha, P. Prior perineural or neonatal treatment with capsaicin does not alter the development of spinal microgliosis induced by peripheral nerve injury. Cell Tissue Res. 2021, 383, 677–692. [Google Scholar] [CrossRef]
- Suter, M.R.; Berta, T.; Gao, Y.J.; Decosterd, I.; Ji, R.R. Large a-fiber activity is required for microglial proliferation and p38 mapk activation in the spinal cord: Different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain. 2009, 5, 53. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Okubo, M.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Kanda, H.; Yagi, H.; Noguchi, K. Macrophage-colony stimulating factor derived from injured primary afferent induces proliferation of spinal microglia and neuropathic pain in rats. PLoS ONE 2016, 11, e0153375. [Google Scholar] [CrossRef]
- Grosu, A.V.; Gheorghe, R.O.; Filippi, A.; Deftu, A.F.; Isler, M.; Suter, M.; Ristoiu, V. Dorsal root ganglia csf1(+) neuronal subtypes have different impact on macrophages and microglia after spared nerve injury. J. Peripher. Nerv. Syst. 2024, 29, 514–527. [Google Scholar] [CrossRef]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Uchida, H.; Nagai, J.; Inoue, M.; Aoki, J.; Ueda, H. Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase a2 and autotaxin in nerve injury-induced neuropathic pain. J. Pharmacol. Exp. Ther. 2010, 333, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, S.; Lu, J.; Thilemann, S.; Wortge, S.; Mobius, W.; Bruttger, J.; Karram, K.; Ruhwedel, T.; Blanfeld, M.; Vardeh, D.; et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat. Commun. 2014, 5, 5472. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Miron, V.E. Microglia regulation of central nervous system myelin health and regeneration. Nat. Rev. Immunol. 2024, 24, 49–63. [Google Scholar] [CrossRef]
- Ueda, H. Lysophosphatidic acid signaling is the definitive mechanism underlying neuropathic pain. Pain 2017, 158 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, Y.; Matsumoto, Y.; Kohno, K.; Nakashima, Y.; Tsuda, M. Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers. Cells 2025, 14, 666. https://doi.org/10.3390/cells14090666
Shibata Y, Matsumoto Y, Kohno K, Nakashima Y, Tsuda M. Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers. Cells. 2025; 14(9):666. https://doi.org/10.3390/cells14090666
Chicago/Turabian StyleShibata, Yuto, Yuki Matsumoto, Keita Kohno, Yasuharu Nakashima, and Makoto Tsuda. 2025. "Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers" Cells 14, no. 9: 666. https://doi.org/10.3390/cells14090666
APA StyleShibata, Y., Matsumoto, Y., Kohno, K., Nakashima, Y., & Tsuda, M. (2025). Microgliosis in the Spinal Dorsal Horn Early After Peripheral Nerve Injury Is Associated with Damage to Primary Afferent Aβ-Fibers. Cells, 14(9), 666. https://doi.org/10.3390/cells14090666





