Abstract
Immunocompetent cells of B lineage function in the humoral immunity system in the adaptive immune responses. B cells differentiate into plasmacytes upon antigen-induced activation and produce different subclasses of immunoglobulins/antibodies. Secreted immunoglobulins not only interact with pathogens to inactivate and neutralize them, but also involve the complement system to exert antibacterial activities and trigger opsonization. Endometrium is a mucosal tissue that lines the mammalian uterus and is indispensable for the establishment of a successful pregnancy. The lymphocytes of B cell lineage are a minority in the human cycling endometrium. Human endometrial B cells have therefore been understudied so far. However, the disorders of the female reproductive tract, including chronic endometritis and endometriosis, have highlighted the importance of further research on the endometrial B cell lineage. This review aims to revisit lymphopoiesis, maturation, commitment, and survival of B cells, shedding light on their physiological and pathological implications in the human endometrium.
1. Introduction
B cells (B lymphocytes) are immunocompetent cells that function in the humoral immunity system in the adaptive immune responses. B cells differentiate into plasmacytes (PCs) upon antigen-induced activation and produce different subclasses of immunoglobulins (Ig)/antibodies. Secreted Ig not only interact with pathogens to inactivate and neutralize them, but also involve the complement system to exert antibacterial activities and trigger opsonization (phagocytosis by antibody-coated pathogens) [1].
Endometrium is a mucosal tissue that lines the mammalian uterus and is indispensable for the establishment of a successful pregnancy [2]. Human endometrium contains various types of immunocompetent cells, including T cells, macrophages, natural killer cells, neutrophils, and eosinophils [3]. Endometrial immunocompetent cells are considered not only to provide a front-line defense against invading pathogens in the uterine cavity, but also to play a role in conditioning the local microenvironment for intact pregnancy [4,5,6].
By contrast, lymphocytes of B cell lineage are a minority in the human cycling endometrium [7]. Human endometrial B cells have therefore been understudied so far. However, recent studies on chronic endometritis (CE) and endometriosis have alerted us to the importance of further research on endometrial B cell lineage [8,9]. This review aims to revisit lymphopoiesis, maturation, commitment, and survival of B cells, shedding light on their physiological and pathological roles in the human endometrium.
2. B Cell Subpopulations
Based on their ontogeny, phenotype, and functions, B cells are categorized into B-1 cells and B-2 cells [10]. B-1 cells participate in the innate immune system and produce Ig that are distinguished by their recognition of self-antigens and repetitive epitopes such as carbohydrates. B-1 cells are further subdivided into B-1a and B-1b subsets, depending on the presence or absence of the inhibitory surface receptor CD5 [11]. While B-1a cells produce Ig that react with molecular features standard to many microbes and play a pivotal role in scavenging senescent cells, B-1b cells contribute to the acquisition of adaptive immune responses against bacteria without the assistance of T cells. Artificial stimulation of Toll-like receptors on B-1a cells downregulates their CD5 expression, suggesting that B-1b cells are the activated form of B-1a cells [11,12]. B-2 cells are present in the secondary lymphoid organs and are thought to be mediators of adaptive immunity. B-2 cells are subclassified into a predominant population of follicular B (FOB) cells and a minor population of marginal zone B (MZB) cells, both of which are able to undergo Ig class switching and differentiate into memory B cells [13]. While FOB cells eventually differentiate into PCs or long-lived memory B cells, MZB cells reside predominantly within the marginal zone of the spleen, lymph nodes, and peripheral blood. MZB cells rapidly respond to invading antigens from the circulation upon encountering them. MZB cells also exhibit B1 cell-like innate properties and functions. In this manuscript, hereinafter, B2 cells, immature B cells, and memory B cells are referred to as B cells [14].
3. B Cell Lymphopoiesis
In mammals, B cells mainly originate in hematopoietic stem cells that reside in the bone marrow and differentiate through a multistep process. Hematopoietic stem cells give rise to B cell progenitors via the stages of lymphomyelo-primed progenitors and common lymphoid progenitors. The maturation of lymphomyelo-primed progenitors into common lymphoid progenitors is induced by Fms-like tyrosine kinase 3 signaling, which upregulates lymphoid-specific genes (E2a, Il7ra, and Ebf1) [15,16] (Figure 1).
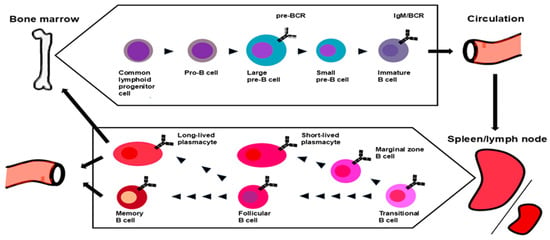
Figure 1.
Current models of B cell lymphopoiesis, maturation, and commitment. B cells originate from the bone marrow hematopoietic precursor cells. Recombination-activating gene (RAG) 1/2–induced rearrangement from the germline initiates at the pro-B-cell stage. V-gene segment rearrangement follows in the early pre-B cell stage. Pre-B cell receptor (BCR)–associated signals silence RAG gene expression, suppress the rearrangement of the second H-chains, and induce cell proliferation. IgM-expressing immature B cells alter the gene expression pattern and prepare for egress into the circulation. Immature B cells enter the spleen as transitional B cells, receive survival signals through B-cell activating factor receptor (BAFF-R), and complete the first stage of development, depending on the specificity of their BCR. Upon contact with antigens and support by B helper neutrophils, MZB cells develop into short-lived PC. FOB cells are activated by antigen binding for development in the germinal centers with the aid of helper T cells. T cell-independent activation promotes the differentiation of FOB or MZB cells to PCs, whereas T cell-dependent activation leads them to memory B cells.
Ikaros is a transcription factor in the Ikaros protein family. Ikaros regulates transcriptional programs via the coordination of six zinc finger domains. While the first four zinc finger domains are essential for DNA binding, the last two facilitate homodimerization and heterodimerization with other members of the Ikaros protein family, comprising Aiolos, Helios, Eos, and Pegasus [17]. Ikaros is expressed in the human bone marrow from the early progenitor stage and plays a crucial role in B lymphopoiesis. The lack or deficiency in Ikaros causes a serious reduction in B cells and/or severe hypogammaglobulinemia [18].
B cell commitment is determined by the expression of paired box protein 5 (PAX5), a transcription factor of which gene transcripts first appear at the pro-B cell stage [19]. PAX5 suppresses the expression of other lineage genes, such as notch1 (T cells) or csfr1 (monocytes/macrophages), in common lymphoid progenitors, whereas it upregulates B cell-specific molecules, including CD19, Ig lambda-like polypeptide-5, and CD79A [20]. The expression of PAX5 is maintained until the transitional phase into PCs [21].
Pre-progenitor (pro)-B cells are the earliest stage of B-lineage cells that proceed to the stages of early pro-B cells, and later into precursor (pre)-B cells. Pre-pro-B cells express transcription factors associated with myeloid lineages, including Runt-related transcription factor 2, interferon regulatory factor (IRF)-8, bone marrow stromal antigen 2, and transcription factor 4. These molecules are silenced in the differentiation process into pro-B cells [22].
E2A (also known as transcription factor 3), SRY-related HMG-box 4, and early B-cell factor 1 expressed in pro-B cells drive the expression of recombination activating gene (RAG) 1 and RAG2 [23]. RAG1 and RAG2 form a complex and initiate the T-cell receptor variable–diversity–joining recombination (V(D)J rearrangement) for the production of Ig heavy chains (IgH) [24]. Terminal deoxynucleotidyl transferase plays a role in the diversity acquisition of Ig repertoire by random nucleotide addition to DNA single strands [25]. Another role of early B-cell factor 1 is VpreB1 (CD179A) induction and Ig lambda-like polypeptide-1 (CD179B) production in pro-B cells and early pre-B cells, which are indispensable for the biosynthesis of surrogate light chains [26]. In the maturation process from pro-B cells to pre-B cells, lymphoid enhancer–binding factor 1, a member of the T cell factor/lymphoid enhancer factor family, is involved in cell survival and proliferation via the Wnt signaling pathway. The expression of lymphoid enhancer-binding factor 1 is turned off in mature B cells [27].
Pre-BII large cells are characterized by cytoplasmic IgH, which is paired with surrogate light chains to form pre-B cell receptors (BCRs). In humans, signaling via pre-BCRs, rather than interleukin (IL)-7 receptors, induces a proliferative burst, leading to clonal expansion of pre-BII large cells [16]. Pre-BCR signaling is also a requisite for the differentiation process from pre-BII large cells to pre-BII small cells by regulating positive and negative selection, developmental progression, and allelic exclusion, which is a phenomenon that pre-BII cells use to ensure that one cell does not express two IgH with different specificities [28,29,30]. In the transition into pre-BII small cells, pre-BII large cells rearrange the Ig light chain (IgL) variable–joining genes. Following the successful rearrangement and expression of IgL, previously synthesized IgH and IgL are eventually assembled into BCRs (IgM) [31,32]. The BCR is transported to and anchored on the plasma membrane to form the complex with CD79a (Ig) and CD79b (Ig), which marks the transitional B cells to immature B cells [31,32].
At the development stage of immature B cells, the cell-surface BCR acquires the ability to bind antigens. In the microenvironment of the bone marrow where immature B cells appear, the antigens that engage BCRs are primarily self-antigens. Ligation of BCRs with self-antigens induces signaling responsible for molecular processes to reduce the self-reactivity of immature B cells (central tolerance) [33]. Central tolerance averts the escape of autoreactive immature B cells from the bone marrow. Under these circumstances, residual autoreactive immature B cells are destined for receptor editing (secondary IgL rearrangement), clonal deletion (apoptosis), or anergic state (low BCR expression and signaling) [34]. The regulatory processes of central tolerance, however, are often leaky. More than half of Ig in the immature compartment remain autoreactive or polyreactive [33,34]. Clinical studies estimated that human B lymphopoiesis requires approximately 6–12 months in patients with rheumatoid arthritis using targeted B cell therapies with anti-CD20 monoclonal antibodies (rituximab), B cell reconstitution after hematopoietic stem cell transplantation, or B cell depletion [35,36,37].
4. B Cell Maturation
Transitional B cells that migrate from the bone marrow enter into peripheral circulation and eventually differentiate into naïve B cells, FOB cells, or MZB cells. Upon encountering antigens, B cells become proliferative, migrate to the border of the T cell zone or interfollicular region of the secondary lymphoid organs (lymph nodes, tonsils, spleen, or mucosal-associated lymphoid tissues), and are activated [38]. A fraction of activated B cells migrate farther to the extrafollicular foci and differentiate into short-lived plasmablasts that secrete low-affinity antibodies [39]. Some activated B cells proliferate, home to the B-cell follicle, and form germinal centers (GCs). GCs are involved in adaptive immune responses against locally presented antigens. Follicular helper T (Th) cells play an important role in this process. T cell-independent activation promotes the differentiation of FOB and MZB cells to PCs, whereas T cell-dependent activation leads them to memory B cells [40]. In T cell-dependent antigen responses, long-lived CD40L/CD40R interactions between T and B cells differentiate into follicular Th cells and PCs, respectively. The GC reaction typically occurs in the secondary lymphoid organs, but similar structures have also been found in the ectopic or tertiary lymphoid structures [41]. In the GC, B cells undergo somatic hypermutation and subsequent clonal selection. While these B cells increase their Ig affinity against antigens, they differentiate into memory B cells or long-lived PCs (LL-PCs) that return to the bone marrow [1].
The maturation process from B cells into PCs is strictly regulated by a network of multiple transcription factors, including upregulation of PC-specific transcription factors [IRF-4, X-box binding protein-1, and B lymphocyte-induced maturation protein-1] and downregulation of B cell-specific ones [PAX5, IRF8, and BTB Domain and CNC Homolog 2 (BACH2)]. Ensuing events followed by these gene transcriptional alterations are increased cell-surface expression of CD27, CD38, CD138, and CD269, along with concomitant reduction in CD19 and CD20. While transcription factor-associated mechanisms of PC differentiation have been studied intensively, the trajectories that convert naïve B cells into PCs and the regulators that control the fate of PCs remain largely unknown [42].
5. B Cell Commitment
Prior to final differentiation into CD138positive nonproliferating PCs, B cells undergo a major change in morphological and epigenomic profiles and become immature and proliferative plasmablasts, the precursor cells of PCs [41]. In the process of the acquisition of Ig secretion potential, the organelles in plasmablasts drastically change. Human circulating plasmablasts are generally defined as CD19positive CD38bright CD27bright cells with high heterogeneity. In vitro study demonstrated that human naïve B cells cultured with IL-2, CD40L, CpG, and anti-IgM Fab’2 proceed to S-phase and display significant gene expression modifications by day 1. These highly proliferative, activated, committed B cells are characterized by the extinction of IL-4/signal transducers and activators of the transcription 6 pathway and Cbl proto-oncogene B ubiquitin ligase expression, downregulation of CD23 on the plasma membrane, along with upregulation of IRF-4. After day 4, under the additional couple of days of maintenance with IL-2, IL-4, and IL-10, a small subset of CD23negative B cells entered the cell cycle after promoting the G1/S transition and differentiated into the plasmablast phenotype [43]. In this process, a striking increase in the expression of the proviral integrations of Moloney virus 2 serine/threonine kinase (PIM2) was identified in these cells, suggesting the role of PIM2 in the B cell commitment [44]. BACH2 is an early regulatory factor that decides the fate and commitment of B cells. Reduction in BACH2 in IgG1 memory B cells accelerates their differentiation into PCs. Following the repression of BACH2, PIM2 expression is stimulated by IL-21, IL-10, IL-6, and signal transducer and activator of the transcription-3 pathway. The high expression of PIM2 at an early stage enables cell cycle entry and G1/S transition of B cells via degradation of cyclin-dependent kinase inhibitor 1B and activation of cell division cycle 25A, promoting the differentiation into plasmablasts. PIM2 also plays a role in protecting cells from apoptosis by suppressing the activation of caspase 3. Finally, an additional 3-day culture with IL-6 and interferon-α led these cells to CD138positive nonproliferating PCs [45].
In mature PCs, the endoplasmic reticulum alters its morphological appearance and peaks in protein production and secretion. These endoplasmic reticulum expansions are mainly led through the inositol-requiring enzyme type-1 pathway by promoting the synthesis of spliced X-box binding protein-1, which, in turn, further activates mitochondrial and endoplasmic reticulum expansions. These unfolded protein responses are also regulated by activating transcription factor 6, an endoplasmic reticulum stress-regulated transmembrane transcription factor, and protein kinase R (PKR)-like endoplasmic reticulum kinase, an endoplasmic reticulum-associated stress sensor protein [43,44,46]. In the course of the final steps of B cell differentiation, mTORC1 plays a pivotal role in the control of the secretory program by PCs, balancing their antibody production and cell survival with the regulation of autophagy to limit endoplasmic reticulum expansions, until amplified and sustained B lymphocyte-induced maturation protein inositol-requiring enzyme type-1/spliced X-box binding protein-1 program takes over. Transcriptomic analysis of B cell commitment to PCs demonstrated that the primary enriched genes in this transition are pro-apoptotic genes associated with cell death (such as BCL2L11 and CASP3) and pro-survival genes related to cell survival (such as BCL2L1, MCL1, and XIAP) [47].
Both memory B cells and LL-PCs are responsible for long-term host immunity against pathogens. While the former cells are recognized as the property that responds to the reinfection of pathogens and their variants, the latter cells are characterized by the protective antibody production [34]. The antigen-driven differentiation from naïve B cells into memory B cells or LL-PCs is induced primally in the B cell follicles and GCs in the secondary lymphoid organs, including lymph nodes, tonsils, spleen, and mucosal-associated lymphoid tissues. First, antigen stimulations via BCRs promote the transition of naïve B cells to GC B cells or short-lived PCs in the B cell follicles. Next, antigen stimulation further drives GC B cells into memory B cells or LL-PCs. Memory B cells are generated in an early immune response, primarily arising from low-affinity cells [48]. Recent studies suggest that the downregulation of Bcl6 and subsequent upregulation of Bcl2 in low-affinity cells may be a key event for their survival [49]. Unlike PC precursors, memory B cell precursors that reside at the periphery of the light zone in the GC are no longer cycling. The transition from GC B cells to memory B cells is thought to include at least three interconnected sequential processes. First, cessation of proliferation in the dark zone is characterized by the stepwise decline in c-Myc expression and active mTORC1. Second, trafficking back to the light zone is accompanied by decay of c-Myc expression and mTORC1 activity, resulting in downregulation of CXC chemokine receptor (CXCR)-4 and migration from the dark zone to the light zone. Third, entrance into the quiescent stage with the acquisition of survival signals is marked by high BACH2 expression required for dampening c-Myc expression and mTORC1 activity [50]. In the following recall responses to antigens, memory B cells differentiate into LL-PCs or re-enter the germinal cell reactions [51].
6. B Cell Survival
The number of naïve B cells is tightly controlled under the regulation of signaling via several receptors, including BCRs and B-cell activating factor (BAFF)/BAFF receptor [52]. Distinct from the activating signals induced by antigen binding, the survival signals via the BCR are ligand-independent ones that involve phosphoinositide 3-kinase (PI3K). The key molecule in B cell survival signals is the PI3Kδ isoform, which is expressed in B cells at a higher level than the other three isoforms [53]. Activating signals via BAFF receptor promote protein synthesis and upregulate the expression of some cell cycle-associated proteins, including cyclin D2, cyclin E, and cyclin-dependent kinase 4 in B cells, leading to set them up for potential proliferation in response to mitogenic stimulation [52]. The major mechanism by which BAFF/BAFF receptor manages B cells is mediated through the noncanonical nuclear factor (NF)-B pathway [54]. The BAFF/BAFF receptor combination induces recruitment of tumor necrosis factor receptor-associated factor (TRAF) 3 to the cytoplasmic domain of BAFF receptor, leading to ubiquitination and degradation of TRAF3 by cellular inhibitor of apoptosis protein-1. This results in NF-B inducing kinase-mediated accumulation and phosphorylation of IκB kinase 1, which in turn promotes the phosphorylation of NF-B2, subsequent procession into transcription factor p52, and entry of p52/transcription factor RelB complexes into the nucleus. BAFF receptor-induced ubiquitination and degradation of TRAF3 also stimulate glucose uptake, anaerobic glycolysis, and oxidative phosphorylation in B cells via upregulation of expression of glucose transporter 1 and hexokinase 2, leading to an increase in their cell size. Additionally, the noncanonical NF-B pathway induces the expression of ovarian tumor deubiquitinase 7B to stabilize TRAF3 for a negative feedback loop [54].
Spleen tyrosine kinase (SYK) also plays a critical role in the survival of B cells [55]. SYK is able to bind to phosphorylated immunoreceptor tyrosine-based activation motifs of CD79A and CD79B of BCRs and undergoes BAFF receptor-mediated activation, which induces signaling via extracellular signal-regulated kinase 5 and PI3K/protein kinase B pathways required for B cell survival. Studies using inducible deletion of SYK from mature B cells demonstrated a severe loss of FOB [56]. BCRs and BAFF receptors were suggested to cooperate with CD19 for B cell survival. CD19 is a transmembrane protein expressed in all human B-lineage cells and acts as a coreceptor/adaptor protein for BCRs. CD19 is essential for BAFF receptor-mediated activation of the protein kinase B pathway and following the suppression of proline-directed serine-threonine kinase glycogen synthase kinase (GSK) 3A/3B, and transcription factor forkhead box protein O1. Intriguingly, genetic loss of both GSK3A and GSK3B in mature B cells leads to the loss of both FOB and MZB. In CD19-mediated signaling pathways, mTORC2 also plays a crucial role in B cell maintenance, as its elimination causes a 50% loss of FOB [57].
Wiskott–Aldrich syndrome protein-interacting protein (WIP) is a key molecule in actin cytoskeleton remodeling. WIP not only binds Wiskott–Aldrich syndrome protein, an activator of actin-related protein 2/3 complex, to prevent it from degradation and support its intracellular distribution, but can act independently of Wiskott–Aldrich syndrome protein by promoting actin polymerization and stabilizing actin filaments linking with the adaptor molecules such as noncatalytic region of tyrosine kinase proteins and growth factor receptor-bound protein 2 [58]. WIP also contributes to the intracellular coupling of BAFFR, BCR, and CD19 and is involved in BAFF receptor-mediated phosphorylation of CD19 and activation of the protein kinase B pathway [59].
CD74 is a surface receptor for macrophage migration inhibitory factor. Signaling via CD74 leads to cleavage and release of the CD74 intracellular domain. The nuclear translocation of the CD74 intracellular domain induces gene expression required for B cell survival [60].
GTPase, IMAP family member 1 (GIMAP1 GTPase) is one of the proteins in the GTP-binding superfamily and immuno-associated nucleotide subfamily of nucleotide-binding proteins. GIMAP1 GTPase plays a critical role in the differentiation and development of mature B cells. Loss of GIMAP1 GTPase leads to the complete absence of FOB and MZB in humans. POU domain class 2-associating factor 1 (POU2AF1/BOB1) is a transcriptional coactivator expressed principally in B cells and regulates Ig production and gene expression for CD20. POU2AF1/BOB1 is upregulated in B cells by BAFF/BAFF receptor signaling via noncanonical and canonical NF-B pathways [61].
The attenuation of cellular stress responses associated with the unfolded protein response is essential for the longevity of PCs. Integrated stress response (ISR) is a cellular response equipped in eukaryotic cells to restore homeostasis against diverse internal or environmental stresses such as hypoxia, amino acid/glucose deprivation, and viral infection [62,63]. ISR is drawing attention due to its contribution to cell survival. In ISR, cells activate a common adaptive pathway. Cellular stress is sensed principally by specialized eukaryotic translation initiation factor 2 (EIF2) kinases, including heme-regulated inhibitor, PKR, PKR-like ER kinase (PERK), and general control nonderepressible 2 kinase [64]. Phosphorylation of a single serine by EIF2 kinases induces conversion of EIF2 to the competitive inhibitor of EIF2B complex, a guanine nucleotide exchange factor for EIF2. Inhibition of EIF2B activity by phosphorylated EIF2 lowers cellular EIF2-GTP·Met-tRNAi ternary complex level, leading to the trigger of ISR [65]. B cell differentiation occurs independently of PERK, whereas heme-regulated inhibitor and general control nonderepressible 2 kinase respond to nutrient deprivation and heme deficiency. Of the four EIF2 kinases, PKR is considered a central key regulator for ISR-mediated translation in PCs by utilizing its double-stranded RNA binding domain and PKR-associated activator recruitment [66].
LL-PCs are possibly capable of surviving until the day the host dies without repeated antigen stimulation. According to the results of the somatic hypermutation studies, LL-PCs have been considered to originate in the GCs. However, the identification and analysis of bone marrow LL-PCs suggest that high affinity is not a strict prerequisite for the longevity of PCs [67,68]. While most PCs are distributed in the bone marrow where they can receive survival signals from their surrounding microenvironment, only a few reach survival niches that support their longevity [69,70]. Interestingly, CXCR4 was found to stimulate the extrafollicular maturation of PCs and contribute to the long-term presence of bone marrow LL-PC [71], suggesting a possible role in the interaction between CXCR4 on LL-PCs and CXC ligand 12 (CXCL12) from bone marrow stromal cells in the determination of their fate [72]. PIM2 is essential for the surface expression of CXCR4 in PCs and their migration toward CXCL12 [44]. Human CD138positive PCs, particularly bone marrow PCs, express a high level of cytoplasmic PIM2, suggesting that bone marrow is a preferable microenvironment for PC survival. Thus, PIM2 may play a pivotal role in ISR regulation in LL-PCs. Interestingly, ISR is potentially suppressed by PIM2, inactivating some ISR-associated kinases, including PKR and PERK [44].
7. B Cell Lineage in the Human Endometrium
7.1. B Cells, Plasmacytes, and Immunoglobulins in Human Nonpathological Endometrium
Human endometrium harbors diverse leucocyte subpopulations including T cells, natural killer cells, macrophages, dendritic cells, and neutrophils. The density and proportion of the endometrial leukocytes fluctuate across the menstrual cycle [2]. Additionally, B cell lineage is a minority in the cycling endometrium in women of reproductive age. B cells account for 2% of whole endometrial cells and only 3% of endometrial lymphocytes [7]. According to their locations, endometrial B cells are classified into two subtypes. First, endometrial single B cells are found scattered throughout the stromal compartment. These endometrial stromal single B cells are resistant to the fluctuations in ovarian steroid levels, as their density is constant throughout the menstrual cycle [73]. Second, some B cells reside in the core of the endometrial lymphoid aggregates, a unique structure in the stratum basalis and deeper part in the stratum functionalis that consists of hundreds of leukocytes, such as inner layer CD4negative CD8positive T cells and outer layer macrophages [74,75]. Contrary to stromal single B cells, lymphoid aggregate–core B cells may be influenced by ovarian steroids, as lymphoid aggregates are more likely to appear in the proliferative phase (before ovulation) than in the secretory phase (after ovulation). Although the significance and function of endometrial lymphoid aggregates remain largely unknown, some studies suggest a role in antigen scavenging and antibody production, similar to GCs in the secondary lymphoid organs. In contrast to other endometrial mononuclear cell subpopulations such as T cells, natural killer cells, and macrophages expressing activated cell markers [high level of CD69 and human leukocyte antigen (HLA)-DR], the majority of endometrial B cells primarily show a rather naïve lymphocyte phenotype with surface expression of IgM and CD22, an inhibitory receptor against BCR signaling, along with a high level of CD19, CD20, CD79A, CD83, CD229, BTG1, and CXCR4 and a moderate level of CD74 and HLA-DR [76]. Flow cytometric analysis discovered CD27positive memory-type B cells in the human endometrium, along with a small number of CD24negative CD38high plasmablast cell types [77].
CD83 is an Ig superfamily glycoprotein expressed in various immunocompetent cells, including B cells. CD83 has one extracellular Ig V-like structure and is anchored on the plasma membrane, and some of them are released as soluble forms. CD83 stabilizes the major histocompatibility complex-class II antigens by negative regulation of the E3 ubiquitin–protein ligases membrane-associated ring-CH-type Finger 1 and 8 [78]. In addition, CD83 was demonstrated to be involved in the longevity of B cells in mice [79]. The expression of CD83 and CD74 may cooperate for the survival of endometrial B cells in the dynamic local microenvironment. Recent studies showed that surface and intracellular expression of CD83 is detectable in endometrial CD19positive B cells, CD11positive dendritic cells, and T cell subpopulations [76]. In vitro stimulation with lipopolysaccharides in cooperation with pregnancy-associated molecules, including estradiol, progesterone, human chorionic gonadotropin, and transforming growth factor-β1 upregulates CD83 expression selectively in endometrial B cells, but not in other endometrial leukocyte subsets and circulating B cells, while dexamethasone reverses it. The hormonal regulation of CD83 expression in endometrial B cells is likely to come from gene expression balancing between this molecule and matrix metalloproteinase 7, supporting the idea that the physiological immunomodulatory role of endometrial B cells in anti-bacterial responses at the fetal–maternal interface during pregnancy [80].
Moreover, endometrial B cells potentially inhibit intrauterine inflammation during gestation and contribute to the prevention of preterm labor. B cells are a minor subpopulation in the decidualized endometrium in early pregnancy, but cells with varying maturation stages are observed in the stromal compartment of the decidua. While all decidualized endometrial stromal B cells express BAFF receptors, decidualized endometrial stromal fibroblasts are capable of producing and secreting BAFF in response to the stimulation by IFN-γ and IFN-α, supporting the idea that the human decidualized endometrial stromal compartment can provide the niche for the survival of local B cells [81]. Indeed, the density of decidualized endometrial stromal B cells slightly increases toward late gestation with some phenotypic and functional changes probably driven by placental hormones [81]. It is noteworthy that the expression level of BAFF in decidualized endometrial stromal fibroblasts is decreased in women with a history of recurrent pregnancy loss compared with those without miscarriage [82]. Interestingly, soluble BAFF receptors are found to be released by decidualized endometrial stromal fibroblasts. The results of in vitro studies suggest that these decidualized endometrial soluble BAFF receptors suppress the proliferation of activated monocytes/macrophages and the secretion of tumor necrosis factor (TNF)-α and IL-6, suggesting their inhibitory role in intrauterine inflammation during gestation [83].
Decidualized endometrial B cells in late pregnancy, particularly at term pregnancy, display higher levels of activated markers of memory B cells than circulating B cells [84]. Recently, mice deficient in B cells were demonstrated to exhibit a lower level of active progesterone-induced blocking factor 1, an anti-inflammatory immunomodulator produced by B cells in the presence of progesterone, in their uteri, and its administration mitigated intrauterine inflammation and reduced preterm labor in these mice. Furthermore, the production of progesterone-induced blocking factor 1 by decidualized endometrial B cells is mediated by IL-33, which in turn suppresses the activation and influx of natural killer cells and neutrophils and the production of proinflammatory cytokines in the decidua [85]. In humans, a decrease in the expression of the IL-33 receptor α chain on decidualized endometrial B cells and a reduction in the production of active progesterone-induced blocking factor 1 in late pregnancy were also associated with preterm labor [85].
Under physiological local conditions, PCs are also minor immunocompetent cells in the stromal compartment in the human cycling endometrium. Approximately 31% of fertile women harbor one or more endometrial stromal plasmacytes (ESPCs) per 10 high power fields under light microscopy [86]. ESPCs are thought to produce Ig subclasses locally. These include heavy chains of IgA1, IgA2, IgM, IgG1, and IgG2, along with light chains of Ig, and J chain, which are necessary for the generation of polymeric Igs, whereas IgG3, IgG4, IgE, and IgD subclasses are not detectable in the nonpathological endometrium [87,88,89]. Two IgA subclasses are expressed constitutively mainly on the apical side of the endometrial surface epithelium, glandular epithelium, and glandular secretion. Similarly, IgM is found on the apical side of the endometrial surface and glandular epithelial areas, but some cells lack its expression, with the variances among individuals. Most IgA- and IgM-bearing endometrial epithelial cells coexpress the J chain and secretory component. These epithelial expression levels of endometrial IgA, IgM, J chain, and secretory components are higher in the proliferative phase compared with the secretory phase. IgG1 and IgG2 are also localized on the apical side of endometrial epithelial cells, with marked variances within and between individuals. Immunoreactivity to IgM, IgA, IgG1, and IgG2 in the endometrial stromal compartment with weaker and sparser immunostaining intensity compared with the endometrial epithelial areas [87].
In addition to the local production by ESPC, monomeric IgA (that lacks J chain), IgG1, and IgG2 are thought to be brought into the endometrial glandular epithelium from the stromal compartment by passive diffusion [89]. Endometrial glandular epithelial cells express HLA-DR across the menstrual cycle regardless of the expression level of the secretory component, implicating the active secretory component-mediated incorporation of polymeric Ig by the endometrial epithelial cells. As shown in other mucosal tissues, these endometrial Ig subclasses display a defensive property against foreign body invasion into the endometrium. Moreover, the menstrual cycle-dependent fluctuation in the expression level of some endometrial Ig subclasses implicates their possible contribution to blastocyst implantation and menstrual mucosal shedding [89].
7.2. B Cells, Plasmacytes, and Immunoglobulins in Human Pathological Endometrium
7.2.1. Chronic Endometritis
CE is a localized inflammatory endometrial disorder that is recognized by an unusual infiltration of ESPCs [90]. CE is mostly asymptomatic or oligosymptomatic with some subtle gynecologic symptoms. According to its silent and nondescriptive symptomatologic nature, CE is often missed both by affected women and experienced gynecologists [91]. The principal pathogens of CE include common bacteria in the female urogenital organs: Mycoplasma (M. genitalium and M. hominis), Ureaplasma (U. urealyticum), Proteus species, Corynebacterium, Gardnerella vaginalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Mycobacterium tuberculosis, and yeasts (Saccharomyces cerevisiae and Candida) [92]. Antibiotic treatment against these microorganisms eradicates ESPCs in CE, although multidrug-resistant CE has recently emerged as a clinical problem, like in other medical fields [93,94,95,96,97,98,99] (Table 1).

Table 1.
Inflammatory and immunologic signature in CE.
The increase in densities of ESPCs in the endometrium with CE seems attributable to the selective recruitment of circulating B cells into the endometrium. We investigated the endometrial expression profiles of the chemokines that can induce the selective migratory responses of peripheral blood B cells and PCs (CCR2, CCR7, CXCR1, CXCR2, CXCR4, and CXCR5 ligands) in CE. Of these seven potential chemokines, CXCL13, a CXCR5 ligand that displays a selective chemotactic activity for B cells, is abnormally expressed in endometrial endothelial cells in women with CE, along with CXCL1 in endometrial epithelial cells. In addition, selectin E, a ligand to endoglycan, a sialomucin presented on peripheral blood B cells, is aberrantly expressed in human endometrial endothelial cells in CE [100] (Figure 2). These findings suggest that these local inflammatory microenvironments in CE induce selective extravasation of B cells, rather than PCs, from circulation into the endometrial stromal compartment via endometrial microvessels and attract them further into the epithelial area. These pro-inflammatory molecules are found to be locally induced in isolated endometrial microvascular endothelial cells (CXCL13 and selectin E) and endometrial epithelial cells (CXCL1) in vitro by microbial antigens such as lipopolysaccharide, a Toll-like receptor 2/4 ligand [100]. Moreover, the concentration of IL-6, a differentiation factor of mature B cells, is significantly higher in the menstrual blood of women with CE than in those without CE, indicating that the inflamed endometrium with CE provides extravasated B cells with the niches for in situ differentiation into PCs [101]. Regarding the local Ig profiles, the densities of IgM, IgA1, IgA2, IgG1, and IgG are higher in the stromal compartment during the proliferative phase in the endometrium with CE than those without CE. The endometrial Ig subclasses in CE are characterized by the highest density of IgMpositive stromal cells and the predominance of IgG2positive stromal cells over IgG1positive counterparts [88].
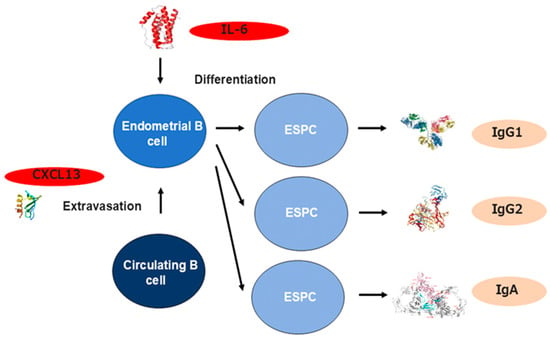
Figure 2.
The mechanism underlying B cell recruitment and differentiation in the endometrium with CE. CXCL13 (a CXCR5 antagonist) and CD62E (a ligand for endoglycan) are abnormally expressed in endometrial endothelial cells with CE and display a selective chemotactic activity for B cells. IL-6 (a B cell differentiation factor) concentration is also elevated in the endometrium and induces the differentiation from extravasated B cells to ESPC to produce Ig.
The concentration of TNF-α is also higher in the menstrual effluents of women with CE compared with those without CE [101]. TNF-α is capable of promoting local estrogen biosynthesis in endometrial glandular epithelial cells via estrogen receptor-α signaling, which potentially alters endometrial epithelium to the proliferative phenotype [102] (Figure 3). Studies support the idea that such local microenvironments in CE play a role in the proliferation and survival of the endometrial cell components. Endometrial micropolyposis is a mucosal finding in CE, which is detectable with hysteroscopy [103,104]. The endometrium with micropolyposis has proliferative characteristics and contains a higher concentration of ESPCs than the nonpathologic endometrium. Additionally, one of the histopathological features of CE is the delayed endometrial differentiation in the secretory phase (after ovulation) when the endometrium has to prepare for the implantation of blastocysts. Approximately one-third of the endometrium with CE displays an “out-of-phase” morphological appearance in glandular and surface epithelial cells, i.e., pseudostratification and/or mitotic nuclei, the findings seen in the proliferative phase (before ovulation) [104]. Indeed, the expression levels of messenger RNAs involved in cellular proliferation (ki-67), anti-apoptosis (bcl2 and bax), and ovarian steroid receptors (esr1, esr2, and pgr) are abnormally raised in the secretory phase endometrium with CE. By contrast, the expression of messenger RNAs potentially associated with embryo receptivity (il11, ccl4, igf1, and casp8) and decidualization (prl and igfbp1) are reduced, implicating that, in the presence of CE, the endometrium is unable to respond enough to ovarian steroid stimulation and to transform its component cells to acquire the receptivity for implanting blastocysts [105,106,107]. These findings support the idea that the endometrium with CE exhibits progesterone resistance, which is often observed in the endometrium with endometriosis [108].
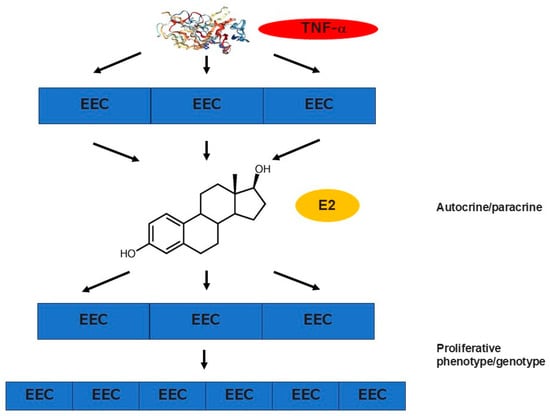
Figure 3.
Proliferative phenotype and progesterone resistance in the endometrium with CE and endometriosis. TNF-α level is elevated in the uterine cavity in both pathological conditions. High concentration of TNF-α promotes local estrogen biosynthesis in endometrial glandular epithelial cells via estrogen receptor-α signaling, which in turn potentially alters the epithelial cells to the proliferative phenotype and provokes their survival. For example, the endometrium with CE is characterized by the delayed endometrial differentiation in the secretory phase (after ovulation) when this mucosal tissue has to prepare for the implantation of blastocysts. In addition, approximately one-third of the endometrium with CE displays an “out-of-phase” morphological appearance in glandular and surface epithelial cells, i.e., pseudostratification and/or mitotic nuclei, the findings seen in the proliferative phase (before ovulation). While the endometrial expression levels of messenger RNAs involved in cellular proliferation (ki-67), anti-apoptosis (bcl2 and bax), and ovarian steroid receptors (esr1, esr2, and pgr) are abnormally raised in the secretory phase, the expression of messenger RNAs potentially associated with embryo receptivity (il11, ccl4, igf1, and casp8) and decidualization (prl and igfbp1) are reduced, supporting the idea that the endometrium is unable to respond enough to ovarian steroid stimulation and to transform its component cells to acquire the receptivity for implanting blastocysts.
7.2.2. Endometriosis
One of the unique cellular immunological characteristics seen in the endometrium in endometriosis is the menstrual cycle-independent behavior of endometrial macrophages. Macrophages are subclassified into M1 types (classically activated macrophages) and M2 counterparts (alternatively activated macrophages). M1 macrophages differentiate by the stimulation of interferon-γ and promote inflammatory responses via antigen presentation and production of interleukin IL-6, IL-12, and TNF-α (Figure 3), whereas M2 macrophages differentiate by the stimulation of IL-4 and inhibit inflammatory responses via the production of IL-10, TNF-α, and arginase-I [109]. While whole macrophages increase in density after ovulation in the nonpathological endometrium, such a postovulatory rise of macrophage density is not observed in the eutopic endometrium with endometriosis. However, the density of CD197positive CD80positive M1 macrophages in the eutopic endometrium decreases from stage I to IV, and that of eutopic endometrial CD163positive CD206positive M2 macrophages increases with the progress of the disease [110]. These locally increased eutopic endometrial M2 macrophages are likely to play a role in the proliferation of other endometrial cells and the induction of mucosal angiogenesis. Such biases toward M2 over M1 macrophages are also seen in the ectopic endometrioid lesions of endometriosis [111,112]. The blocking of the actions of IL-6 with antagonistic antibodies can reduce the shift from M1 to M2 counterparts, indicating the putative role of IL-6 in this alteration of local immune responses [111]. In addition, CD16negative CD56bright natural killer cells, which are unusual lymphocyte subpopulations in circulating blood and other organs, increase in number in the endometrium following pituitary luteinizing hormone surge toward the mid/late-secretory-phase endometrium. In the eutopic endometrium with endometriosis, the postovulatory rise of these unique natural killer cells is also seen, but their cytolytic activity is impaired compared with those without endometriosis [113].
Autoimmunity has long been believed to be associated with the onset and progression of endometriosis, but its entity is yet elusive. While multiple studies point out an appearance of the autoantibodies of IgG1 and IgG2 subclasses against endometrial antigens are detectable in the serum of women with endometriosis, the molecules involved in the autoimmunity in endometriosis remain to be determined [114]. The immunological feature of B cell lineage in the eutopic endometrium of women with endometriosis is the appearance of ESPC and CD5positive CD20positive HLA-DRpositive B cells [115], which are commonly found in the endometrium with CE. Although early reports demonstrate that the expression level of IgG in the eutopic endometrium with endometriosis is higher than in those without endometriosis, their subclasses were not detailed. IgG deposits and C3 component of the complement cascade are detectable in the ectopic endometrioid lesions in endometriosis, but their IgG subclasses are not described [116].
BCL6 is a transcriptional repressor that regulates follicular Th cell proliferation and contributes to the development of GC. BCL6 is expressed in the human nonpathological endometrium throughout the menstrual cycle, with a slight increase in the mid-secretory phase. The expression level of BCL6 in the secretory phase was found to be higher in the eutopic endometrium in women with endometriosis and unexplained infertility than in that of fertile women [117]. High BCL6 expression is also reported to be associated with poor reproductive outcomes in in vitro fertilization–embryo transfer cycles in women with unexplained infertility [118]. Additionally, the endometrium in infertile women exposed to more exogenous progesterone had a significantly lower likelihood of high BCL6 expression [119]. BCL6 may therefore be a candidate biomarker for endometriosis and endometrial dysfunction, including progesterone resistance. However, the clinical significance of the medical intervention for infertile women with high BCL6 expression remains controversial. While some studies demonstrated that treatment with leuprorelin, a gonadotropin-releasing hormone agonist, and laparoscopy improved the reproductive outcomes in these women, others found no correlation between BCL6 expression level and live birth rate among normal ovarian responders [120].
Endometriosis lesions are characterized by the infiltration of PC, many of which produce IgM, and macrophages that produce BAFF. Additionally, BAFF concentration was found to be elevated in the serum of endometriosis patients. These findings suggest BAFF-responsive PCs interact with retrograde menstrual tissues to give rise to endometriosis lesions [121]. Intriguingly, a recent report identified mature tertiary lymphoid structures in the ectopic endometrioid lesions on the ovary and fallopian tube in women with endometriosis. These mature tertiary lymphoid structures contained CD3positive CD8positive T cells, CD79apositive B cells, CD208positive dendritic cells, CD21positive follicular dendritic cells, and PNAdpositive high endothelial venules, along with immature tertiary lymphoid structures (lacking follicular dendritic cell networks and high endothelial venules) in the eutopic endometrium with or without endometriosis [122].
Furthermore, recent seminal research demonstrated a pathogenic role of Fusobacterium, strictly anaerobic Gram-negative rod bacteria, in the development of endometriosis in humans. Fusobacterium infiltrate into the human endometrial stroma and activate transforming growth factor-βsignaling, provoking the transition from quiescent stromal fibroblasts to transgelin-expressing myofibroblasts. These myoblasts acquire the ability to proliferate, adhere, and migrate, leading to the formation of the ectopic endometriotic lesions [123]. Antibiotic treatment with metronidazole and/or chloramphenicol prevented the establishment of endometriosis in the Fusobacterium-inoculated model mice. Further studies are warranted to elucidate the relationship between B cells and endometriosis [123,124].
7.2.3. Repeated Implantation Failure/Recurrent Pregnancy Loss/Impaired Endometrial Receptivity
Repeated implantation failure (RIF) is an infertile condition where women undergoing IVF-ET treatment fail to have any positive pregnancy tests despite the transfer of at least three good-quality embryos. The major cause of RIF is thought to be chromosomal abnormalities of transferred embryos, but many studies suggest defects in endometrial receptivity, including immunological factors, in this pathology [125]. CE is detectable in 7–34% of infertile women with a history of RIF [126]. A study using single-cell transcriptome profiling demonstrated the selective recruitment of XCR1+ B cells into the endometrium by the action of high levels of various chemokines and endometrial CD49apositive CXCR4positive CCR9positive type 2 natural killer cells in infertile women with a history of RIF [127]. In 2023, the European Society of Human Reproduction and Embryology Working Group issued Good Practice Recommendations on RIF, in which assessment for CE can be considered for infertile women with a history of RIF following IVF-ET, and antibiotic treatment can also be considered if CE is found in these women [128]. Moreover, CE is identifiable in 9–13% of women who experienced unexplained early recurrent (two or more) pregnancy losses. A multicenter, double-blind, randomized clinical trial, termed the CERM (CE and Recurrent Miscarriage) Study, was initiated in the United Kingdom to determine if preconceptual treatment with oral doxycycline administration is effective for increasing live births in women with a history of two or more consecutive first-trimester pregnancy losses [129].
Controlled ovarian stimulation is an indispensable tool in assisted reproductive technology to obtain more mature oocytes available for in vitro fertilization and intracytoplasmic sperm injection. Controlled ovarian stimulation is thought to pose potential detrimental harms on endometrial receptivity, at least in part, due to excessive estradiol exposure and premature progesterone rise [130,131]. Moreover, a prospective cohort study demonstrated that controlled ovarian stimulation brings about not only morphological dyssynchrony and aberrant gene expression profiles, but also unusual endometrial immunocompetent cells. For example, in the periovulatory period, controlled ovarian stimulation induces the infiltration of endometrial monocytes/macrophages, which increase in number during the mid-to-late secretory phase in the natural ovulatory cycle, indicating the “over-advancement” in the local immunologic microenvironment. Additionally, in the window of implantation during the mid-secretory phase, controlled ovarian stimulation provokes uncommon invasion of endometrial B cells and reduction in CD4positive T cells, which is a mucosal cellular immunologic condition similar to CE [132]. Additionally, the proportion of endometrial CD79positive B cells was found to be higher in infertile women with a history of RIF undergoing a failed conception in the subsequent embryo transfer cycles than in those who achieved a successful pregnancy [133].
8. Conclusions
A growing body of evidence is accumulating to demonstrate that immunocompetent cells of the B cell lineage are present in the human endometrium under physiological conditions. In several gynecologic diseases that are associated with infertility, the number or density of endometrial B cells/ESPCs is increasing. One of the current questions on endometrial B cell lineage is that we do not have effective ways to distinguish pathologic B cells from physiologic counterparts. Single-cell sequencing analysis prevails in various medical fields. The introduction of these new technologies, in combination with conventional flow cytometry and immunohistochemistry, is awaited to gain our understanding of endometrial B cells in mucosal integrity and pathology. Moreover, little is clear about the functions and roles of endometrial leukocyte aggregates and central B cells. The elucidation of these unique immunologic structures, mainly located in the endometrial basal layer, has the potential to break through the understanding of the mechanisms underlying embryo implantation and fetal–maternal interface formation. Finally, the threshold for ESPC density remains undetermined to define the histopathologic diagnosis of CE.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
I thank Naoko Kobayashi, Yuko Morita, Atsumi Hamazaki, Shinji Murayama, Takako Mihara, and Masaya Mihara (Infertility Center, Iryouhoujin Kouseikai Mihara Hospital), Tadahiro Yasuo (Department of Obstetrics and Gynecology, Otsu City Hospital), and Takeshi Yamaguchi (Infertility Center, Daigo Watanabe Clinic) for their contributions to the studies.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Morgan, D.; Tergaonkar, V. Unraveling B cell trajectories at single cell resolution. Trends Immunol. 2022, 43, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.D.; Wuidar, V.; Zielonka, M.; Pequeux, C. Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview. Cells 2024, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Yasuo, T.; Tada, Y.; Hayashi, T.; Iwaki, Y.; Karita, M.; Funabiki, M.; Taguchi, S.; Spillers, D.; Nakamura, Y.; et al. Unusual Inflammation in Gynecologic Pathology Associated with Defective Endometrial Receptivity. Histol. Histopathol. 2014, 29, 1113–1127. [Google Scholar]
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Bulmer, J.N. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J. Reprod. Immunol. 2011, 88, 156–164. [Google Scholar] [CrossRef]
- Moffett, A.; Shreeve, N. Local immune recognition of trophoblast in early human pregnancy: Controversies and questions. Nat. Rev. Immunol. 2023, 23, 222–235. [Google Scholar] [CrossRef]
- Shen, M.; O’Donnell, E.; Leon, G.; Kisovar, A.; Melo, P.; Zondervan, K.; Granne, I.; Southcombe, J. The Role of Endometrial B Cells in Normal Endometrium and Benign Female Reproductive Pathologies: A Systematic Review. Hum. Reprod. Open 2022, 2022, hoab043. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of Chronic Endometritis in Repeated Unexplained Implantation Failure and the IVF Success Rate after Antibiotic Therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Commonalities and Disparities between Endometriosis and Chronic Endometritis: Therapeutic Potential of Novel Antibiotic Treatment Strategy against Ectopic Endometrium. Int. J. Mol. Sci. 2023, 24, 2059. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Dorshkind, K. B-1 B cell development in the fetus and adult. Immunity. 2012, 36, 13–21. [Google Scholar] [CrossRef]
- Savage, H.P.; Kläsener, K.; Smith, F.L.; Luo, Z.; Reth, M.; Baumgarth, N. TLR induces reorganization of the IgM-BCR complex regulating murine B-1 cell responses to infections. eLife 2019, 8, e46997. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Kaer, L.V. Role of canonical and noncanonical autophagy pathways in shaping the life journey of B cells. Front. Immunol. 2024, 15, 1426204. [Google Scholar] [CrossRef]
- Palm, A.E.; Kleinau, S. Marginal zone B cells: From housekeeping function to autoimmunity? J. Autoimmun. 2021, 119, 102627. [Google Scholar] [CrossRef]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B cells, antibodies, and more. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Boller, S.; Grosschedl, R. The Regulatory Network of B-Cell Differentiation: A Focused View of Early B-Cell Factor 1 Function. Immunol. Rev. 2014, 261, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, F.M.P.; Janowska, I.; Menafra, R.; de Gier, M.; Korzhenevich, J.; Pico-Knijnenburg, I.; Khatri, I.; Schulz, A.S.; Kuijpers, T.W.; Lankester, A.C.; et al. IL-7 receptor signaling drives human B-cell progenitor differentiation and expansion. Blood 2023, 142, 113. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, A.; Georgopoulos, K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 1996, 15, 5358–5369. [Google Scholar] [CrossRef]
- Schwickert, T.A.; Tagoh, H.; Gültekin, S.; Dakic, A.; Axelsson, E.; Minnich, M.; Ebert, A.; Werner, B.; Roth, M.; Cimmino, L.; et al. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat. Immunol. 2014, 15, 283–293. [Google Scholar] [CrossRef]
- Urbánek, P.; Wang, Z.Q.; Fetka, I.; Wagner, E.F.; Busslinger, M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 1994, 79, 901–912. [Google Scholar] [CrossRef]
- Souabni, A.; Cobaleda, C.; Schebesta, M.; Busslinger, M. Pax5 promotes b lymphopoiesis and blocks t cell development by repressing notch1. Immunity 2002, 17, 781–793. [Google Scholar] [CrossRef]
- Tagoh, H.; Ingram, R.; Wilson, N.; Salvagiotto, G.; Warren, A.J.; Clarke, D.; Busslinger, M.; Bonifer, C. The mechanism of repression of the myeloid-specific c-fms gene by Pax5 during B lineage restriction. EMBO J. 2006, 25, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.; Bashford-Rogers, R.J.M.; Bhute, V.J.; Penalver, A.; Ferdinand, J.R.; Stewart, B.J.; Smith, J.E.G.; Deobagkar-Lele, M.; Giudice, G.; Connor, T.M.; et al. Dynamic regulation of hypoxia-inducible factor-1alpha activity is essential for normal B cell development. Nat. Immunol. 2020, 21, 1408–1420. [Google Scholar] [CrossRef]
- Romanow, W.J.; Langerak, A.W.; Goebel, P.; Wolvers-Tettero, I.L.; van Dongen, J.J.; Feeney, A.J.; Murre, C. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol. Cell 2000, 5, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Mandel, E.M.; Pott, S.; Györy, I.; Firner, S.; Liu, E.T.; Grosschedl, R. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription-independent poising of chromatin. Immunity 2010, 32, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.L.; Gilfillan, S.; Thai, T.H.; Kearney, J.F. Terminal deoxynucleotidyl transferase and repertoire development. Immunol. Rev. 2000, 175, 150–157. [Google Scholar] [CrossRef]
- Reya, T.; O’Riordan, M.; Okamura, R.; Devaney, E.; Willert, K.; Nusse, R.; Grosschedl, R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000, 13, 15–24. [Google Scholar] [CrossRef]
- Schilham, M.W.; Oosterwegel, M.A.; Moerer, P.; Ya, J.; de Boer, P.A.; van de Wetering, M.; Verbeek, S.; Lamers, W.H.; Kruisbeek, A.M.; Cumano, A. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature 1996, 380, 711–714. [Google Scholar] [CrossRef]
- Puel, A.; Ziegler, S.F.; Buckley, R.H.; Leonard, W.J. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 1998, 20, 394–397. [Google Scholar] [CrossRef]
- Mårtensson, I.-L.; Almqvist, N.; Grimsholm, O.; Bernardi, A.I. The pre-B cell receptor checkpoint. FEBS Lett. 2010, 584, 2572–2579. [Google Scholar] [CrossRef]
- Ye, J.; McCray, S.K.; Clarke, S.H. The transition of pre-BI to pre-BII cells is dependent on the VH structure of the mu/surrogate L chain receptor. EMBO J. 1996, 15, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Korzhenevich, J.; Janowska, I.; van der Burg, M.; Rizzi, M. Human and mouse early B cell development: So similar but so different. Immunol. Lett. 2023, 261, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santana-Sánchez, P.; Vaquero-García, R.; Legorreta-Haquet, M.V.; Chávez-Sánchez, L.; Chávez-Rueda, A.K. Hormones and B-Cell Development in Health and Autoimmunity. Front. Immunol. 2024, 15, 1385501. [Google Scholar] [CrossRef]
- Kyewski, B.; Klein, L. A central role for central tolerance. Annu. Rev. Immunol. 2006, 24, 571–606. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef]
- Leandro, M.J.; Cambridge, G.; Ehrenstein, M.R.; Edwards, J.C.W. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 613–620. [Google Scholar] [CrossRef]
- Roll, P.; Palanichamy, A.; Kneitz, C.; Dorner, T.; Tony, H.P. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, G.; Perry, H.C.; Nogueira, L.; Serre, G.; Parsons, H.M.; De La Torre, I.; Dickson, M.C.; Leandro, M.J.; Edwards, J.C. The effect of B-cell depletion therapy on serological evidence of B-cell and plasmablast activation in patients with rheumatoid arthritis over multiple cycles of rituximab treatment. J. Autoimmun. 2014, 50, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Song, W.; Craft, J. T Follicular Helper Cell Heterogeneity. Annu. Rev. Immunol. 2023, 42, 127–152. [Google Scholar] [CrossRef]
- Inoue, T.; Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 2024, 24, 5–17. [Google Scholar] [CrossRef]
- Neri, P. Plasma cells’ fate: It is a complex “orchestra”. Blood. 2024, 144, 466–467. [Google Scholar] [CrossRef]
- Caron, G.; Hussein, M.; Kulis, M.; Delaloy, C.; Chatonnet, F.; Pignarre, A.; Avner, S.; Lemarié, M.; Mahé, E.A.; Verdaguer-Dot, N.; et al. Cell-Cycle-Dependent Reconfiguration of the DNA Methylome during Terminal Differentiation of Human B Cells into Plasma Cells. Cell Rep. 2015, 13, 1059–1071. [Google Scholar] [CrossRef]
- Haas, M.; Fest, T. Final step of B-cell differentiation into plasmablasts; the right time to activate plasma cell PIM2 kinase. Immunol. Lett. 2023, 258, 45–50. [Google Scholar] [CrossRef]
- Kulis, M.; Merkel, A.; Heath, S.; Queirós, A.C.; Schuyler, R.P.; Castellano, G.; Beekman, R.; Raineri, E.; Esteve, A.; Clot, G.; et al. Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat. Genet. 2015, 47, 746. [Google Scholar] [CrossRef]
- Ricci, D.; Gidalevitz, T.; Argon, Y. The special unfolded protein response in plasma cells. Immunol. Rev. 2021, 303, 35–51. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Mechanisms That Determine Plasma Cell Lifespan and the Duration of Humoral Immunity. Immunol. Rev. 2010, 236, 125–138. [Google Scholar] [CrossRef]
- Alaterre, E.; Ovejero, S.; Bret, C.; Dutrieux, L.; Sika, D.; Perez, R.F.; Espéli, M.; Fest, T.; Cogné, M.; Martin-Subero, J.I.; et al. Integrative Single-Cell Chromatin and Transcriptome Analysis of Human Plasma Cell Differentiation. Blood 2024, 144, 496–509. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid Nanoparticles Enhance the Efficacy of MRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Toboso-Navasa, A.; Gunawan, A.; Morlino, G.; Nakagawa, R.; Taddei, A.; Damry, D.; Patel, Y.; Chakravarty, P.; Janz, M.; Kassiotis, G.; et al. Restriction of Memory B Cell Differentiation at the Germinal Center B Cell Positive Selection Stage. J. Exp. Med. 2020, 217, e20191933. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Cyster, J.G. Transcriptional regulation of memory B cell differentiation. Nat. Rev. Immunol. 2021, 21, 209–220. [Google Scholar] [CrossRef]
- Schweighoffer, E.; Tybulewicz, V.L. Signalling for B cell survival. Curr. Opin. Cell Biol. 2018, 51, 8–14. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-receptor in B cell selection and survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Hu, H.; Brittain, G.C.; Chang, J.H.; Puebla-Osorio, N.; Jin, J.; Zal, A.; Xiao, Y.; Cheng, X.; Chang, M.; Fu, Y.X.; et al. OTUD7B controls non-canonical NF-κB activation through deubiquitination of TRAF3. Nature 2013, 494, 371–374. [Google Scholar] [CrossRef]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Schweighoffer, E.; Tybulewicz, V.L. BAFF signaling in health and disease. Curr. Opin. Immunol. 2021, 71, 124–131. [Google Scholar] [CrossRef]
- Jellusova, J.; Cato, M.H.; Apgar, J.R.; Ramezani-Rad, P.; Leung, C.R.; Chen, C.; Richardson, A.D.; Conner, E.M.; Benschop, R.J.; Woodgett, J.R.; et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat. Immunol. 2017, 18, 303–312. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ramirez-Komo, J.A.; Park, H.; Iritani, B.M. Control of B lymphocyte development and functions by the mTOR signaling pathways. Cytokine Growth Factor Rev. 2017, 35, 47–62. [Google Scholar] [CrossRef]
- Keppler, S.J.; Gasparrini, F.; Burbage, M.; Aggarwal, S.; Frederico, B.; Geha, R.S.; Way, M.; Bruckbauer, A.; Batista, F.D. Wiskott-Aldrich syndrome interacting protein deficiency uncovers the role of the co-receptor CD19 as a generic hub for PI3 kinase signaling in B cells. Immunity 2015, 43, 660–673. [Google Scholar] [CrossRef]
- Starlets, D.; Gore, Y.; Binsky, I.; Haran, M.; Harpaz, N.; Shvidel, L.; Becker-Herman, S.; Berrebi, A.; Shachar, I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 2006, 107, 4807–4816. [Google Scholar] [CrossRef]
- Webb, L.M.; Datta, P.; Bell, S.E.; Kitamura, D.; Turner, M.; Butcher, G.W. GIMAP1 is essential for the survival of naive and activated B cells in vivo. J. Immunol. 2016, 196, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Marintchev, A.; Ito, T. eIF2B and the integrated stress response: A structural and mechanistic view. Biochemistry 2020, 59, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. The role of eIF2 phosphorylation in cell and organismal physiology: New roles for well-known actors. Biochem. J. 2022, 479, 1059–1082. [Google Scholar] [CrossRef]
- Kalinin, A.; Zubkova, E.; Menshikov, M. Integrated Stress Response (ISR) Pathway: Unraveling Its Role in Cellular Senescence. Int. J. Mol. Sci. 2023, 24, 17423. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.N.; Jiang, H.-Y.; Wek, R.C.; Brewer, J.W. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol. Immunol. 2008, 45, 1035–1043. [Google Scholar] [CrossRef]
- Khamyath, M.; Melhem, H.; Balabanian, K.; Espéli, M. New Insights into the Mechanisms Regulating Plasma Cell Survival and Longevity. Curr. Opin. Immunol. 2024, 88, 102442. [Google Scholar] [CrossRef]
- Alouche, N.; Bonaud, A.; Rondeau, V.; Hussein-Agha, R.; Nguyen, J.; Bisio, V.; Khamyath, M.; Crickx, É.; Setterblad, N.; Dulphy, N.; et al. Hematologic disorder–associated Cxcr4 gain-of-function mutation leads to uncontrolled extrafollicular immune response. Blood 2021, 137, 3050–3063. [Google Scholar] [CrossRef]
- Liu, X.; Yao, J.; Zhao, Y.; Wang, J.; Qi, H. Heterogeneous plasma cells and long-lived subsets in response to immunization, autoantigen and microbiota. Nat. Immunol. 2022, 23, 1564–1576. [Google Scholar] [CrossRef]
- Benet, Z.; Jing, Z.; Fooksman, D.R. Plasma cell dynamics in the bone marrow niche. Cell Rep. 2021, 34, 108733. [Google Scholar] [CrossRef]
- Robinson, M.J.; Ding, Z.; Dowling, M.R.; Hill, D.L.; Webster, R.H.; McKenzie, C.; Pitt, C.; O’Donnell, K.; Mulder, J.; Brodie, E.; et al. Intrinsically determined turnover underlies broad heterogeneity in plasma-cell lifespan. Immunity 2023, 56, 1596–1612.e4. [Google Scholar] [CrossRef]
- Koike, T.; Fujii, K.; Kometani, K.; Butler, N.S.; Funakoshi, K.; Yari, S.; Kikuta, J.; Ishii, M.; Kurosaki, T.; Ise, W. Progressive Differentiation toward the Long-Lived Plasma Cell Compartment in the Bone Marrow. J. Exp. Med. 2023, 220, e20221717. [Google Scholar] [CrossRef]
- Klentzeris, L.D.; Bulmer, J.N.; Warren, A.; Morrison, L.; Li, T.C.; Cooke, I.D. Endometrial lymphoid tissue in the timed endometrial biopsy: Morphometric and immunohistochemical aspects. Am. J. Obstet. Gynecol. 1992, 167, 667–674. [Google Scholar] [CrossRef]
- Yeaman, G.R.; Guyre, P.M.; Fanger, M.W.; Collins, J.E.; White, H.D.; Rathbun, W.; Orndorff, K.A.; Gonzalez, J.; Stern, J.E.; Wira, C.R. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J. Leukoc. Biol. 1997, 61, 427–435. [Google Scholar] [CrossRef]
- Mettler, L.; Jürgensen, A.; Volkov, N.I.; Kulakov, V.; Parwaresch, M.R. lmmunohistochemical profile of endometrium in patients with genital endometriosis. Diagn. Ther. Endosc. 1997, 3, 127–145. [Google Scholar] [CrossRef]
- Shen, M.; Child, T.; Mittal, M.; Sarodey, G.; Salim, R.; Granne, I.; Southcombe, J.H. B Cell Subset Analysis and Gene Expression Characterization in Mid-Luteal Endometrium. Front. Cell Dev. Biol. 2021, 9, 709280. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.-S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent Pregnancy Loss Is Associated with a Pro-Senescent Decidual Response during the Peri-Implantation Window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef]
- Bannard, O.; McGowan, S.J.; Ersching, J.; Ishido, S.; Victora, G.D.; Shin, J.S.; Cyster, J.G. Ubiquitin-mediated fluctuations in MHC class II facilitate efficient germinal center B cell responses. J. Exp. Med. 2016, 213, 993–1009. [Google Scholar] [CrossRef]
- Liu, H.; Jain, R.; Guan, J.; Vuong, V.; Ishido, S.; La Gruta, N.; Gray, D.; Villadangos, J.A.; Mintern, J.D. Ubiquitin ligase MARCH 8 cooperates with CD83 to control surface MHC II expression in thymic epithelium and CD4 T cell selection. J. Exp. Med. 2016, 213, 1695–1703. [Google Scholar] [CrossRef]
- Krupa, P.; Wein, H.; Zemmrich, L.S.; Zygmunt, M.; Muzzio, D.O. Pregnancy-related factors induce immune tolerance through regulation of sCD83 release. Front. Immunol. 2024, 15, 1452879. [Google Scholar] [CrossRef]
- Lundell, A.C.; Nordstrom, I.; Andersson, K.; Lundqvist, C.; Telemo, E.; Nava, S.; Kaipe, H.; Rudin, A. IFN type I and II induce BAFF secretion from human decidual stromal cells. Sci. Rep. 2017, 7, 39904. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Qu, X.; Yang, M.; Zhang, W.; Liang, L.; Shao, Q.; Kong, B. Expression of BAFF in the Trophoblast and Decidua of Normal Early Pregnant Women and Patients with Recurrent Spontaneous Miscarriage. Chin. Med. J. 2008, 121, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.P.; Zhang, Y.; Wang, Q.J.; Xu, X.F.; Zhang, H.; Yang, Y.M.; Mao, H.T.; Gao, W.J.; Song, B.F.; Kong, B.H.; et al. Soluble BAFF-R Produced by Decidual Stromal Cells Plays an Inhibitory Role in Monocytes and Macrophages. Reprod. Biomed. Online 2012, 24, 654–663. [Google Scholar] [CrossRef][Green Version]
- Bartmann, C.; Segerer, S.E.; Rieger, L.; Kapp, M.; Sutterlin, M.; Kammerer, U. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am. J. Reprod. Immunol. 2014, 71, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Faucette, A.N.; Pawlitz, M.D.; Pei, B.; Goyert, J.W.; Zhou, J.Z.; El-Hage, N.G.; Deng, J.; Lin, J.; Yao, F.; et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat. Med. 2017, 23, 128–135. [Google Scholar] [CrossRef]
- McQueen, D.B.; Maniar, K.P.; Hutchinson, A.; Confino, R.; Bernardi, L.; Pavone, M.E. Redefining chronic endometritis: The importance of endometrial stromal changes. Fertil. Steril. 2021, 116, 855–861. [Google Scholar] [CrossRef]
- Bjercke, S.; Brandtzaeg, P. Glandular distribution of immunoglobulins, J chain, secretory component, and HLA—DR in the human endometrium throughout the menstrual cycle. Hum. Reprod. 1993, 8, 1420–1425. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Hayashi, T.; Taguchi, S.; Funabiki, M.; Nakamura, Y. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am. J. Reprod. Immunol. 2014, 72, 386–391. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Mucosal Immunity in the Female Genital Tract. J. Reprod. Immunol. 1997, 36, 23–50. [Google Scholar] [CrossRef]
- Vitagliano, A.; Laganà, A.S.; De Ziegler, D.; Cicinelli, R.; Santarsiero, C.M.; Buzzaccarini, G.; Chiantera, V.; Cicinelli, E.; Marinaccio, M. Chronic Endometritis in Infertile Women: Impact of Untreated Disease, Plasma Cell Count and Antibiotic Therapy on IVF Outcome—A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2250. [Google Scholar] [CrossRef]
- Yasuo, T.; Kitaya, K. Challenges in Clinical Diagnosis and Management of Chronic Endometritis. Diagnostics 2022, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; de Ziegler, D.; Nicoletti, R.; Colafiglio, G.; Saliani, N.; Resta, L.; Rizzi, D.; de Vito, D. Chronic Endometritis: Correlation among Hysteroscopic, Histologic, and Bacteriologic Findings in a Prospective Trial with 2190 Consecutive Office Hysteroscopies. Fertil. Steril. 2008, 89, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Matsubayashi, H.; Takaya, Y.; Nishiyama, R.; Yamaguchi, K.; Takeuchi, T.; Ishikawa, T. Live Birth Rate Following Oral Antibiotic Treatment for Chronic Endometritis in Infertile Women with Repeated Implantation Failure. Am. J. Reprod. Immunol. 2017, 78, e12719. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Solouki, S.; Sarig-Meth, T.; Vega, M.G.; Albertini, D.F.; Darmon, S.K.; Deligdisch, L.; Barad, D.H.; Gleicher, N. Systemic inflammation and autoimmunity in women with chronic endometritis. Am. J. Reprod. Immunol. 2016, 75, 672–677. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Hillier, S.L.; Meyn, L.A.; Amortegui, A.J.; Sweet, R.L. Subclinical Pelvic Inflammatory Disease and Infertility. Obstet. Gynecol. 2012, 120, 37–43. [Google Scholar] [CrossRef]
- Song, D.; He, Y.; Wang, Y.; Liu, Z.; Xia, E.; Huang, X.; Xiao, Y.; Li, T.C. Impact of Antibiotic Therapy on the Rate of Negative Test Results for Chronic Endometritis: A Prospective Randomized Control Trial. Fertil. Steril. 2021, 115, 1549–1556. [Google Scholar] [CrossRef]
- Kitaya, K.; Tanaka, S.E.; Sakuraba, Y.; Ishikawa, T. Multi-drug-resistant chronic endometritis in infertile women with repeated implantation failure: Trend over the decade and pilot study for third-line oral antibiotic treatment. J. Assist. Reprod. Genet. 2022, 39, 1839–1848. [Google Scholar] [CrossRef]
- Kitaya, K.; Ishikawa, T. Lincomycin administration against persistent multi-drug-resistant chronic endometritis in infertile women with a history of repeated implantation failure. Appl. Microbiol. 2022, 2, 554–560. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Guido, G.; Frallonardo, L.; Pennazzi, L.; Bevilacqua, M.; Locantore, P.; Vitagliano, A.; Saracino, A.; Cicinelli, E. Chronic Endometritis and Antimicrobial Resistance: Towards a Multidrug-Resistant Endometritis? An Expert Opinion. Microorganisms 2025, 13, 197. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Aberrant Expression of Selectin E, CXCL1, and CXCL13 in Chronic Endometritis. Mod. Pathol. 2010, 23, 1136–1146. [Google Scholar] [CrossRef]
- Tortorella, C.; Piazzolla, G.; Matteo, M.; Pinto, V.; Tinelli, R.; Sabbà, C.; Fanelli, M.; Cicinelli, E. Interleukin-6, Interleukin-1β, and Tumor Necrosis Factor α in Menstrual Effluents as Biomarkers of Chronic Endometritis. Fertil. Steril. 2014, 101, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Gori, I.; Pellegrini, C.; Staedler, D.; Russell, R.; Jan, C.; Canny, G.O. Tumor Necrosis Factor-α Activates Estrogen Signaling Pathways in Endometrial Epithelial Cells via Estrogen Receptor. Mol. Cell. Endocrinol. 2011, 345, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Zappimbulso, V.; Tartagni, M.; Saliani, N. Endometrial Micropolyps at Fluid Hysteroscopy Suggest the Existence of Chronic Endometritis. Hum. Reprod. 2005, 20, 1386–1389. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Tada, Y.; Taguchi, S.; Funabiki, M.; Hayashi, T.; Nakamura, Y. Local mononuclear cell infiltrates in infertile patients with endometrial macropolyps versus micropolyps. Hum. Reprod. 2012, 27, 3474–3480. [Google Scholar] [CrossRef]
- Di Pietro, C.; Cicinelli, E.; Guglielmino, M.R.; Ragusa, M.; Farina, M.; Palumbo, M.A.; Cianci, A. Altered transcriptional regulation of cytokines, growth factors and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am. J. Reprod. Immunol. 2013, 69, 509–517. [Google Scholar] [CrossRef]
- Mishra, K.; Wadhwa, N.; Guleria, K.; Agarwal, S. ER, PR and Ki-67 expression status in granulomatous and chronic non-specific endometritis. J. Obstet. Gynecol. Res. 2008, 34, 371–378. [Google Scholar] [CrossRef]
- Pickartz, H.; Beckmann, R.; Fleige, B.; Düe, W.; Gerdes, J.; Stein, H. Steroid receptors and proliferative activity in non-neoplastic and neoplastic endometria. Virchows Arch. A Pathol. Anat. Histopathol. 1990, 417, 163–171. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, G. Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int. J. Mol. Sci. 2023, 24, 6992. [Google Scholar] [CrossRef]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Wu, D.; Zheng, L.; Takahashi, K.; Suginami, H.; et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol. 2014, 73, 221–231. [Google Scholar] [CrossRef]
- Poli-Neto, O.B.; Meola, J.; Rosa-e-Silva, J.C.; Tiezzi, D. Transcriptome meta-analysis reveals differences of immune profile between eutopic endometrium from stage I-II and III-IV endometriosis independently of hormonal milieu. Sci. Rep. 2020, 10, 313. [Google Scholar] [CrossRef]
- Vallvé-Juanico, J.; Santamaria, X.; Vo, K.C.; Houshdaran, S.; Giudice, L.C. Macrophages display proinflammatory phenotypes in the eutopic endometrium of women with endometriosis with relevance to an infectious etiology of the disease. Fertil. Steril. 2019, 112, 1118–1128. [Google Scholar] [CrossRef]
- Nie, M.-F.; Xie, Q.; Wu, Y.-H.; He, H.; Zou, L.-J.; She, X.-L.; Wu, X.-Q. Serum and ectopic endometrium from women with endometriosis modulate macrophage M1/M2 polarization via the Smad2/Smad3 pathway. J. Immunol. Res. 2018, 2018, 6285813. [Google Scholar] [CrossRef]
- Laganà, A.S.; Salmeri, F.M.; Frangež, H.B.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. 2020, 36, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, O.A.; Wheatcroft, N.; Weetman, A.P.; Cooke, I.D. The prevalence of endometrial immunoglobulin G antibodies in patients with endometriosis. Hum. Reprod. 1995, 10, 1214–1219. [Google Scholar] [CrossRef]
- Mathur, S.; Garza, D.E.; Smith, L.F. Endometrial autoantigens eliciting immunoglobulin (Ig) G, IgA, and IgM responses in endometriosis. Fertil. Steril. 1990, 54, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Weed, J.C.; Arquembourg, P.C. Endometriosis: Can it produce an autoimmune response resulting in infertility? Clin. Obstet. Gynecol. 1980, 23, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Evans-Hoeker, E.; Lessey, B.A.; Jeong, J.W.; Savaris, R.F.; Palomino, W.A.; Yuan, L.; Schammel, D.P.; Young, S.L. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women with Endometriosis. Reprod. Sci. 2016, 23, 1234–1241. [Google Scholar] [CrossRef]
- Almquist, L.D.; Likes, C.E.; Stone, B.; Brown, K.R.; Savaris, R.; Forstein, D.A.; Miller, P.B.; Lessey, B.A. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: A cohort study. Fertil. Steril. 2017, 108, 1063–1069. [Google Scholar] [CrossRef]
- Huang, D.; Chan, M.; Solomon, M.; Cedars, M.I.; Giudice, L.C.; Cakmak, H. B-cell lymphoma 6 expression significantly differs by the uterine preparation method used for frozen embryo transfer. Fertil. Steril. 2023, 120, 305–311. [Google Scholar] [CrossRef]
- Klimczak, A.M.; Herlihy, N.S.; Scott, C.S.; Hanson, B.M.; Kim, J.G.; Titus, S.; Seli, E.; Scott, R.T., Jr. B-cell lymphoma 6 expression is not associated with live birth in a normal responder in vitro fertilization population. Fertil. Steril. 2022, 117, 351–358. [Google Scholar] [CrossRef]
- Hever, A.; Roth, R.B.; Hevezi, P.; Marin, M.E.; Acosta, J.A.; Acosta, H.; Rojas, J.; Herrera, R.; Grigoriadis, D.; White, E.; et al. Human Endometriosis Is Associated with Plasma Cells and Overexpression of B Lymphocyte Stimulator. Proc. Natl. Acad. Sci. USA 2007, 104, 12451–12456. [Google Scholar] [CrossRef] [PubMed]
- Zutautas, K.B.; Yolmo, P.; Xu, M.; Childs, T.; Koti, M.; Tayade, C. Tertiary Lymphoid Structures in Endometriosis. F&S Sci. 2024, 5, 335–341. [Google Scholar]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Ito, M.; et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Cheng, M.; Parnell, L.A.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic Therapy with Metronidazole Reduces Endometriosis Disease Progression in Mice: A Potential Role for Gut Microbiota. Hum. Reprod. 2019, 34, 1106–1116. [Google Scholar] [CrossRef]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent Implantation Failure: Definition and Management. Reprod. BioMed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Yasuo, T.; Yamaguchi, T. Bridging the Diagnostic Gap between Histopathologic and Hysteroscopic Chronic Endometritis with Deep Learning Models. Medicina 2024, 60, 972. [Google Scholar] [CrossRef]
- Lai, Z.-Z.; Wang, Y.; Zhou, W.-J.; Liang, Z.; Shi, J.-W.; Yang, H.-L.; Xie, F.; Chen, W.-D.; Zhu, R.; Zhang, C.; et al. Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics 2022, 12, 6527–6547. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Working Group on Recurrent Implantation Failure; Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar]
- Odendaal, J.; Black, N.; Bouliotis, G.; Guck, J.; Underwood, M.; Fisher, J.; Quenby, S. Preconceptual Administration of Doxycycline in Women with Recurrent Miscarriage and Chronic Endometritis: Protocol for the Chronic Endometritis and Recurrent Miscarriage (CERM) Trial, a Multicentre, Double-Blind, Placebo-Controlled, Adaptive Randomised Trial with an Embedded Translational Substudy. BMJ Open 2023, 13, e081470. [Google Scholar]
- Harvey, A.J.; Willson, B.E.; Surrey, E.S.; Gardner, D.K. Ovarian Stimulation Protocols: Impact on Oocyte and Endometrial Quality and Function. Fertil. Steril. 2025, 123, 10–21. [Google Scholar] [CrossRef]
- Lawrenz, B.; Melado, L.; Fatemi, H. Premature Progesterone Rise in ART-Cycles. Reprod. Biol. 2018, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chemerinski, A.; Shen, M.; Valero-Pacheco, N.; Zhao, Q.; Murphy, T.; George, L.; Lemenze, A.; Sherman, L.; Heller, D.; Chen, X.; et al. The Impact of Ovarian Stimulation on the Human Endometrial Microenvironment. Hum. Reprod. 2024, 39, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, R.; Parvanov, D.; Vidolova, N.; Ruseva, M.; Handzhiyska, M.; Arsov, K.; Decheva, I.; Metodiev, D.; Moskova-Doumanova, V.; Stamenov, G. Endometrial immune cell ratios and implantation success in patients with recurrent implantation failure. J. Reprod. Immunol. 2023, 156, 103816. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).