Memory T Cell Subsets Expressing Tissue Homing Receptors and Chemokine Levels in Human Tegumentary Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Ethics Statement
2.3. Blood Sampling Procedure
2.4. Anti-T. Cruzi Antibody Analysis
2.5. Chemokine Receptor Determination
2.6. Soluble Leishmania Antigens (SLAs)
2.7. In Vitro PBMC Cultures
2.8. Chemokine and Cytokine Quantification
2.9. Statistical Analysis
3. Results
3.1. Peripheral CLA, CCR4, CCR6, CCR3, and CCR10 Expression on Total CD4+ and CD8+ T Cells in TL Patients
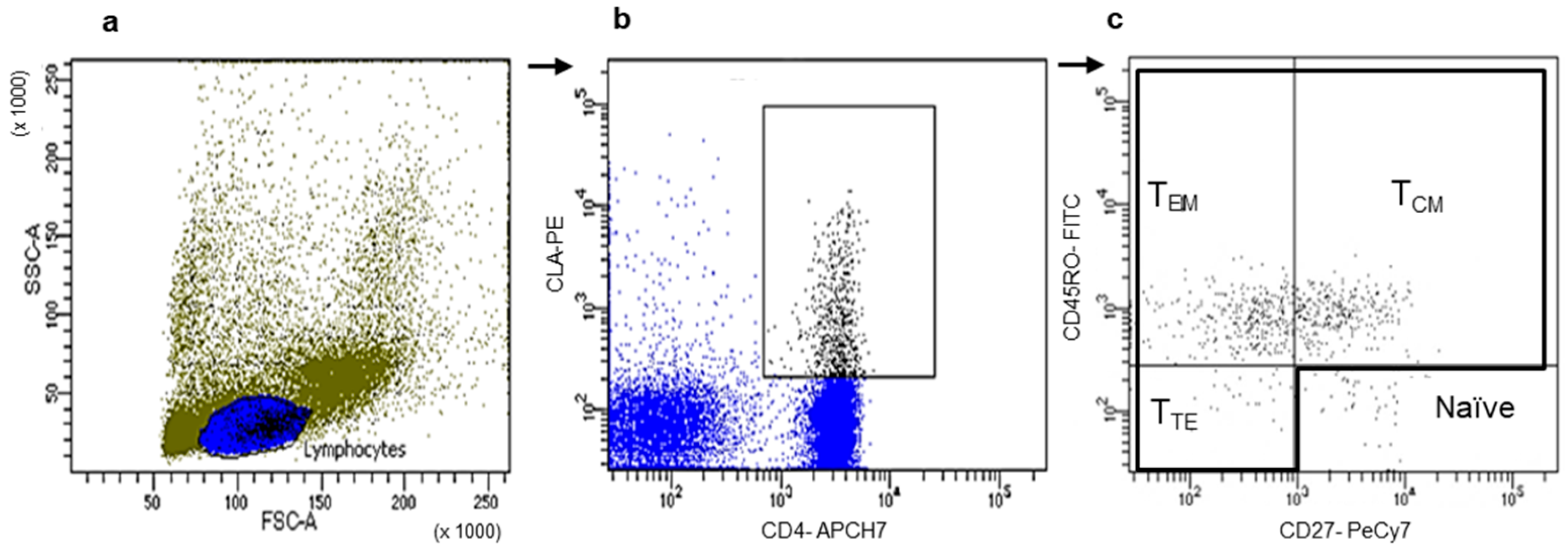
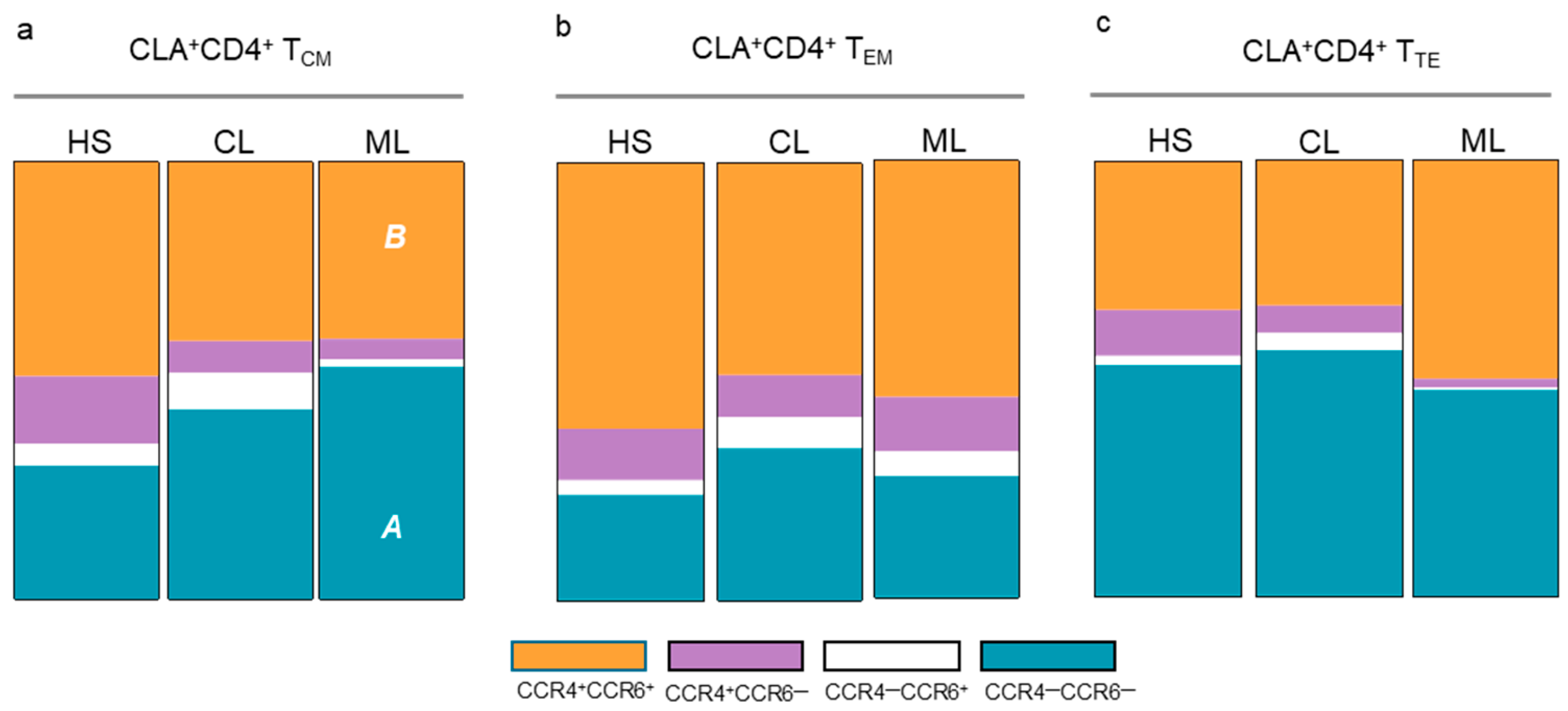
3.2. Memory T Cell Populations Expressing Tissue Tropism Receptors
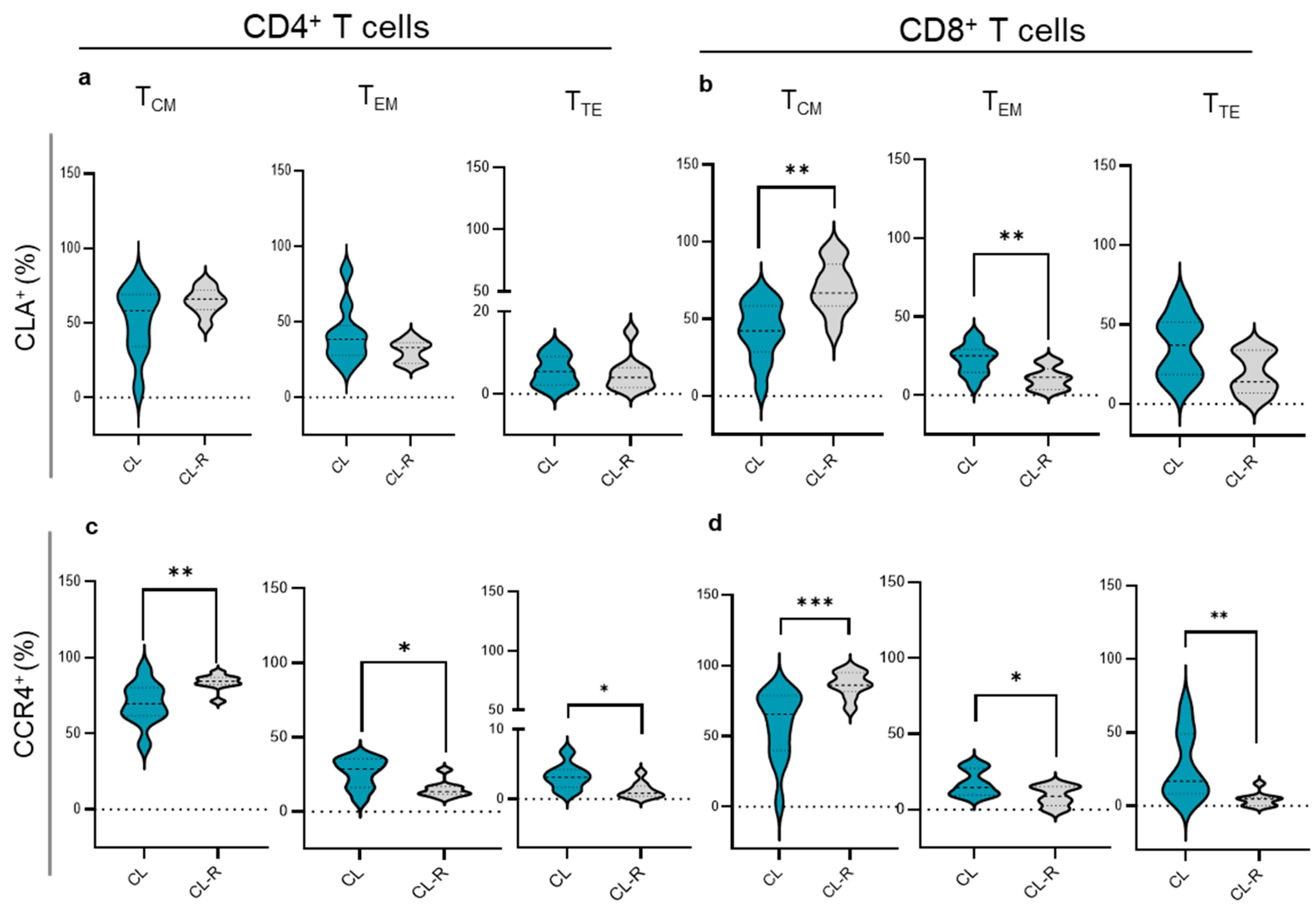
3.3. Memory T Cell Subsets in CL Patients Recovered from Their Cutaneous Lesions
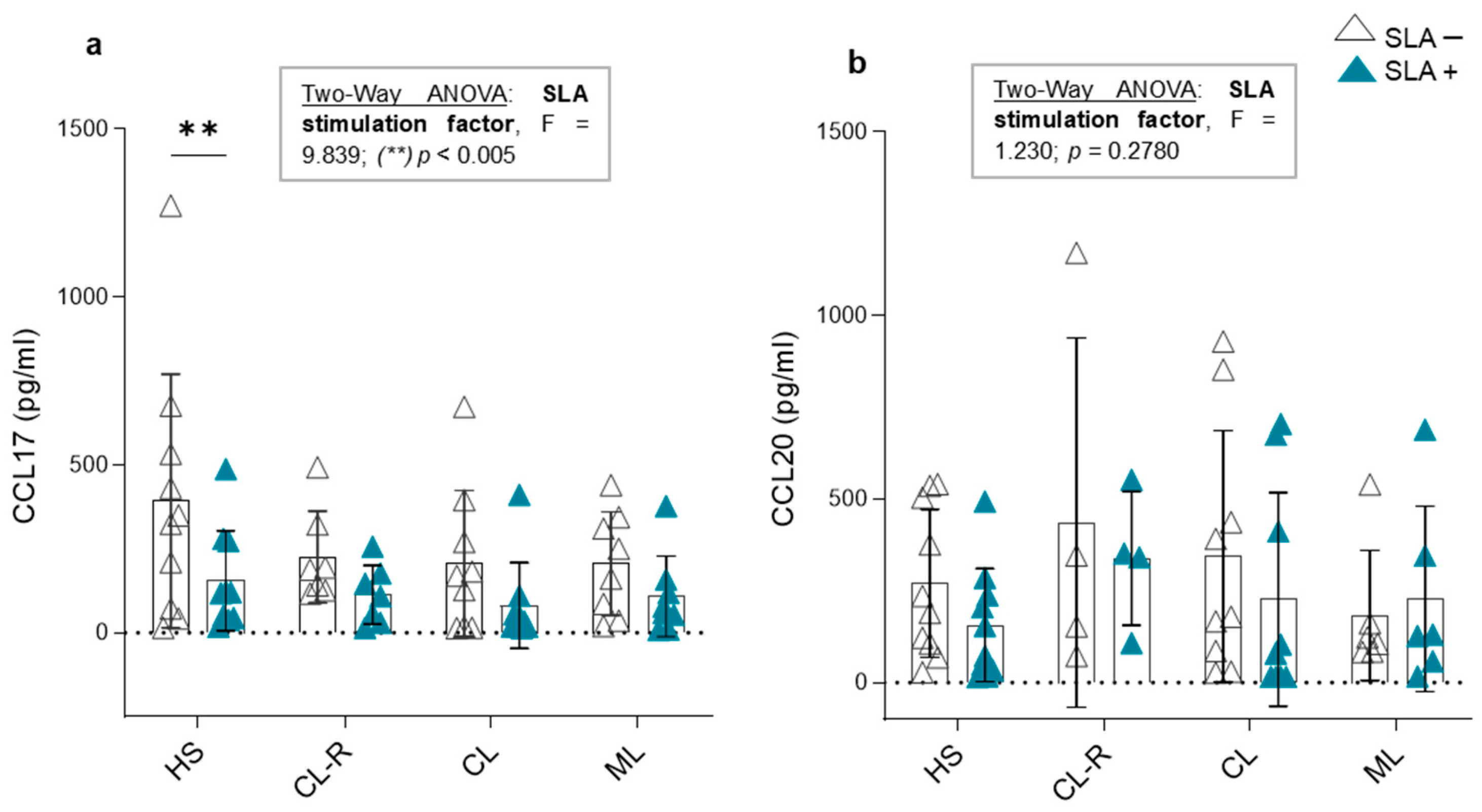
3.4. Plasma Concentrations of CCL17 and CCL20 Chemokines
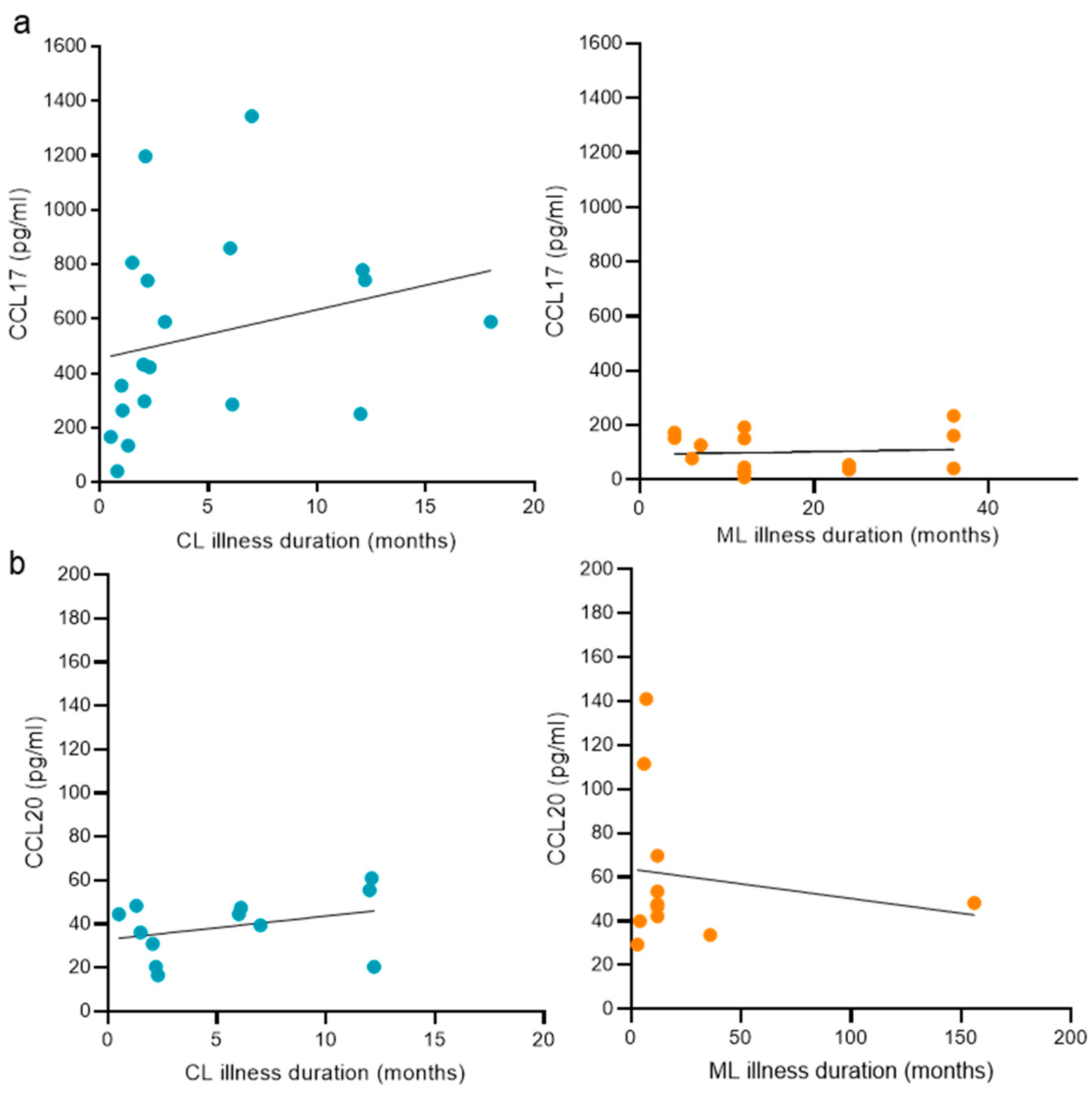
3.5. The Duration of the Disease and Its Relation with Plasma Chemokine Concentrations Targeting Skin and Nasopharyngeal Mucosa
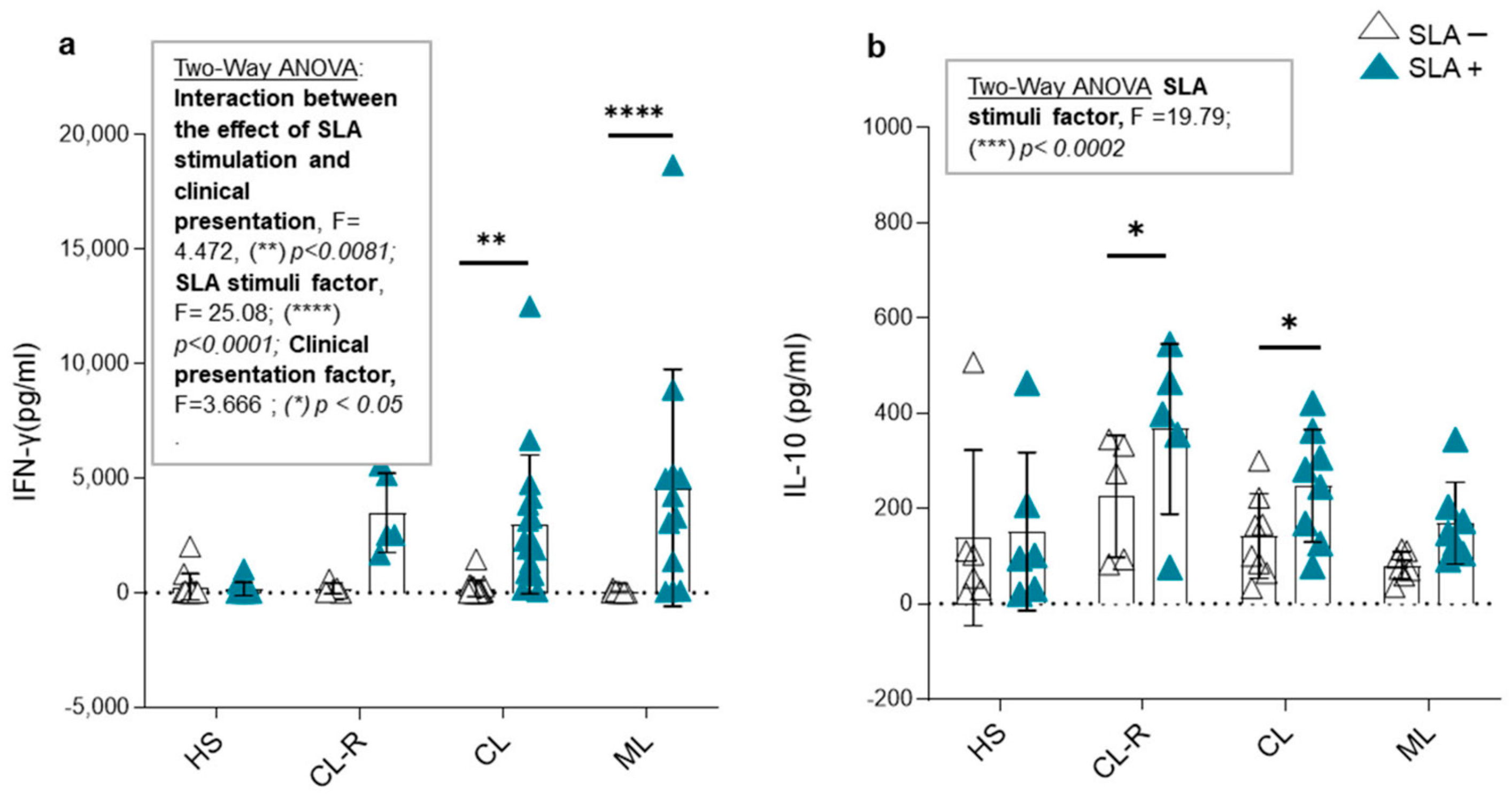
3.6. CCL17 and CCL20 Production in Culture Supernatants and Cytokine Modulation in Response to Leishmania Antigens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TL | Tegumentary leishmaniasis |
| CL | Active cutaneous leishmaniasis patients |
| ML | Mucosal leishmaniasis patients |
| CL-R | Recovered cutaneous leishmaniasis patients |
| HS | Healthy subjects |
| PBMCs | Peripheral blood mononuclear cells |
| SLA | Soluble Leishmania antigens |
| TCM | Central memory T cells |
| TEM | Effector memory T cells |
| TTE | Terminal effector T cells |
References
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the Host-Pathogen Interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Amato, V.S.; Tuon, F.F.; Bacha, H.A.; Neto, V.A.; Nicodemo, A.C. Mucosal Leishmaniasis: Current Scenario and Prospects for Treatment. Acta Trop. 2008, 105, 1–9. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef]
- Parodi, C.; García Bustos, M.F.; Barrio, A.; Ramos, F.; González Prieto, A.G.; Mora, M.C.; Baré, P.; Basombrío, M.A.; de Elizalde de Bracco, M.M. American Tegumentary Leishmaniasis: T-Cell Differentiation Profile of Cutaneous and Mucosal Forms—Co-Infection with Trypanosoma Cruzi. Med. Microbiol. Immunol. 2016, 205, 353–369. [Google Scholar] [CrossRef]
- Morgado, F.N.; Schubach, A.; Rosalino, C.M.V.; Quintella, L.P.; Santos, G.; Salgueiro, M.; Conceição-Silva, F. Is the in Situ Inflammatory Reaction an Important Tool to Understand the Cellular Immune Response in American Tegumentary Leishmaniasis? Br. J. Dermatol. 2008, 158, 50–58. [Google Scholar] [CrossRef]
- García Bustos, M.F.; González-Prieto, G.; Ramos, F.; Mora, M.C.; Hashiguchi, Y.; Parodi, C.; Basombrío, M.A.; Moreno, S.; Monroig, S.; Beckar, J.; et al. Clinical and Epidemiological Features of Leishmaniasis in Northwestern-Argentina through a Retrospective Analysis of Recent Cases. Acta Trop. 2016, 154, 125–132. [Google Scholar] [CrossRef]
- Novais, F.O.; Scott, P. CD8+ T Cells in Cutaneous Leishmaniasis: The Good, the Bad, and the Ugly. Semin. Immunopathol. 2015, 37, 251–259. [Google Scholar] [CrossRef]
- de Oliveira, B.C.; da Silva, A.A.; de Andrade Cavalcante, M.K.; de Brito, M.E.F.; de Castro, M.C.A.B.; de Medeiros, V.L.S.; de Freitas e Silva, R.; Pereira, V.R.A. Central and Effector Memory Human CD4+ and CD8+ T Cells during Cutaneous Leishmaniasis and after In Vitro Stimulation with Leishmania (Viannia) Braziliensis Epitopes. Vaccines 2023, 11, 158. [Google Scholar] [CrossRef]
- de Araújo, F.F.; Costa-Silva, M.F.; Pereira, A.A.S.; Rêgo, F.D.; Pereira, V.H.S.; de Souza, J.P.; Fernandes, L.O.B.; Martins-Filho, O.A.; Gontijo, C.M.F.; Peruhype-Magalhães, V.; et al. Chemokines in Leishmaniasis: Map of Cell Movements Highlights the Landscape of Infection and Pathogenesis. Cytokine 2021, 147, 155339. [Google Scholar] [CrossRef]
- de Oliveira Mendes-Aguiar, C.; Vieira-Gonçalves, R.; Guimarães, L.H.; de Oliveira-Neto, M.P.; Carvalho, E.M.; Da-Cruz, A.M. Effector Memory CD4+ T Cells Differentially Express Activation Associated Molecules Depending on the Duration of American Cutaneous Leishmaniasis Lesions. Clin. Exp. Immunol. 2016, 185, 202–209. [Google Scholar] [CrossRef]
- Palmeiro, M.R.; Morgado, F.N.; Valete-Rosalino, C.M.; Martins, A.C.; Moreira, J.; Quintella, L.P.; de Oliveira Schubach, A.; Conceição-Silva, F. Comparative Study of the in Situ Immune Response in Oral and Nasal Mucosal Leishmaniasis. Parasite Immunol. 2012, 34, 23–31. [Google Scholar] [CrossRef]
- Lechner, A.; Ritter, U.; Varona, R.; Marquez, G.; Bogdan, C.; Körner, H. Protective Immunity and Delayed Type Hypersensitivity Reaction Are Uncoupled in Experimental Leishmania Major Infection of CCR6-Negative Mice. Microbes Infect. 2007, 9, 291–299. [Google Scholar] [CrossRef]
- Barth, T.; Schmidt, D.; Botteron, C.; Nguyen, T.T.T.; Ritter, U.; Männel, D.N.; Lechner, A. An Early Reduction in Treg Cells Correlates with Enhanced Local Inflammation in Cutaneous Leishmaniasis in CCR6-Deficient Mice. PLoS ONE 2012, 7, e44499. [Google Scholar] [CrossRef]
- Díaz, N.L.; Zerpa, O.; Tapia, F.J. Chemokines and Chemokine Receptors Expression in the Lesions of Patients with American Cutaneous Leishmaniasis. Memórias Inst. Oswaldo Cruz 2013, 108, 446–452. [Google Scholar] [CrossRef]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences, 7th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Lwanga, S.K.; Lemeshow, S. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Bracamonte, M.E. Estrategias Noveles de ADN Recombinante Aplicadas al Inmunodiagnóstico de Las Leishmaniasis En Argentina. Doctoral Dissertation, National University of Tucumán, San Miguel de Tucumán, Argentina, 2021. [Google Scholar]
- Conceição-Silva, F.; Leite-Silva, J.; Morgado, F.N. The Binomial Parasite-Host Immunity in the Healing Process and in Reactivation of Human Tegumentary Leishmaniasis. Front. Microbiol. 2018, 9, 1308. [Google Scholar] [CrossRef]
- Morgado, F.N.; Schubach, A.; Vasconcellos, E.; Azeredo-Coutinho, R.B.; Valete-Rosalino, C.M.; Quintella, L.P.; Santos, G.; Salgueiro, M.; Palmeiro, M.R.; Conceição-Silva, F. Signs of an in Situ Inflammatory Reaction in Scars of Human American Tegumentary Leishmaniasis. Parasite Immunol. 2010, 32, 285–295. [Google Scholar] [CrossRef]
- Martínez-Valencia, A.J.; Daza-Rivera, C.F.; Rosales-Chilama, M.; Cossio, A.; Casadiego Rincón, E.J.; Desai, M.M.; Saravia, N.G.; Gómez, M.A. Clinical and Parasitological Factors in Parasite Persistence after Treatment and Clinical Cure of Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005713. [Google Scholar] [CrossRef]
- de Souza, R.M.; de Andrade Junior, H.F.; Duarte, M.I.S.; Braz, L.M.A.; de Oliveira Schubach, A.; Silva, F.C.; Amato, V.S. Reactivation of Cutaneous and Mucocutaneous Tegumentary Leishmaniasis in Rheumatoid Arthritis Patients: An Emerging Problem? Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e6. [Google Scholar] [CrossRef]
- Nicolàs, L.S.D.S.; Czarnowicki, T.; Akdis, M.; Pujol, R.M.; Lozano-Ojalvo, D.; Leung, D.Y.M.; Guttman-Yassky, E.; Santamaria-Babí, L.F. CLA+ Memory T Cells in Atopic Dermatitis: CLA+ T Cells and Atopic Dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2024, 79, 15–25. [Google Scholar] [CrossRef]
- Shin, J.U.; Abaci, H.E.; Herron, L.; Guo, Z.; Sallee, B.; Pappalardo, A.; Jackow, J.; Wang, E.H.C.; Doucet, Y.; Christiano, A.M. Recapitulating T Cell Infiltration in 3D Psoriatic Skin Models for Patient-Specific Drug Testing. Sci. Rep. 2020, 10, 4123. [Google Scholar] [CrossRef]
- Peru, S.; Prochazkova-Carlotti, M.; Cherrier, F.; Velazquez, J.; Richard, E.; Idrissi, Y.; Cappellen, D.; Azzi-Martin, L.; Pham-Ledard, A.; Beylot-Barry, M.; et al. Cutaneous Lymphocyte Antigen Is a Potential Therapeutic Target in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2022, 142, 3243–3252.e10. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Jo, Y.; Matsumoto, T.; Yada, S.; Fujisawa, K.; Esaki, M.; Onai, N.; Matsushima, K.; Iida, M. CCR4 is an up-regulated chemokine receptor of peripheral blood memory CD4+ T cells in Crohn’s disease. Clin. Exp. Immunol. 2003, 132, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska-Wojdylo, M.; Wenzel, J.; Gaffal, E.; Steitz, J.; Roszkiewicz, J.; Bieber, T.; Tüting, T. Absence of CD26 Expression on Skin-Homing CLA+ CD4+ T Lymphocytes in Peripheral Blood Is a Highly Sensitive Marker for Early Diagnosis and Therapeutic Monitoring of Patients with Sézary Syndrome. Clin. Exp. Dermatol. 2005, 30, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Haus, J.M.; Chen, L.; Wu, S.C.; Urao, N.; Koh, T.J.; Minshall, R.D. CCL28-Induced CCR10/ENOS Interaction in Angiogenesis and Skin Wound Healing. FASEB J. 2020, 34, 5838–5850. [Google Scholar] [CrossRef]
- Fujimoto, S.; Uratsuji, H.; Saeki, H.; Kagami, S.; Tsunemi, Y.; Komine, M.; Tamaki, K. CCR4 and CCR10 Are Expressed on Epidermal Keratinocytes and Are Involved in Cutaneous Immune Reaction. Cytokine 2008, 44, 172–178. [Google Scholar] [CrossRef]
- Xiong, N.; Fu, Y.; Hu, S.; Xia, M.; Yang, J. CCR10 and Its Ligands in Regulation of Epithelial Immunity and Diseases. Protein Cell 2012, 3, 571–580. [Google Scholar] [CrossRef]
- Skovdahl, H.K.; Van Beelen Granlund, A.; Østvik, A.E.; Bruland, T.; Bakke, I.; Torp, S.H.; Damas, J.K.; Sandvik, A.K. Expression of CCL20 and Its Corresponding Receptor CCR6 Is Enhanced in Active Inflammatory Bowel Disease, and TLR3 Mediates CCL20 Expression in Colonic Epithelial Cells. PLoS ONE 2015, 10, e0141710. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Eri, R. Pleiotropic Immune Functions of Chemokine Receptor 6 in Health and Disease. Medicines 2018, 5, 69. [Google Scholar] [CrossRef]
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 Axis as a Therapeutic Target for Autoimmune Diseases. Autoimmun. Rev. 2021, 20, 102846. [Google Scholar] [CrossRef]
- Ito, T.; Carson, W.F.; Cavassani, K.A.; Connett, J.M.; Kunkel, S.L. CCR6 as a Mediator of Immunity in the Lung and Gut. Exp. Cell Res. 2011, 317, 613–619. [Google Scholar] [CrossRef]
- Kennedy-Crispin, M.; Billick, E.; Mitsui, H.; Gulati, N.; Fujita, H.; Gilleaudeau, P.; Sullivan-Whalen, M.; Johnson-Huang, L.M.; Suárez-Farĩas, M.; Krueger, J.G. Human Keratinocytes’ Response to Injury Upregulates CCL20 and Other Genes Linking Innate and Adaptive Immunity. J. Investig. Dermatol. 2012, 132, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Elnabawi, Y.A.; Garshick, M.S.; Tawil, M.; Barrett, T.J.; Fisher, E.A.; Lo Sicco, K.; Neimann, A.L.; Scher, J.U.; Krueger, J.; Berger, J.S. CCL20 in Psoriasis: A Potential Biomarker of Disease Severity, Inflammation, and Impaired Vascular Health. J. Am. Acad. Dermatol. 2021, 84, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S.; Körner, H. The CCR6-CCL20 Axis in Humoral Immunity and T-B Cell Immunobiology. Immunobiology 2019, 224, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Carvalho, R.; Mendes-Aguiar, C.O.; Oliveira-Neto, M.P.; Covas, C.J.F.; Bertho, Á.L.; Da-Cruz, A.M.; Gomes-Silva, A. Leishmania Braziliensis-Reactive T Cells Are down-Regulated in Long-Term Cured Cutaneous Leishmaniasis, but the Renewal Capacity of T Effector Memory Compartments Is Preserved. PLoS ONE 2013, 8, e81529. [Google Scholar] [CrossRef]
- Egui, A.; Ledesma, D.; Pérez-Antón, E.; Montoya, A.; Gómez, I.; Robledo, S.M.; Infante, J.J.; Vélez, I.D.; López, M.C.; Thomas, M.C. Phenotypic and Functional Profiles of Antigen-Specific CD4+ and CD8+ T Cells Associated with Infection Control in Patients with Cutaneous Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 393. [Google Scholar] [CrossRef]
- Jaigirdar, S.A.; MacLeod, M.K.L. Development and Function of Protective and Pathologic Memory CD4 T Cells. Front. Immunol. 2015, 6, 456. [Google Scholar] [CrossRef]
- Novais, F.O.; Carvalho, L.P.; Graff, J.W.; Beiting, D.P.; Ruthel, G.; Roos, D.S.; Betts, M.R.; Goldschmidt, M.H.; Wilson, M.E.; de Oliveira, C.I.; et al. Cytotoxic T Cells Mediate Pathology and Metastasis in Cutaneous Leishmaniasis. PLoS Pathog. 2013, 9, e1003504. [Google Scholar] [CrossRef]
- Sacramento, L.A.; Amorim, C.F.; Lombana, C.G.; Beiting, D.; Novais, F.; Carvalho, L.P.; Carvalho, E.M.; Scott, P. CCR5 Promotes the Migration of Pathological CD8+ T Cells to the Leishmanial Lesions. PLoS Pathog. 2024, 20, e1012211. [Google Scholar] [CrossRef]
- Campanelli, A.P.; Brodskyn, C.I.; Boaventura, V.; Silva, C.; Roselino, A.M.; Costa, J.; Saldanha, A.C.; Rodrigues de Freitas, L.A.Ô.; De Oliveira, C.I.; Barral-Netto, M.; et al. Chemokines and Chemokine Receptors Coordinate the Inflammatory Immune Response in Human Cutaneous Leishmaniasis. Hum. Immunol. 2010, 71, 1220–1227. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública de La República Argentina. InfoLEG—Información Legislativa y Documental—Ministerio de Justicia y Derechos Humanos—Presidencia de La Nación de La República Argentina. In Resolución 386/2004: Manual de Procedimientos Para El Nivel Gerencial y Profesional Sobre Leshmaniosis; Ministerio de Salud Pública de La República Argentina: Buenos Aires, Argentina, 2004. [Google Scholar]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.D.; Ghoreschi, K. T Cell Pathology in Skin Inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y. Thymus and Activation-Regulated Chemokine as a Clinical Biomarker in Atopic Dermatitis. J. Dermatol. 2014, 41, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sengupta, R.; Mukhopadhyay, D.; Braun, C.; Mitra, S.; Roy, S.; Kanti Das, N.; Chatterjee, U.; von Stebut-Borschitz, E.; Chatterjee, M. Impaired Activation of Lesional CD8+ T-Cells Is Associated with Enhanced Expression of Programmed Death-1 in Indian Post Kala-Azar Dermal Leishmaniasis. Sci. Rep. 2019, 9, 762. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, S.C.; Lee, M.H.; Oh, H.M.; Choi, E.Y.; Choi, E.J.; Yun, K.J.; Seo, G.S.; Kim, S.W.; Lee, J.G.; et al. Increased Expression of MIP-3α/CCL20 in Peripheral Blood Mononuclear Cells from Patients with Ulcerative Colitis and Its down-Regulation by Sulfasalazine and Glucocorticoid Treatment. Inflamm. Bowel Dis. 2005, 11, 1070–1079. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Eri, R. Modulation of the CCR6-CCl20 Axis: A Potential Therapeutic Target in Inflammation and Cancer. Medicina 2018, 54, 88. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Tsuji, G.; Nakahara, T.; Furue, M. The CCL20 and CCR6 Axis in Psoriasis. Scand. J. Immunol. 2020, 91, e12846. [Google Scholar] [CrossRef]
- Glennie, N.D.; Scott, P. Memory T Cells in Cutaneous Leishmaniasis. Cell. Immunol. 2016, 309, 50–54. [Google Scholar] [CrossRef]
- Scorza, B.M.; Wacker, M.A.; Messingham, K.; Kim, P.; Klingelhutz, A.; Fairley, J.; Wilson, M.E. Differential Activation of Human Keratinocytes by Leishmania Species Causing Localized or Disseminated Disease. J. Investig. Dermatol. 2017, 137, 2149–2156. [Google Scholar] [CrossRef]
- Pinheiro, N.F.; Hermida, M.D.R.; Macedo, M.P.; Mengel, J.; Bafica, A.; Dos-Santos, W.L.C. Leishmania Infection Impairs Β1-Integrin Function and Chemokine Receptor Expression in Mononuclear Phagocytes. Infect. Immun. 2006, 74, 3912–3921. [Google Scholar] [CrossRef]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef]
- Zaph, C.; Uzonna, J.; Beverley, S.M.; Scott, P. Central Memory T Cells Mediate Long-Term Immunity to Leishmania Major in the Absence of Persistent Parasites. Nat. Med. 2004, 10, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.M.; Cruz, I.; Picado, A.; Van Bocxlaer, K.; Croft, S.L. Leishmaniasis Immunopathology—Impact on Design and Use of Vaccines, Diagnostics and Drugs. Semin. Immunopathol. 2020, 42, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, I.; Cierpka, S. Interferon Gamma in the Etiology of Atherosclerosis and Periodontitis. Thromb. Res. 2010, 126, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.D.S.; Boaventura, V.; Ribeiro Cardoso, C.; Tavares, N.; Lordelo, M.J.; Noronha, A.; Costa, J.; Borges, V.M.; De Oliveira, C.I.; Van Weyenbergh, J.; et al. CD8+ Granzyme B+—Mediated Tissue Injury vs. CD4+IFNγ+ -Mediated Parasite Killing in Human Cutaneous Leishmaniasis. J. Investig. Dermatol. 2013, 133, 1533–1540. [Google Scholar] [CrossRef]
- da Silva Santos, C.; Brodskyn, C.I. The Role of CD4 and CD8 T Cells in Human Cutaneous Leishmaniasis. Front. Public Health 2014, 2, 165. [Google Scholar] [CrossRef]


| Study Groups | N | Age (Years) | Male/ Female | Subjects with Detectable Anti-T. cruzi Antibodies (%) | Cutaneous/Mucosal Illness Duration (months) | Time of Complete Recovery (months) |
|---|---|---|---|---|---|---|
| Cutaneous leishmaniasis (CL) | 32 | 40 [12–72] | 26/6 | 9.3 | 2 [0.5–18] | NA |
| Cutaneous leishmaniasis recovered (CL-R) | 13 | 42 [19–72] | 9/4 | 6.25 | NA | 31 [2–72] |
| Mucosal leishmaniasis (LM) | 24 | 57 [27–86] | 17/7 | 41.66 | 36 [12–156] | NA |
| Healthy subjects (HS) | 17 | 37 [29–61] | 10/7 | 0 | NA | NA |
| Cell Marker | CD4+ T Cells (%) | CD8+ T Cells (%) | ||||
|---|---|---|---|---|---|---|
| HS | CL | ML | HS | CL | ML | |
| CLA | 10.30 [7.70–12.80] | 10.30 [6.70–28.80] | 14.80 [7.30–32.80] * | 4.65 [2.00–8.70] | 3.80 [1.30–13.40] | 8.70 [1.30–11.60] |
| CCR4 | 14.50 [8.00–30.50] | 13.30 [4.30–24.80] | 18.40 [7.50–49.30] ** | 2.60 [1.50–6.00] | 3.10 [0.80–6.50] | 3.40 [1.70–9.00] |
| CCR6 | 6.70 [2.70–9.90] | 2.40 [0.50–11.80] | 8.40 [0.40–19.70] ≠ | 1.90 [0.70–7.70] | 1.70 [0.70–3.40] | 3.30 [0.50–9.60] ≠ |
| CCR3 | 0.90 [0.50–3.10] | 1.45 [0.50–3.80] | 2.10 [0.80–4.10] € | 0.70 [0.30–2.90] | 1.40 [0.40–3.80] | 2.60 [1.20–3.10] € |
| CCR10 | 6.15 [4.60–9.70] | 6.30 [3.20–17.30] | 8.10 [4.60–13.30] | 2.40 [1.50–4.40] | 2.50 [1.50–6.40] | 3.75 [1.60–7.70] ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, J.; García Bustos, M.F.; Ragone, P.; Marco, J.D.; Barroso, P.; Mesías, A.C.; Basombrío, M.; Occhionero, M.; Ramos, F.; Laucella, S.A.; et al. Memory T Cell Subsets Expressing Tissue Homing Receptors and Chemokine Levels in Human Tegumentary Leishmaniasis. Cells 2025, 14, 604. https://doi.org/10.3390/cells14080604
Pimentel J, García Bustos MF, Ragone P, Marco JD, Barroso P, Mesías AC, Basombrío M, Occhionero M, Ramos F, Laucella SA, et al. Memory T Cell Subsets Expressing Tissue Homing Receptors and Chemokine Levels in Human Tegumentary Leishmaniasis. Cells. 2025; 14(8):604. https://doi.org/10.3390/cells14080604
Chicago/Turabian StylePimentel, Julia, M. Fernanda García Bustos, Paula Ragone, Jorge D. Marco, Paola Barroso, Andrea Cecilia Mesías, Mercedes Basombrío, María Occhionero, Federico Ramos, Susana Adriana Laucella, and et al. 2025. "Memory T Cell Subsets Expressing Tissue Homing Receptors and Chemokine Levels in Human Tegumentary Leishmaniasis" Cells 14, no. 8: 604. https://doi.org/10.3390/cells14080604
APA StylePimentel, J., García Bustos, M. F., Ragone, P., Marco, J. D., Barroso, P., Mesías, A. C., Basombrío, M., Occhionero, M., Ramos, F., Laucella, S. A., Pérez Brandán, C., & Parodi, C. (2025). Memory T Cell Subsets Expressing Tissue Homing Receptors and Chemokine Levels in Human Tegumentary Leishmaniasis. Cells, 14(8), 604. https://doi.org/10.3390/cells14080604






