L-Tryptophan-Rich Diet Alleviates High-Intensity-Exercise-Induced Liver Dysfunction via the Metabolite Indole-3-Acetic Acid and AhR Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Experimental Protocol
2.2.1. Experiment #1
2.2.2. Experiment #2
2.2.3. Experiment #3
2.2.4. Experiment #4
2.2.5. Study Design Subsection
2.3. Non-Targeted Metabolomics and Targeted Tryptophan Metabolites Assay
2.4. Metabolomics Data Processing and Analysis
2.5. Cell Culture and Treatment
2.6. ALT and AST Measurements
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Determination of Hepatic Parameters
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Western Blot Analysis
2.11. Histological Staining and Immunofluorescence Analysis
2.12. Detection of Intracellular Reactive Oxygen Species (ROS)
2.13. Molecular Docking
2.14. Statistical Analysis
3. Results
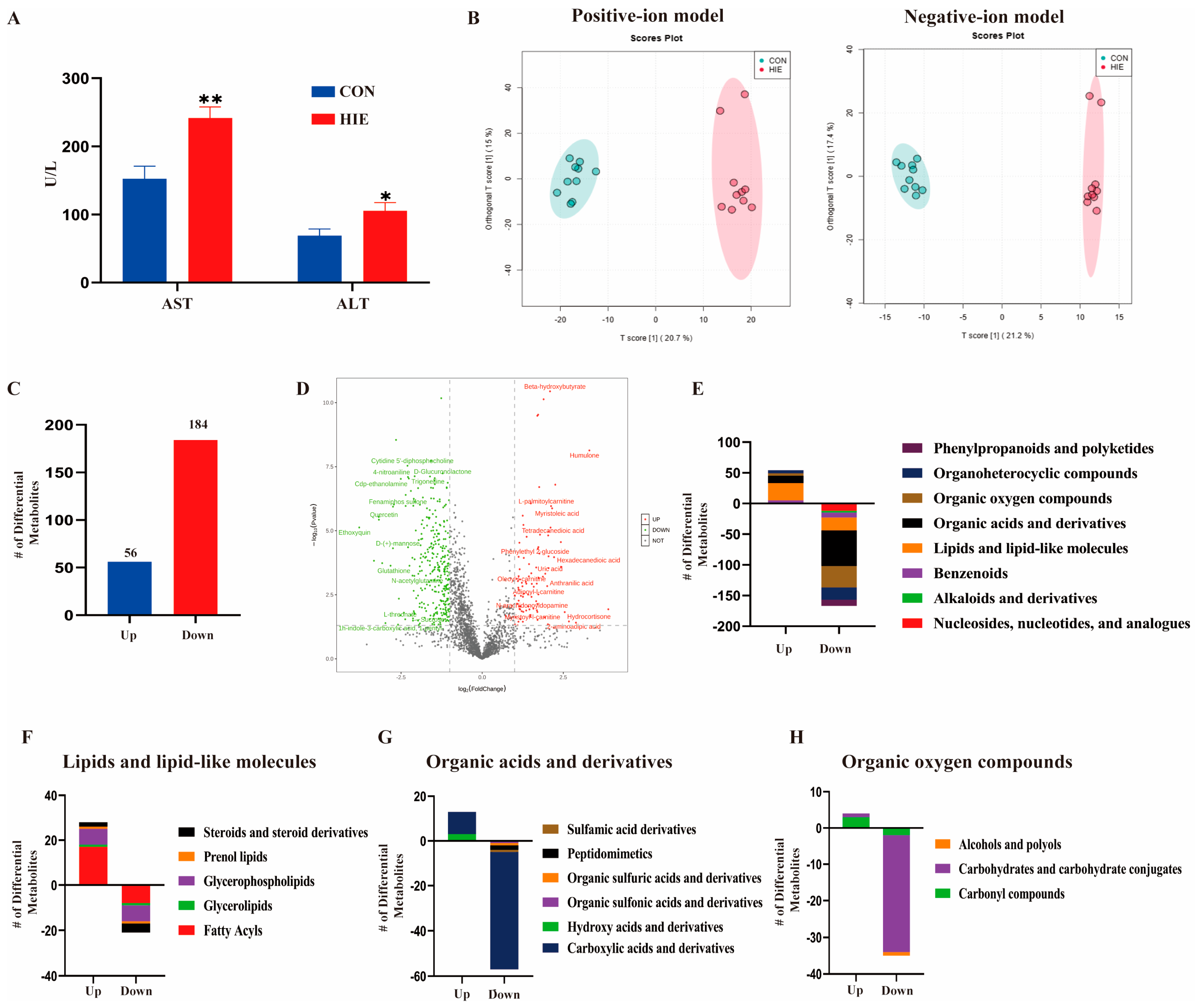
3.1. HIE Induced Aggravated Liver Dysfunction and Metabolic Disturbance
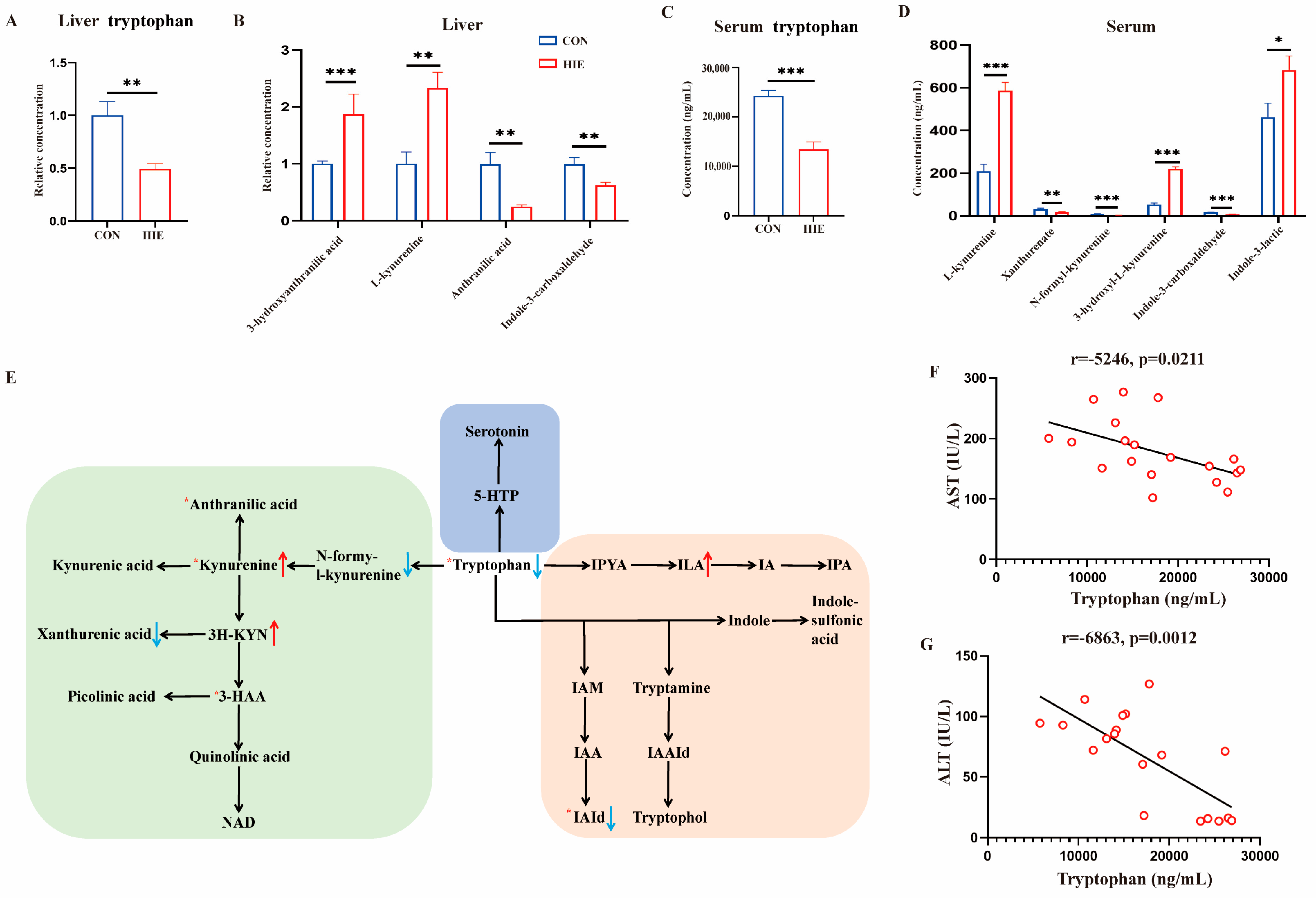
3.2. HIE-Induced Tryptophan Metabolic Disorder in Liver, Serum and L-Tryptophan-Rich Diet Ameliorated HIE-Induced Liver Dysfunction
3.3. L-Tryptophan-Rich Diet Significantly Reduced HIE-Induced Liver Oxidative Stress and Inflammatory Response
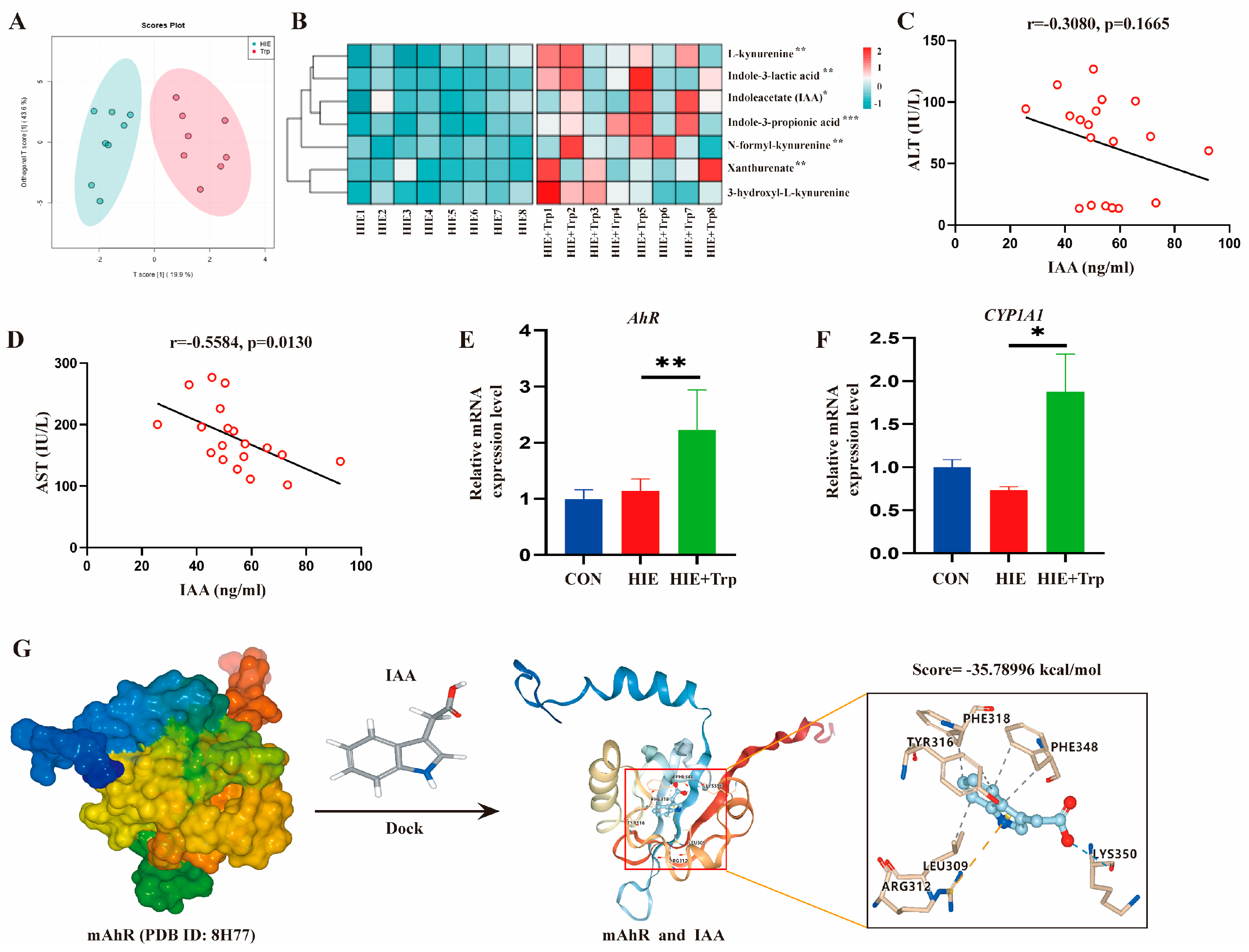
3.4. L-Tryptophan-Rich Diet Improved HIE-Induced Liver Dysfunction Associated with the Increased Tryptophan Metabolite IAA and AhR Activation
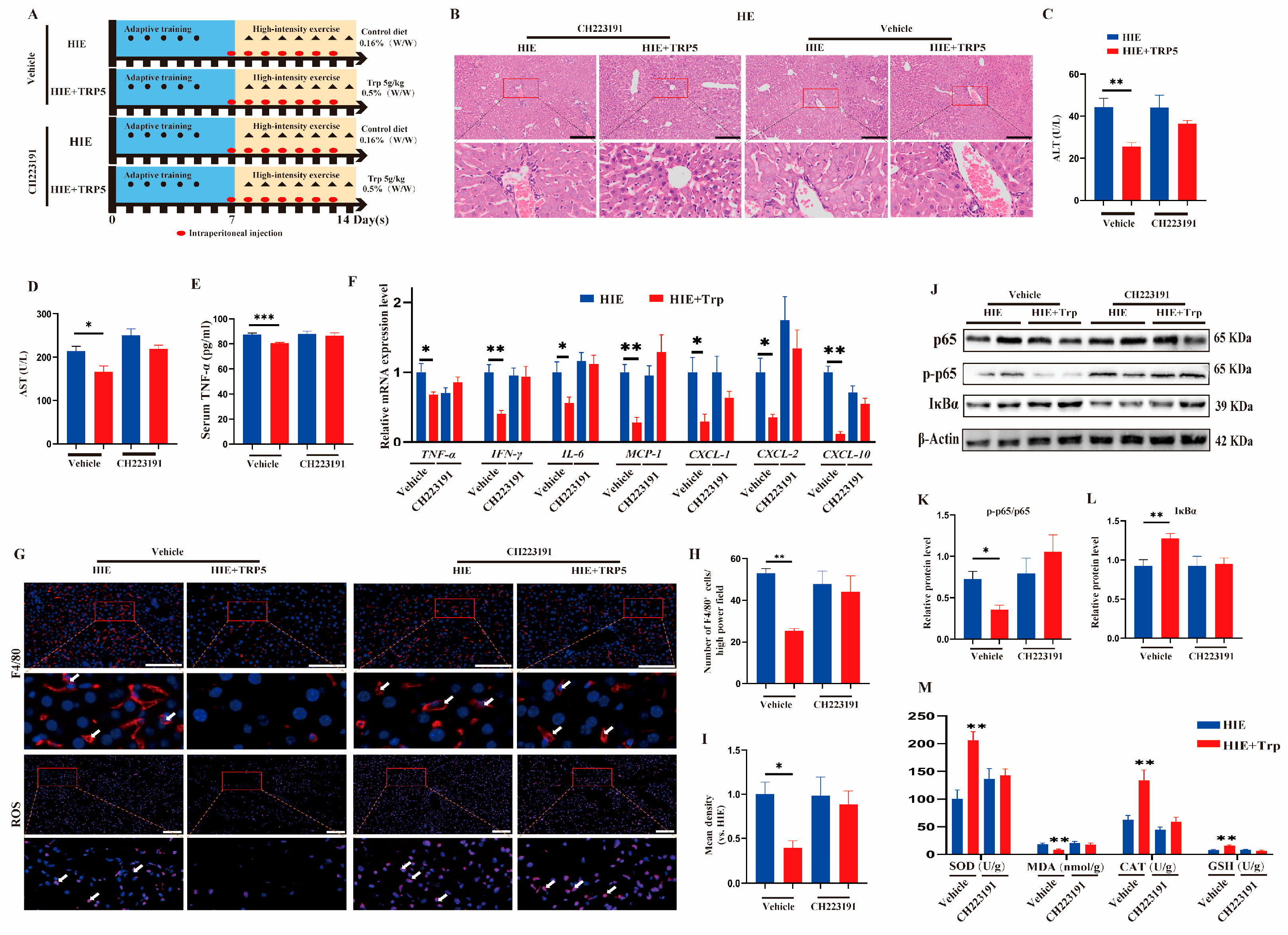
3.5. The Effect of an L-Tryptophan-Rich Diet on Improving HIE-Induced Liver Dysfunction Was Also Abrogated by the AhR Inhibitor CH223191
3.6. IAA Intervention Improved LPS-Induced Hepatocyte Injury In Vitro and HIE-Induced Liver Dysfunction In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lin, W.-T.; Hsu, F.-L.; Tsai, P.-W.; Hou, C.-C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2010, 108, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Wu, W.-T.; Yang, F.-L.; Chiu, Y.-H.; Peng, T.-C.; Hsu, B.-G.; Liao, K.-W.; Lee, R.-P. Effects of freshwater clam extract supplementation on time to exhaustion, muscle damage, pro/anti-inflammatory cytokines, and liver injury in rats after exhaustive exercise. Molecules 2013, 18, 3825–3838. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Phillipose, J.; Ahmed, M.; Deeb, L. Athlete’s Hepatitis in a Young Healthy Marathon Runner. Case Rep. Gastroenterol. 2018, 12, 176–181. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiology. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- McGettigan, B.; McMahan, R.; Orlicky, D.; Burchill, M.; Danhorn, T.; Francis, P.; Cheng, L.L.; Golden-Mason, L.; Jakubzick, C.V.; Rosen, H.R. Dietary Lipids Differentially Shape Nonalcoholic Steatohepatitis Progression and the Transcriptome of Kupffer Cells and Infiltrating Macrophages. Hepatology 2019, 70, 67–83. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; Shirakawa, H. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef]
- Bray, T.M.; Emmerson, K.S. Putative mechanisms of toxicity of 3-methylindole: From free radical to pneumotoxicosis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 91–115. [Google Scholar] [CrossRef]
- Mascanfroni, I.D.; Takenaka, M.C.; Yeste, A.; Patel, B.; Wu, Y.; Kenison, J.E.; Siddiqui, S.; Basso, A.S.; Otterbein, L.E.; Pardoll, D.M.; et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med. 2015, 21, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yi, L.; Lang, H.; Zhang, J.; Zhang, Q.; Yu, L.; Zhu, J.; Mi, M. Dihydromyricetin-Encapsulated Liposomes Inhibit Exhaustive Exercise-Induced Liver Inflammation by Orchestrating M1/M2 Macrophage Polarization. Front. Pharmacol. 2022, 13, 887263. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Jeong, D.Y.; Kim, J.G.; Lee, S. The Acute Effects of Swimming Exercise on PGC-1α-FNDC5/Irisin-UCP1 Expression in Male C57BL/6J Mice. Metabolites 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, L.; Zhou, M.; Hou, P.; Yi, L.; Mi, M. Dihydromyricetin ameliorates liver fibrosis via inhibition of hepatic stellate cells by inducing autophagy and natural killer cell-mediated killing effect. Nutr. Metab. 2021, 18, 64. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Zhang, B.; Hang, Y.; Xu, L.; Chen, Y.; Xie, Q.; Zhao, Q.; Zhang, L.; Li, G.; et al. Cryo-EM structure of the cytosolic AhR complex. Structure 2023, 31, 295–308 e294. [Google Scholar] [CrossRef]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Kackley, M.L.; Short, J.A.; Hyde, P.N.; LaFountain, R.A.; Buga, A.; Miller, V.J.; Dickerson, R.M.; Sapper, T.N.; Barnhart, E.C.; Krishnan, D.; et al. A Pre-Workout Supplement of Ketone Salts, Caffeine, and Amino Acids Improves High-Intensity Exercise Performance in Keto-Naïve and Keto-Adapted Individuals. J. Am. Coll. Nutr. 2020, 39, 290–300. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Montagnana, M.; Salvagno, G.L.; Banfi, G.; Guidi, G.C. Significant variation of traditional markers of liver injury after a half-marathon run. Eur. J. Intern. Med. 2011, 22, e36–e38. [Google Scholar] [CrossRef]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med. Open 2018, 4, 2. [Google Scholar] [CrossRef]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Pastori, D.; Angelico, F. The role of nutraceuticals for the treatment of non-alcoholic fatty liver disease. Br. J. Clin. Pharmacol. 2017, 83, 88–95. [Google Scholar] [CrossRef]
- Williams, N.C.; Killer, S.C.; Svendsen, I.S.; Jones, A.W. Immune nutrition and exercise: Narrative review and practical recommendations. Eur. J. Sport. Sci. 2019, 19, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Turner, J.; Del Mar, C. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst. Rev. 2002, CD003198. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Ciocan, D.; Hugot, C.; Spatz, M.; Dupeux, M.; Houron, C.; Lievin-Le Moal, V.; Puchois, V.; Ferrere, G.; Trainel, N.; et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 2021, 70, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Hendrikx, T.; Duan, Y.; Wang, Y.; Oh, J.-H.; Alexander, L.M.; Huang, W.; Stärkel, P.; Ho, S.B.; Gao, B.; Fiehn, O.; et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019, 68, 1504–1515. [Google Scholar] [CrossRef]
- Li, M.; Ding, Y.; Wei, J.; Dong, Y.; Wang, J.; Dai, X.; Yan, J.; Chu, F.; Zhang, K.; Meng, F.; et al. Gut microbiota metabolite indole-3-acetic acid maintains intestinal epithelial homeostasis through mucin sulfation. Gut Microbes 2024, 16, 2377576. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity—Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef]
- Liu, J.R.; Miao, H.; Deng, D.Q.; Vaziri, N.D.; Li, P.; Zhao, Y.Y. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol. Life Sci. 2021, 78, 909–922. [Google Scholar] [CrossRef]



| Target Gene | Primers (5′→3′) |
|---|---|
| TNF-α | F: 5′-ATGTCTCAGCCTCTTCTCATTC-3′ |
| R: 5′-GCTTGTCACTCGAATTTTGAGA-3′ | |
| IL-6 | F: 5′-CTCCCAACAGACCTGTCTATAC-3′ |
| R: 5′-CCATTGCACAACTCTTTTCTCA-3′ | |
| IL-10 | F: 5′-GCTCCAAGACCAAGGTGTCT-3′ |
| R: 5′-CGGAGAGAGGTACAAACGAGG-3′ | |
| CXCL-1 | F: 5′-CGCCTATCGCCAATGAGC-3′ |
| R: 5′-AGCTTCAGGGTCAAGGCAA-3′ | |
| CXCL-2 | F: 5′-GAAGTCATAGCCACTCTCAAGG-3′ |
| R: 5′-CCTCCTTTCCAGGTCAGTTAGC-3′ | |
| CXCL-10 | F: 5′-GAAATTATTCCTGCAAGCCAATTT-3′ |
| R: 5′-TCACCCTTCTTTTTCATTGTAGCA-3′ | |
| MCP-1 | F: 5′-TCCCAATGAGTAGGCTGGAG-3′ |
| R: 5′-TCTGGACCCATTCCTTCTTG-3′ | |
| IFN-γ | F: 5′-CTGGAGGAACTGGCAAAAGGATGG-3′ |
| R: 5′-GACGCTTATGTTGTTGCTGATGGC-3′ | |
| β-actin | F: 5′-CTACCTCATGAAGATCCTGACC-3′ |
| R: 5′-CACAGCTTCTCTTTGATGTCAC-3′ |
| Superclass | Name | Class | Subclass | VIP | Fold Change | p-Value |
|---|---|---|---|---|---|---|
| Beta-hydroxybutyrate | Hydroxy acids and derivatives | Beta hydroxy acids and derivatives | 2.81 | 4.26 | <0.001 | |
| Malonic acid | Carboxylic acids and derivatives | Dicarboxylic acids and derivatives | 1.63 | 0.36 | <0.001 | |
| Ser-Pro-Arg | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 1.64 | 0.34 | <0.001 | |
| Pro-hyp | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 1.25 | 0.49 | <0.001 | |
| Organic acids | Carbamoylaspartate | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 1.30 | 0.31 | <0.001 |
| and derivatives | Asparagine | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 2.31 | 0.47 | <0.001 |
| Lys-Val | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 1.11 | 2.41 | <0.001 | |
| Histidine | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 8.33 | 0.41 | <0.001 | |
| L-thyronine | Carboxylic acids and derivatives | Amino acids, peptides, and analogues | 3.29 | 0.27 | <0.001 | |
| Jasplakinolide | Peptidomimetics | Hybrid peptides | 2.96 | 0.27 | <0.001 | |
| Lactulose | Prenol lipids | Diterpenoids | 1.82 | 0.23 | <0.001 | |
| D-Maltose | Fatty Acyls | Fatty acyl glycosides | 1.80 | 0.23 | <0.001 | |
| L-palmitoylcarnitine | Glycerophospholipids | Glycerophosphoinositol phosphates | 2.71 | 4.36 | <0.001 | |
| Organic acids | Myristoleic acid | Fatty Acyls | Fatty acid esters | 2.19 | 4.46 | <0.001 |
| and derivatives | Isomaltose | Fatty Acyls | Fatty acids and conjugates | 6.67 | 0.32 | <0.001 |
| 1-hexadecyl-sn-glycero-3-phosphocholine | Glycerophospholipids | Glycerophosphoinositol phosphates | 3.49 | 3.44 | <0.001 | |
| Tetradecanedioic acid | Glycerophospholipids | Glycerophosphocholines | 1.25 | 4.10 | <0.001 | |
| Aminocaproic acid | Fatty Acyls | Fatty acids and conjugates | 1.03 | 0.50 | <0.001 | |
| 1-(1,2-dihexanoylphosphatidyl)inositol-4-phosphate | Fatty Acyls | Fatty acyl glycosides | 2.62 | 0.19 | <0.001 | |
| 1-(1,2r-dioctanoylphosphatidyl)inositol-3,4-bisphosphate | Fatty Acyls | Fatty acyl glycosides | 2.26 | 0.24 | <0.001 | |
| Humulone | Organooxygen compounds | Carbonyl compounds | 3.39 | 9.88 | <0.001 | |
| D-Glucuronate | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 10.17 | 0.34 | <0.001 | |
| L-Sorbose | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 1.15 | 0.26 | <0.001 | |
| Stachyose | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 2.11 | 0.33 | <0.001 | |
| Organic oxygen | Maltotetraose | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 12.53 | 0.32 | <0.001 |
| compounds | Glutaraldehyde | Organooxygen compounds | Carbonyl compounds | 1.45 | 0.44 | <0.001 |
| Ribitol | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 1.66 | 0.27 | <0.001 | |
| Lactose | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 2.86 | 0.36 | <0.001 | |
| Tubuloside a | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 2.49 | 0.28 | <0.001 | |
| D-psicose | Organooxygen compounds | Carbohydrates and carbohydrate conjugates | 1.42 | 0.44 | <0.001 | |
| D-Glucuronolactone | Lactones | Gamma butyrolactones | 1.95 | 0.33 | <0.001 | |
| Dihydrouracil | Diazines | Pyrimidines and pyrimidine derivatives | 3.30 | 0.39 | <0.001 | |
| 4-pyridoxic acid | Pyridines and derivatives | Pyridinecarboxylic acids and derivatives | 2.18 | 0.56 | <0.001 | |
| Thiamine | Diazines | Pyrimidines and pyrimidine derivatives | 5.62 | 0.57 | <0.001 | |
| Organoheterocy | Ethoxyquin | Quinolines and derivatives | Quinolones and derivatives | 1.84 | 0.07 | <0.001 |
| clic compounds | Enrofloxacin | Quinolines and derivatives | Quinoline carboxylic acids | 1.57 | 0.29 | <0.001 |
| Pheniramine | Pyridines and derivatives | Pheniramines | 1.28 | 1.86 | <0.001 | |
| L-gulono-1,4-lactone | Lactones | Gamma butyrolactones | 4.17 | 0.59 | <0.001 | |
| Cytosine | Diazines | Pyrimidines and pyrimidine derivatives | 3.73 | 0.55 | <0.001 |
| Name | AST | ALT | ||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| Indoleacetate | −0.6504 | 0.022 * | −0.5467 | 0.0659 |
| L-kynurenine | −0.5039 | 0.0949 | −0.3307 | 0.2937 |
| Indole-3-propionic acid | −0.4644 | 0.1282 | −0.5568 | 0.0601 |
| Indole-3-lactic acid | −0.5059 | 0.0933 | −0.1851 | 0.5647 |
| Xanthurenate | −0.5033 | 0.0953 | 0.08369 | 0.7959 |
| N-formyl-kynurenine | −0.3793 | 0.224 | −0.4298 | 0.1632 |
| 3-hydroxyl-L-kynurenine | −0.3308 | 0.2937 | 0.01773 | 0.9564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Hou, P.; Lang, H.; Xia, Y.; Bai, Q.; Yao, Y.; Yi, L.; Mi, M. L-Tryptophan-Rich Diet Alleviates High-Intensity-Exercise-Induced Liver Dysfunction via the Metabolite Indole-3-Acetic Acid and AhR Activation. Cells 2025, 14, 605. https://doi.org/10.3390/cells14080605
Wang D, Hou P, Lang H, Xia Y, Bai Q, Yao Y, Yi L, Mi M. L-Tryptophan-Rich Diet Alleviates High-Intensity-Exercise-Induced Liver Dysfunction via the Metabolite Indole-3-Acetic Acid and AhR Activation. Cells. 2025; 14(8):605. https://doi.org/10.3390/cells14080605
Chicago/Turabian StyleWang, Dawei, Pengfei Hou, Hedong Lang, Yundong Xia, Qian Bai, Yu Yao, Long Yi, and Mantian Mi. 2025. "L-Tryptophan-Rich Diet Alleviates High-Intensity-Exercise-Induced Liver Dysfunction via the Metabolite Indole-3-Acetic Acid and AhR Activation" Cells 14, no. 8: 605. https://doi.org/10.3390/cells14080605
APA StyleWang, D., Hou, P., Lang, H., Xia, Y., Bai, Q., Yao, Y., Yi, L., & Mi, M. (2025). L-Tryptophan-Rich Diet Alleviates High-Intensity-Exercise-Induced Liver Dysfunction via the Metabolite Indole-3-Acetic Acid and AhR Activation. Cells, 14(8), 605. https://doi.org/10.3390/cells14080605





