Evolution of Allogeneic Stem Cell Transplantation: Main Focus on AML
Abstract
1. Introduction
2. Transplant Indications
2.1. Indication for Transplantation in AML
- t(6;9)(p23.3;q34.1)/DEK::NUP214;
- t(v;11q23.3)/KMT2A-rearranged (excluding partial tandem duplication);
- t(9;22)(q34.1;q11.2)/BCR::ABL1;
- t(8;16)(p11.2;p13.3)/KAT6A::CREBBP;
- inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/GATA2, MECOM (EVI1);
- t(3q26.2;v)/MECOM (EVI1)-rearranged;
- −5 or del(5q), −7, −17 or abn(17p);
- complex karyotype and monosomal karyotype.
2.2. Indication for Transplantation in Myelodysplastic Syndrome (MDS)
2.3. Indication for Transplantation in Myelofibrosis
2.4. Indication for Transplantation in Chronic Myelomonocytic Leukemia (CMML)
3. Transplant Survival Improvement
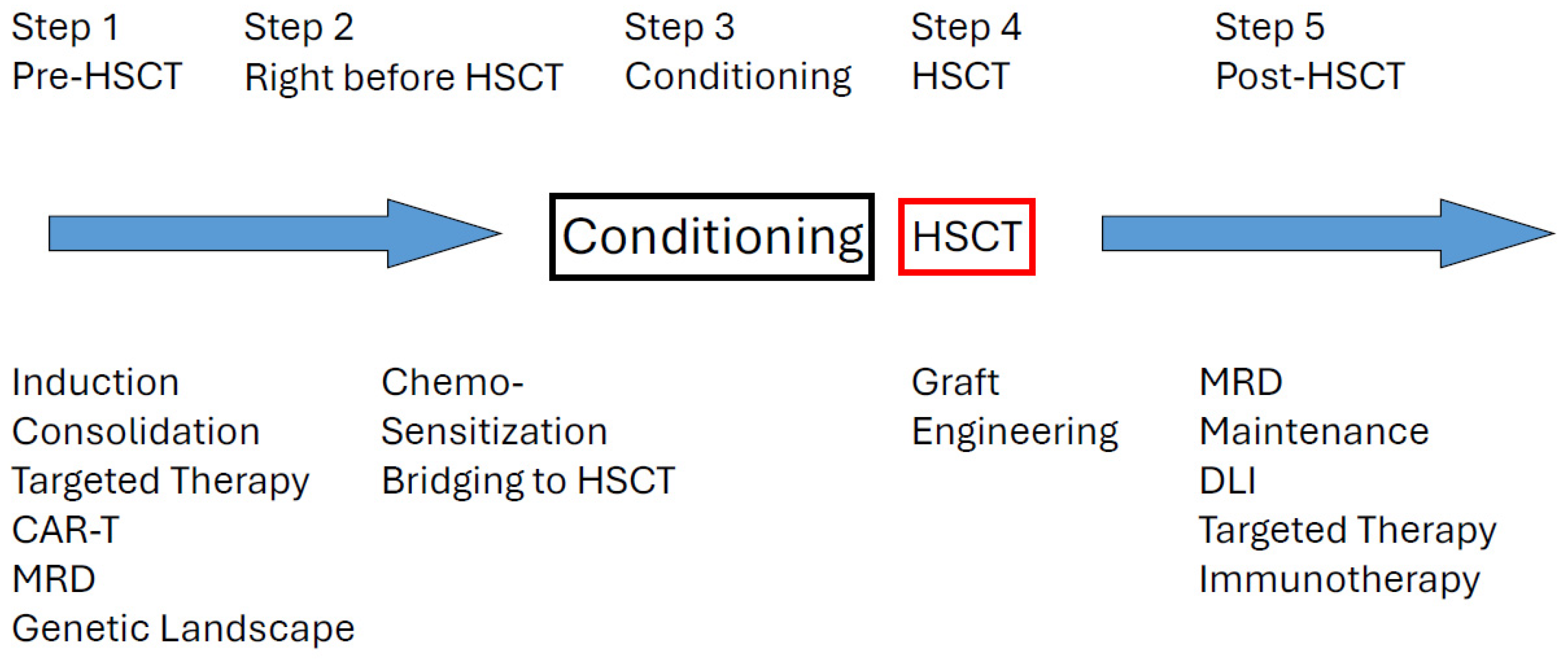
4. Challenges in Transplant Practice
4.1. MRD Positive Diseases
4.1.1. Defining MRD Sensitivity
4.1.2. Approaches for MRD-Positive Disease
4.2. TP53 Mutations
4.3. Non-Remission AML
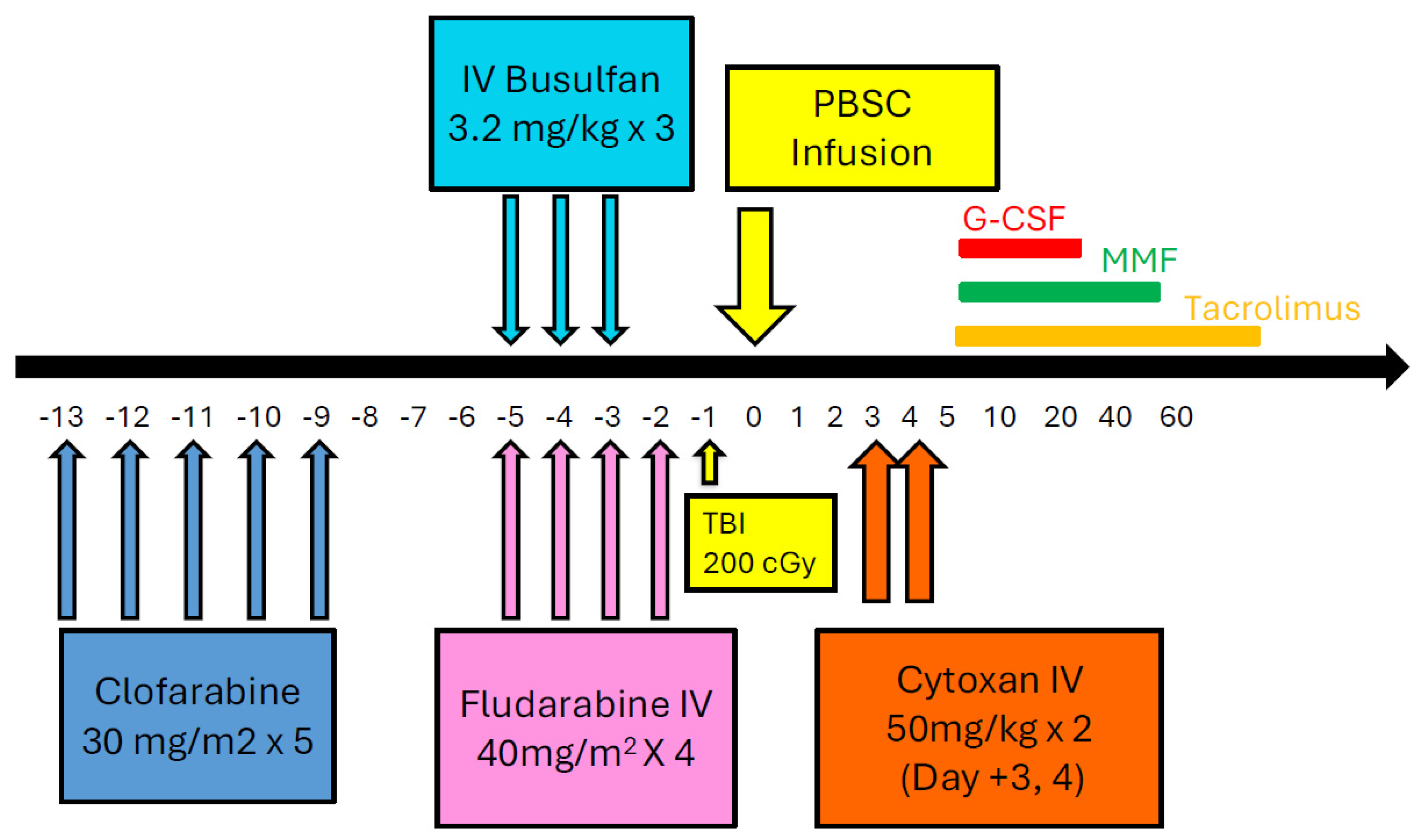
4.3.1. CloBu4 Trial and Outcomes
4.3.2. Pre-Conditioning Approach
4.3.3. Clofarabine Preconditioning
4.4. Post-Transplant Maintenance Strategies
| Agents | Drug Name | Author (Study ID) | Disease | N, Study Design | Results | p-Value |
|---|---|---|---|---|---|---|
| Flt3 inhibitors | Sorafenib | Burchert et al. [72] (SORMAIN /DRKS00000591) | FLT3-ITD+ AML | Sorafenib (N = 43) Placebo (N = 40), Phase II | 2-year RFS 85% in Sorafenib 53.3% in Placebo | <0.01 |
| Xuan et al. [78] (NCT02474290) | FLT3-ITD+ AML | Sorafenib (N = 100) Placebo (n = 102), Phase III | 1-year RR 7% in Sorafenib 24.5% in Placebo | <0.01 | ||
| Gilteritinib | Levis et al. [74] (BMT−CTN 1506 /NCT02997202) | FLT3-ITD+ AML | Gilteritinib (N = 178) Placebo (N = 178), Phase III | 2-year RFS 77.2% in Gilteritinib 69.9% in Placebo | 0.052 | |
| Midostaurin | Maziarz et al. [73] (RADIUS /NCT01883362) | FLT3-ITD+ AML | Midostaurin (N = 16) SOC (N = 14), Phase II | 18-month RFS 89% in Midostaurin 76% in SOC | 0.27 | |
| Quizartinib | Sandmaier et al. [79] (2689-CL-0011/(NCT01468467) | FLT3-ITD+ AML | N = 13, Phase I | RR 7.7% | - | |
| Erba et al. [75] (QuANTUM-First/NCT02668653) | FLT3-ITD+ AML | Quizartinib (N = 268) Placebo (N = 271), Phase III | Median OS 31.9 months in Quizartinib 15.1 months in Placebo | 0.03 | ||
| Crenolanib | Oran et al. [80] (NCT02400255) | FLT3-ITD/TKD+ AML | N = 30, Phase II | 5-year OS 69% 5-year RFS 69.7% | - | |
| IDH1/2 inhibitors | Ivosidenib | Fathi et al. [81] (NCT03564821) | IDH1(R132)-mutant AML | N = 18, Phase I | 1-year RR 19% | - |
| Enasidenib | Fathi et al. [82] (NCT03515512) | IDH2-mutant AML, MDS or CMML | N = 23, Phase I | 2-year OS 74% 2-year PFS 69% 2-year RR 16% | - | |
| Salhotra et al. [83] (NCT03728335) | AML with IDH2 mutation | N = 15, Phase I | 2-year OS 100% 2-year LFS 100% | - | ||
| Bcl-2 inhibitor | Venetoclax | Kent et al. [84] | AML | N = 49, Phase II | 1-year OS 70% 1-year PFS 67% 1-year RR 20% | - |
| Wei et al. [85] (ChiCTR1900025374) | AML/MDS | N = 20, AML (N = 17) MDS (N = 3), Phase II | 2-year RFS 84.7% (median follow-up 598 days, treated with low-dose decitabine) | - | ||
| Gracia et al. [86] (NCT03613532) | AML/MDS/MPN | N = 27, AML (N = 10), MDS (N = 16), MPN (N = 1), Phase I | 2-year OS 67% 2-year PFS 59% 2-year RR 41% | - | ||
| Hypomethylating agents | 5-azacytidine (AZA) | Oran et al. [67] (NCT00887068) | AML/MDS | AZA (N = 93) Control (N = 94), Phase III | Median RFS 2.07 years in AZA 1.28 years in control | 0.14 |
| Keruakous et al. [68] | AML | AZA (N = 31) Control (N = 18), Phase II | RR 25.8% in AZA 66.7% in Control | <0.01 | ||
| Oral formulation of AZA (CC-486) | De Lima et al. [87] (NCT01835587) | AML/MDS | 7 days per cycle (n = 7), 14 days per cycle (n = 23), Phase I | 1-year RFS 54% in 7-day and 72% in 14-day CC-486 dosing | - | |
| Decitabine (DAC) | Pusic et al. [88] (NCT00986804) | AML/MDS | N = 22, Phase I | 2-year OS 56% 2-year DFS 48% 2-year RR 28% | - | |
| Gao et al. [69] (ChiCTR-IIR-16008182) | AML | G-Dec (n = 100) Non-G-Dec (n = 102), Phase II | 2-year RR 15% in the G-Dec 38.3% in the non–G-Dec | <0.01 | ||
| Ma et al. [70] | AML | DAC = 21 Control = 63, Retrospective study | 3-year RFS 94.1% in DAC 55% in control | <0.01 |
4.5. Role of Consolidation Chemotherapy in the Era of Reduced Intensity Conditioning
4.6. How PTCy Changed the Transplant Practice
4.7. Advances in GVHD Prophylaxis Beyond PTCy
4.8. Transplant for Elderly AML Patients Aged 70 and Above
4.9. Improvements in Supportive Care and Management of Complications
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://cibmtr.org/CIBMTR/Resources/Center-Specific-Survival-Analysis (accessed on 13 February 2025).
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Fenwarth, L.; Vasseur, L.; Duployez, N.; Gardin, C.; Terré, C.; Lambert, J.; de Botton, S.; Celli-Lebras, K.; Turlure, P.; Cluzeau, T.; et al. Prognostic Impact of Monoallelic Versus Biallelic TP53 Alterations in Intensively-Treated Adults AML Patients: A Retrospective Study from the ALFA Group. Blood 2022, 140, 737–738. [Google Scholar] [CrossRef]
- Stengel, A.; Haferlach, T.; Baer, C.; Hutter, S.; Meggendorfer, M.; Kern, W.; Haferlach, C. Specific subtype distribution with impact on prognosis of TP53 single-hit and double-hit events in AML and MDS. Blood Adv. 2023, 7, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Montalban-Bravo, G.; Hwang, H.; Ning, J.; Franquiz, M.J.; Kanagal-Shamanna, R.; Patel, K.P.; DiNardo, C.D.; Ravandi, F.; Garcia-Manero, G.; et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv. 2020, 4, 5681–5689. [Google Scholar] [CrossRef]
- Grob, T.; Al Hinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L.; Biemond, B.J.; Breems, D.A.; Maertens, J.; van Marwijk Kooy, M.; et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. Jama 2009, 301, 2349–2361. [Google Scholar] [CrossRef]
- Bornhäuser, M.; Schliemann, C.; Schetelig, J.; Röllig, C.; Kramer, M.; Glass, B.; Platzbecker, U.; Burchert, A.; Hänel, M.; Müller, L.P.; et al. Allogeneic Hematopoietic Cell Transplantation vs Standard Consolidation Chemotherapy in Patients With Intermediate-Risk Acute Myeloid Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Lee, S.J.; Greenberg, P.; Deeg, H.J.; Perez, W.S.; Anasetti, C.; Bolwell, B.J.; Cairo, M.S.; Gale, R.P.; Klein, J.P.; et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004, 104, 579–585. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Caramazza, D.; Vaidya, R.; George, G.; Begna, K.; Schwager, S.; Van Dyke, D.; Hanson, C.; Wu, W.; Pardanani, A.; et al. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011, 29, 392–397. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Elena, C.; Galli, A.; Such, E.; Meggendorfer, M.; Germing, U.; Rizzo, E.; Cervera, J.; Molteni, E.; Fasan, A.; Schuler, E.; et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016, 128, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Onida, F.; Gagelmann, N.; Chalandon, Y.; Kobbe, G.; Robin, M.; Symeonidis, A.; de Witte, T.; Itzykson, R.; Jentzsch, M.; Platzbecker, U.; et al. Management of adult patients with CMML undergoing allo-HCT: Recommendations from the EBMT PH&G Committee. Blood 2024, 143, 2227–2244. [Google Scholar] [CrossRef]
- Gooley, T.A.; Chien, J.W.; Pergam, S.A.; Hingorani, S.; Sorror, M.L.; Boeckh, M.; Martin, P.J.; Sandmaier, B.M.; Marr, K.A.; Appelbaum, F.R.; et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef]
- Shouval, R.; Fein, J.A.; Labopin, M.; Kroger, N.; Duarte, R.F.; Bader, P.; Chabannon, C.; Kuball, J.; Basak, G.W.; Dufour, C.; et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: A European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019, 6, e573–e584. [Google Scholar] [CrossRef]
- Bolanos-Meade, J.; Logan, B.R.; Alousi, A.M.; Antin, J.H.; Barowski, K.; Carter, S.L.; Goldstein, S.C.; Hexner, E.O.; Horowitz, M.M.; Lee, S.J.; et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood 2014, 124, 3221–3227, quiz 3335. [Google Scholar] [CrossRef]
- Holtan, S.G.; DeFor, T.E.; Lazaryan, A.; Bejanyan, N.; Arora, M.; Brunstein, C.G.; Blazar, B.R.; MacMillan, M.L.; Weisdorf, D.J. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015, 125, 1333–1338. [Google Scholar] [CrossRef]
- Kurosawa, S.; Yamaguchi, T.; Miyawaki, S.; Uchida, N.; Kanamori, H.; Usuki, K.; Yamashita, T.; Watanabe, M.; Yakushiji, K.; Yano, S.; et al. A Markov decision analysis of allogeneic hematopoietic cell transplantation versus chemotherapy in patients with acute myeloid leukemia in first remission. Blood 2011, 117, 2113–2120. [Google Scholar] [CrossRef]
- Broers, A.E.C.; de Jong, C.N.; Bakunina, K.; Hazenberg, M.D.; van Marwijk Kooy, M.; de Groot, M.R.; van Gelder, M.; Kuball, J.; van der Holt, B.; Meijer, E.; et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: Results of the prospective randomized HOVON-96 trial. Blood Adv. 2022, 6, 3378–3385. [Google Scholar] [CrossRef]
- Bolaños-Meade, J.; Hamadani, M.; Wu, J.; Al Malki, M.M.; Martens, M.J.; Runaas, L.; Elmariah, H.; Rezvani, A.R.; Gooptu, M.; Larkin, K.T.; et al. Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis. N. Engl. J. Med. 2023, 388, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; Al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.; Klein, J.P.; He, W.; Cahn, J.Y.; Cairo, M.; Camitta, B.M.; Kamble, R.; Copelan, E.; de Lima, M.; Gupta, V.; et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J. Clin. Oncol. 2010, 28, 3730–3738. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Sapienza, G.; Tringali, S.; Rotolo, C.; Patti, C.; Mule, A.; Calafiore, V.; Santoro, A.; Castagna, L. Allogeneic Stem Cell Transplantation in Refractory Acute Myeloid Leukaemia. Cells 2024, 13, 755. [Google Scholar] [CrossRef]
- Yanada, M.; Yamasaki, S.; Kondo, T.; Kawata, T.; Harada, K.; Uchida, N.; Doki, N.; Yoshihara, S.; Katayama, Y.; Eto, T.; et al. Allogeneic hematopoietic cell transplantation for patients with acute myeloid leukemia not in remission. Leukemia 2024, 38, 513–520. [Google Scholar] [CrossRef]
- Ali, N.; Othus, M.; Rodriguez-Arboli, E.; Orvain, C.; Milano, F.; Sandmaier, B.M.; Davis, C.; Basom, R.S.; Appelbaum, F.R.; Walter, R.B. Measurable residual disease as predictor of post-day +100 relapses after allografting in adult AML. Blood Adv. 2025, 9, 558–570. [Google Scholar] [CrossRef]
- Walter, R.B. Perspective on measurable residual disease testing in acute myeloid leukemia. Leukemia 2024, 38, 10–13. [Google Scholar] [CrossRef]
- Rodríguez-Arbolí, E.; Othus, M.; Freeman, S.D.; Buccisano, F.; Ngai, L.L.; Thomas, I.; Palmieri, R.; Cloos, J.; Johnson, S.; Meddi, E.; et al. Optimal prognostic threshold for measurable residual disease positivity by multiparameter flow cytometry in acute myeloid leukemia (AML). Leukemia 2024, 38, 2266–2269. [Google Scholar] [CrossRef]
- Freeman, S.D. Delving the depths of measurable residual disease negativity in acute myeloid leukemia. Haematologica 2022, 107, 2776–2778. [Google Scholar] [CrossRef] [PubMed]
- Blachly, J.S.; Walter, R.B.; Hourigan, C.S. The present and future of measurable residual disease testing in acute myeloid leukemia. Haematologica 2022, 107, 2810–2822. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.A.; Wood, B.L.; Othus, M.; Hourigan, C.S.; Ustun, C.; Linden, M.A.; DeFor, T.E.; Malagola, M.; Anthias, C.; Valkova, V.; et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis. Haematologica 2017, 102, 865–873. [Google Scholar] [CrossRef]
- Naik, S.; Rakszawski, K.; Zheng, H.; Claxton, D.; Minagawa, K.; Mineishi, S. Clofarabine Preconditioning followed by Allogeneic Transplant Using TBI and Post-Transplant Cyclophosphamide for Relapsed Refractory Leukemia. Int. J. Mol. Sci. 2024, 25, 957. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Badar, T.; Atallah, E.; Shallis, R.M.; Goldberg, A.D.; Patel, A.; Abaza, Y.; Bewersdorf, J.P.; Saliba, A.N.; Correia, G.S.C.; Murthy, G.; et al. Outcomes of TP53-mutated AML with evolving frontline therapies: Impact of allogeneic stem cell transplantation on survival. Am. J. Hematol. 2022, 97, E232–E235. [Google Scholar] [CrossRef]
- Badar, T.; Atallah, E.; Shallis, R.; Saliba, A.N.; Patel, A.; Bewersdorf, J.P.; Grenet, J.; Stahl, M.; Duvall, A.; Burkart, M.; et al. Survival of TP53-mutated acute myeloid leukemia patients receiving allogeneic stem cell transplantation after first induction or salvage therapy: Results from the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND). Leukemia 2023, 37, 799–806. [Google Scholar] [CrossRef]
- Sallman, D.A.; Stahl, M. TP53-Mutated Acute Myeloid Leukemia: How Can We Improve Outcomes? Blood 2024, in press. [Google Scholar] [CrossRef]
- Versluis, J.; Lindsley, R.C. Transplant for TP53-mutated MDS and AML: Because we can or because we should? Hematol. Am. Soc. Hematol. Educ. Program. 2022, 2022, 522–527. [Google Scholar] [CrossRef]
- Nawas, M.T.; Kosuri, S. Utility or futility? A contemporary approach to allogeneic hematopoietic cell transplantation for TP53-mutated MDS/AML. Blood Adv. 2024, 8, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; Labopin, M.; Craddock, C.; Cornelissen, J.J.; Labussiere-Wallet, H.; Wagner-Drouet, E.M.; Van Gorkom, G.; Schaap, N.P.M.; Kroger, N.M.; Veelken, J.H.; et al. Additional cytogenetic features determine outcome in patients allografted for TP53 mutant acute myeloid leukemia. Cancer 2022, 128, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Murdock, H.M.; Kim, H.T.; Denlinger, N.; Vachhani, P.; Hambley, B.; Manning, B.S.; Gier, S.; Cho, C.; Tsai, H.K.; McCurdy, S.; et al. Impact of diagnostic genetics on remission MRD and transplantation outcomes in older patients with AML. Blood 2022, 139, 3546–3557. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Chilkulwar, A.; Saliba, R.M.; Chen, J.; Rondon, G.; Patel, K.P.; Khogeer, H.; Shah, A.R.; Randolph, B.V.; Perez, J.M.R.; et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood 2018, 131, 2989–2992. [Google Scholar] [CrossRef]
- Marvin-Peek, J.; Mason, E.F.; Kishtagari, A.; Jayani, R.V.; Dholaria, B.; Kim, T.K.; Engelhardt, B.G.; Chen, H.; Strickland, S.; Savani, B.; et al. TP53 Mutations Are Associated with Increased Infections and Reduced Hematopoietic Cell Transplantation Rates in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Transplant. Cell Ther. 2023, 29, 390.e1–390.e10. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef]
- Byrne, M.T.; Kurian, T.J.; Patel, D.A.; Tamari, R.; Hong, S.; Abdelhakim, H.; Klein, V.; Rojas, P.; Madhavan, R.; Kent, A.; et al. Non-Relapse Mortality in TP53-Mutated MDS/AML—A Multi-Center Collaborative Study. Blood 2021, 138, 2922. [Google Scholar] [CrossRef]
- Chan, O.; Hunter, A.; Talati, C.; Sallman, D.A.; Asghari, H.; Song, J.; Hussaini, M.; Bejanyan, N.; Elmariah, H.; Kuykendall, A.T.; et al. Impact of TP53 gene Mutation Clearance and Conditioning Intensity on Outcome in MDS or AML Patients Prior to Allogeneic Stem Cell Transplantation. Blood 2019, 134, 149. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef]
- Cluzeau, T.; Sebert, M.; Rahme, R.; Cuzzubbo, S.; Lehmann-Che, J.; Madelaine, I.; Peterlin, P.; Beve, B.; Attalah, H.; Chermat, F.; et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myelodysplasies (GFM). J. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar] [CrossRef]
- Mishra, A.; Tamari, R.; DeZern, A.E.; Byrne, M.T.; Gooptu, M.; Chen, Y.B.; Deeg, H.J.; Sallman, D.; Gallacher, P.; Wennborg, A.; et al. Eprenetapopt Plus Azacitidine After Allogeneic Hematopoietic Stem-Cell Transplantation for. J. Clin. Oncol. 2022, 40, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.G.; Vyas, P.; Kambhampati, S.; Al Malki, M.M.; Larson, R.A.; Asch, A.S.; Mannis, G.; Chai-Ho, W.; Tanaka, T.N.; Bradley, T.J.; et al. Tolerability and Efficacy of the Anticluster of Differentiation 47 Antibody Magrolimab Combined With Azacitidine in Patients With Previously Untreated AML: Phase Ib Results. J. Clin. Oncol. 2023, 41, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Al Malki, M.M.; Asch, A.S.; Wang, E.S.; Jurcic, J.G.; Bradley, T.J.; Flinn, I.W.; Pollyea, D.A.; Kambhampati, S.; Tanaka, T.N.; et al. Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study. J. Clin. Oncol. 2023, 41, 2815–2826. [Google Scholar] [CrossRef]
- Ravandi, F.; Cortes, J.; Faderl, S.; O’Brien, S.; Garcia-Manero, G.; Verstovsek, S.; Santos, F.P.S.; Shan, J.; Brandt, M.; de Lima, M.; et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010, 116, 5818–5823. [Google Scholar] [CrossRef]
- Othus, M.; Appelbaum, F.R.; Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Nevill, T.; Brandwein, J.; Larson, R.A.; Stiff, P.J.; Walter, R.B.; et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.; Labopin, M.; Pillai, S.; Finke, J.; Bunjes, D.; Greinix, H.; Ehninger, G.; Steckel, N.K.; Zander, A.R.; Schwerdtfeger, R.; et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011, 25, 808–813. [Google Scholar] [CrossRef]
- Ferguson, P.; Craddock, C. Allogeneic transplantation in primary refractory AML. Bone Marrow Transplant. 2017, 52, 950–951. [Google Scholar] [CrossRef]
- Magenau, J.; Tobai, H.; Pawarode, A.; Braun, T.; Peres, E.; Reddy, P.; Kitko, C.; Choi, S.; Yanik, G.; Frame, D.; et al. Clofarabine and busulfan conditioning facilitates engraftment and provides significant antitumor activity in nonremission hematologic malignancies. Blood 2011, 118, 4258–4264. [Google Scholar] [CrossRef][Green Version]
- Magenau, J.; Westervelt, P.; Khaled, S.; McGuirk, J.; Hari, P.; Eapen, M.; Becker, P.S.; Parkin, B.; Braun, T.; Logan, B.; et al. A multicenter trial of myeloablative clofarabine and busulfan conditioning for relapsed or primary induction failure AML not in remission at the time of allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017, 52, 59–65. [Google Scholar] [CrossRef]
- Schmid, C.; Schleuning, M.; Schwerdtfeger, R.; Hertenstein, B.; Mischak-Weissinger, E.; Bunjes, D.; Harsdorf, S.V.; Scheid, C.; Holtick, U.; Greinix, H.; et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006, 108, 1092–1099. [Google Scholar] [CrossRef]
- Platzbecker, U.; Thiede, C.; Füssel, M.; Geissler, G.; Illmer, T.; Mohr, B.; Hänel, M.; Mahlberg, R.; Krümpelmann, U.; Weissinger, F.; et al. Reduced intensity conditioning allows for up-front allogeneic hematopoietic stem cell transplantation after cytoreductive induction therapy in newly-diagnosed high-risk acute myeloid leukemia. Leukemia 2006, 20, 707–714. [Google Scholar] [CrossRef]
- Stelljes, M.; Middeke, J.M.; Bug, G.; Wagner-Drouet, E.M.; Müller, L.P.; Schmid, C.; Krause, S.W.; Bethge, W.; Jost, E.; Platzbecker, U.; et al. Remission induction versus immediate allogeneic haematopoietic stem cell transplantation for patients with relapsed or poor responsive acute myeloid leukaemia (ASAP): A randomised, open-label, phase 3, non-inferiority trial. Lancet Haematol. 2024, 11, e324–e335. [Google Scholar] [CrossRef]
- Locke, F.L.; Artz, A.; Rich, E.; Zhang, Y.; van Besien, K.; Stock, W. Feasibility of clofarabine cytoreduction before allogeneic transplant conditioning for refractory AML. Bone Marrow Transplant. 2010, 45, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.S.; Publicover, A.; Hill, K.; Hurlock, C.; Newman, J.; McKeag, N.; Main, S.; Launders, H.; Orchard, K.H. Early Experience with Clofarabine Pre-Conditioning Prior to Full Intensity or Reduced Toxicity Allogeneic Stem Cell Transplantation: A Promising New Therapy for the Treatment of Refractory Acute Leukaemia. Blood 2012, 120, 4511. [Google Scholar] [CrossRef]
- Rakszawski, K.; Miki, K.; Claxton, D.; Wagner, H.; Shike, H.; Mineishi, S.; Naik, S. Clofarabine followed by haploidentical stem cell transplant using fludarabine, busulfan, and total-body irradiation with post-transplant cyclophosphamide in non-remission AML. Int. J. Hematol. 2018, 108, 348–350. [Google Scholar] [CrossRef]
- Oran, B.; de Lima, M.; Garcia-Manero, G.; Thall, P.F.; Lin, R.; Popat, U.; Alousi, A.M.; Hosing, C.; Giralt, S.; Rondon, G.; et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020, 4, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Keruakous, A.R.; Holter-Chakrabarty, J.; Schmidt, S.A.; Khawandanah, M.O.; Selby, G.; Yuen, C. Azacitidine Maintenance Therapy Post-Allogeneic Stem Cell Transplantation in Poor-Risk Acute Myeloid Leukemia. Hematol. Oncol. Stem Cell Ther. 2023, 16, 52–60. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Wang, S.; Kong, P.; Su, Y.; Hu, J.; Jiang, M.; Bai, H.; Lang, T.; Wang, J.; et al. Effect of rhG-CSF Combined With Decitabine Prophylaxis on Relapse of Patients With High-Risk MRD-Negative AML After HSCT: An Open-Label, Multicenter, Randomized Controlled Trial. J. Clin. Oncol. 2020, 38, 4249–4259. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, C.; Dai, H.; Yin, J.; Li, Z.; Chen, J.; Qiu, H.; Sun, A.; Miao, M.; Fu, C.; et al. Maintenance therapy with decitabine after allogeneic hematopoietic stem cell transplantation to prevent relapse of high-risk acute myeloid leukemia. Bone Marrow Transplant. 2020, 55, 1206–1208. [Google Scholar] [CrossRef]
- Appelbaum, F.R. Maintenance therapy after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Best. Pract. Res. Clin. Haematol. 2019, 32, 101109. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Levis, M.; Patnaik, M.M.; Scott, B.L.; Mohan, S.R.; Deol, A.; Rowley, S.D.; Kim, D.D.H.; Hernandez, D.; Rajkhowa, T.; et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2021, 56, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Gilteritinib as Post-Transplant Maintenance for AML with Internal Tandem Duplication Mutation of FLT3. J. Clin. Oncol. 2024, 42, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Erba, H.P.; Montesinos, P.; Kim, H.J.; Patkowska, E.; Vrhovac, R.; Zak, P.; Wang, P.N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond. Engl. 2023, 401, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Zhang, M.J.; Bacigalupo, A.A.; Bashey, A.; Appelbaum, F.R.; Aljitawi, O.S.; Armand, P.; Antin, J.H.; Chen, J.; Devine, S.M.; et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015, 126, 1033–1040. [Google Scholar] [CrossRef]
- Mehta, R.S.; Saliba, R.M.; Chen, J.; Rondon, G.; Hammerstrom, A.E.; Alousi, A.; Qazilbash, M.; Bashir, Q.; Ahmed, S.; Popat, U.; et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br. J. Haematol. 2016, 173, 444–455. [Google Scholar] [CrossRef]
- Xuan, L.; Wang, Y.; Huang, F.; Fan, Z.; Xu, Y.; Sun, J.; Xu, N.; Deng, L.; Li, X.; Liang, X.; et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: An open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020, 21, 1201–1212. [Google Scholar] [CrossRef]
- Sandmaier, B.M.; Khaled, S.; Oran, B.; Gammon, G.; Trone, D.; Frankfurt, O. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am. J. Hematol. 2018, 93, 222–231. [Google Scholar] [CrossRef]
- Oran, B.; Marin, D.; Bashir, Q.; Ahmed, S.; Olson, A.L.; Popat, U.; Kebriaei, P.; Shpall, E.J.; Jain, V.; Champlin, R.E. Long-Term Follow-up of Crenolanib Maintenance after Allogeneic Transplantation in Newly Diagnosed and Relapsed FLT3 Mutated AML. Blood 2024, 144, 1497. [Google Scholar] [CrossRef]
- Fathi, A.T.; Kim, H.T.; Soiffer, R.J.; Levis, M.J.; Li, S.; Kim, A.S.; DeFilipp, Z.; El-Jawahri, A.; McAfee, S.L.; Brunner, A.M.; et al. Multicenter Phase I Trial of Ivosidenib as Maintenance Treatment Following Allogeneic Hematopoietic Cell Transplantation for IDH1-Mutated Acute Myeloid Leukemia. Clin. Cancer Res. 2023, 29, 2034–2042. [Google Scholar] [CrossRef]
- Fathi, A.T.; Kim, H.T.; Soiffer, R.J.; Levis, M.J.; Li, S.; Kim, A.S.; Mims, A.S.; DeFilipp, Z.; El-Jawahri, A.; McAfee, S.L.; et al. Enasidenib as maintenance following allogeneic hematopoietic cell transplantation for IDH2-mutated myeloid malignancies. Blood Adv. 2022, 6, 5857–5865. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Bejanyan, N.; Yang, D.; Mokhtari, S.; Al Malki, M.M.; Sandhu, K.S.; Faramand, R.; Aldoss, I.; Artz, A.S.; Aribi, A.; et al. Multicenter Pilot Clinical Trial of Enasidenib As Maintenance Therapy after Allogeneic Hematopoietic Cell Transplantation in Patients with Acute Myeloid Leukemia (AML) Carrying IDH2 Mutations. Blood 2022, 140, 1890–1892. [Google Scholar] [CrossRef]
- Kent, A.; Schwartz, M.; McMahon, C.; Amaya, M.; Smith, C.A.; Tobin, J.; Marciano, K.; Rezac, R.; Bosma, G.; Pollyea, D.A.; et al. Venetoclax is safe and tolerable as post-transplant maintenance therapy for AML patients at high risk for relapse. Bone Marrow Transplant. 2023, 58, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiong, X.; Li, X.; Lu, W.; He, X.; Jin, X.; Sun, R.; Lyu, H.; Yuan, T.; Sun, T.; et al. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome. Cancer Sci. 2021, 112, 3636–3644. [Google Scholar] [CrossRef]
- Garcia, J.S.; Kim, H.T.; Murdock, H.M.; Ansuinelli, M.; Brock, J.; Cutler, C.S.; Gooptu, M.; Ho, V.T.; Koreth, J.; Nikiforow, S.; et al. Prophylactic maintenance with venetoclax/azacitidine after reduced-intensity conditioning allogeneic transplant for high-risk MDS and AML. Blood Adv. 2024, 8, 978–990. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.; Oran, B.; Champlin, R.E.; Papadopoulos, E.B.; Giralt, S.A.; Scott, B.L.; William, B.M.; Hetzer, J.; Laille, E.; Hubbell, B.; et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2018, 24, 2017–2024. [Google Scholar] [CrossRef]
- Pusic, I.; Choi, J.; Fiala, M.A.; Gao, F.; Holt, M.; Cashen, A.F.; Vij, R.; Abboud, C.N.; Stockerl-Goldstein, K.E.; Jacoby, M.A.; et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2015, 21, 1761–1769. [Google Scholar] [CrossRef]
- Tallman, M.S.; Rowlings, P.A.; Milone, G.; Zhang, M.J.; Perez, W.S.; Weisdorf, D.; Keating, A.; Gale, R.P.; Geller, R.B.; Laughlin, M.J.; et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood 2000, 96, 1254–1258. [Google Scholar]
- Yeshurun, M.; Labopin, M.; Blaise, D.; Cornelissen, J.J.; Sengeloev, H.; Vindelov, L.; Kuball, J.; Chevallier, P.; Craddock, C.; Socie, G.; et al. Impact of postremission consolidation chemotherapy on outcome after reduced-intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia in first complete remission: A report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2014, 120, 855–863. [Google Scholar] [CrossRef]
- Yeshurun, M.; Wolach, O. When should patients receive consolidation chemotherapy before allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first complete remission? Curr. Opin. Hematol. 2018, 25, 75–80. [Google Scholar] [CrossRef]
- Appelbaum, F.R. Consolidation chemotherapy prior to hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Best. Pract. Res. Clin. Haematol. 2016, 29, 365–371. [Google Scholar] [CrossRef]
- Rashidi, A.; Linden, M.A.; DeFor, T.E.; Warlick, E.; Bejanyan, N.; Yohe, S.; Weisdorf, D.J.; Ustun, C. History of consolidation is prognostic in acute myeloid leukemia patients undergoing allogeneic hematopoietic cell transplantation in minimal residual disease-negative first complete remission. Am. J. Hematol. 2017, 92, 1032–1036. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Xiao, L.; Kadia, T.; Daver, N.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Menghrajani, K.; Derkach, A.; Chan, A.; Xiao, W.; Glass, J.; King, A.C.; Daniyan, A.F.; Famulare, C.; Cuello, B.M.; et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021, 5, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef]
- Brodsky, R.A.; Petri, M.; Smith, B.D.; Seifter, E.J.; Spivak, J.L.; Styler, M.; Dang, C.V.; Brodsky, I.; Jones, R.J. Immunoablative high-dose cyclophosphamide without stem-cell rescue for refractory, severe autoimmune disease. Ann. Intern. Med. 1998, 129, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Al-Homsi, A.S.; Roy, T.S.; Cole, K.; Feng, Y.; Duffner, U. Post-Transplant High-Dose Cyclophosphamide for the Prevention of Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 604–611. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Ganguly, S.; Zahurak, M.; Bolaños-Meade, J.; Thoburn, C.; Perkins, B.; Fuchs, E.J.; Jones, R.J.; Hess, A.D.; Luznik, L. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci. Transl. Med. 2013, 5, 211ra157. [Google Scholar] [CrossRef]
- Wachsmuth, L.P.; Patterson, M.T.; Eckhaus, M.A.; Venzon, D.J.; Gress, R.E.; Kanakry, C.G. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J. Clin. Investig. 2019, 129, 2357–2373. [Google Scholar] [CrossRef]
- Ikegawa, S.; Meguri, Y.; Kondo, T.; Sugiura, H.; Sando, Y.; Nakamura, M.; Iwamoto, M.; Maeda, Y.; Matsuoka, K.-I. PTCy ameliorates GVHD by restoring regulatory and effector T-cell homeostasis in recipients with PD-1 blockade. Blood Adv. 2019, 3, 4081–4094. [Google Scholar] [CrossRef]
- Mancusi, A.; Piccinelli, S.; Velardi, A.; Pierini, A. CD4+FOXP3+ Regulatory T Cell Therapies in HLA Haploidentical Hematopoietic Transplantation. Front. Immunol. 2019, 10, 2901. [Google Scholar] [CrossRef] [PubMed]
- Cioccio, J.; Rakszawski, K.; Zheng, H.; Nickolich, M.; Naik, S.; Wirk, B.; Rybka, W.; Ehmann, W.; Silar, B.; Vajdic, C.; et al. Post-Transplant Cyclophosphamide As GVHD Prophylaxis Eliminates GVHD Mortality and Improves Overall Survival in Alternative Donor Allogeneic Stem Cell Transplant. Transplant. Cell. Ther. 2021, 27, S241–S242. [Google Scholar] [CrossRef]
- Jamy, O.; Innis-Shelton, R.; Bal, S.; Paluri, R.; Salzman, D.; Di Stasi, A.; Costa, L.; Meredith, R.; Lamb, L.; Minagawa, K.; et al. Phase II clinical trial of one dose of post-transplant cyclophosphamide for graft versus host disease prevention following myeloablative, peripheral blood stem cell, matched-unrelated donor transplantation. Am. J. Hematol. 2021, 96, E396–E398. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, S.R.; Hyder, M.A.; Sabina, R.; DeVries, A.; Chalupa, K.; Berryman, N.; Bernhardt, J.; Cusmano, A.C.; Chai, A.; Schwartz, R.; et al. Interim Results of a Phase I/II Trial of Intermediate-Dose Post-Transplantation Cyclophosphamide after Reduced Intensity Conditioned HLA-Mismatched Bone Marrow Transplantation for Older or Unfit Patients. Transplant. Cell. Ther. 2024, 30, S2–S3. [Google Scholar] [CrossRef]
- Slade, M.; Goldsmith, S.; Romee, R.; DiPersio, J.F.; Dubberke, E.R.; Westervelt, P.; Uy, G.L.; Lawrence, S.J. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl. Infect. Dis. 2017, 19, e12629. [Google Scholar] [CrossRef]
- Irene, G.-C.; Albert, E.; Anna, B.-V.; Rahinatu, A.; Silvana, N.; Silvana, S.; Ana, G.; Jordi, L.; Carolina, C.A.; Miquel, G.; et al. Patterns of infection and infectious-related mortality in patients receiving post-transplant high dose cyclophosphamide as graft-versus-host-disease prophylaxis: Impact of HLA donor matching. Bone Marrow Transplant. 2021, 56, 818–827. [Google Scholar] [CrossRef]
- Fayard, A.; Daguenet, E.; Blaise, D.; Chevallier, P.; Labussière, H.; Berceanu, A.; Yakoub-Agha, I.; Socié, G.; Charbonnier, A.; Suarez, F.; et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: A study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant. 2019, 54, 1586–1594. [Google Scholar] [CrossRef]
- Cieri, N.; Oliveira, G.; Greco, R.; Forcato, M.; Taccioli, C.; Cianciotti, B.; Valtolina, V.; Noviello, M.; Vago, L.; Bondanza, A.; et al. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood 2015, 125, 2865–2874. [Google Scholar] [CrossRef]
- Zulch, E.; Inoue, Y.; Cioccio, J.; Rakszawski, K.; Songdej, N.; Nickolich, M.; Zheng, H.; Naik, S.; Rybka, W.; Ehmann, C.; et al. Impact of post-transplant cyclophosphamide and splenomegaly on primary graft failure and multi-lineage cytopenia after allogeneic hematopoietic cell transplantation. Leuk. Res. 2024, 143, 107530. [Google Scholar] [CrossRef]
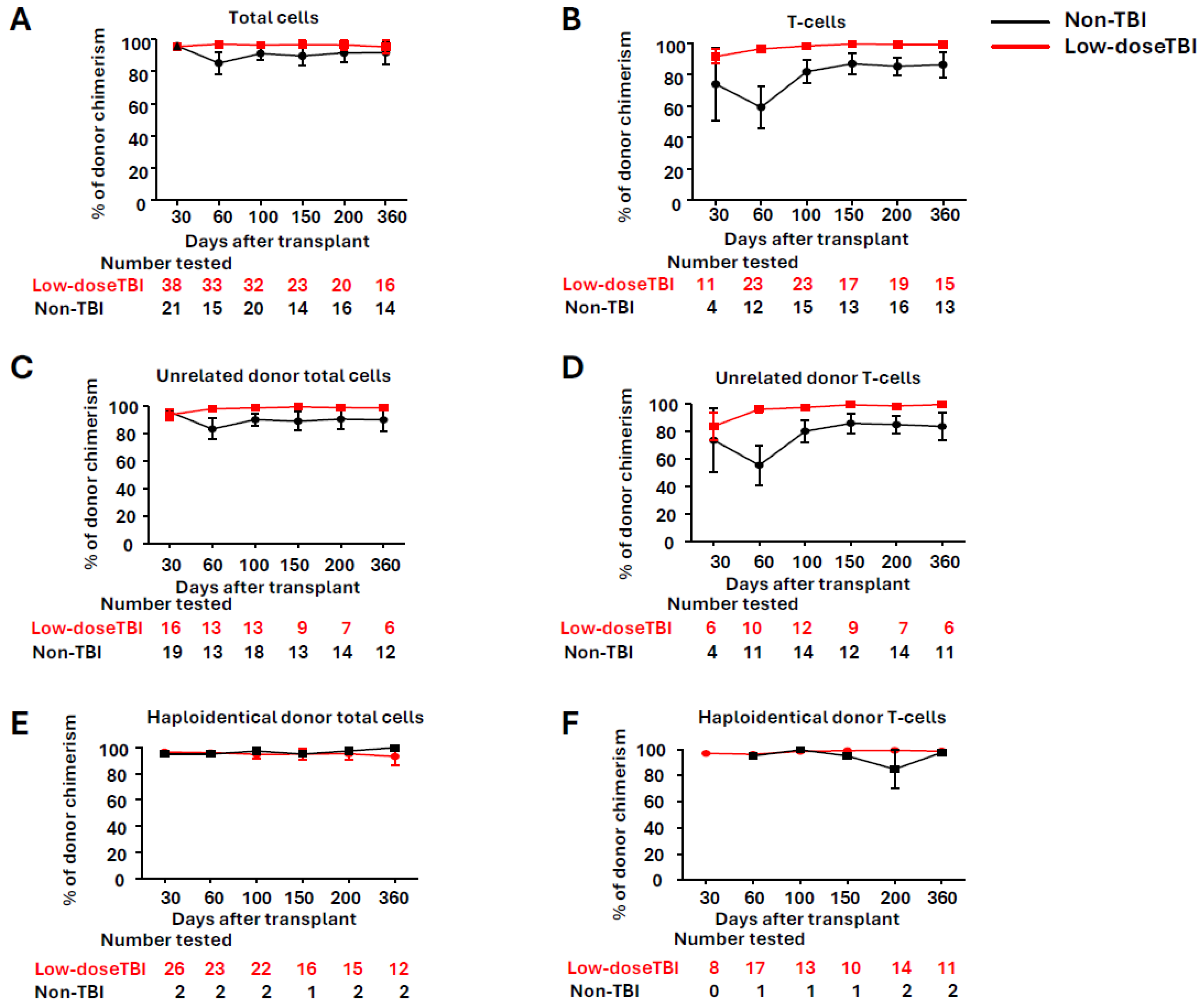
- Shah, N.; Cioccio, J.; Rakszawski, K.; Zheng, H.; Nickolich, M.; Naik, S.; Wirk, B.; Rybka, W.; Ehmann, C.; Silar, B.; et al. Low-dose total body irradiation promotes T-cells donor chimerism in reduced-intensity/non-myeloablative allogeneic stem cell transplant with post-transplant cyclophosphamide. Leuk. Res. 2022, 123, 106969. [Google Scholar] [CrossRef]
- Hexner, E.O.; DeFilipp, Z. Update in GVHD Prophylaxis: Novel Pharmacologic and Graft Manipulation Strategies. Am. J. Hematol. 2025, 100, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Brissot, E.; Labopin, M.; Moiseev, I.; Cornelissen, J.J.; Meijer, E.; Van Gorkom, G.; Rovira, M.; Ciceri, F.; Griskevicius, L.; Blaise, D.; et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J. Hematol. Oncol. 2020, 13, 87. [Google Scholar] [CrossRef]
- Nagler, A.; Labopin, M.; Swoboda, R.; Schroeder, T.; Hamladji, R.M.; Griskevicius, L.; Salmenniemi, U.; Rambaldi, A.; Mielke, S.; Kulagin, A.; et al. Post-transplant cyclophosphamide, calcineurin inhibitor, and mycophenolate mofetil compared to anti-thymocyte globulin, calcineurin inhibitor, and methotrexate combinations as graft-versus-host disease prophylaxis post allogeneic stem cell transplantation from sibling and unrelated donors in patients with acute myeloid leukemia: A study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2024, 59, 1012–1021. [Google Scholar] [CrossRef]
- Ngwube, A.; Rangarajan, H.; Shah, N. Role of abatacept in the prevention of graft-versus-host disease: Current perspectives. Ther. Adv. Hematol. 2023, 14, 20406207231152644. [Google Scholar] [CrossRef]
- Watkins, B.; Qayed, M.; McCracken, C.; Bratrude, B.; Betz, K.; Suessmuth, Y.; Yu, A.; Sinclair, S.; Furlan, S.; Bosinger, S.; et al. Phase II Trial of Costimulation Blockade With Abatacept for Prevention of Acute GVHD. J. Clin. Oncol. 2021, 39, 1865–1877. [Google Scholar] [CrossRef]
- Kean, L.S.; Burns, L.J.; Kou, T.D.; Kapikian, R.; Lozenski, K.; Langston, A.; Horan, J.T.; Watkins, B.; Qayed, M.; Bratrude, B.; et al. Abatacept for acute graft-versus-host disease prophylaxis after unrelated donor hematopoietic cell transplantation. Blood 2024, 144, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.H.; Hoeg, R.; Moroz, A.; Xei, B.; Wu, H.-H.; Pawar, R.; Heydari, K.; Miklos, D.B.; Shiraz, P.; Muffly, L.S.; et al. Orca-T, a Precision Treg-Engineered Donor Product, Prevents Acute Gvhd with Less Immunosuppression in an Early Multicenter Experience with Myeloablative HLA-Matched Transplants. Blood 2020, 136, 47–48. [Google Scholar] [CrossRef]
- Hoeg, R.T.; Moroz, A.; Gandhi, A.; Muffly, L.; Shiraz, P.; Oliai, C.; Mehta, R.S.; Srour, S.A.; McGuirk, J.P.; Waller, E.K.; et al. Orca-T Results in High Gvhd-Free and Relapse-Free Survival Following Myeloablative Conditioning for Hematological Malignancies: Results of a Single Center Phase 2 and a Multicenter Phase 1b Study. Blood 2021, 138, 98. [Google Scholar] [CrossRef]
- Meyer, E.H.; Srour, S.A.; Oliai, C.; Hoeg, R.T.; Gandhi, A.P.; Muffly, L.S.; Mehta, R.S.; Waller, E.K.; Lowsky, R.; Patel, S.S.; et al. Observational Comparison of Overall Survival between Phase 1b Orca-T and Registry-Based Post-Transplant Cyclophosphamide Patients. Transplant. Cell. Ther. Off. Publ. Am. Soc. Transplant. Cell. Ther. 2025, 31, S108–S109. [Google Scholar] [CrossRef]
- Srour, S.; Pantin, J.; Patel, S.; Faramand, R.; Gandhi, A.; Salhotra, A.; Oliai, C.; Dholaria, B.; Pavlova, A.; McClellan, S.; et al. AML-521 Treatment of Acute Myeloid Leukemia With Orca-T. Clin. Lymphoma Myeloma Leuk. 2024, 24, S317. [Google Scholar] [CrossRef]
- Abedi, M.; Srour, S.A.; Salhotra, A.; Hoeg, R.T.; Waller, E.K.; Choe, H.; Pavlova, A.; Zharkevich, T.; McClellan, J.S.; Fernhoff, N.B.; et al. Preliminary Safety and Efficacy of Myeloablative Orca-Q with No GvHD Prophylaxis for Treatment of Advanced Hematologic Malignancies. Blood 2024, 144, 382. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Colditz, G.A.; DiPersio, J.F. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol. Blood Marrow Transplant. 2016, 22, 651–657. [Google Scholar] [CrossRef]
- Tey, S.K.; Lane, S.W. Better the cure you know: Why patients with AML ≥ 60 years of age should be offered early allogeneic stem cell transplantation. Blood Adv. 2022, 6, 1619–1622. [Google Scholar] [CrossRef]
- Ustun, C.; Le-Rademacher, J.; Wang, H.L.; Othus, M.; Sun, Z.; Major, B.; Zhang, M.J.; Storrick, E.; Lafky, J.M.; Chow, S.; et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): An alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia 2019, 33, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Ringden, O.; Boumendil, A.; Labopin, M.; Canaani, J.; Beelen, D.; Ehninger, G.; Niederwieser, D.; Finke, J.; Stelljes, M.; Gerbitz, A.; et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients Age > 69 Years with Acute Myelogenous Leukemia: On Behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Iqbal, Q.; Amin, M.K.; Kasaiean, A.; Oskouie, I.M.; Warraich, S.Z.; Yu, J.; Anwar, I.; Jaglal, M.; Mushtaq, M.U. Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged 70 Years and Older: A Systematic Review and Meta-Analysis. Transplant. Cell Ther. 2025, 31, 172.e1–172.e13. [Google Scholar] [CrossRef] [PubMed]
- Olin, R.L.; Fretham, C.; Pasquini, M.C.; Arora, M.; Bhatt, V.R.; Derman, B.; Giralt, S.A.; Huang, L.W.; Koll, T.; Lee, S.M.; et al. Geriatric assessment in older alloHCT recipients: Association of functional and cognitive impairment with outcomes. Blood Adv. 2020, 4, 2810–2820. [Google Scholar] [CrossRef]
- Artz, A.; Logan, B.R.; Saber, W.; Geller, N.; Bellach, A.; Kou, J.; Wood, W.; McCarty, J.M.; Knight, T.G.; Runaas, L.; et al. The Composite Health Risk Assessment Model (CHARM) to Predict 1-Year Non-Relapse Mortality (NRM) Among Older Recipients of Allogeneic Transplantation: A Prospective BMT-CTN Study 1704. Blood 2023, 142, 109. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Russo, D.; Schmitt, M.; Pilorge, S.; Stelljes, M.; Kawakita, T.; Teal, V.L.; Haber, B.; Bopp, C.; Dadwal, S.S.; Badshah, C. Efficacy and safety of extended duration letermovir prophylaxis in recipients of haematopoietic stem-cell transplantation at risk of cytomegalovirus infection: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Haematol. 2024, 11, e127–e135. [Google Scholar] [CrossRef]
- Elmaagacli, A.H.; Steckel, N.K.; Koldehoff, M.; Hegerfeldt, Y.; Trenschel, R.; Ditschkowski, M.; Christoph, S.; Gromke, T.; Kordelas, L.; Ottinger, H.D.; et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: Evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011, 118, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Leisenring, W.M.; Xie, H.; Walter, R.B.; Mielcarek, M.; Sandmaier, B.M.; Riddell, S.R.; Boeckh, M. CMV reactivation after allogeneic HCT and relapse risk: Evidence for early protection in acute myeloid leukemia. Blood 2013, 122, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Elmaagacli, A.H.; Koldehoff, M. Cytomegalovirus replication reduces the relapse incidence in patients with acute myeloid leukemia. Blood 2016, 128, 456–459. [Google Scholar] [CrossRef]
- Morishita, T.; Martin, P.J.; Inamoto, Y. Treatment Response in Individual Organs Affected by Chronic Graft-Versus-Host Disease. Cells 2025, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.E. Prediction and Prognostication of Acute Graft-Versus-Host Disease by MAGIC Biomarkers. Am. J. Hematol. 2025, 100 (Suppl. S3), 5–13. [Google Scholar] [CrossRef]
- Zeiser, R.; Bubnoff, N.v.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. New Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Zeiser, R.; Polverelli, N.; Ram, R.; Hashmi, S.K.; Chakraverty, R.; Middeke, J.M.; Musso, M.; Giebel, S.; Uzay, A.; Langmuir, P.; et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2021, 385, 228–238. [Google Scholar] [CrossRef]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Cutler, C.; Lee, S.J.; Arai, S.; Rotta, M.; Zoghi, B.; Lazaryan, A.; Ramakrishnan, A.; DeFilipp, Z.; Salhotra, A.; Chai-Ho, W.; et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar Study. Blood 2021, 138, 2278–2289. [Google Scholar] [CrossRef]
- Wolff, D.; Cutler, C.; Lee, S.J.; Pusic, I.; Bittencourt, H.; White, J.; Hamadani, M.; Arai, S.; Salhotra, A.; Perez-Simon, J.A.; et al. Axatilimab in Recurrent or Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2024, 391, 1002–1014. [Google Scholar] [CrossRef]
- McDonald, G.B.; Sharma, P.; Matthews, D.E.; Shulman, H.M.; Thomas, D.E. Venocclusive Disease of the Liver after Bone Marrow Transplantation: Diagnosis, Incidence, and Predisposing Factors. Hepatology 1984, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Lee, K.S.; Beschorner, W.E.; Vogel, V.G.; Grochow, L.B.; Braine, H.G.; Vogelsang, G.B.; Sensenbrenner, L.L.; Santos, G.W.; Saral, R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987, 44, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; Blaise, D.; Brissot, E.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 2023, 58, 749–754. [Google Scholar] [CrossRef]
- Nishida, M.; Kahata, K.; Hayase, E.; Shigematsu, A.; Sato, M.; Kudo, Y.; Omotehara, S.; Iwai, T.; Sugita, J.; Shibuya, H.; et al. Novel Ultrasonographic Scoring System of Sinusoidal Obstruction Syndrome after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 1896–1900. [Google Scholar] [CrossRef]
- Kernan, N.A.; Grupp, S.; Smith, A.R.; Arai, S.; Triplett, B.; Antin, J.H.; Lehmann, L.; Shore, T.; Ho, V.T.; Bunin, N.; et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br. J. Haematol. 2018, 181, 816–827. [Google Scholar] [CrossRef]
- Grupp, S.A.; Corbacioglu, S.; Kang, H.J.; Teshima, T.; Khaw, S.L.; Locatelli, F.; Maertens, J.; Stelljes, M.; Stepensky, P.; Lopez, P.; et al. Defibrotide plus best standard of care compared with best standard of care alone for the prevention of sinusoidal obstruction syndrome (HARMONY): A randomised, multicentre, phase 3 trial. Lancet Haematol. 2023, 10, e333–e345. [Google Scholar] [CrossRef]

| Molecular (NGS-Based, PCR-Based) | Multi-Color Flow Cytometry | Molecular Mutation Panel (NGS-Based) | Fluorescence In Situ Hybridization | Cytogenetics | |
|---|---|---|---|---|---|
| Targets | NPM1, FLT3-ITD, CBFB-MYH11, PML-RARα, etc. | Aberrant immunophenotypes | Myeloid panel (Detect around 50 leukemia-related genes) | KMT2A-MLL, Del17p, +8, −5q, etc. | NA |
| Strength | ▪ Disease-specific ▪ Very sensitive (up to 10−6) | Relatively sensitive (up to 10−3) | Comprehensive | Easily accessible | ▪ Easily accessible ▪ Comprehensive structural variant analysis |
| Weakness | ▪ Available only for limited mutations/translocations ▪ May fail to track MRD when clonal evolution occurs | ▪ Cell viability is critical ▪ Needs 500 K to 1 million CD45+ cells to achieve 0.1% sensitivity ▪ Less specific | ▪ Relatively low sensitivity (up to 10−2) ▪ Detection of clonal hematopoiesis of indeterminate potential (CHIP) mutations may not indicate relapse | ▪ Relatively low sensitivity (up to 10−2) ▪ Fail to detect short length of deletion or translocation | ▪ Hard to identify cryptic structural variances ▪ Low sensitivity (can analyze only 20 metaphase cells) |
| Modality | Suppress Donor T-Cells | Suppress Host T-Cells | Effect to Prevent Graft Failure | Problems | Comments |
|---|---|---|---|---|---|
| Intensifying Conditioning | No | Yes | Good | Toxicity | - |
| ATG | Yes | Yes | ? | Infection | Works in T-cell depleted transplant |
| Alemtuzumab | Yes | Yes | ? | Infection | Works in T-cell depleted transplant |
| TBI | No | Yes | Good | Toxicity | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Cioccio, J.; Mineishi, S.; Minagawa, K. Evolution of Allogeneic Stem Cell Transplantation: Main Focus on AML. Cells 2025, 14, 572. https://doi.org/10.3390/cells14080572
Inoue Y, Cioccio J, Mineishi S, Minagawa K. Evolution of Allogeneic Stem Cell Transplantation: Main Focus on AML. Cells. 2025; 14(8):572. https://doi.org/10.3390/cells14080572
Chicago/Turabian StyleInoue, Yoshitaka, Joseph Cioccio, Shin Mineishi, and Kentaro Minagawa. 2025. "Evolution of Allogeneic Stem Cell Transplantation: Main Focus on AML" Cells 14, no. 8: 572. https://doi.org/10.3390/cells14080572
APA StyleInoue, Y., Cioccio, J., Mineishi, S., & Minagawa, K. (2025). Evolution of Allogeneic Stem Cell Transplantation: Main Focus on AML. Cells, 14(8), 572. https://doi.org/10.3390/cells14080572






