Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animal Care, and Institutional/Ethical Approvals
2.2. Biospecimens
2.3. Cell Lines
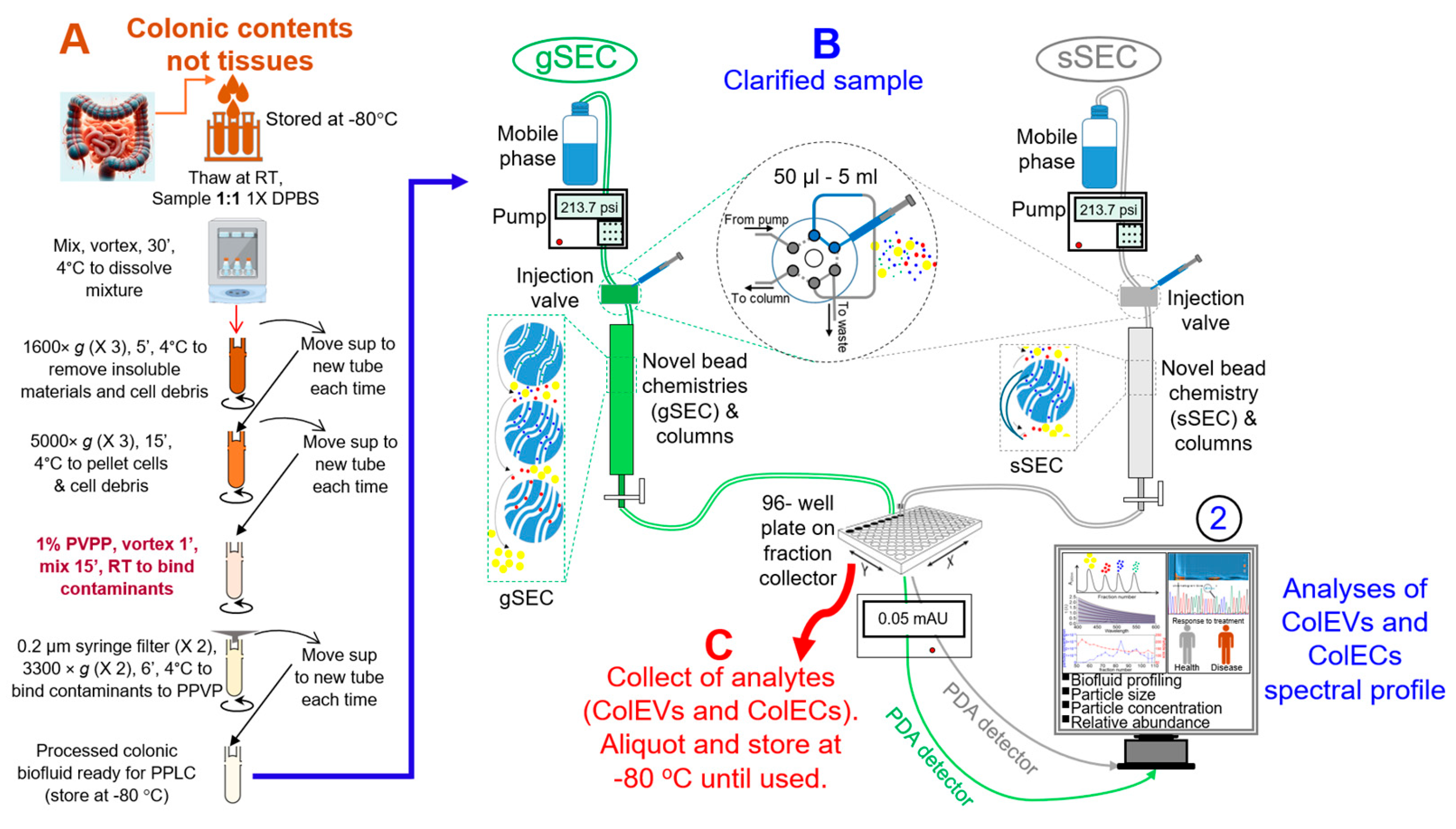
2.4. Preprocessing of Colonic Contents (Thawing and Pre-Treatment with PVPP) and Isolation of ColEVs
2.5. Nano Tracking Analysis (NTA)
2.6. Labeling of ColEVs and Uptake of EVs by TZM-GFP and JLAT-TAT-GFP Cells
2.7. Western Blot
2.8. Transmission Electron Microscopy (TEM)
2.9. Activation of HIV LTR Promoter
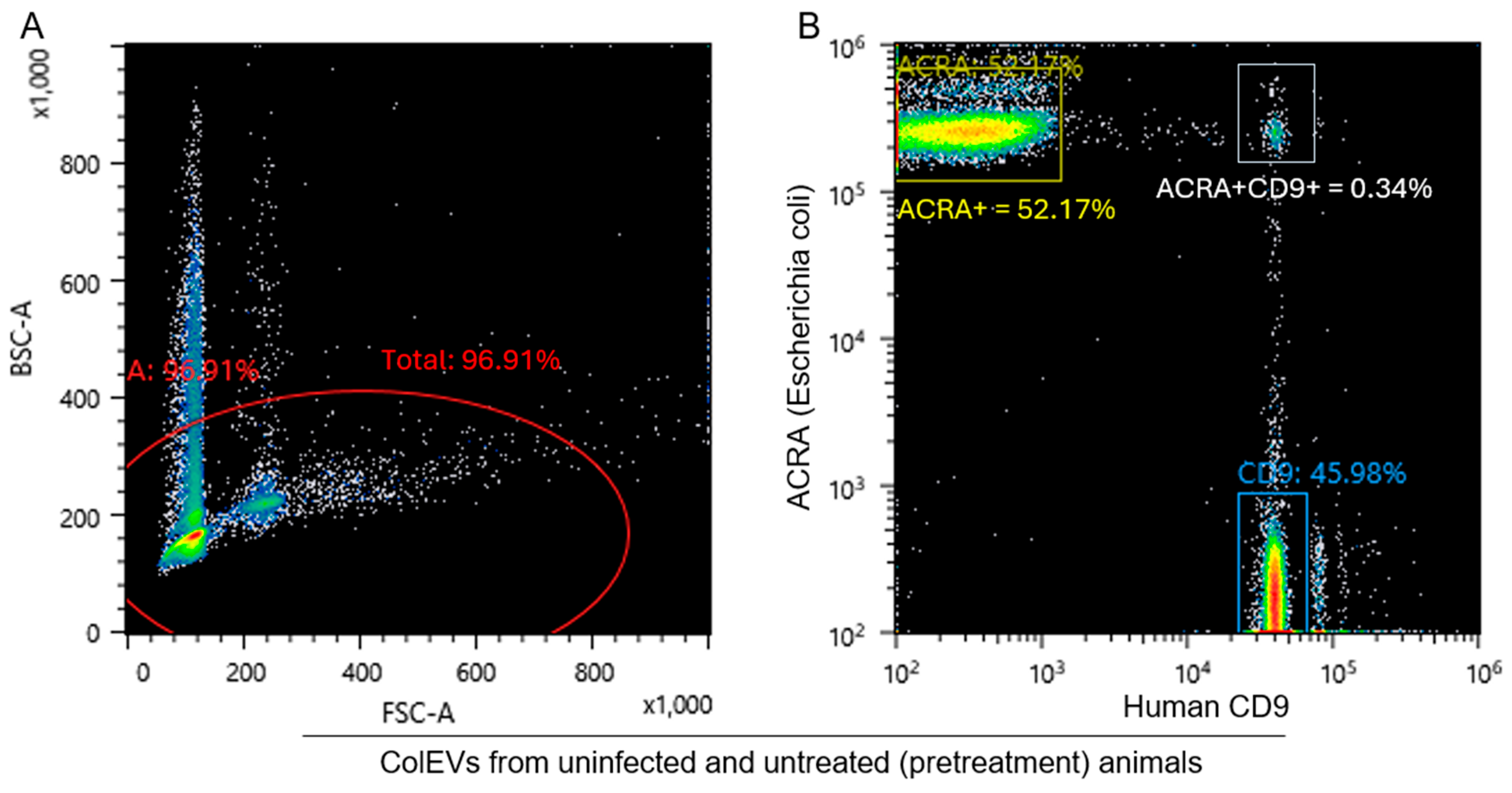
2.10. Sorting Colonic EVs Using Flow Cytometry
2.11. Normalization Approach for Cell-Based Assays
2.12. Statistical Analysis
3. Results
3.1. Development and Overview of the Protocol
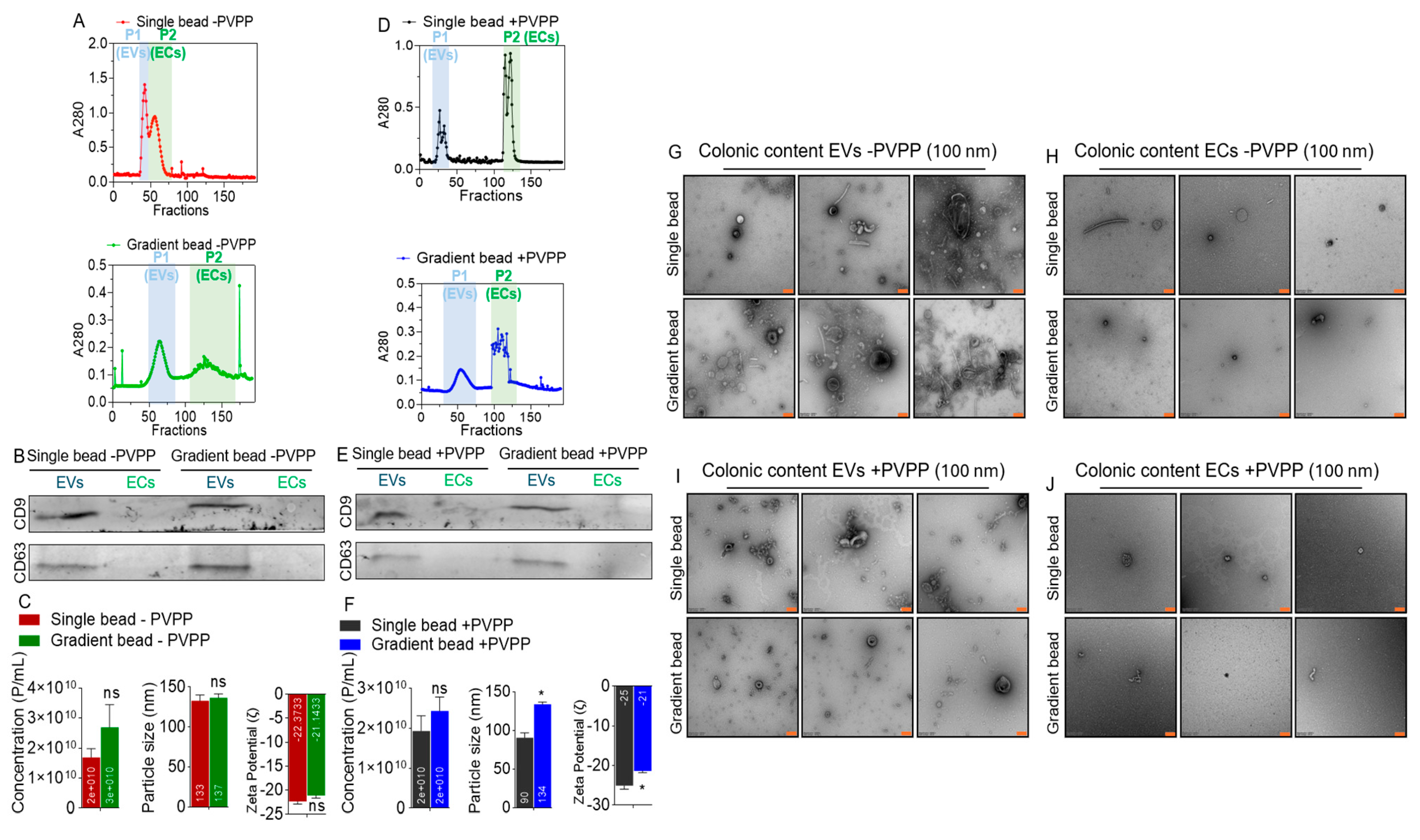
3.2. EVs but Not ECs Are Present in Colonic Contents of Rhesus Macaques
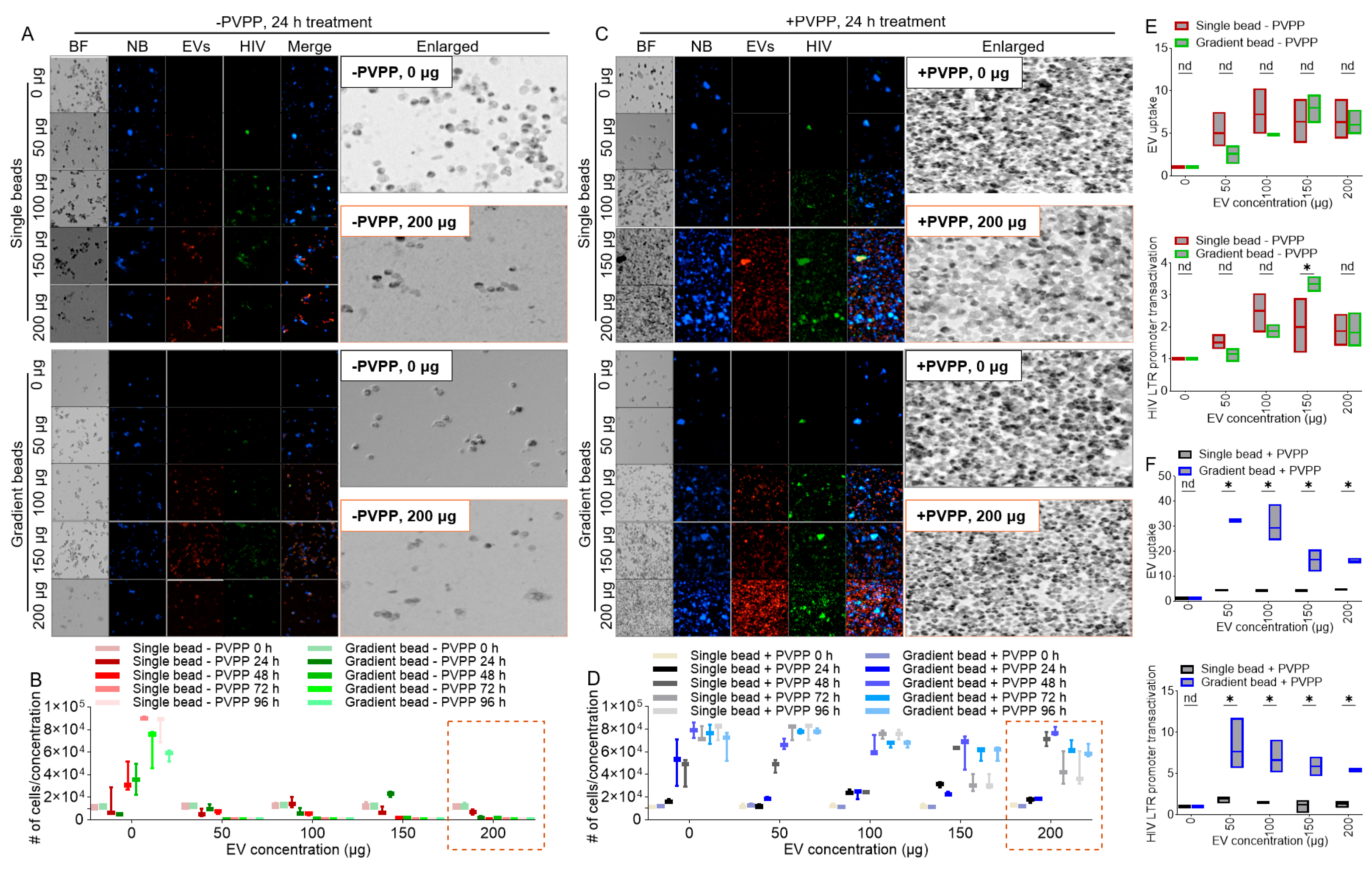
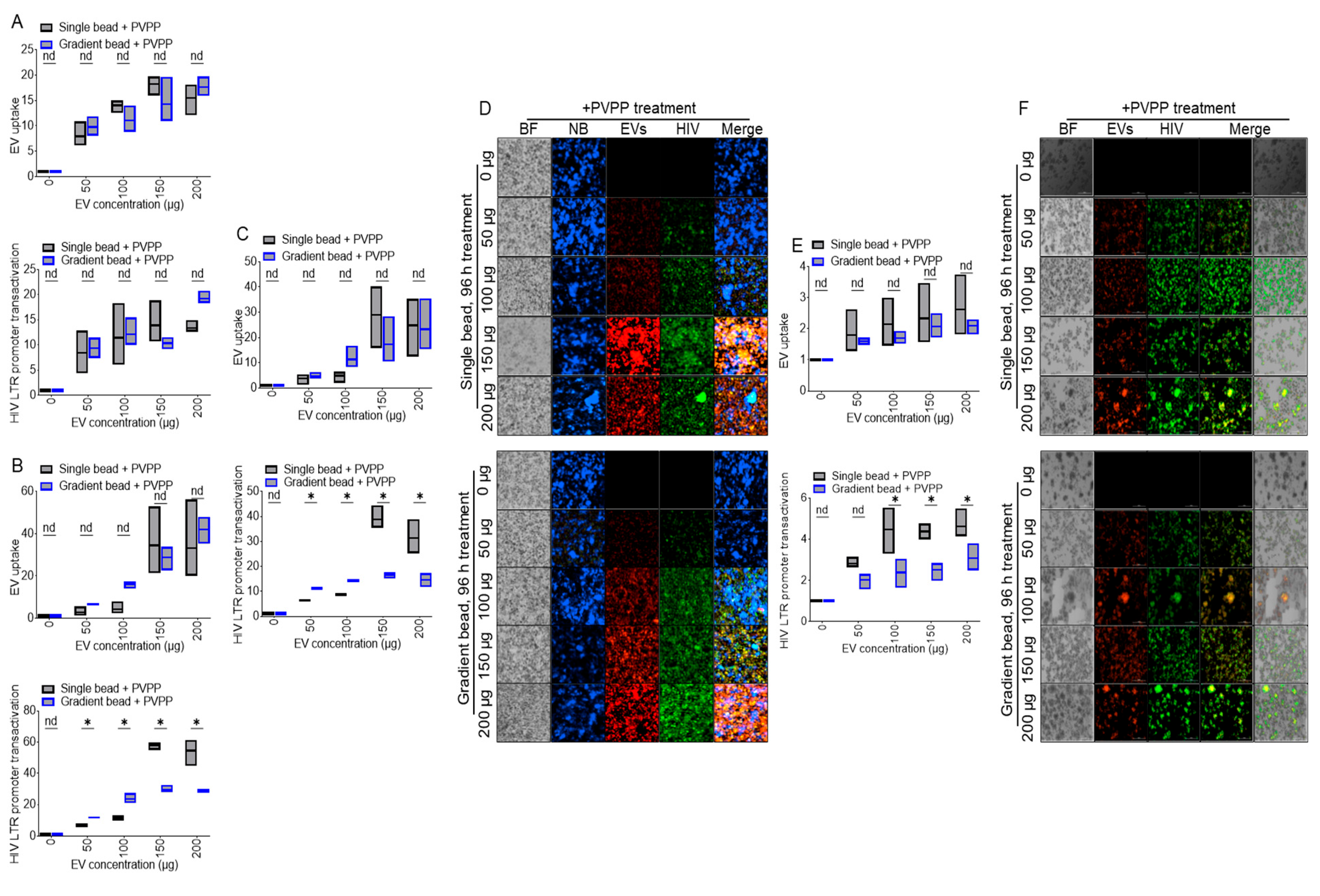
3.3. PVPP Facilitates Isolation of Pure ColEVs That Are Internalized by Human T Cells
3.4. ColEVs Activate HIV LTR Promoter
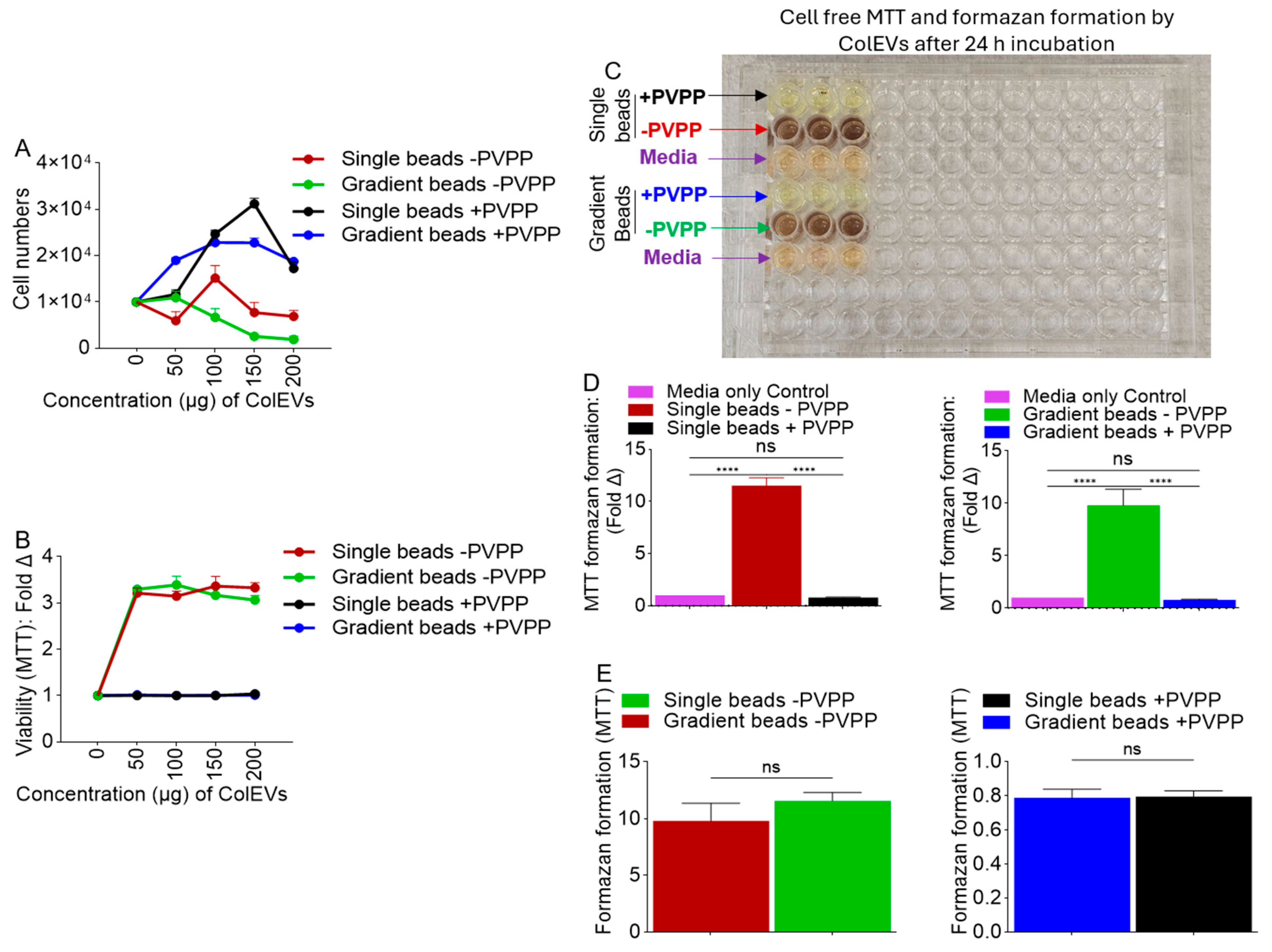
3.5. ColEVs Isolated in the Absence of PVPP Interfere with MTT Tetrazolium Reduction
3.6. Direct Reductive Potential of MTT Absorbance by ColEVs -PVPP in a Cell-Free System
3.7. ColEVs Isolated with Single Beads Are More Effective in Transactivating HIV LTR Promoter
3.8. ColEVs Are Originate from Both Bacteria and Host Particles
4. Discussion and Application of the Protocol
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, J.L.; Stapleton, J.T.; Okeoma, C.M. Vehicles of intercellular communication: Exosomes and HIV-1. J. Gen. Virol. 2019, 100, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenback, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Rafie, C.; Lai, S.; Sales, S.; Page, B.; Campa, A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J. Acquir. Immune Defic. Syndr. 2009, 50, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Valadi, H. Cell to cell signalling via exosomes through esRNA. Cell Adhes. Migr. 2007, 1, 156–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Daniel, R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. AIDS 2016, 30, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kopcho, S.; McDew-White, M.; Naushad, W.; Mohan, M.; Okeoma, C.M. SIV Infection Regulates Compartmentalization of Circulating Blood Plasma miRNAs within Extracellular Vesicles (EVs) and Extracellular Condensates (ECs) and Decreases EV-Associated miRNA-128. Viruses 2023, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Kopcho, S.; McDew-White, M.; Naushad, W.; Mohan, M.; Okeoma, C.M. Alterations in Abundance and Compartmentalization of miRNAs in Blood Plasma Extracellular Vesicles and Extracellular Condensates during HIV/SIV Infection and Its Modulation by Antiretroviral Therapy (ART) and Delta-9-Tetrahydrocannabinol (Δ9-THC). Viruses 2023, 15, 623. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; McDew-White, M.; Madeira, M.M.; Tranquille, M.A.; Tsirka, S.E.; Mohan, M.; Okeoma, C.M. Chronic delta-9-tetrahydrocannabinol (THC) treatment counteracts SIV-induced modulation of proinflammatory microRNA cargo in basal ganglia-derived extracellular vesicles. J. Neuroinflamm. 2022, 19, 225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.D.; Zhao, J.; Yang, X.; Zhou, B.W.; Yan, Z.; Liu, W.F.; Li, C.; Liu, K.X. Gut-Derived Exosomes Mediate Memory Impairment After Intestinal Ischemia/Reperfusion via Activating Microglia. Mol. Neurobiol. 2021, 58, 4828–4841. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Kopcho, S.; Mohan, M.; Okeoma, C.M. Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues. Cells 2020, 9, 2243. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: Their emerging role in tumor heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madison, M.N.; Jones, P.H.; Okeoma, C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 2015, 482, 189–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madison, M.N.; Welch, J.L.; Okeoma, C.M. Isolation of Exosomes from Semen for in vitro Uptake and HIV-1 Infection Assays. Bio Protoc. 2017, 7, e2216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welch, J.L.; Kaddour, H.; Schlievert, P.M.; Stapleton, J.T.; Okeoma, C.M. Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-kB/Sp1/Tat circuitry. J. Virol. 2018, 92, e00731-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welch, J.L.; Kaddour, H.; Winchester, L.; Fletcher, C.V.; Stapleton, J.T.; Okeoma, C.M. Semen Extracellular Vesicles From HIV-1-Infected Individuals Inhibit HIV-1 Replication In Vitro, and Extracellular Vesicles Carry Antiretroviral Drugs In Vivo. J. Acquir. Immune Defic. Syndr. 2020, 83, 90–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dantas-Pereira, L.; Menna-Barreto, R.; Lannes-Vieira, J. Extracellular Vesicles: Potential Role in Remote Signaling and Inflammation in Trypanosoma cruzi-Triggered Disease. Front. Cell Dev. Biol. 2021, 9, 798054. [Google Scholar] [CrossRef]
- Madeira, R.P.; Dal’Mas Romera, L.M.; De Cássia Buck, P.; Mady, C.; Ianni, B.M.; Torrecilhas, A.C. New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response. J. Immunol. Res. 2021, 2021, 6650670. [Google Scholar] [CrossRef]
- Moreira, L.R.; Serrano, F.R.; Osuna, A. Extracellular vesicles of Trypanosoma cruzi tissue-culture cell-derived trypomastigotes: Induction of physiological changes in non-parasitized culture cells. PLoS Neglected Trop. Dis. 2019, 13, e0007163. [Google Scholar] [CrossRef]
- Torró, L.M.d.P.; Moreira, L.R.; Osuna, A. Extracellular vesicles in chagas disease: A new passenger for an old disease. Front. Microbiol. 2018, 9, 1190. [Google Scholar] [CrossRef]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G., Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016, 7, 86999–87015. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Sun, W.; Zhang, Q.; Gu, T.; Li, G. Plasma exosomes as novel biomarker for the early diagnosis of gastric cancer. Cancer Biomark. 2018, 21, 805–812. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
- Oves, M.; Qari, H.A.; Felemban, N.M.; Khan, A.A.P.; Rehan, M.; Tabrez, S.; Ahmed, F.; Haque, A.; Khan, M.S.; Khan, J.M.; et al. Exosomes: A Paradigm in Drug Development against Cancer and Infectious Diseases. J. Nanomater. 2018, 2018, 6895464. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Xiang, D.; Nguyen, T.N.G.; Tran, T.T.D.; Chen, Q.; Yin, W.; Zhang, Y.; Kong, L.; Duan, A.; Chen, K.; et al. Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics 2020, 10, 3849–3866. [Google Scholar] [CrossRef]
- Xie, X.; Wu, H.; Li, M.; Chen, X.; Xu, X.; Ni, W.; Lu, C.; Ni, R.; Bao, B.; Xiao, M. Progress in the application of exosomes as therapeutic vectors in tumor-targeted therapy. Cytotherapy 2019, 21, 509–524. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef] [PubMed]
- Alhamwe, B.A.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma-More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 346–356. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Roncal, C.; Orbe, J.; Blanco-Colio, L.M. Role of Extracellular Vesicles as Potential Diagnostic and/or Therapeutic Biomarkers in Chronic Cardiovascular Diseases. Front. Cell Dev. Biol. 2022, 10, 813885. [Google Scholar] [CrossRef]
- Fu, H.; Hu, D.; Zhang, L.; Tang, P. Role of extracellular vesicles in rheumatoid arthritis. Mol. Immunol. 2018, 93, 125–132. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Capra, J.; Rilla, K.; Lehenkari, P.; Oikari, S.; Kääriäinen, T.; Joukainen, A.; Kröger, H.; Paakkonen, T.; Matilainen, J.; et al. Characterization of hyaluronan-coated extracellular vesicles in synovial fluid of patients with osteoarthritis and rheumatoid arthritis. BMC Musculoskelet. Disord. 2021, 22, 247. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, M.; Lu, Q. Extracellular Vesicles in Rheumatoid Arthritis and Systemic Lupus Erythematosus: Functions and Applications. Front. Immunol. 2021, 11, 575712. [Google Scholar] [CrossRef]
- Chun, H.J.; Reis, R.L.; Motta, A.; Khang, G. Biomimicked Biomaterials: Advances in Tissue Engineering and Regenerative Medicine; Springer: Singapore, 2020. [Google Scholar]
- Hao, Z.C.; Lu, J.; Wang, S.Z.; Wu, H.; Zhang, Y.T.; Xu, S.G. Stem cell-derived exosomes: A promising strategy for fracture healing. Cell Prolif. 2017, 50, e12359. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, Y. The Development of Extracellular Vesicle-Integrated Biomaterials for Bone Regeneration. Adv. Exp. Med. Biol. 2020, 1250, 97–108. [Google Scholar] [CrossRef]
- Han, N.D.; Cheng, J.; Delannoy-Bruno, O.; Webber, D.; Terrapon, N.; Henrissat, B.; Rodionov, D.A.; Arzamasov, A.A.; Osterman, A.L.; Hayashi, D.K.; et al. Microbial liberation of N-methylserotonin from orange fiber in gnotobiotic mice and humans. Cell 2022, 185, 2495–2509.e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez, F.A.; Kaddour, H.; Lyu, Y.; Preece, C.; Cohen, J.; Baer, L.; Stopeck, A.T.; Thompson, P.; Okeoma, C.M. Blood plasma derived extracellular vesicles (BEVs): Particle purification liquid chromatography (PPLC) and proteomic analysis reveals BEVs as a potential minimally invasive tool for predicting response to breast cancer treatment. Breast Cancer Res. Treat. 2022, 196, 423–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaddour, H.; Lyu, Y.; Shouman, N.; Mohan, M.; Okeoma, C.M. Development of Novel High-Resolution Size-Guided Turbidimetry-Enabled Particle Purification Liquid Chromatography (PPLC): Extracellular Vesicles and Membraneless Condensates in Focus. Int. J. Mol. Sci. 2020, 21, 5361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaddour, H.; Tranquille, M.; Okeoma, C.M. The Past, the Present, and the Future of the Size Exclusion Chromatography in Extracellular Vesicles Separation. Viruses 2021, 13, 2272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopal, C.; Rehmanji, M. PVPP–the route to effective beer stabilization. Brew. Guard. 2000, 129, 1–4. [Google Scholar]
- Durán-Lara, E.F.; Ávila-Salas, F.; Galaz, S.; John, A.; Maricán, A.; Gutiérrez, M.; Nachtigall, F.M.; Gonzalez-Nilo, F.D.; Santos, L.S. Nano-detoxification of organophosphate agents by PAMAM derivatives. J. Braz. Chem. Soc. 2015, 26, 580–591. [Google Scholar] [CrossRef]
- Laborde, B.; Moine-Ledoux, V.; Richard, T.; Saucier, C.; Dubourdieu, D.; Monti, J.P. PVPP-polyphenol complexes: A molecular approach. J. Agric. Food Chem. 2006, 54, 4383–4389. [Google Scholar] [CrossRef] [PubMed]
- Gayon, P.R.; Glories, Y.; Maujean, A.; Dudourdieu, D. Handbook of Enology–The Chemistry of Wine Stabilization and Treatments; John Wiley and Sons: Chichester, UK, 2006. [Google Scholar]
- Castro, R.I.; Forero-Doria, O.; Guzmán, L.; Laurie, V.F.; Valdés, O.; Ávila-Salas, F.; López-Cortés, X.; Santos, L.S. New polymer for removal of wine phenolics: Poly(N-(3-(N-isobutyrylisobutyramido)-3-oxopropyl)acrylamide) (P-NIOA). Food Chem. 2016, 213, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Gludish, D.W.; Boliar, S.; Caldwell, S.; Tembo, D.L.; Chimbayo, E.T.; Jambo, K.C.; Mwandumba, H.C.; Russell, D.G. TZM-gfp cells: A tractable fluorescent tool for analysis of rare and early HIV-1 infection. Sci. Rep. 2020, 10, 19900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jordan, A.; Bisgrove, D.; Verdin, E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003, 22, 1868–1877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jordan, A.; Defechereux, P.; Verdin, E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 2001, 20, 1726–1738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaddour, H.; Lyu, Y.; Welch, J.L.; Paromov, V.; Mandape, S.N.; Sakhare, S.S.; Pandhare, J.; Stapleton, J.T.; Pratap, S.; Dash, C.; et al. Proteomics Profiling of Autologous Blood and Semen Exosomes from HIV-infected and Uninfected Individuals Reveals Compositional and Functional Variabilities. Mol. Cell. Proteom. 2020, 19, 78–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Abdullahi, A.D.; Unban, K.; Saenjum, C.; Kodchasee, P.; Kangwan, N.; Thananchai, H.; Shetty, K.; Khanongnuch, C. Antibacterial activities of Miang extracts against selected pathogens and the potential of the tannin-free extracts in the growth inhibition of Streptococcus mutans. PLoS ONE 2024, 19, e0302717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, B.; Ren, P. Reduction of MTT by flavonoids in the absence of cells. Colloids Surf. B Biointerfaces 2005, 45, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Irmer, B.; Chandrabalan, S.; Maas, L.; Bleckmann, A.; Menck, K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers 2023, 15, 1307. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Yang, D.; Xu, W.; Qian, H. Extracellular vesicles: Emerging roles, biomarkers and therapeutic strategies in fibrotic diseases. J. Nanobiotechnol. 2023, 21, 164. [Google Scholar] [CrossRef]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, J.N.; Zeng, Z.G.; Wang, H.N.; Yang, T.; Zhang, P.J.; Li, Y.L.; Zhang, A.Y.; Fan, W.Q.; Zhang, Y.; Yang, X.; et al. An effective method for isolation of DNA from pig faeces and comparison of five different methods. J. Microbiol. Methods 2008, 75, 432–436. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Re-evaluation of polyvinylpyrrolidone (E 1201) and polyvinylpolypyrrolidone (E 1202) as food additives and extension of use of polyvinylpyrrolidone (E 1201). EFSA J. 2020, 18, e06215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gil, M.; Louazil, P.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Effect of polyvinylpolypyrrolidone treatment on rosés wines during fermentation: Impact on color, polyphenols and thiol aromas. Food Chem. 2019, 295, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic Building Blocks for the Assembly of Functional Materials. Angew. Chem. Int. Ed. Engl. 2019, 58, 1904–1927. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyanwu, N.C.J.; Premadasa, L.S.; Naushad, W.; Okeoma, B.C.; Mohan, M.; Okeoma, C.M. Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV. Cells 2025, 14, 568. https://doi.org/10.3390/cells14080568
Anyanwu NCJ, Premadasa LS, Naushad W, Okeoma BC, Mohan M, Okeoma CM. Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV. Cells. 2025; 14(8):568. https://doi.org/10.3390/cells14080568
Chicago/Turabian StyleAnyanwu, Nneoma C. J., Lakmini S. Premadasa, Wasifa Naushad, Bryson C. Okeoma, Mahesh Mohan, and Chioma M. Okeoma. 2025. "Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV" Cells 14, no. 8: 568. https://doi.org/10.3390/cells14080568
APA StyleAnyanwu, N. C. J., Premadasa, L. S., Naushad, W., Okeoma, B. C., Mohan, M., & Okeoma, C. M. (2025). Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV. Cells, 14(8), 568. https://doi.org/10.3390/cells14080568






