Defective Intracellular Insulin/IGF-1 Signaling Elucidates the Link Between Metabolic Defect and Autoimmunity in Vitiligo
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Skin Biopsies and Cell Cultures
2.3. Cell Culture Treatments
2.4. Reporter Cell Lines Stimulation to Assess the Secreted Embryonic Alkaline Phosphatase (SEAP) and Luciferase Reporter Enzyme Detection
2.5. Protein and Advanced Glycation End Product Determination by Sandwich ELISA
2.6. Direct Total Cell Counts by Flow Cytometry
2.7. Detection of Intracellular ROS Levels
2.8. Measurement of Mitochondrial Membrane Potential (ψm)
2.9. Mitochondrial Mass Measurement
2.10. ATP Measurement
2.11. RNA Extraction and Quantitative Real-Time PCR
2.12. Western Blot Analysis
2.13. Glucose Up-Take Determnation
2.14. Statistical Analysis
3. Results
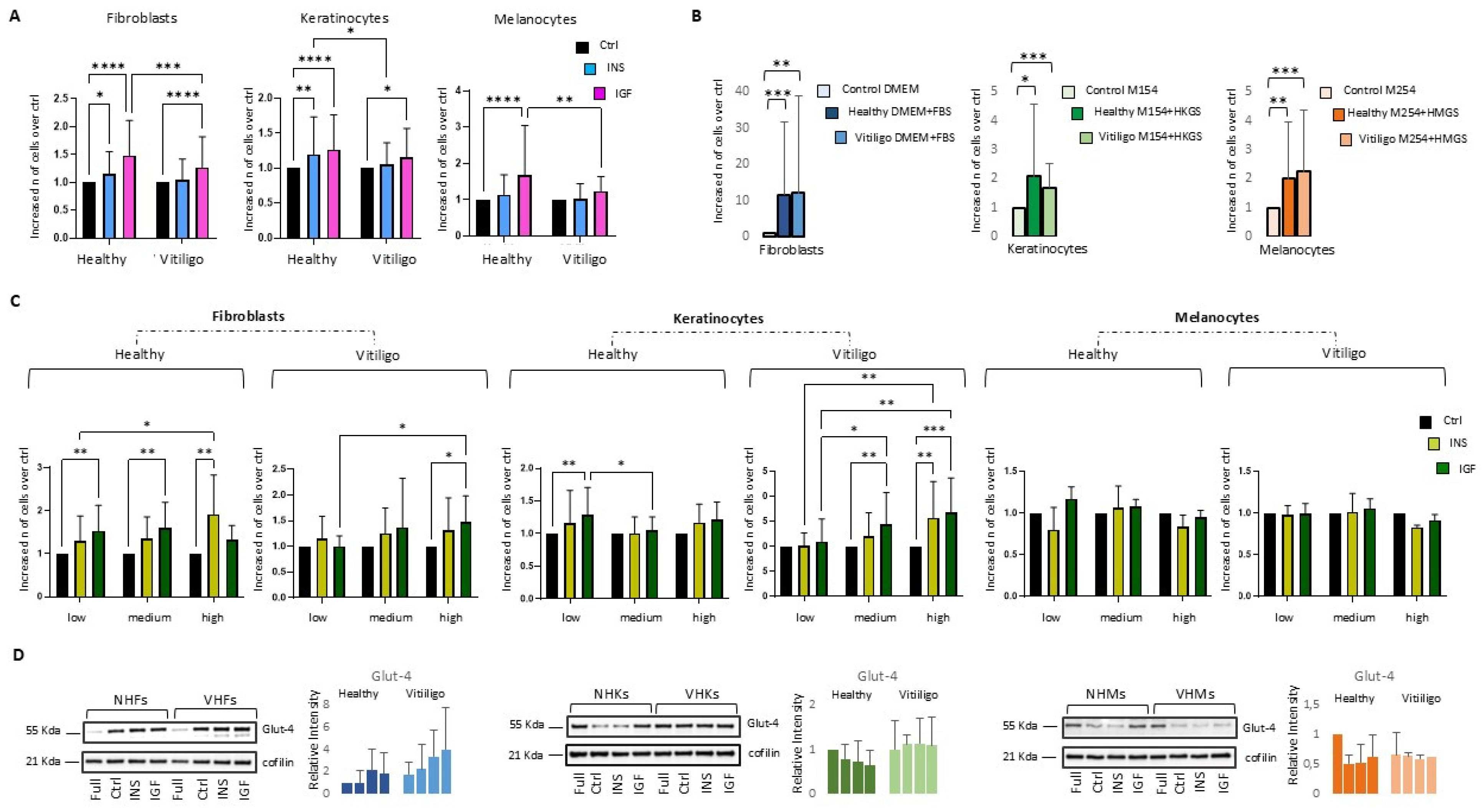
3.1. IGF-1- and Insulin-Dependent Mitogenic Activity Is Attenuated in Vitiligo Cells
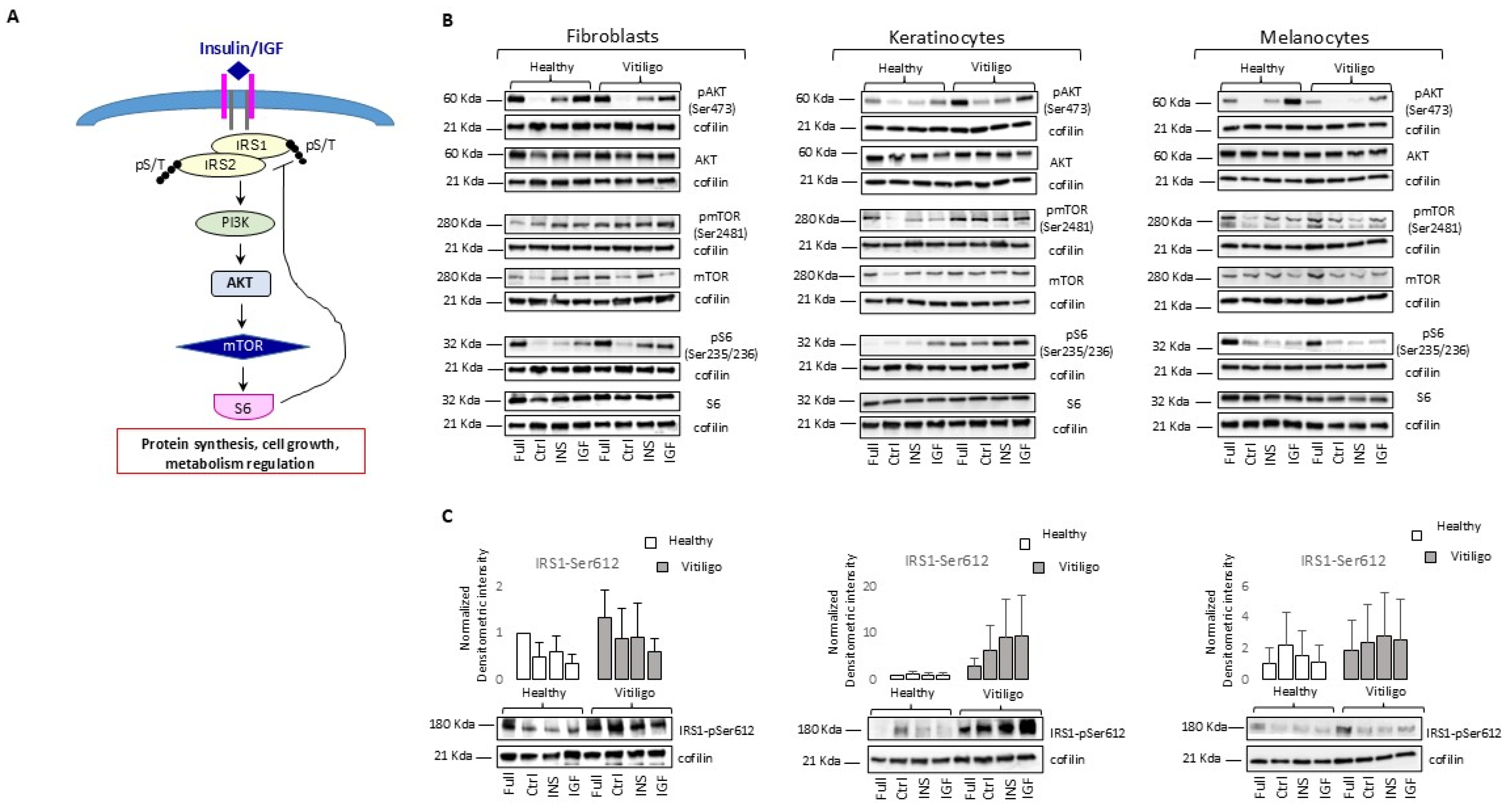
3.2. Molecular Impairment of IGF-1/Ins Signaling in Vitiligo Cells
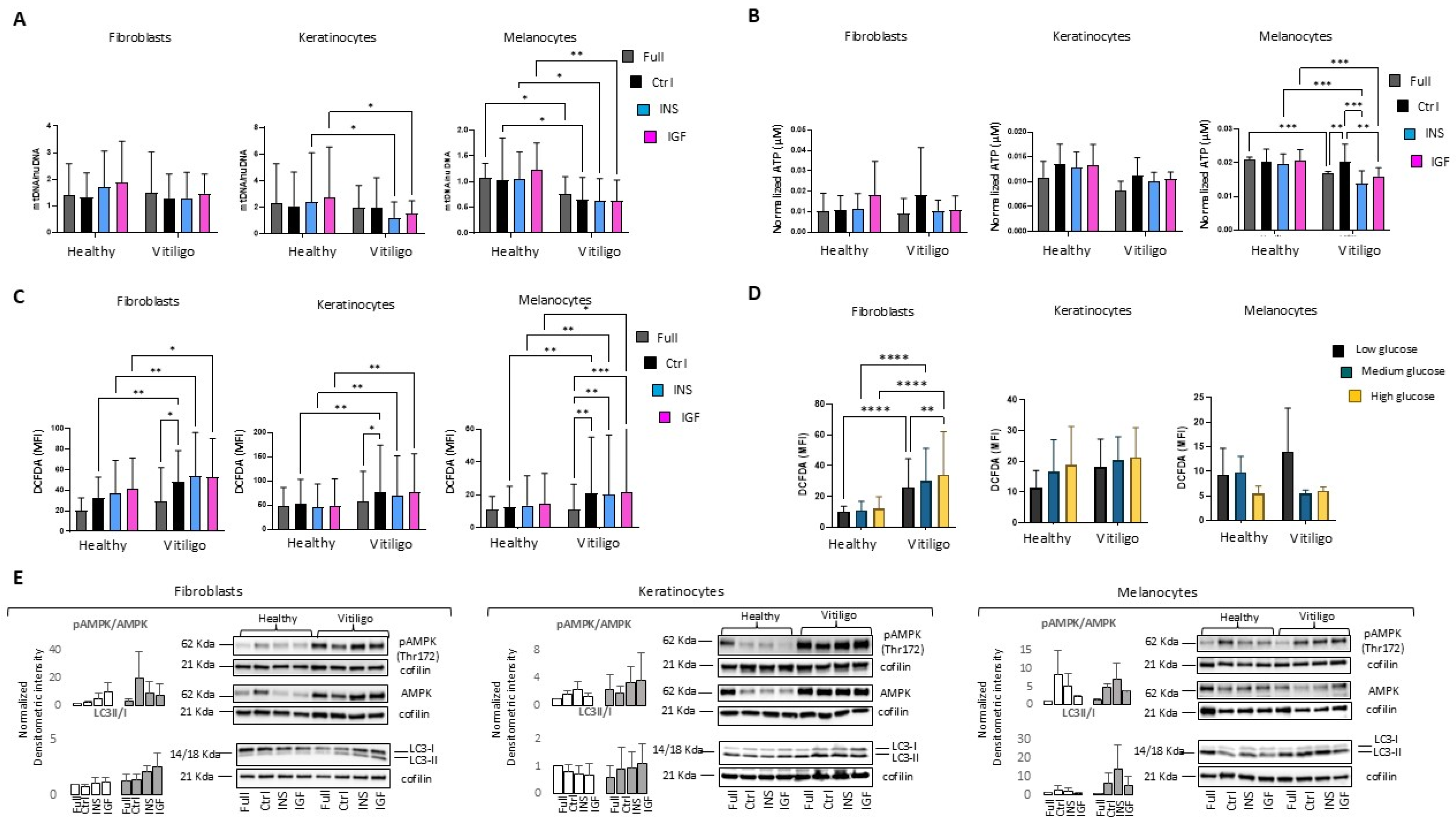
3.3. Impairment of Insulin/IGF-1 Intracellular Metabolic Activities Exacerbate Metabolic Stress in Vitiligo Cells
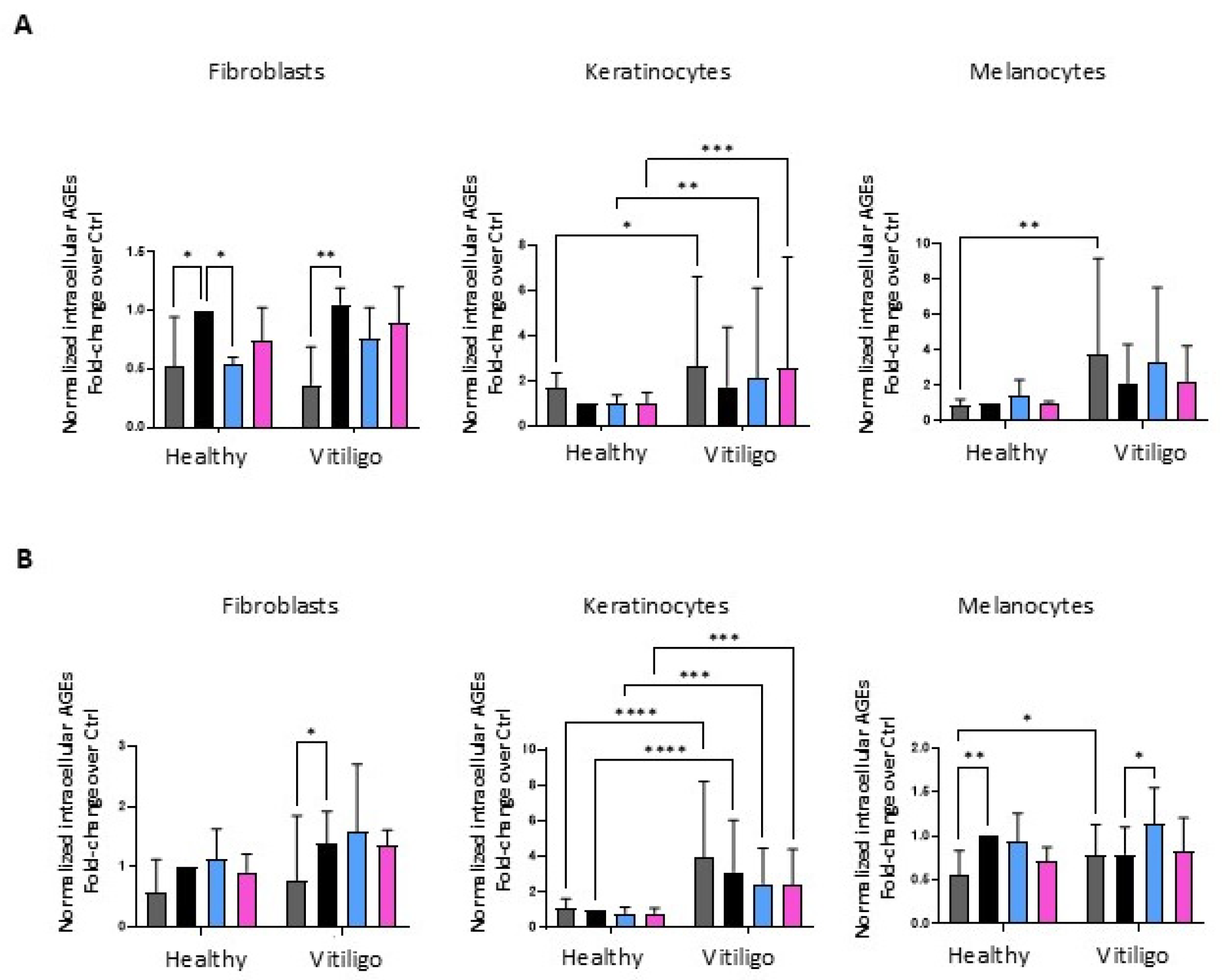
3.4. Unbalanced Glucose Metabolic Pathways Cause the Overproduction of Endogenous Advanced Glycation End Products in Vitiligo Keratinocytes
3.5. Insulin and IGF-1/Ins Stimulation Supports a Pro-Inflammatory Phenotype in Vitiligo Keratinocytes
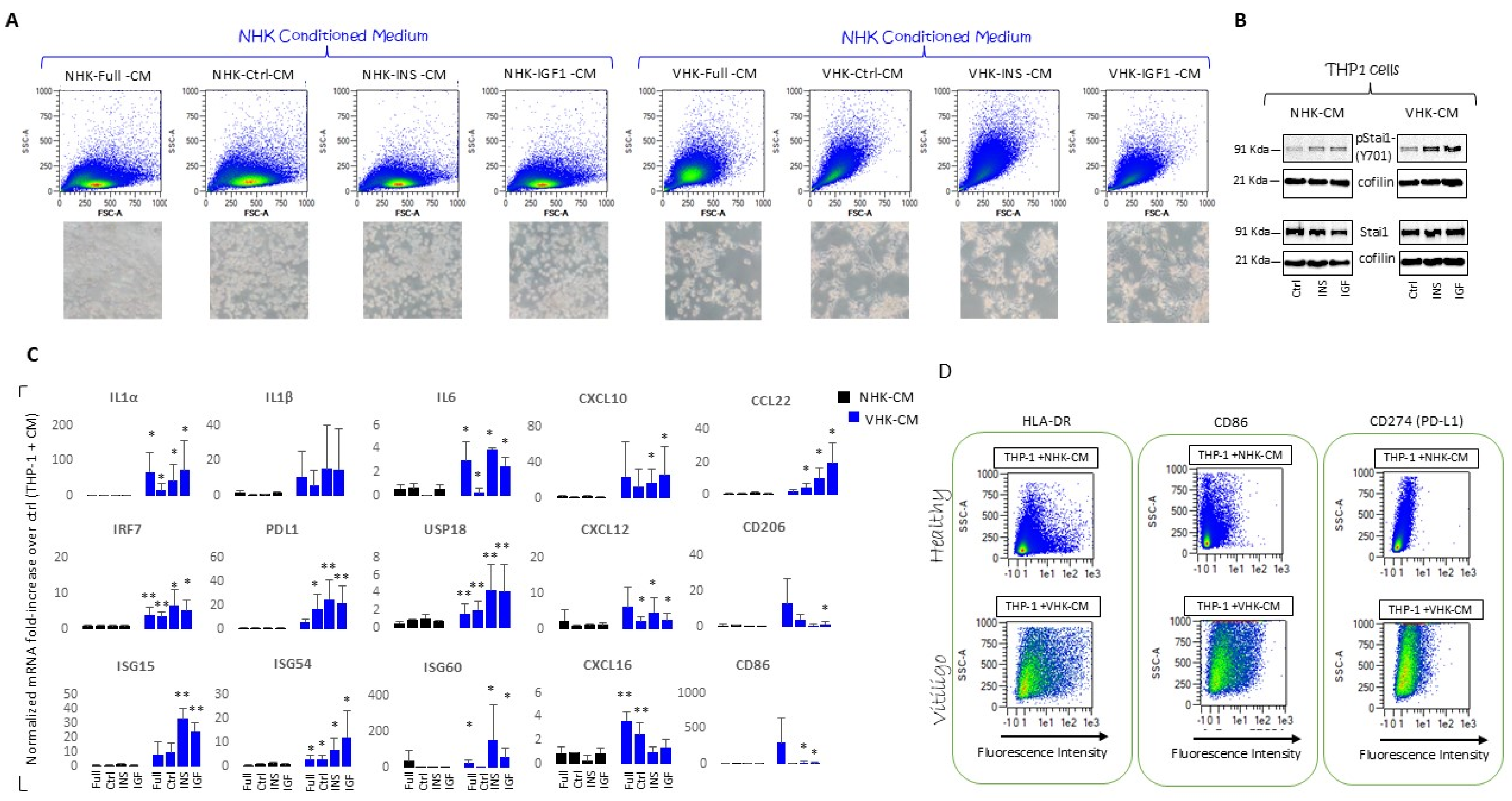
3.6. THP-1 Monocytes Are Differentiated into Macrophage by Incubation with VHKs Conditioned Medium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Gayyar, M.A.; Helmy, M.E.; Amer, E.R.; Elsaied, M.A.; Gaballah, M.A. Antimelanocyte Antibodies: A Possible Role in Patients with Vitiligo. Indian J. Dermatol. 2020, 65, 33–37. [Google Scholar] [PubMed]
- Mustafa, A.I.; Hamed, A.M.; Kadah, A.S.; Fawzy, E.M.; El Shimi, O.S. A notorious trio, metabolic syndrome and vitiligo. Indian Dermatol. Online J. 2023, 14, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.; Kemp, E.H.; Gawkrodger, D.J. Patho-Immunological Mechanisms of Vitiligo: The Role of the Innate and Adaptive Immunities and Environmental Stress Factors. Clin. Exp. Immunol. 2022, 207, 27–43. [Google Scholar] [CrossRef]
- Speeckaert, R.; Caelenberg, E.V.; Belpaire, A.; Speeckaert, M.M.; Geel, N.V. Vitiligo: From Pathogenesis to treatment. J. Clin. Med. 2024, 13, 5225. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Ottaviani, M.; Kovacs, D.; Mirabilii, S.; Brown, D.A.; Cota, C.; Migliano, E.; Bastonini, E.; Bellei, B.; Cardinali, G.; et al. Energetic Mitochondrial Failing in Vitiligo and Possible Rescue by Cardiolipin. Sci. Rep. 2017, 7, 13663–13665. [Google Scholar] [CrossRef]
- Yu, R.; Broady, R.; Huang, Y.; Wang, Y.; Yu, J.; Gao, M.; Levings, M.; Wei, S.; Zhang, S.; Xu, A.; et al. Transcriptome Analysis Reveals Markers of Aberrantly Activated Innate Immunity in Vitiligo Lesional and Non-Lesional Skin. PLoS ONE 2012, 7, e51040. [Google Scholar] [CrossRef]
- Badri, A.M.; Todd, P.M.; Garioch, J.J.; Gudgeon, J.E.; Stewart, D.G.; Goudie, R.B. An Immunohistological Study of Cutaneous Lymphocytes in Vitiligo. J. Pathol. 1993, 170, 149–155. [Google Scholar] [CrossRef]
- Mantovani, S.; Garbelli, S.; Palermo, B.; Campanelli, R.; Brazzelli, V.; Borroni, G.M.; Martinetti, F.; Benvenuto, G.; Merlini, G.R.; della Cuna, G.R.; et al. Molecular and functional bases of self-antogen recognition in long-term persistent melanocyte-specific CD8+ T cells in one vitiligo patient. J. Investig. Dermatol. 2003, 121, 308–314. [Google Scholar] [CrossRef][Green Version]
- Harris, J.E.; Harris, T.H.; Weninger, W.; Wherry, E.J.; Hunter, C.A.; Turka, L.A. A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-Γ for Autoreactive CD8⁺ T-Cell Accumulation in the Skin. J. Investig. Dermatol. 2012, 132, 1869–1876. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Le, Q.; Tong, J.; Wang, H. The IFN-gamma-CXCL9/CXCL10-CXCR3 axis in vitiligo: Pathological mechanism and treatment. Eur. J. Immunol. 2024, 54, e2250281. [Google Scholar] [CrossRef]
- Richmond, J.M.; Bangari, D.S.; Essien, K.I.; Currimbhoy, S.D.; Groom, J.R.; Pandya, A.G.; Youd, M.E.; Luster, A.D.; Harris, J.E. Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J. Investig. Dermatol. 2017, 137, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Tulic, M.K.; Cavazza, E.; Cheli, Y.; Jacquel, A.; Luci, C.; Cardot-Leccia, N.; Hadhiri-Bzioueche, H.; Abbe, P.; Gesson, M.; Sormani, L.; et al. Innate lumphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat. Commun. 2019, 10, 2178. [Google Scholar] [CrossRef]
- Murfaca, G.; Colombo, B.M.; Puppo, F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Inter. Emerg. 2011, 6, 487–495. [Google Scholar] [CrossRef]
- Singh, R.K.; Lee, K.M.; Vujkovic-Cvijin, I.; Ucmak, D.; Farahnik, B.; Abrouk, M.; Nakamura, M.; Zhu, T.H.; Bhutani, T.; Wei, M.; et al. The role of IL-17 in vitiligo: A review. Autoimmun. Rev. 2016, 15, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.S.; Dwivedi, M.; Begum, R. Decreased suppression of CD8(+) and CD4(+) T cells by peripheral regulatory T cells in generalized vitiligo due to reduced NFATC1 and FOXP3 proteins. Exp. Dermatol. 2020, 29, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Steitz, J.; Wenzel, J.; Gaffal, E.; Tuting, T. Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: Implications for the pathophysiology of vitiligo. Eur. J. Cell Biol. 2004, 83, 797–803. [Google Scholar] [CrossRef]
- Kroll, T.M.; Bommiasamy, H.; Boissy, R.E.; Herandez, C.; Nickoloff, B.J.; Mestril, R.; Le Poole, C. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: Relevance to vitiligo. J. Investig. Dermatol. 2005, 124, 798–806. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Wei, G.; Mao, H.; Liu, R.; He, Y. Damage-associated molecular patterns in vitiligo: Igniter fuse from oxidative stress to melanocyte loss. Redox Rep. 2022, 27, 193–199. [Google Scholar] [CrossRef]
- Dell’Anna, M.L.; Ottaviani, M.; Bellei, B.; Albanesi, V.; Cossarizza, A.; Rossi, L.; Picardo, M. Membrane lipid defects are responsible for the generation of reactive oxygen species in peripheral blood mononuclear cells from vitiligo patients. J. Cell Physiol. 2010, 223, 187–193. [Google Scholar] [CrossRef]
- Jian, Z.; Li, K.; Song, P.; Zhu, G.; Zhu, L.; Cui, T.; Liu, B.; Tang, L.; Wang, X.; Wang, G.; et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014, 134, 2221–2230. [Google Scholar] [CrossRef]
- Böhm, M. More evidence for catalase as a pathogenetic cornerstone in vitiligo. Exp. Dermatol. 2017, 177, 1477. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Salem, M.A.; Holtz, S.; Panske, A. Basic evidence for epidermal H2O2/ONOO−-mediated oxidation/nitration in segmental vitiligo is supported by repigmentation of skin and eyelashes after reduction of epidermal H2O2 with topical NB-UVB-activated pseudocatalase PC-KUS. FASEB J. 2013, 27, 3113–3122. [Google Scholar] [CrossRef]
- Papaccio, F.; Bellei, B.; Ottaviani, M.; D’Arino, A.; Truglio, M.; Caputo, S.; Cigliana, G.; Sciuto, L.; Migliano, E.; Pacifico, A.; et al. A Possible Modulator of Vitiligo Metabolic Impairment: Rethinking a PPARgamma Agonist. Cells 2022, 11, 3583. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, S.; Zhang, W.; Dai, W.; Cui, T.; Wang, G.; Gao, T.; Li, C. Dysregulated Autophagy Increased Melanocyte Sensitivity to H2O2-Induced Oxidative Stress in Vitiligo. Sci. Rep. 2017, 7, 42394. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, Y.; Huang, X.; Cheng, H.; Seifert, O. The Difference in Expression of Autophagy-Related Proteins in Lesional and Perilesional Skin in Adult Patients with Active and Stable Generalized Vitiligo-A Cross-Sectional Pilot Study. Indian J. Dermatol. 2021, 66, 331–336. [Google Scholar] [CrossRef]
- Weiland, D.; Brachvogel, B.; Hornig-Do, H.T.; Neuhaus, J.F.G.; Holzer, T.; Tobin, D.J.; Niessen, C.M.; Wiesner, R.J.; Baris, O.R. Imbalance of mitochondrial respiratory chain complexes in the epidermis induces severe skin inflammation. J. Investig. Dermatol. 2018, 138, 132–140. [Google Scholar] [CrossRef]
- Van der Burgh, R.; Boes, M. Mitochondria in autoinflammation: Cause, mediator or bystander? Trends Endocrinol. Metab. 2015, 26, 263–271. [Google Scholar] [CrossRef]
- Bastonini, E.; Bellei, B.; Filoni, A.; Kovacs, D.; Iacovelli, P.; Picardo, M. Involvement of non-melanocytic skin cells in vitiligo. Exp. Dermatol. 2019, 28, 667–673. [Google Scholar] [CrossRef]
- D’Arino, A.; Picardo, M.; Truglio, M.; Pacifico, A.; Iacovelli, P. Metabolic Comorbidities in Vitiligo: A Brief Review and Report of New Data from a Single-Center Experience. Int. J. Mol. Sci. 2021, 22, 8820. [Google Scholar] [CrossRef]
- Aryanian, Z.; Shirzadian, A.; Farzaneh, S.; Goodarzi, A.; Azizpour, A.; Hatami, P. Metabolic Derangement in Patients with Vitiligo: A Cross-Sectional Study. J. Investig. Med. 2022, 70, 963–966. [Google Scholar] [CrossRef]
- Li, G.; Qu, B.; Zheng, T.; Cheng, Y.; Li, P.; Liu, Z.; Zhao, J. Assessing the causal effect of genetically predicted metabolites and metabolic pathways on vitiligo: Evidence from mendelian randomization and animal experiments. J. Steroid Biochem. Mol. Biol. 2025, 247, 106677. [Google Scholar] [CrossRef]
- Hu, S.; Che, Y.; Cai, J.; Chen, S.; Gao, R.; Huang, X. Diabetes, glycemic profile and risk of vitiligo: A mendelian randomization study. Skin Res. Technol. 2024, 30, e13787. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Bastonini, E.; Briganti, S.; Ottaviani, M.; D’Arino, A.; Truglio, M.; Sciuto, L.; Zaccarini, M.; Pacifico, A.; Cota, C.; et al. Altered Epidermal Proliferation, Differentiation, and Lipid Composition: Novel Key Elements in the Vitiligo Puzzle. Sci. Adv. 2022, 8, eabn9299. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Ottaviani, M.; Truglio, M.; D’Arino, A.; Caputo, S.; Pacifico, A.; Iacovelli, P.; Di Nardo, A.; Picardo, M.; Bellei, B. Markers of metabolic abnormalities in vitiligo patients. Int. J. Mol. Sci. 2024, 25, 10201. [Google Scholar] [CrossRef]
- He, M.J.; Ran, D.L.; Zhang, Z.Y.; Fu, D.S.; He, Q.; Zhang, H.Y.; Mao, Y.; Zhao, P.Y.; Yin, G.W.; Zhang, J.A. Exploring the roles and potential therapeutic strategies of inflammation and metabolism in the pathogenesis of vitiligo: A mendelian randomization and bioinformatics-based investigation. Front. Genet. 2024, 15, 1385339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, M.; Li, L. Identifying the genetic association between diabetes mellitus and the risk of vitiligo. Clin. Cosmet. Investig. Dermatol. 2024, 13, 2261–2271. [Google Scholar] [CrossRef]
- Afkhami-Ardekani, M.; Ghadiri-Anari, A.; Ebrahimzadeh-Ardakani, M.; Zaji, N. Prevalence of vitiligo among type 2 diabetic patients in an Iranian population. Int. J. Dermatol. 2014, 53, 956–958. [Google Scholar] [CrossRef]
- Chang, H.C.; Lin, M.H.; Huang, Y.C.; Hou, T.Y. The association between vitiligo and diabetes mellitus: A systemic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 81, 1442–1445. [Google Scholar] [CrossRef]
- Karadag, A.S.; Tutal, E.; Ertugrul, D.T. Insulin resistance is increased in patients with vitiligo. Acta Derm. Venereol. 2011, 91, 541–544. [Google Scholar] [CrossRef]
- Calderon-DuPont, D.; Torre-Villalvazo, I.; Diaz-Villasenon, A. Is insulin resistance tissue-dependent and substrate-specific? The role of white adipose tissue and skeletal muscle. Biochimie 2023, 204, 48–68. [Google Scholar] [CrossRef]
- Boucher, J.; Tseng, Y.; Kahn, C.R. Insulin and Insulin-Like Growth Factor-1 Receptors Act as Ligand-Specific Amplitude Modulators of a Common Pathway Regulating Gene Transcription. J. Biol. Chem. 2010, 285, 17235–17245. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Involvement of Insulin-Like Growth Factor-I in the Control of Glucose Homeostasis. Curr. Opin. Pharmacol. 2006, 6, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R.; Moses, A.C.; Sommer, A.; Jacobson, W.; Rogol, A.D.; Sleevi, M.R.; Allan, G. Rh/IGF-I/rhIGFBP-3 Administration to Patients with Type 2 Diabetes Mellitus Reduces Insulin Requirements while also Lowering Fasting Glucose. Growth Horm. IGF Res. 2005, 15, 265–274. [Google Scholar] [CrossRef]
- Siddle, K.; Urso, B.; Niesler, C.A.; Cope, D.L.; Molina, L.; Surinya, K.H.; Soos, M.A. Specificity in Ligand Binding and Intracellular Signalling by Insulin and Insulin-Like Growth Factor Receptors. Biochem. Soc. Trans. 2001, 29, 513–525. [Google Scholar] [CrossRef]
- Pittelkow, M.R.; Shipley, G.D. Serum-free culture of normal human melanocytes: Growth kinetics and growth factor requirements. J. Cell Physiol. 1989, 140, 565–576. [Google Scholar] [CrossRef]
- Tavakkol, A.; Elder, J.T.; Griffiths, C.E.; Cooper, K.D.; Talwar, H.; Fisher, G.J.; Keane, K.M.; Foltin, S.K.; Voorhees, J.J. Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J. Investig. Dermatol. 1992, 99, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.R.; Russo, V.C.; McFarlane, A.C.; Wraight, C.J.; Werther, G.A. Interaction between growth hormone, insulin-like growth factor I, and basic fibroblast growth factor in melanocytes growth. J. Clin. Endocrinol. Metab. 1999, 84, 1638–1644. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zimmer, M.A.; Guardia, T.; Callahan, S.J.; Mondal, C.; Martino, J.D.; Takagi, T.; Fennell, M.; Garippa, R.; Campbell, N.R.; et al. Distant insulin signaling regulates vertebrate pigmentation though the Sheddase Bace2. Dev. Cell 2018, 45, 580–594. [Google Scholar] [CrossRef]
- Hermanns-Lê, T.; Scheen, A.; Piérard, G.E. Acanthosis nigricans associated with insulin resistance: Pathophysiology and management. Am. J. Clin. Dermatol. 2004, 5, 199–203. [Google Scholar] [CrossRef]
- Spravchikov, N.; Sizyakov, G.; Gartsbein, M.; Accili, D.; Tennenbaum, T.; Wertheimer, E. Glucose effects on skin keratinocytes: Implocation for diabetes skin complication. Diabetes 2021, 50, 1627–1635. [Google Scholar] [CrossRef]
- Monaco, S.; Illario, M.; Rusciano, M.R.; Gragnaniello, G.; Di Spigna, G.; Leggiero, E.; Pastore, L.; Fenzi, G.; Rossi, G.; Vitale, M. Insulin stimulates fibroblast proliferation through calciumcalmodulin-dependent kinase II. Cell Cycle 2009, 8, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Hann, S.K.; Park, Y.K.; Kim, H.I. Culture of melanocytes obtained from normal and vitiligo subjects. Yonsei Med. J. 1992, 33, 344–350. [Google Scholar] [CrossRef]
- Dell’anna, M.L.; Cario-André, M.; Bellei, B.; Taieb, A.; Picardo, M. In vitro research on vitiligo: Strategies, principles, methodological options and common pitfalls. Exp. Dermatol. 2012, 21, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Hugl, S.R.; White, M.F.; Rhodes, C.J. Insulin-like growth factor I (IGF-1)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose. J. Biol. Chem. 1998, 273, 17771–17779. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Makino, N.; Matsuda, A.; Ikeda, Y.; Kakizaki, Y.; Saito, Y.; Ueno, Y.; Kawata, S. High Glucose Accelerates Cell Proliferation and Increases the Secretion and mRNA Expression of Osteopontin in Human Pancreatic Duct Epithelial Cells. Int. J. Mol. Sci. 2017, 18, 807. [Google Scholar] [CrossRef]
- Cheng, Z.; Tseng, Y.; White, M.F. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol. Metab. 2010, 21, 589–598. [Google Scholar] [CrossRef]
- Leontieva, O.V.; Demidenko, Z.N.; Blagosklonny, M.V. Rapamycin reverses insulin resistance (IR) in high-glucose medium without causing IR in normoglycemic medium. Cell Death Dis. 2014, 5, e1214. [Google Scholar] [CrossRef][Green Version]
- Widlansky, M.E.; Wang, J.; Shenouda, S.M.; Hagen, T.M.; Smith, A.R.; Kizhakekuttu, T.J.; Kluge, M.A.; Weihrauch, D.; Gutterman, D.D.; Vita, J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res. 2010, 156, 15–25. [Google Scholar] [CrossRef]
- Chen, J.; Chernatynskaya, A.V.; Li, J.W.; Kimbrell, M.R.; Cassidy, R.J.; Perry, D.J.; Muir, A.B.; Atkinson, A.; Brusko, T.M.; Mathews, C.E. T cells display mitochondria hyperpolarization in human type 1 diabetes. Sci. Rep. 2017, 7, 10835. [Google Scholar] [CrossRef]
- Guan, X.; Yan, Q.; Wang, D.; Du, G.; Zhou, J. IGF-1signaling regulates mitochondrial remodeling during myogenic differentiation. Nutrients 2022, 14, 1249. [Google Scholar] [CrossRef]
- Riis, S.; Murray, J.B.; O’Connor, R. IGF-1 Signalling regulates mitochondria dynamics and turnover through a conserved GSK-3β-Nrf2-BNIP3 pathway. Cells 2020, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanism of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Basters, A.; Knobeloch, K.; Fritz, G. USP18-a multifunctional component in the interferon response. Biosci. Rep. 2018, 38, BSR20180250. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, S.; Wang, S.; Sun, Y.; Lu, X.; Li, H.; Li, G. IGF-1 via PI3K/AKT/S6K signaling pathway protects DRG neurons with high glucose-induced toxicity. Open Life Sci. 2019, 14, 502–514. [Google Scholar] [CrossRef]
- Zarpellon, P.S.; Murat, C.; Leão, R.M. Reduction in mitochondrial ATP synthesis mimics the effect of low glucose in depolarizing neurons from the subpostremal nucleus of the solitary tract of rats. J. Bioenerg. Biomembr. 2024, 56, 483–493. [Google Scholar] [CrossRef]
- Lee, E.Y.; Bae, H.C.; Lee, H.; Jang, Y.; Park, Y.H.; Kim, J.H.; Ryu, W.I.; Choi, B.H.; Kim, J.H.; Jeong, S.H.; et al. Intracellular ROS levels determine the apoptotic potential of keratinocyte by Quantum Dot via blockade of AKT-Phosphorylation. Exp. Dermatol. 2017, 26, 1046–1052. [Google Scholar] [CrossRef]
- Shi, J.; Han, C.; Chen, D.; Trivedi, H.M.; Bangash, H.I. High Glucose Induces Late Differentiation and Death of Human Oral Keratinocytes. Curr. Issues Mol. Biol. 2022, 44, 4015–4027. [Google Scholar] [CrossRef]
- Berge, U.; Behrens, J.; Rattan, S.I.S. Sugar-induced premature aging and altered differentiation in human epidermal keratinocytes. Ann. N. Y. Acad. Sci. 1100, 524–529. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, C.; Xu, P.; Wu, S.; Fu, X.; Xia, W.; Yao, M. AGEs Induced Autophagy Impairs Cutaneous Wound Healing via Stimulating Macrophage Polarization to M1 in Diabetes. Sci. Rep. 2016, 6, 36416. [Google Scholar] [CrossRef]
- Liu, J.; Man, W.Y.; Lv, C.Z.; Song, S.P.; Shi, Y.J.; Elias, P.M.; Man, M.Q. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol. Physiol. 2010, 23, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, W.; Qiu, Y.; Yu, C.; Wang, R.; Li, C. Role of glucose metabolism reprogramming in keratinocytes in the link between psoriasis and metabolic syndrome. Int. Immunopharmacol. 2024, 30, 112704. [Google Scholar] [CrossRef] [PubMed]
- Gergely, P.; Grossman, C.; Niland, B.; Puskas, F.; Neupane, H.; Allam, F.; Banki, K.; Phillips, P.E.; Perl, A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupis erythematosus. Arthritis Rheum. 2002, 46, 175–190. [Google Scholar] [CrossRef]
- Murata, N.; Nishimura, K.; Harada, N.; Kitakaze, T.; Yoshihara, E.; Inui, H.; Yamaji, R. Insulin reduces endoplasmic reticulum stress-induced apoptosis by decreasing mitochondrial hyperpolarization and caspase-12 in INS-1 pancreatic β-cells. Physiol. Rep. 2024, 12, e16106. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Yeo, J.H.; Park, S.; Bae, Y.J.; Know, I.J.; Seong, S.H.; Lee, J.; Oh, S.H. ISG15-USP18 Dysregulation by Oxidative Stress Promotes IFN-γ Secretion from CD8+ T Cells in Vitiligo. J. Investig. Dermatol. 2024, 144, 272–283. [Google Scholar] [CrossRef]
- Saferding, V.; Blüml, S. Innate immunity as the trigger of systemic autoimmune diseases. J. Autoimmun. 2020, 110, 102382. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, M.; Yang, H.; Qu, R.; Qiu, Y.; Hao, J.; Bi, H.; Guo, D. Regulatory Mechanism of M1/M2 Macrophage Polarization in the Development of Autoimmune Diseases. Mediat. Inflamm. 2023, 8821610. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Sain, N.; Hooda, V.; Singh, A.; Gupta, S.; Arava, S.; Sharma, A. Macrophage inhibitory factor alters the functionality of macrophages and their involvement in disease pathogenesis of active generalized vitiligo patients. Cytokine 2024, 176, 156516. [Google Scholar] [CrossRef] [PubMed]

| Marker | NHK Full | NHK st | NHK Ins | NHK IGF-1 | VHK Full | VHK st | VHK Ins | VHK IGF-1 |
|---|---|---|---|---|---|---|---|---|
| CD86 | 8.0 ± 8 | 4.7 ± 1 | 4.0 ± 1 | 4.7 ± 2 | 29.3 ± 13 | 25.0 ± 20 | 24.0 ± 25 | 31.0 ± 22 |
| HLA-DR | 36.0 ± 9 | 33.5 ± 6 | 32.0 ± 5 | 37.7 ± 2 | 51.5 ± 8 | 61.7 ± 16 | 55.75 ± 12 | 53.5 ± 17 |
| CD206 | 20.5 ± 15 | 6.5 ± 1 | 4.0 ± 1 | 6.0 ± 1 | 47.0 ± 17 | 33.5 ± 14 | 32.5 ± 18 | 32.0 ± 20 |
| CD163 | 21.0 ± 20 | 3.0 ± 2 | 2.0 ± 1 | 3.0 ± 2 | 40.0 ± 6 | 12.0 ± 1 | 11.5 ± 2 | 11.0 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, S.; Papaccio, F.; Marrapodi, R.; Lopez, G.; Iacovelli, P.; Pacifico, A.; Migliano, E.; Cota, C.; Di Nardo, A.; Picardo, M.; et al. Defective Intracellular Insulin/IGF-1 Signaling Elucidates the Link Between Metabolic Defect and Autoimmunity in Vitiligo. Cells 2025, 14, 565. https://doi.org/10.3390/cells14080565
Caputo S, Papaccio F, Marrapodi R, Lopez G, Iacovelli P, Pacifico A, Migliano E, Cota C, Di Nardo A, Picardo M, et al. Defective Intracellular Insulin/IGF-1 Signaling Elucidates the Link Between Metabolic Defect and Autoimmunity in Vitiligo. Cells. 2025; 14(8):565. https://doi.org/10.3390/cells14080565
Chicago/Turabian StyleCaputo, Silvia, Federica Papaccio, Ramona Marrapodi, Gianluca Lopez, Paolo Iacovelli, Alessia Pacifico, Emilia Migliano, Carlo Cota, Anna Di Nardo, Mauro Picardo, and et al. 2025. "Defective Intracellular Insulin/IGF-1 Signaling Elucidates the Link Between Metabolic Defect and Autoimmunity in Vitiligo" Cells 14, no. 8: 565. https://doi.org/10.3390/cells14080565
APA StyleCaputo, S., Papaccio, F., Marrapodi, R., Lopez, G., Iacovelli, P., Pacifico, A., Migliano, E., Cota, C., Di Nardo, A., Picardo, M., & Bellei, B. (2025). Defective Intracellular Insulin/IGF-1 Signaling Elucidates the Link Between Metabolic Defect and Autoimmunity in Vitiligo. Cells, 14(8), 565. https://doi.org/10.3390/cells14080565








