The Growth of Soybean (Glycine max) Under Salt Stress Is Modulated in Simulated Microgravity Conditions
Abstract
1. Introduction
2. Materials and Methods
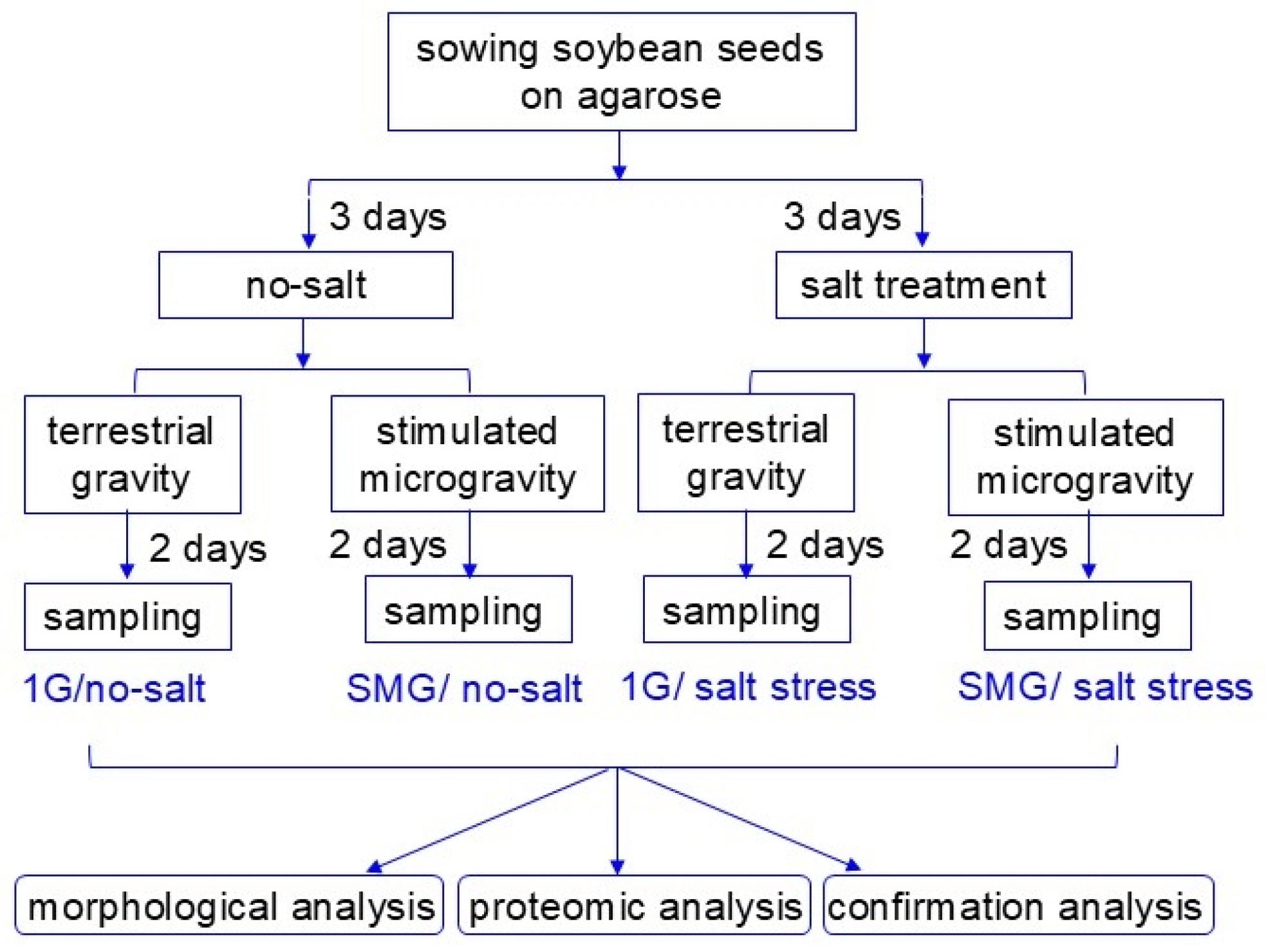
2.1. Plant Material, Salt Application, and Simulated Microgravity Treatment
2.2. Protein Extraction and Concentration Measurement
2.3. Protein Enrichment, Reduction, Alkylation, and Digestion
2.4. Protein Identification Using nanoLC-MS/MS
2.5. Analysis of MS Data
2.6. Comparative Analysis of Proteins Using MS Data
2.7. Analysis Using Immunoblotting
2.8. Statistical Analysis
3. Results
3.1. Morphological Changes of Soybean Induced by Simulated Microgravity Condition Under Salt Stress
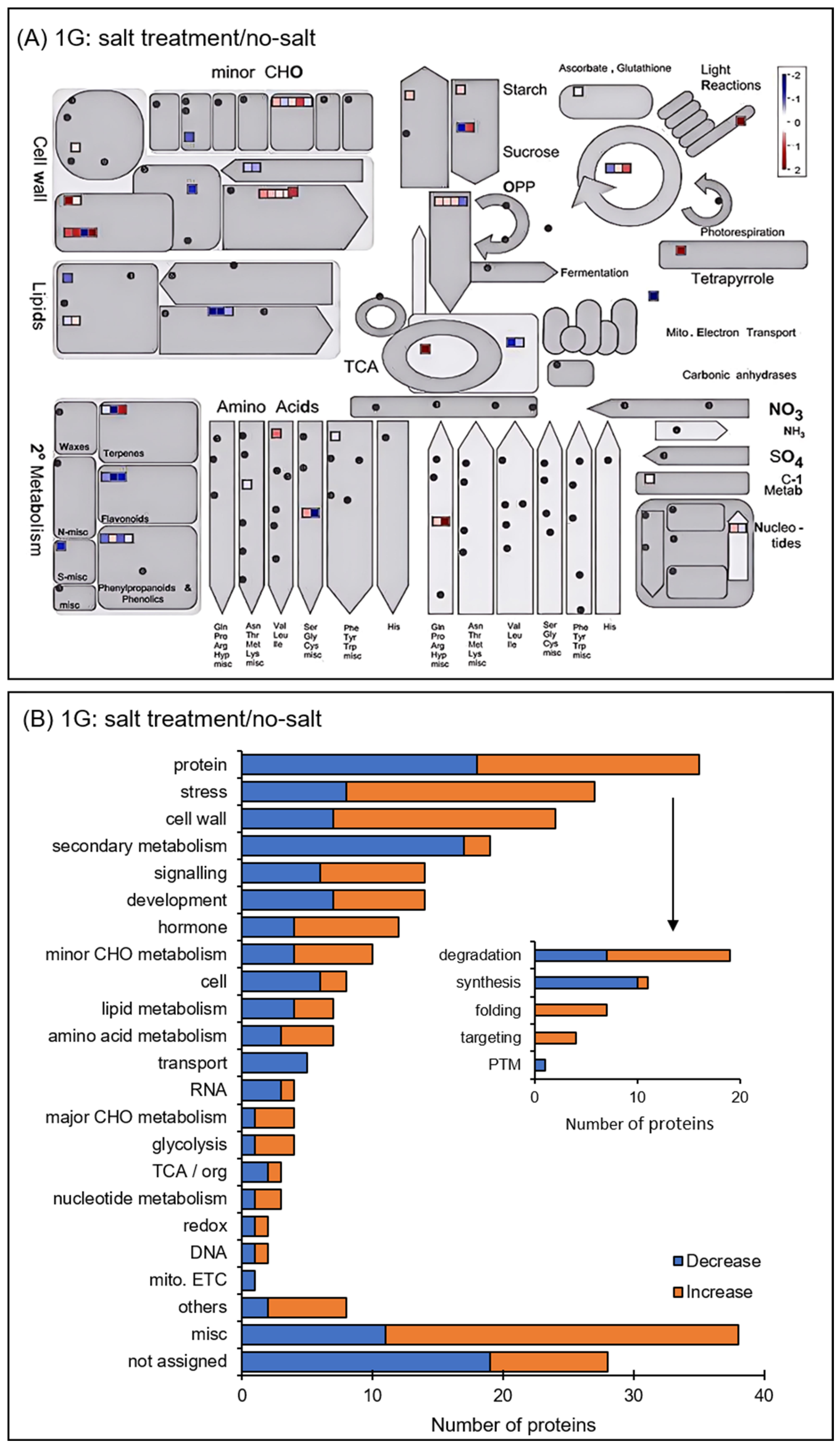
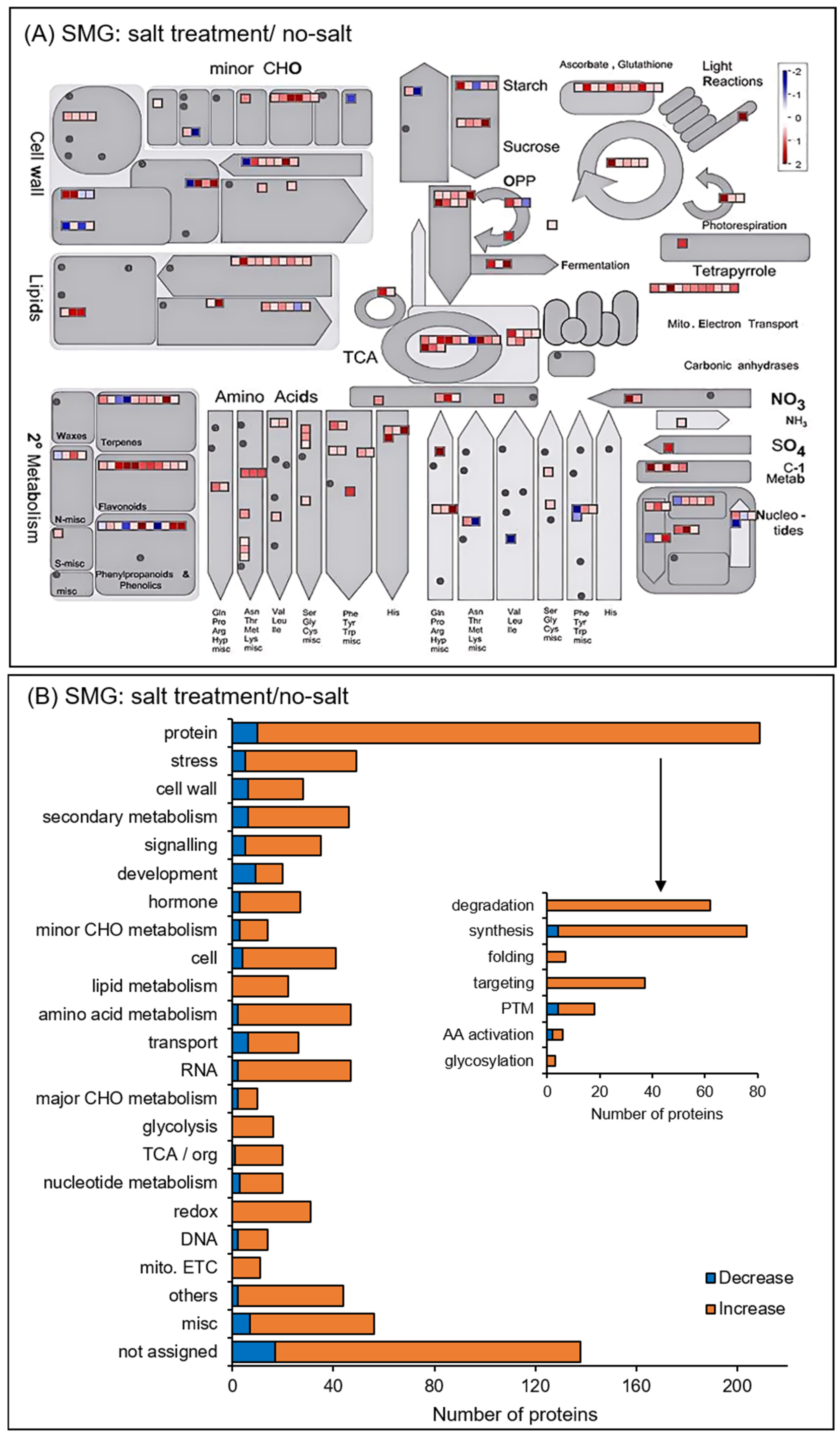
3.2. Identification and Functional Classification of Proteins in Soybean Root Treated with Simulated Microgravity Under Salt Stress
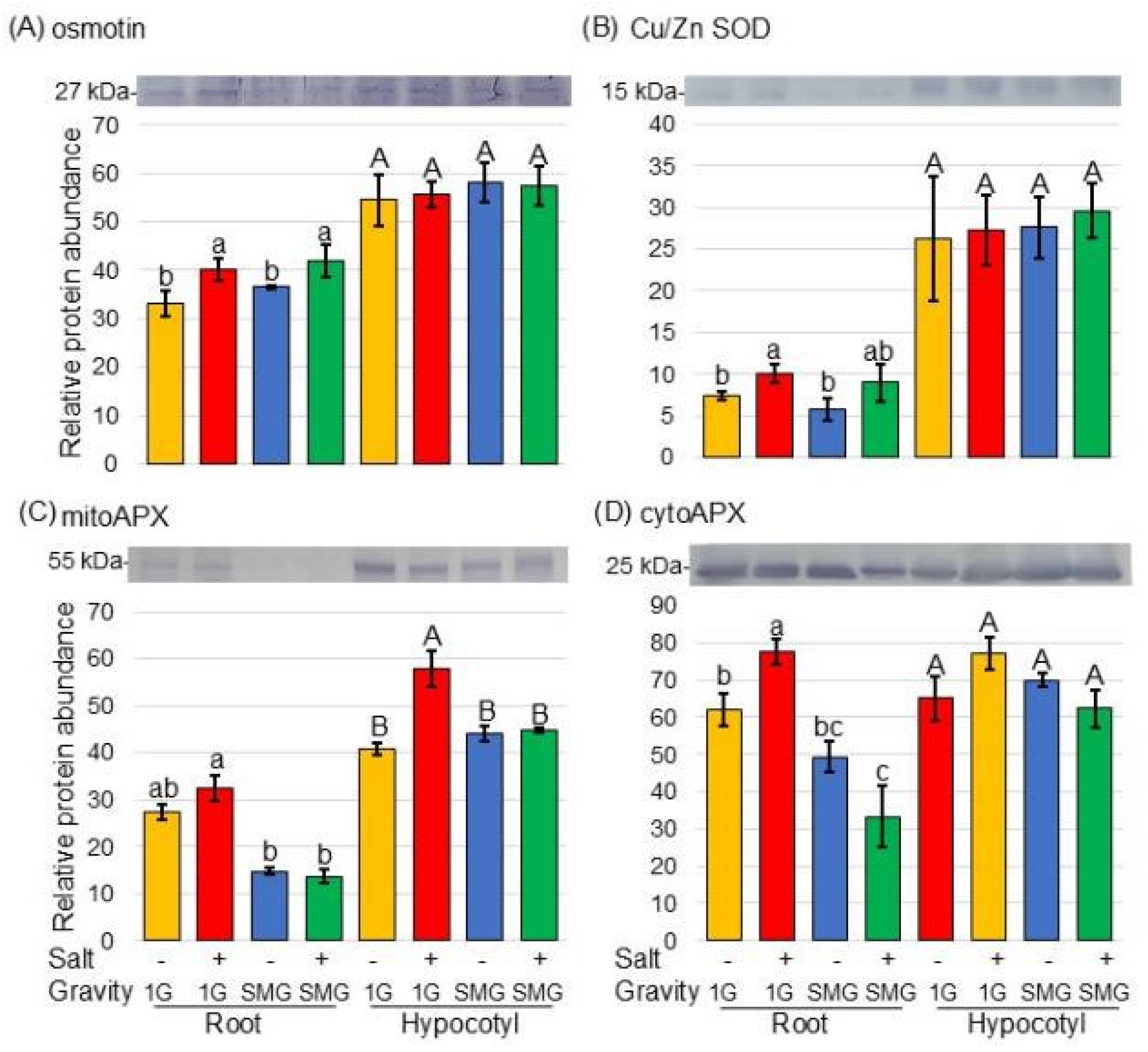
3.3. Protein Accumulation Altered in Soybean Treated with Simulated Microgravity Under Salt Stress
4. Discussion
4.1. Simulated Microgravity Mitigates the Negative Growth Effects of Salt in Soybean
4.2. Simulated Microgravity Treatment Has Positive Effect on Soybean Growth Through Phytohormone
4.3. Simulated Microgravity Treatment Decreases ROS Production Even Under Salt Stress
4.4. Simulated Microgravity Treatment Alters Membrane-Localized Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| APX | Ascorbate peroxidase |
References
- Muhammad, A.; Khan, M.H.U.; Kong, X.; Zheng, S.; Bai, N.; Li, L.; Zhang, N.; Muhammad, S.; Li, Z.; Zhang, X.; et al. Rhizospheric crosstalk: A mechanistic overview of how plant secondary metabolites alleviate abiotic stresses. Plant Sci. 2025, 354, 112431. [Google Scholar] [PubMed]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar]
- Mo, M.; Yokawa, K.; Wan, Y.; Baluška, F. How and why do root apices sense light under the soil surface? Front. Plant Sci. 2015, 6, 775. [Google Scholar]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- de Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Zheng, C.; Ma, H.; Zeng, M.; Yang, X. Root system architecture plasticity with beneficial rhizosphere microbes: Current findings and future perspectives. Microbiol. Res. 2025, 292, 128028. [Google Scholar]
- Jan, M.; Muhammad, S.; Jin, W.; Zhong, W.; Zhang, S.; Lin, Y.; Zhou, Y.; Liu, J.; Liu, H.; Munir, R.; et al. Modulating root system architecture: Crosstalk between auxin and phytohormones. Front. Plant Sci. 2024, 15, 1343928. [Google Scholar]
- Kuya, N.; Nishijima, R.; Kitomi, Y.; Kawakatsu, T.; Uga, Y. Transcriptome profiles of rice roots under simulated microgravity conditions and following gravistimulation. Front. Plant Sci. 2023, 14, 1193042. [Google Scholar]
- Nakamura, M.; Nishimura, T.; Morita, M.T. Gravity sensing and signal conversion in plant gravitropism. J. Exp. Bot. 2019, 70, 3495–3506. [Google Scholar]
- Furutani, M.; Hirano, Y.; Nishimura, T.; Nakamura, M.; Taniguchi, M.; Suzuki, K.; Oshida, R.; Kondo, C.; Sun, S.; Kato, K.; et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 2020, 11, 76. [Google Scholar]
- Han, H.; Adamowski, M.; Qi, L.; Alotaibi, S.S.; Friml, J. PIN-mediated polar auxin transport regulations in plant tropic responses. New Phytol. 2021, 232, 510–522. [Google Scholar]
- Li, L.; Chen, H.; Alotaibi, S.S.; Pěnčík, A.; Adamowski, M.; Novák, O.; Friml, J. RALF1 peptide triggers biphasic root growth inhibition upstream of auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121058119. [Google Scholar] [CrossRef]
- Choi, W.G.; Barker, R.J.; Kim, S.H.; Swanson, S.J.; Gilroy, S. Variation in the transcriptome of different ecotypes of Arabidopsis thaliana reveals signatures of oxidative stress in plant responses to spaceflight. Am. J. Bot. 2019, 106, 123–136. [Google Scholar]
- Fengler, S.; Spirer, I.; Neef, M.; Ecke, M.; Nieselt, K.; Hampp, R. A whole-genome microarray study of Arabidopsis thaliana semisolid callus cultures exposed to microgravity and nonmicrogravity related spaceflight conditions for 5 days on board of Shenzhou 8. BioMed Res. Int. 2015, 2015, 547495. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrink, J.P.; Herranz, R.; Poehlman, W.L.; Alex Feltus, F.; Villacampa, A.; Ciska, M.; Javier Medina, F.; Kiss, J.Z. RNA-seq analyses of Arabidopsis thaliana seedlings after exposure to blue-light phototropic stimuli in microgravity. Am. J. Bot. 2019, 106, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Subramanian, A.; Pattathil, S.; Correll, M.J.; Kiss, J.Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 2017, 104, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.; Land, E.S.; Toennisson, T.A.; Doherty, C.J.; Perera, I.Y. Uncovering transcriptional responses to fractional gravity in Arabidopsis roots. Life 2021, 11, 1010. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Jiang, Y.; Wang, H.; Zhou, L.; Li, F.; Wang, L.; Jiang, D.; Chen, F.; Chen, S. Transcription factor CmHSFA4-CmMYBS3 complex enhances salt tolerance in chrysanthemum by repressing CmMYB121 expression. Plant Physiol. 2024, 195, 3119–3135. [Google Scholar]
- Komatsu, S.; Nishiuchi, T. Proteomic Analysis to Understand the Promotive Effect of Ethanol on Soybean Growth under Salt Stress. Biology 2024, 13, 861. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamanouchi, S.; Takeuchi, K.; Takahashi, S.; Tashiro, M.; Hidema, J.; Higashitani, A.; Adachi, T.; Zhang, S.; Guirguis, F.N.L.; et al. Combined environment simulator for low-dose-rate radiation and partial gravity of moon and mars. Life 2020, 10, 274. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Komatsu, S.; Han, C.; Nanjo, Y.; Altaf-Un-Nahar, M.; Wang, K.; He, D.; Yang, P. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013, 12, 4769–4784. [Google Scholar]
- Li, X.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Yamaguchi, T.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the effect of plant-derived smoke on soybean during recovery from flooding stress. J. Proteom. 2018, 181, 238–248. [Google Scholar] [CrossRef]
- Komatsu, S.; Maruyama, J.; Furuya, T.; Yin, X.; Yamaguchi, H.; Hitachi, K.; Miyashita, N.; Tsuchida, K.; Tani, M. Proteomic and biological analyses reveal the effect on growth under flooding stress of chickpea irradiated with millimeter waves. J. Proteome Res. 2021, 20, 4718–4727. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Nagel, A.; Thimm, O.; Redestig, H.; Blaesing, O.E.; Palacios Rofas, N.; Selbig, J.; Hannemann, J.; Piques, M.C.; Steinhauser, D.; et al. Extension of the visualization tool mapman to allow statistical analysis of arrays, display of corresponding genes, and comparison with known databases. Plant Physiol. 2005, 138, 1195–1204. [Google Scholar] [CrossRef]
- Usadel, B.; Poree, F.; Nagel, A.; Lohse, M.; Czedik-Eysenberg, A.; Stitt, M. A guide to using mapman to visualize and compare omics data in plants: A case study in the crop species, maize. Plant Cell Environ. 2009, 32, 1211–1229. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Konishi, H.; Ishiguro, K.; Komatsu, S. A proteomics approach towards understanding blast fungus infection of rice grown under different levels of nitrogen fertilization. Proteomics 2001, 1, 1162–1171. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamamoto, A.; Nakamura, T.; Nouri, M.Z.; Nanjo, Y.; Nishizawa, K.; Furukawa, K. Comprehensive analysis of mitochondria in roots and hypocotyls of soybean under flooding stress using proteomics and metabolomics techniques. J. Proteome Res. 2011, 10, 3993–4004. [Google Scholar] [CrossRef]
- Komatsu, S.; Masuda, T.; Abe, K. Phosphorylation of a protein (pp56) is related to the regeneration of rice cultured suspension cells. Plant Cell Physiol. 1996, 37, 748–753. [Google Scholar] [CrossRef]
- Villacampa, A.; Sora, L.; Herranz, R.; Medina, F.J.; Ciska, M. Analysis of graviresponse and biological effects of vertical and horizontal clinorotation in Arabidopsis thaliana root tip. Plants 2021, 10, 734. [Google Scholar] [CrossRef]
- Millar, K.D.L.; Johnson, C.M.; Edelmann, R.E.; Kiss, J.Z. An endogenous growth pattern of roots is revealed in seedlings grown in microgravity. Astrobiology 2011, 11, 787–797. [Google Scholar] [CrossRef]
- Karahara, I.; Suto, T.; Yamaguchi, T.; Yashiro, U.; Tamaoki, D.; Okamoto, E.; Yano, S.; Tanigaki, F.; Shimazu, T.; Kasahara, H.; et al. Vegetative and reproductive growth of Arabidopsis under microgravity conditions in space. J. Plant Res. 2020, 133, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T.; Soga, K. New aspects of gravity responses in plant cells. Int. Rev. Cytol. 2003, 229, 209–244. [Google Scholar]
- Matia, I.; Gonzalez-Camacho, F.; Herranz, R.; Kiss, J.Z.; Gasset, G.; van Loon, J.J.; Marco, R.; Medina, F.J. Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J. Plant Physiol. 2010, 167, 184–193. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Mori, R.; Saiki, M.; Nakamura, Y.; Wakabayashi, K.; Kamisaka, S. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol. 2002, 43, 1067–1071. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 2002, 215, 1040–1046. [Google Scholar] [CrossRef]
- Soga, K.; Yamazaki, C.; Kamada, M.; Tanigawa, N.; Kasahara, H.; Yano, S.; Kojo, K.H.; Kutsuna, N.; Kato, T.; Hashimoto, T.; et al. Modification of growth anisotropy and cortical microtubule dynamics in Arabidopsis hypocotyls grown under microgravity conditions in space. Physiol. Plant. 2018, 162, 135–144. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Hoson, T.; Kotake, T.; Kojima, M.; Sakakibara, H.; Yamazaki, T.; Higashibata, A.; Ishioka, N.; Shimazu, T.; et al. Persistence of plant hormone levels in rice shoots grown under microgravity conditions in space: Its relationship to maintenance of shoot growth. Physiol. Plant. 2017, 161, 285–293. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.H.; Xie, J.Y.; Zheng, H.Q. Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft. Planta 2015, 241, 475–488. [Google Scholar] [CrossRef]
- Hilaire, E.; Peterson, B.V.; Guikema, J.A.; Brown, C.S. Clinorotation affects morphology and ethylene production in soybean seedlings. Plant Cell Physiol. 1996, 37, 929–934. [Google Scholar] [CrossRef][Green Version]
- Land, E.S.; Sheppard, J.; Doherty, C.J.; Perera, I.Y. Conserved plant transcriptional responses to microgravity from two consecutive spaceflight experiments. Front. Plant Sci. 2024, 14, 1308713. [Google Scholar] [CrossRef]
- Yamazaki, C.; Yamazaki, T.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Uheda, E.; Oka, M.; Kamada, M.; Shimazu, T.; Kasahara, H.; et al. Comprehensive analyses of plant hormones in etiolated pea and maize seedlings grown under microgravity conditions in space: Relevance to the International Space Station experiment “Auxin Transport”. Life Sci. Space Res. 2023, 36, 138–146. [Google Scholar]
- Barker, R.; Kruse, C.P.S.; Johnson, C.; Saravia-Butler, A.; Fogle, H.; Chang, H.S.; Trane, R.M.; Kinscherf, N.; Villacampa, A.; Manzano, A.; et al. Meta-analysis of the space flight and microgravity response of the Arabidopsis plant transcriptome. NPJ Microgravity 2023, 9, 21. [Google Scholar] [PubMed]
- Hausmann, N.; Fengler, S.; Hennig, A.; Franz-Wachtel, M.; Hampp, R.; Neef, M. Cytosolic calcium, hydrogen peroxide and related gene expression and protein modulation in Arabidopsis thaliana cell cultures respond immediately to altered gravitation: Parabolic flight data. Plant Biol. 2013, 16, 120–128. [Google Scholar] [PubMed]
- Barjaktarović, Ž.; Nordheim, A.; Lamkemeyer, T.; Fladerer, C.; Madlung, J.; Hampp, R. Time-course of changes in amounts of specific proteins upon exposure to hyper-g, 2-D clinorotation, and 3-D random positioning of Arabidopsis cell cultures. J. Exp. Bot. 2007, 58, 4357–4363. [Google Scholar]
- Olanrewaju, G.O.; Haveman, N.J.; Naldrett, M.J.; Paul, A.L.; Ferl, R.J.; Wyatt, S.E. Integrative transcriptomics and proteomics profiling of Arabidopsis thaliana elucidates novel mechanisms underlying spaceflight adaptation. Front. Plant Sci. 2023, 14, 1260429. [Google Scholar]
- De Francesco, S.; Le Disquet, I.; Pereda-Loth, V.; Tisseyre, L.; De Pascale, S.; Amitrano, C.; Carnero Diaz, E.; De Micco, V. Combined effects of microgravity and chronic low-dose gamma radiation on Brassica rapa microgreens. Plants 2024, 14, 64. [Google Scholar] [CrossRef]
- Mazars, C.; Brière, C.; Grat, S.; Pichereaux, C.; Rossignol, M.; Pereda-Loth, V.; Eche, B.; Boucheron-Dubuisson, E.; Le Disquet, I.; Medina, F.J.; et al. Microsome-associated proteome modifications of Arabidopsis seedlings grown on board the International Space Station reveal the possible effect on plants of space stresses other than microgravity. Plant Signal. Behav. 2014, 9, e29637. [Google Scholar]
- Kamada, M.; Oka, M.; Miyamoto, K.; Uheda, E.; Yamazaki, C.; Shimazu, T.; Sano, H.; Kasahara, H.; Suzuki, T.; Higashibata, A.; et al. Microarray profile of gene expression in etiolated Pisum sativum seedlings grown under microgravity conditions in space: Relevance to the International Space Station experiment “Auxin Transport”. Life Sci. Space Res. 2020, 26, 55–61. [Google Scholar]
- Ha, H.J.; Subburaj, S.; Kim, Y.S.; Kim, J.B.; Kang, S.Y.; Lee, G.J. Molecular characterization and identification of calnexin 1 as a radiation biomarker from Tradescantia BNL4430. Plants 2020, 9, 387. [Google Scholar] [CrossRef]
- Valdez, B.C.; Li, Y.; Murray, D.; Liu, Y.; Nieto, Y.; Bashir, Q.; Qazilbash, M.H.; Andersson, B.S. Panobinostat and venetoclax enhance the cytotoxicity of gemcitabine, busulfan, and melphalan in multiple myeloma cells. Exp. Hematol. 2020, 81, 32–41. [Google Scholar]
- Komatsu, S.; Hamada, K.; Furuya, T.; Nishiuchi, T.; Tani, M. Membrane Proteomics to Understand Enhancement Effects of Millimeter-Wave Irradiation on Wheat Root under Flooding Stress. Int. J. Mol. Sci. 2023, 24, 9014. [Google Scholar] [CrossRef] [PubMed]
- Sarwat, M.; Naqvi, A.R. Heterologous expression of rice calnexin (OsCNX) confers drought tolerance in Nicotiana tabacum. Mol. Biol. Rep. 2013, 40, 5451–5464. [Google Scholar] [PubMed]
- Chevet, E.; Smirle, J.; Cameron, P.H.; Thomas, D.Y.; Bergeron, J.J. Calnexin phosphorylation: Linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin. Cell Dev. Biol. 2010, 21, 486–490. [Google Scholar] [PubMed]
- Vizcaíno, J.A.; Côté, R.G.; Csordas, A.; Dianes, J.A.; Fabregat, A.; Foster, J.M.; Griss, J.; Alpi, E.; Birim, M.; Contell, J.; et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: Status in 2013. Nucleic Acids Res. 2013, 41, D1063–D1069. [Google Scholar]
- Okuda, S.; Watanabe, Y.; Moriya, Y.; Kawano, S.; Yamamoto, T.; Matsumoto, M.; Takami, T.; Kobayashi, D.; Araki, N.; Yoshizawa, A.C.; et al. jPOSTTrepo: An international standard data repository for proteomes. Nucleic Acids Res. 2017, 45, D1107–D1111. [Google Scholar]




| Difference | Accession | Protein Names | Gene Ontology |
|---|---|---|---|
| Phytohormone-related proteins | |||
| 0.6143 | F6KBT6 | Allene oxide cyclase 6 | Jasmonic acid biosynthetic process |
| −0.8150 | I1LY51 | Oxidored_FMN protein | Jasmonic acid biosynthetic process |
| −2.6814 | I1JNN8 | Dirigent protein | Jasmonic acid biosynthetic process |
| −0.9307 | A0A0R0H3J2 | Serine/threonine-protein phosphatase | Brassinosteroid mediated signaling pathway |
| −1.5529 | I1JJV5 | Protein kinase | Brassinosteroid mediated signaling pathway |
| 0.5960 | C6TIU6 | 7-dehydrocholesterol reductase | Brassinosteroid biosynthetic process |
| −2.0043 | K7MV36 | UBR-type domain-containing protein | Auxin polar transport |
| −0.6056 | D3G9M7 | Calcium dependent protein kinase | Abscisic acid-activated signaling pathway |
| −1.2503 | I1K6C6 | Serine/threonine-protein kinase | Abscisic acid-activated signaling pathway |
| −3.8083 | I1KBT7 | Calcium-dependent protein kinase | Abscisic acid-activated signaling pathway |
| −1.5482 | E6YBW4 | Pathogenesis-related protein 10 | Abscisic acid-activated signaling pathway |
| −1.2505 | I1MRM5 | Bet_v_1 domain-containing protein | Abscisic acid-activated signaling pathway |
| −0.6948 | I1KE09 | Bet_v_1 domain-containing protein | Abscisic acid-activated signaling pathway |
| Ubiquitin/proteasome-related proteins | |||
| 0.6168 | C6TG97 | Proteasome subunit alpha type | Ubiquitin-dependent protein |
| −0.8034 | I1NB01 | MPN domain-containing protein | Ubiquitin-dependent protein |
| −1.0399 | I1K657 | RPN13_C domain-containing protein | Ubiquitin-dependent protein |
| −1.3676 | I1J8D4 | Ubiquitin-dependent protein | Ubiquitin-dependent protein |
| −1.4256 | I1K1Z8 | PCI domain-containing protein | Ubiquitin-dependent protein |
| −1.5579 | I1JCA6 | Uncharacterized protein | Ubiquitin-dependent protein |
| −1.6108 | C6T2F1 | UBIQUITIN_CONJUGAT_2 | Ubiquitin-dependent protein |
| −3.1095 | I1JQF4 | MPN domain-containing protein | Ubiquitin-dependent protein |
| 0.9386 | C6T574 | Ubiquitin carboxyl-terminal hydrolase | Protein deubiquitination |
| −0.6865 | A0A0R0L2F3 | Ubiquitinyl hydrolase 1 | Protein deubiquitination |
| −0.8156 | I1KGF2 | Ubiquitin carboxyl-terminal hydrolase | Protein deubiquitination |
| −1.1460 | I1NIJ5 | Ubiquitin carboxyl-terminal hydrolase | Protein deubiquitination |
| −1.7558 | I1LSZ2 | Ubiquitin carboxyl-terminal hydrolase | Protein deubiquitination |
| −1.4141 | I1L9D9 | USP domain-containing protein | Protein deubiquitination |
| −1.5029 | I1MF26 | CULLIN_2 domain-containing protein | Proteasome-ubiquitin-dependent protein |
| −1.3441 | K7KAA3 | RING-type domain-containing protein | Proteasome-ubiquitin-dependent protein |
| −0.6033 | I1LS70 | 26S proteasome non-ATPas | Proteasome-ubiquitin-dependent protein |
| −0.8114 | I1KQE8 | 26S proteasome non-ATPase | Proteasome-ubiquitin-dependent protein |
| −1.7403 | I1K5H2 | 26S proteasome non-ATPase | Proteasome-ubiquitin-dependent protein |
| −0.7464 | I1LMP2 | MPN domain-containing protein | Proteasome-ubiquitin-dependent protein |
| −0.7495 | I1J8K2 | VWFA domain-containing protein | Proteasome assembly |
| −0.8726 | I1JCF9 | VWFA domain-containing protein | Proteasome assembly |
| −0.6417 | I1KDR0 | 26S proteasome non-ATPase | Proteasome assembly |
| −2.4357 | A0A0R0G8R3 | Proteasome component Ecm29 | Proteasome assembly |
| −1.3209 | I1N058 | Proteasome activator subunit 4 | Proteasomal ubiquitin catabolic process |
| 0.8760 | I1JBH2 | Proteasome subunit alpha type | Proteasomal protein catabolic process |
| 0.6471 | I1N783 | Proteasome subunit alpha type | Proteasomal protein catabolic process |
| −0.6180 | I1MA63 | Proteasome subunit beta | Proteasomal protein catabolic process |
| −0.7445 | A0A0R4J2M8 | Proteasome subunit alpha type | Proteasomal protein catabolic process |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, S.; Misaki, H.; Zhu, W.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Higashitani, A. The Growth of Soybean (Glycine max) Under Salt Stress Is Modulated in Simulated Microgravity Conditions. Cells 2025, 14, 541. https://doi.org/10.3390/cells14070541
Komatsu S, Misaki H, Zhu W, Yamaguchi H, Hitachi K, Tsuchida K, Higashitani A. The Growth of Soybean (Glycine max) Under Salt Stress Is Modulated in Simulated Microgravity Conditions. Cells. 2025; 14(7):541. https://doi.org/10.3390/cells14070541
Chicago/Turabian StyleKomatsu, Setsuko, Haruka Misaki, Wei Zhu, Hisateru Yamaguchi, Keisuke Hitachi, Kunihiro Tsuchida, and Atsushi Higashitani. 2025. "The Growth of Soybean (Glycine max) Under Salt Stress Is Modulated in Simulated Microgravity Conditions" Cells 14, no. 7: 541. https://doi.org/10.3390/cells14070541
APA StyleKomatsu, S., Misaki, H., Zhu, W., Yamaguchi, H., Hitachi, K., Tsuchida, K., & Higashitani, A. (2025). The Growth of Soybean (Glycine max) Under Salt Stress Is Modulated in Simulated Microgravity Conditions. Cells, 14(7), 541. https://doi.org/10.3390/cells14070541








