Extracellular Vesicles in Asthma: Intercellular Cross-Talk in TH2 Inflammation
Abstract
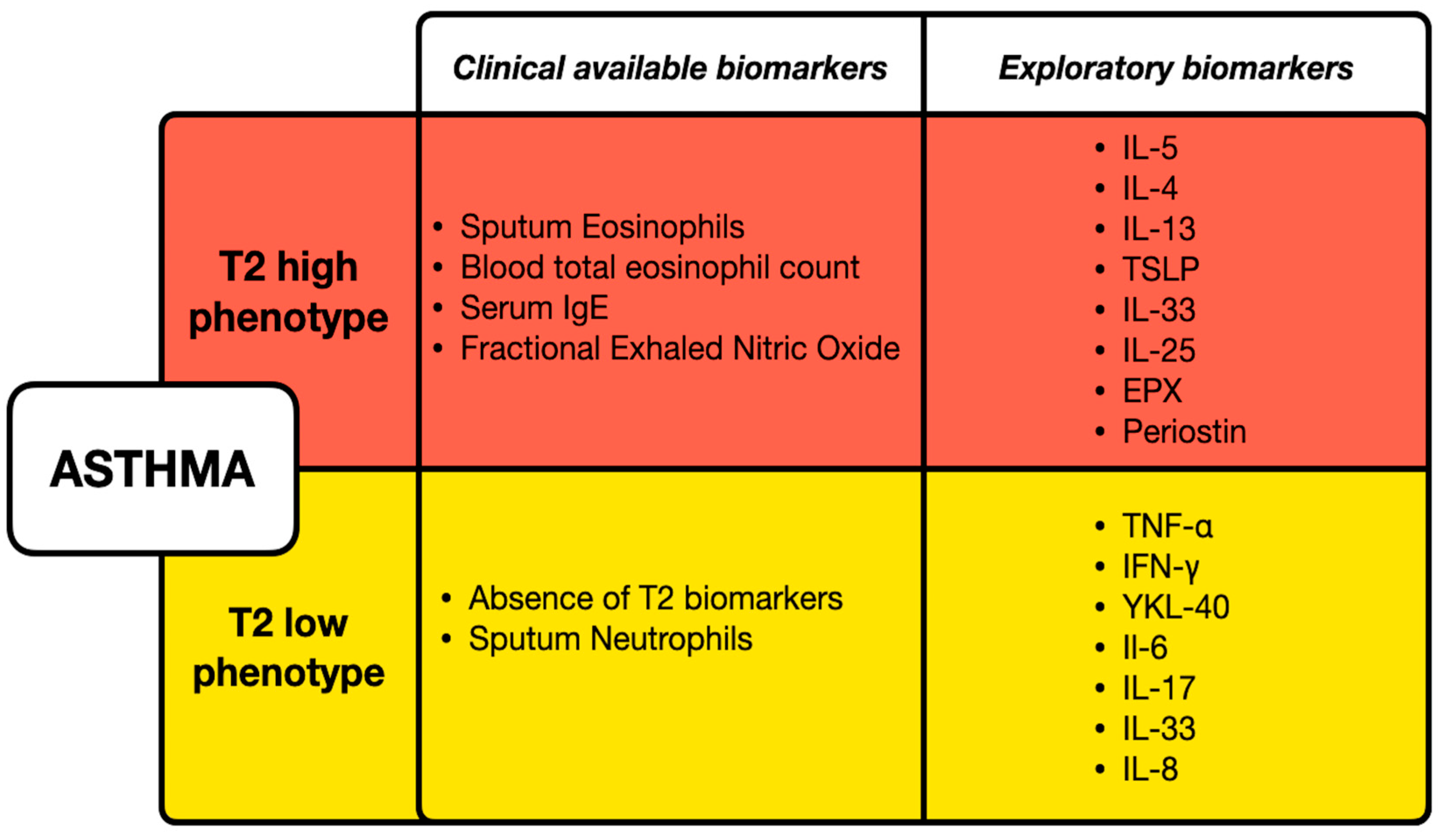
1. Introduction
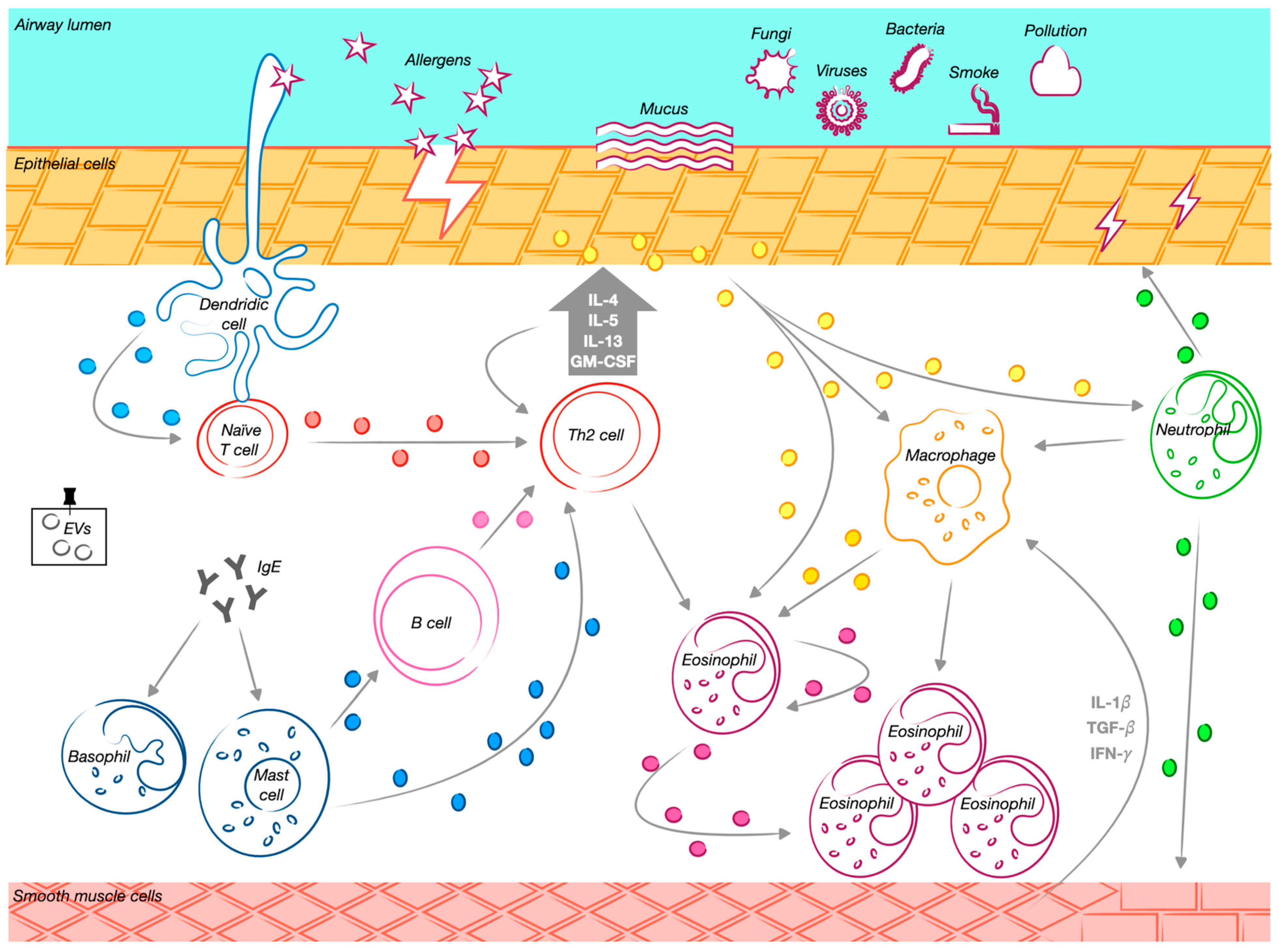
2. The Cellular Drivers in Asthma Inflammation and EVs’ Related Role
2.1. TH2-High Asthma-Related Cells
2.1.1. Epithelial Cell-Derived EVs
2.1.2. Eosinophilic-Derived EVs
2.1.3. Lymphocyte-Derived EVs
2.1.4. Mast Cell-Derived EVs
2.1.5. Dendritic Cell-Derived EVs
2.2. TH2-Low Asthma-Related Cells
2.2.1. Neutrophil-Derived EVs
2.2.2. Macrophage-Derived EVs
3. The Paradigm of Mesenchymal Stem Cell-Derived EVs
| Cell Type | Main Findings | References |
|---|---|---|
| Epithelial cells | Exosomes derived from AECs can cause inflammation by increasing IL-8 and LTC4. | [31] |
| BECs play a pivotal role in exosome-driven cell-to-cell communication and promote the proliferation and infiltration of undifferentiated macrophages. | [43] | |
| AEC-derived exosomes with intertwined filamentous formations on their surface lead to airway inflammation remodeling. | [44] | |
| Exosomes secreted by OVA-induced AECs promote CD4+ T cell differentiation in Th2-like cells. | [45] | |
| AEC-derived exosomes with CNTN1 protein play a significant role in modulating allergic responses via DCs. | [46] | |
| Mechanical stress leads to TF expression and its transport via exosomes in normal BECs. | [47] | |
| Exosomes can transport TF between different cells, linking it to asthma. | [48,49] | |
| TF expression levels were higher in asthmatic patients, and TGF-β plays an important role in asthma mechanical stress. | [50] | |
| TGF-β2 expression in exosomes was reduced in severe asthmatic patients. | [51,52] | |
| TGF-β2 secreted from exosomes has a regulatory effect in cell proliferation. | [52] | |
| Epithelium-derived exosomes secrete miRNA involved in asthma development. | [53] | |
| MiR-34a regulates the functions of dendritic cells and their maturation, targeting the Wnt pathway. | [54] | |
| MiR-92b is involved in epithelial-to-mesenchymal transition. | [55] | |
| Eosinophils | Eosinophil-derived exosomes mediate immune responses and structural changes. | [60,61] |
| Eosinophil-derived exosomes significantly influence asthma pathogenesis. | [36] | |
| EVs from the eosinophils of asthmatic patients increase NO and ROS production. Patients enhance chemotaxis, upregulate cell adhesion molecules, and upregulate integrin α2 in eosinophils. | [62,63] | |
| EVs from eosinophils contribute to the inflammatory response and structural changes in the lungs. | [64,65] | |
| Eosinophil-derived exosomes alter gene expression in various cell types, including lung cells, contributing to asthma pathology. | [63,64] | |
| Eosinophils express exosomal markers such as CD63 and CD9. | [66] | |
| The stimulation of eosinophils with IFN-γ enhanced exosome production, particularly in asthma patients. | [61] | |
| Eosinophils from asthmatic patients have a greater production of exosomes. | [63] | |
| Eosinophil-derived exosomes promote inflammation related with asthma. | [68] | |
| Eosinophil-derived exosomes contribute to airway structural changes. | [64] | |
| Lymphocytes | B cell-derived exosomes exhibit the features of their originating cells and present HSP70, important for DC maturation. | [72,74] |
| B cell-derived exosomes can present antigen peptides to T cells, inducing the release of proinflammatory cytokines. | [73] | |
| Antigen-presenting cell (APC)-derived exosomes are significant contributors to T cell activation. | [75] | |
| Two mechanisms through which B cell-derived exosomes activate T cells are direct stimulation and through the involvement of APC. | [76,77,78] | |
| B lymphocyte-produced exosomes stimulate the release of cytokines IL-5 and IL-13. | [80,81] | |
| Activated T cells release exosomes upon activation. | [29,82] | |
| T cell-derived exosomes trigger mast cell activation and degranulation, cytokine release, tissue remodeling, and increasing airway reactivity. | [83] | |
| T cell-derived exosomes inhibit CD8+ T lymphocyte activity. | [84] | |
| T cell-derived exosomes shape an optimal environment for immune cell operations, mediating communications to enhance immune response. | [59,85] | |
| T cell EVs are able to activate MC degranulation and the release of cytokines. | [86] | |
| Th2 cells promote eosinophil survival through the inhibition of apoptosis. | [87] | |
| Mast cells | MC-derived exosomes carry immune-related factors, which play an important role in immunity. | [92,93] |
| Mast cell-derived exosomes secrete miR-21, which promotes oxidative stress and inflammation in asthmatic mice. | [94] | |
| BMMC-derived exosomes could activate immune cells without direct contact, suggesting the mobilization of B and T cells into lungs. | [95] | |
| MCs can exchange RNA with each other through EVs. | [96] | |
| MC-derived exosomes enhance the ability of DCs to present antigens to T cells and regulate T lymphocyte activation. | [29] | |
| BMMC-derived exosomes are able to lower IgE levels and block mast cell activation. | [97] | |
| MC-derived EVs convey a protective message under oxidative stress, decreasing mortality. | [98] | |
| miR-21 released from MC-derived exosomes enhances oxidative stress and triggers inflammatory reactions in asthmatic mice. | [94] | |
| Exosomes activated by IgE from MCs exacerbate atherosclerosis by inducing endothelial dysfunction through the circular RNA CDR1as, linking asthma with atherosclerosis. | [99] | |
| Dendritic cells | DC-derived exosomes can activate allergen-specific Th2 cells. | [29] |
| DC exosomes specifically activate OVA-targeted CD8+ T cells and promote OVA-specific IgG antibody production. | [104] | |
| DC-derived exosomes carry enzymes necessary for producing leukotrienes. | [105,106] | |
| DC-derived exosomes, when packed with chemotactic eicosanoids, promote inflammation and granulocyte migration in vitro. | [106] | |
| Various subsets of pulmonary DCs have been identified, each contributing differently to asthma pathogenesis. | [107,108] | |
| Neutrophils | Neutrophil-derived exosomes play a role in regulating changes in airway smooth muscle structure. | [113,114] |
| OVA-induced airway epithelium-derived exosomes increase AHR and trigger the accumulation/activation of macrophages, neutrophils, and eosinophils. | [115] | |
| Neutrophil-derived EVs disrupt epithelial cell connections. | [116] | |
| Exosomes released by neutrophils contribute to airway structural changes, promoting the migration and proliferation of ASMCs in response to LPS. | [117] | |
| Neutrophil-derived exosomes containing elastase contribute significantly to airway inflammation. | [118] | |
| Macrophages | Macrophage-derived exosomes have a significant impact on T1 immune reactions. | [126,127] |
| In SSRA, M1 macrophages release high levels of inflammatory molecules, contributing to neutrophil-rich infiltration, AHR, and airway structural changes. | [128] | |
| Exosomes secreted from human macrophages have a proinflammatory role in asthma and contain enzymes that favor LTC4 production. | [106] | |
| Exosomes secreted by M2 macrophages reduce lung inflammation and asthma progression through the action of miR-370. | [129] | |
| Mesenchymal stem cells | MSC-EVs exhibit therapeutic effects similar to MSCs but with reduced risks of immune rejection, tumorigenicity, and pulmonary embolism. | [131] |
| EVsderived from mesenchymal stem cells have a similar therapeutic effect to their parental cells. | [132] | |
| EVsderived from mesenchymal stem cells impact immune cells and inhibit airway remodeling. | [13] | |
| ASCs and other MSCs can reduce allergic airway inflammation in bronchial asthma mouse models. | [133] | |
| ASC-derived EVs immunomodulatory effects are thought to involve the suppression of Th2 cytokine production in airway allergic inflammation. | [134] | |
| AD-MSC-derived exosomes showed beneficial effects on ovalbumin-induced allergic asthma. | [135] | |
| MSC-derived extracellular vesicles have been proposed as a promising alternative to MSCs for treatments such as asthma. | [136] | |
| BMMC-derived exosomes highly expressed a specific miRNA, miR-223-3p, known to be associated with high inflammation and the exacerbation of asthma. | [137] | |
| MSC-derived exosomal miR-1470 induces the expression of P27KIP1 in asthmatic patients, promoting the differentiation of CD4+CD25+FOXP3+ Tregs. | [138] | |
| BMMC-derived exosomes contain miR-188, which has a negative effect on airway remodeling and lung injury. | [139] | |
| hUCMSC-derived EVs have a therapeutic effect in SSRA, with an action on the NF-kB and PI3K/AKT signaling pathways. | [140] | |
| Migrasomes secreted from hUCMSCs play a role in the protective effect of hUCMSCs in asthma. | [141] | |
| Hypo-EVs are able to reduce airway inflammation and remodeling in asthmatic mice. | [142] | |
| Hypo-EVs have a therapeutic effect on epithelial barriers both in vivo and in vitro. | [143] | |
| The therapeutic mechanisms of MSC-EVs can be categorized into several key pathways in the context of asthma treatment. | [144] |
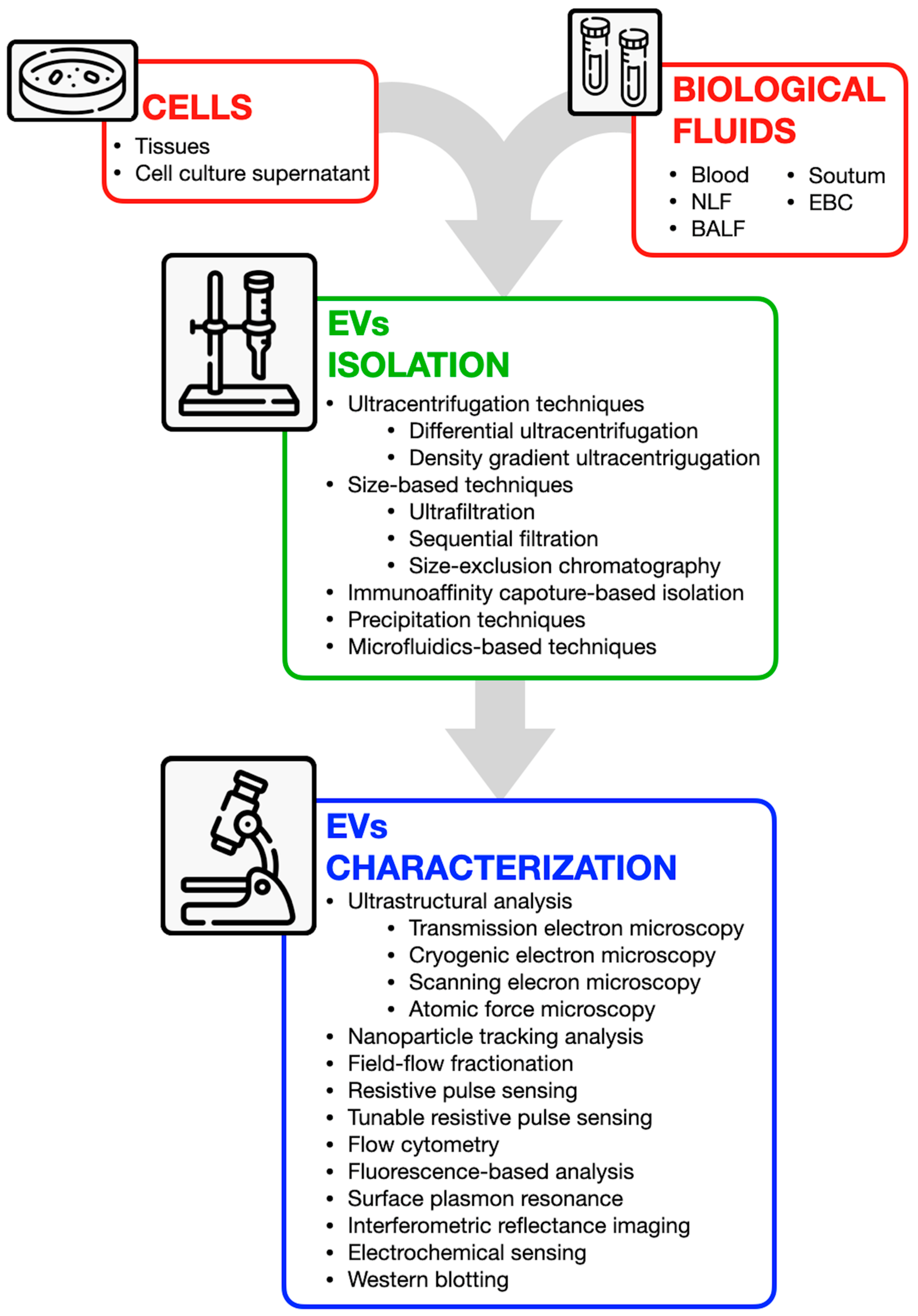
4. Methods for EVs Analysis: Available Tools, Potential, and Challenges
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD-MSCs | Adipose tissue-derived mesenchymal stromal cell |
| AERD | Aspirin-exacerbated respiratory disease |
| AHR | Airway hyperresponsiveness |
| APC | Antigen-presenting cell |
| ASCs | Adipose-derived stem cells |
| ASM | Airway smooth muscle |
| BALF | Bronchoalveolar lavage fluid |
| BECs | Bronchial epithelial cells |
| BMMCs | Bone marrow-derived mesenchymal stem cells |
| BSMCs | Bronchial smooth muscle cells |
| circ | Circular |
| cysLT | Cysteinyl leukotrienes |
| DCs | Dendritic cells |
| DRMs | Detergent-resistant membrane microdomains |
| ECP | Eosinophil cationic protein |
| EDN | Eosinophil-derived neurotoxin |
| EPX | Eosinophil peroxidase |
| ETM | Epithelial-to-mesenchymal transition |
| EVs | Extracellular vesicles |
| FeNO | Nitric oxide |
| FGM1 | Fibroblast growth factor 1 |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| hUCMSCs | Human umbilical cord mesenchymal stem cells |
| Hypo-EVs | Hypoxic hUCMSC-EVs |
| IFN-γ | Interferon-gamma |
| IgE | Immunoglobulin E |
| ISEV | International Society for Extracellular Vesicles |
| LAMP1 | Liposomal-associated membrane protein 1 |
| LFA1 | Lymphocyte function-associated antigen-1 |
| lnc | Long noncoding |
| LPS | Lipopolysaccharide |
| MBP | Major basic protein |
| MCs | Mast cells |
| MHC | Major histocompatibility complex |
| miRNAs | MicroRNAs |
| MSC-EVs | MSC-derived extracellular vesicles |
| MSCs | Mesenchymal stem cells |
| NHBE | Normal human bronchial epithelial cells |
| NO | Nitric oxide |
| NTA | Nanoparticle tracking analysis |
| PDCs | Plasmacytoid |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| SSRA | Severe steroid-resistant asthma |
| TCR | T cell receptor |
| TEM | Transmission electron microscopy |
| TF | Tissue factor |
| Tregs | Regulatory T cells |
| UCMSC-EVs | Umbilical cord mesenchymal stem cell-derived EVs |
References
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Guida, G.; Bertolini, F.; Di Stefano, A.; Carriero, V. Phenotype Overlap in the Natural History of Asthma. Eur. Respir. Rev. 2023, 32, 220201. [Google Scholar] [CrossRef]
- Popović-Grle, S.; Štajduhar, A.; Lampalo, M.; Rnjak, D. Biomarkers in Different Asthma Phenotypes. Genes 2021, 12, 801. [Google Scholar] [CrossRef]
- Ozyigit, L.P.; Morita, H.; Akdis, M. Innate Lymphocyte Cells in Asthma Phenotypes. Clin. Transl. Allergy 2015, 5, 23. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.-S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef]
- Srinivasan, A.; Sundar, I.K. Recent Updates on the Role of Extracellular Vesicles in the Pathogenesis of Allergic Asthma. Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 127–147. [Google Scholar] [CrossRef]
- Sugita, K.; Soyka, M.B.; Wawrzyniak, P.; Rinaldi, A.O.; Mitamura, Y.; Akdis, M.; Akdis, C.A. Outside-in Hypothesis Revisited: The Role of Microbial, Epithelial, and Immune Interactions. Ann. Allergy Asthma Immunol. 2020, 125, 517–527. [Google Scholar] [CrossRef]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef]
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic Endotypes and Phenotypes of Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440. [Google Scholar] [CrossRef]
- Porpodis, K.; Tsiouprou, I.; Apostolopoulos, A.; Ntontsi, P.; Fouka, E.; Papakosta, D.; Vliagoftis, H.; Domvri, K. Eosinophilic Asthma, Phenotypes-Endotypes and Current Biomarkers of Choice. J. Pers. Med. 2022, 12, 1093. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Chen, Y.-F.; Luh, F.; Ho, Y.-S.; Yen, Y. Exosomes: A Review of Biologic Function, Diagnostic and Targeted Therapy Applications, and Clinical Trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Purghè, B.; Manfredi, M.; Ragnoli, B.; Baldanzi, G.; Malerba, M. Exosomes in Chronic Respiratory Diseases. Biomed. Pharmacother. 2021, 144, 112270. [Google Scholar] [CrossRef]
- Modani, S.; Tomar, D.; Tangirala, S.; Sriram, A.; Mehra, N.K.; Kumar, R.; Khatri, D.K.; Singh, P.K. An Updated Review on Exosomes: Biosynthesis to Clinical Applications. J. Drug Target. 2021, 29, 925–940. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of Sphingolipids in the Biogenesis and Biological Activity of Extracellular Vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.-N.; Chen, J.; Zhang, C.-J.; Xie, X.-J.; Liao, D.-F.; Qin, L. The Crosstalk: Exosomes and Lipid Metabolism. Cell Commun. Signal 2020, 18, 119. [Google Scholar] [CrossRef]
- Vatrella, A.; Maglio, A.; Pelaia, C.; Ciampo, L.; Pelaia, G.; Vitale, C. Eosinophilic Inflammation: An Appealing Target for Pharmacologic Treatments in Severe Asthma. Biomedicines 2022, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, J.; Yang, L.; Dai, J.; He, L. Recent Progress in Exosome Research: Isolation, Characterization and Clinical Applications. Cancer Gene Ther. 2023, 30, 1051–1065. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.-H. Clinical Applications of Exosomes: A Critical Review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Cañas, J.A.; Sastre, B.; Rodrigo-Muñoz, J.M.; Del Pozo, V. Exosomes: A New Approach to Asthma Pathology. Clin. Chim. Acta 2019, 495, 139–147. [Google Scholar] [CrossRef]
- Mortaz, E.; Alipoor, S.D.; Varahram, M.; Jamaati, H.; Garssen, J.; Mumby, S.E.; Adcock, I.M. Exosomes in Severe Asthma: Update in Their Roles and Potential in Therapy. Biomed. Res. Int. 2018, 2018, 2862187. [Google Scholar] [CrossRef]
- Sastre, B.; Cañas, J.A.; Rodrigo-Muñoz, J.M.; Del Pozo, V. Novel Modulators of Asthma and Allergy: Exosomes and MicroRNAs. Front. Immunol. 2017, 8, 826. [Google Scholar] [CrossRef]
- Torregrosa Paredes, P.; Esser, J.; Admyre, C.; Nord, M.; Rahman, Q.K.; Lukic, A.; Rådmark, O.; Grönneberg, R.; Grunewald, J.; Eklund, A.; et al. Bronchoalveolar Lavage Fluid Exosomes Contribute to Cytokine and Leukotriene Production in Allergic Asthma. Allergy 2012, 67, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Alashkar Alhamwe, B.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma—More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Q.; Du, T.; Gabriel, A.N.A.; Wang, X.; Sun, L.; Li, X.; Xu, K.; Jiang, X.; Zhang, Y. The Potential Roles of Exosomes in Chronic Obstructive Pulmonary Disease. Front. Med. 2021, 7, 618506. [Google Scholar] [CrossRef]
- Gangadaran, P.; Madhyastha, H.; Madhyastha, R.; Rajendran, R.L.; Nakajima, Y.; Watanabe, N.; Velikkakath, A.K.G.; Hong, C.M.; Gopi, R.V.; Muthukalianan, G.K.; et al. The Emerging Role of Exosomes in Innate Immunity, Diagnosis and Therapy. Front. Immunol. 2022, 13, 1085057. [Google Scholar] [CrossRef]
- Tienda-Vázquez, M.A.; Hanel, J.M.; Márquez-Arteaga, E.M.; Salgado-Álvarez, A.P.; Scheckhuber, C.Q.; Alanis-Gómez, J.R.; Espinoza-Silva, J.I.; Ramos-Kuri, M.; Hernández-Rosas, F.; Melchor-Martínez, E.M.; et al. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells 2023, 12, 1625. [Google Scholar] [CrossRef]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Gil-Martínez, M.; Sastre, B.; Pozo, V. del Exosomes: A Key Piece in Asthmatic Inflammation. Int. J. Mol. Sci. 2021, 22, 963. [Google Scholar] [CrossRef]
- Caminati, M.; Pham, D.L.; Bagnasco, D.; Canonica, G.W. Type 2 Immunity in Asthma. World Allergy Organ. J. 2018, 11, 13. [Google Scholar] [CrossRef]
- Alladina, J.; Medoff, B.D.; Cho, J.L. Innate Immunity and Asthma Exacerbations: Insights from Human Models. Immunol. Rev. 2025, 330, e70016. [Google Scholar] [CrossRef]
- Duffus, E.K.; Holguin, F.; Rastogi, D. Non-T2 Asthma. Curr. Opin. Pulm. Med. 2025, 31, 287–293. [Google Scholar] [CrossRef]
- Calvén, J.; Ax, E.; Rådinger, M. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Wang, M.; Chen, Z.; Yan, Y.; Gu, W.; Tan, J.; Jiang, W.; Ji, W. Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology 2019, 86, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pilette, C. Role of Exosomes in Allergic Asthma: Signaling Platforms between the Epithelium and Type 2 Immunity. J. Allergy Clin. Immunol. 2021, 148, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory Role of Epithelial Cell-Derived Exosomes in Allergic Airway Inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203.e14. [Google Scholar] [CrossRef]
- Kesimer, M.; Gupta, R. Physical Characterization and Profiling of Airway Epithelial Derived Exosomes Using Light Scattering. Methods 2015, 87, 59–63. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, M.; Xie, J.; Jiao, L.; Ding, Y.; Luo, Y. Exposure to Ephedrine Attenuates Th1/Th2 Imbalance Underlying OVA-Induced Asthma through Airway Epithelial Cell-Derived Exosomal Lnc-TRPM2-AS. Chin. J. Nat. Med. 2024, 22, 530–540. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Q.; Tang, W.; Wu, Y.; Lv, J.; Sun, L.; Shi, G.; Wu, M.; Qu, J.; Di, C.; et al. Epithelial Exosomal Contactin-1 Promotes Monocyte-Derived Dendritic Cell-Dominant T-Cell Responses in Asthma. J. Allergy Clin. Immunol. 2021, 148, 1545–1558. [Google Scholar] [CrossRef]
- Mitchel, J.A.; Antoniak, S.; Lee, J.-H.; Kim, S.-H.; McGill, M.; Kasahara, D.I.; Randell, S.H.; Israel, E.; Shore, S.A.; Mackman, N.; et al. IL-13 Augments Compressive Stress-Induced Tissue Factor Expression in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Park, J.-A.; Sharif, A.S.; Tschumperlin, D.J.; Lau, L.; Limbrey, R.; Howarth, P.; Drazen, J.M. Tissue Factor-Bearing Exosome Secretion from Human Mechanically Stimulated Bronchial Epithelial Cells in Vitro and in Vivo. J. Allergy Clin. Immunol. 2012, 130, 1375–1383. [Google Scholar] [CrossRef]
- Zareba, L.; Szymanski, J.; Homoncik, Z.; Czystowska-Kuzmicz, M. EVs from BALF—Mediators of Inflammation and Potential Biomarkers in Lung Diseases. Int. J. Mol. Sci. 2021, 22, 3651. [Google Scholar] [CrossRef]
- Mwase, C.; Schworer, S.A.; Gilmore, R.C.; Khan, F.; Dy, A.B.C.; Haber, A.L.; Boucher, R.C., Jr.; Randell, S.H.; Mackman, N.; Park, J.-A. TGF-β Receptor-Dependent Tissue Factor Release and Proteomic Profiling of Extracellular Vesicles from Mechanically Compressed Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2025. [Google Scholar] [CrossRef]
- Chen, X.; Corry, D.B.; Li, E. Mechanisms of Allergy and Adult Asthma. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Haj-Salem, I.; Plante, S.; Gounni, A.S.; Rouabhia, M.; Chakir, J. Fibroblast-Derived Exosomes Promote Epithelial Cell Proliferation through TGF-Β2 Signalling Pathway in Severe Asthma. Allergy 2018, 73, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human Airway Epithelial Extracellular Vesicle miRNA Signature Is Altered upon Asthma Development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yang, Y.; Chen, S.; Xia, F.; Sun, D.; Fang, D.; Xiong, S.; Jin, L.; Zhang, J. MiR-34a Promotes DCs Development and Inhibits Their Function on T Cell Activation by Targeting WNT1. Oncotarget 2017, 8, 17191–17201. [Google Scholar] [CrossRef]
- Loffredo, L.F.; Abdala-Valencia, H.; Anekalla, K.R.; Cuervo-Pardo, L.; Gottardi, C.J.; Berdnikovs, S. Beyond Epithelial-to-Mesenchymal Transition: Common Suppression of Differentiation Programs Underlies Epithelial Barrier Dysfunction in Mild, Moderate, and Severe Asthma. Allergy 2017, 72, 1988–2004. [Google Scholar] [CrossRef]
- Long, C.M.; Lukomska, E.; Marshall, N.B.; Nayak, A.; Anderson, S.E. Potential Inhibitory Influence of miRNA 210 on Regulatory T Cells during Epicutaneous Chemical Sensitization. Genes 2016, 8, 9. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic Mechanisms in Asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef]
- Gigon, L.; Yousefi, S.; Karaulov, A.; Simon, H.-U. Mechanisms of Toxicity Mediated by Neutrophil and Eosinophil Granule Proteins. Allergol. Int. 2021, 70, 30–38. [Google Scholar] [CrossRef]
- Sangaphunchai, P.; Todd, I.; Fairclough, L.C. Extracellular Vesicles and Asthma: A Review of the Literature. Clin. Exp. Allergy 2020, 50, 291–307. [Google Scholar] [CrossRef]
- Barnes, P.J. Th2 Cytokines and Asthma: An Introduction. Respir. Res. 2001, 2, 64. [Google Scholar] [CrossRef]
- Mazzeo, C.; Cañas, J.A.; Zafra, M.P.; Rojas Marco, A.; Fernández-Nieto, M.; Sanz, V.; Mittelbrunn, M.; Izquierdo, M.; Baixaulli, F.; Sastre, J.; et al. Exosome Secretion by Eosinophils: A Possible Role in Asthma Pathogenesis. J. Allergy Clin. Immunol. 2015, 135, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.; Vera, E.; Marin, J.M.; Sanz-Rubio, D. Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases. Int. J. Mol. Sci. 2021, 22, 583. [Google Scholar] [CrossRef] [PubMed]
- Cañas, J.A.; Sastre, B.; Mazzeo, C.; Fernández-Nieto, M.; Rodrigo-Muñoz, J.M.; González-Guerra, A.; Izquierdo, M.; Barranco, P.; Quirce, S.; Sastre, J.; et al. Exosomes from Eosinophils Autoregulate and Promote Eosinophil Functions. J. Leukoc. Biol. 2017, 101, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Cañas, J.A.; Sastre, B.; Rodrigo-Muñoz, J.M.; Fernández-Nieto, M.; Barranco, P.; Quirce, S.; Sastre, J.; Del Pozo, V. Eosinophil-Derived Exosomes Contribute to Asthma Remodelling by Activating Structural Lung Cells. Clin. Exp. Allergy 2018, 48, 1173–1185. [Google Scholar] [CrossRef]
- Rodrigo-Muñoz, J.M.; Gil-Martínez, M.; Sastre, B.; Del Pozo, V. Emerging Evidence for Pleiotropism of Eosinophils. Int. J. Mol. Sci. 2021, 22, 7075. [Google Scholar] [CrossRef]
- Mahmudi-Azer, S.; Downey, G.P.; Moqbel, R. Translocation of the Tetraspanin CD63 in Association with Human Eosinophil Mediator Release. Blood 2002, 99, 4039–4047. [Google Scholar] [CrossRef]
- Akuthota, P.; Melo, R.C.N.; Spencer, L.A.; Weller, P.F. MHC Class II and CD9 in Human Eosinophils Localize to Detergent-Resistant Membrane Microdomains. Am. J. Respir. Cell Mol. Biol. 2012, 46, 188–195. [Google Scholar] [CrossRef]
- Sastre, B.; Rodrigo-Muñoz, J.M.; Garcia-Sanchez, D.A.; Cañas, J.A.; Del Pozo, V. Eosinophils: Old Players in a New Game. J. Investig. Allergol. Clin. Immunol. 2018, 28, 289–304. [Google Scholar] [CrossRef]
- van Oosterhout, A.J.M.; Bloksma, N. Regulatory T-Lymphocytes in Asthma. Eur. Respir. J. 2005, 26, 918–932. [Google Scholar] [CrossRef]
- Inclan-Rico, J.M.; Ponessa, J.J.; Siracusa, M.C. Contributions of Innate Lymphocytes to Allergic Responses. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 175. [Google Scholar] [CrossRef]
- Lindell, D.M.; Berlin, A.A.; Schaller, M.A.; Lukacs, N.W. B Cell Antigen Presentation Promotes Th2 Responses and Immunopathology during Chronic Allergic Lung Disease. PLoS ONE 2008, 3, e3129. [Google Scholar] [CrossRef] [PubMed]
- Satitsuksanoa, P.; Iwasaki, S.; Boersma, J.; Bel Imam, M.; Schneider, S.R.; Chang, I.; van de Veen, W.; Akdis, M. B Cells: The Many Facets of B Cells in Allergic Diseases. J. Allergy Clin. Immunol. 2023, 152, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Khawar, M.B.; Habiba, U.; Afzal, H.; Hamid, S.E.; Rafiq, M.; Abbasi, M.H.; Sheikh, N.; Abaidullah, R.; Asif, Z.; et al. Diagnostic and Therapeutic Value of EVs in Lungs Diseases and Inflammation. Mol. Biol. Rep. 2023, 51, 26. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of Heat Shock Proteins in B-Cell Exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef]
- Admyre, C.; Bohle, B.; Johansson, S.M.; Focke-Tejkl, M.; Valenta, R.; Scheynius, A.; Gabrielsson, S. B Cell-Derived Exosomes Can Present Allergen Peptides and Activate Allergen-Specific T Cells to Proliferate and Produce TH2-like Cytokines. J. Allergy Clin. Immunol. 2007, 120, 1418–1424. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Paulie, S.; Gabrielsson, S. Direct Exosome Stimulation of Peripheral Human T Cells Detected by ELISPOT. Eur. J. Immunol. 2006, 36, 1772–1781. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Vincent-Schneider, H.; Stumptner-Cuvelette, P.; Lankar, D.; Pain, S.; Raposo, G.; Benaroch, P.; Bonnerot, C. Exosomes Bearing HLA-DR1 Molecules Need Dendritic Cells to Efficiently Stimulate Specific T Cells. Int. Immunol. 2002, 14, 713–722. [Google Scholar] [CrossRef]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune Cells-Derived Exosomes Function as a Double-Edged Sword: Role in Disease Progression and Their Therapeutic Applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Calvo, V.; Izquierdo, M. Inducible Polarized Secretion of Exosomes in T and B Lymphocytes. Int. J. Mol. Sci. 2020, 21, 2631. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Peng, H.; Huyan, T.; Cacalano, N.A. Exosomes: Versatile Nano Mediators of Immune Regulation. Cancers 2019, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, L.N.; Alonso, M.A. Biogenesis and Function of T Cell-Derived Exosomes. Front. Cell Dev. Biol. 2016, 4, 84. [Google Scholar] [CrossRef]
- Shefler, I.; Salamon, P.; Mekori, Y.A. Extracellular Vesicles as Emerging Players in Intercellular Communication: Relevance in Mast Cell-Mediated Pathophysiology. Int. J. Mol. Sci. 2021, 22, 9176. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Li, W.; Chibbar, R.; Xiong, S.; Xiang, J. CD4+ T Cell-Released Exosomes Inhibit CD8+ Cytotoxic T-Lymphocyte Responses and Antitumor Immunity. Cell Mol. Immunol. 2011, 8, 23–30. [Google Scholar] [CrossRef]
- Harker, J.A.; Lloyd, C.M. T Helper 2 Cells in Asthma. J. Exp. Med. 2023, 220, e20221094. [Google Scholar] [CrossRef]
- Shefler, I.; Salamon, P.; Reshef, T.; Mor, A.; Mekori, Y.A. T Cell-Induced Mast Cell Activation: A Role for Microparticles Released from Activated T Cells. J. Immunol. 2010, 185, 4206–4212. [Google Scholar] [CrossRef]
- Bunn, K.E.; Giese-Byrne, B.G.; Pua, H.H. Th2 Cell Extracellular Vesicles Promote Eosinophil Survival through the Cytokine Cargo IL-3 and Prolong Airway Eosinophilia. bioRxiv 2024, 2024.07.23.600647. [Google Scholar] [CrossRef]
- Maurer, M.; Theoharides, T.; Granstein, R.D.; Bischoff, S.C.; Bienenstock, J.; Henz, B.; Kovanen, P.; Piliponsky, A.M.; Kambe, N.; Vliagoftis, H.; et al. What Is the Physiological Function of Mast Cells? Exp. Dermatol. 2003, 12, 886–910. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast Cell-Mediated Immune Regulation in Health and Disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef]
- Deho’, L.; Monticelli, S. Human Mast Cells and Mastocytosis: Harnessing microRNA Expression as a New Approach to Therapy? Arch. Immunol. Ther. Exp. 2010, 58, 279–286. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Lecce, M.; Molfetta, R.; Milito, N.D.; Santoni, A.; Paolini, R. FcεRI Signaling in the Modulation of Allergic Response: Role of Mast Cell-Derived Exosomes. Int. J. Mol. Sci. 2020, 21, 5464. [Google Scholar] [CrossRef] [PubMed]
- Merluzzi, S.; Betto, E.; Ceccaroni, A.A.; Magris, R.; Giunta, M.; Mion, F. Mast Cells, Basophils and B Cell Connection Network. Mol. Immunol. 2015, 63, 94–103. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, Q.; Zhang, Y. MicroRNA-21 Released from Mast Cells-Derived Extracellular Vesicles Drives Asthma in Mice by Potentiating Airway Inflammation and Oxidative Stress. Am. J. Transl. Res. 2021, 13, 7475–7491. [Google Scholar]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.C.; Peronet, R.; David, B.; Namane, A.; Mécheri, S. Mast Cell-Dependent B and T Lymphocyte Activation Is Mediated by the Secretion of Immunologically Active Exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of mRNAs and microRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Xie, G.; Yang, H.; Peng, X.; Lin, L.; Wang, J.; Lin, K.; Cui, Z.; Li, J.; Xiao, H.; Liang, Y.; et al. Mast Cell Exosomes Can Suppress Allergic Reactions by Binding to IgE. J. Allergy Clin. Immunol. 2018, 141, 788–791. [Google Scholar] [CrossRef]
- Eldh, M.; Ekström, K.; Valadi, H.; Sjöstrand, M.; Olsson, B.; Jernås, M.; Lötvall, J. Exosomes Communicate Protective Messages during Oxidative Stress; Possible Role of Exosomal Shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Liu, S.; Xue, Y.; Li, Z.; Wang, T.; Jiao, L.; An, Q.; Liu, B.; Wang, J.; et al. Exosomes from IgE-Stimulated Mast Cells Aggravate Asthma-Mediated Atherosclerosis Through circRNA CDR1as-Mediated Endothelial Cell Dysfunction in Mice. Arterioscler. Thromb. Vasc. Biol. 2024, 44, e99–e115. [Google Scholar] [CrossRef]
- Sabado, R.L.; Bhardwaj, N. Directing Dendritic Cell Immunotherapy towards Successful Cancer Treatment. Immunotherapy 2010, 2, 37–56. [Google Scholar] [CrossRef]
- Gaurav, R.; Agrawal, D.K. Clinical View on the Importance of Dendritic Cells in Asthma. Expert. Rev. Clin. Immunol. 2013, 9, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, O.; Mayer, J.U.; Munoz-Erazo, L.; Ronchese, F. Dendritic Cells in Th2 Immune Responses and Allergic Sensitization. Immunol. Cell Biol. 2020, 98, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Theofani, E.; Tsitsopoulou, A.; Morianos, I.; Semitekolou, M. Severe Asthmatic Responses: The Impact of TSLP. Int. J. Mol. Sci. 2023, 24, 7581. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from Antigen-Pulsed Dendritic Cells Induce Stronger Antigen-Specific Immune Responses than Microvesicles in vivo. Sci. Rep. 2017, 7, 17095. [Google Scholar] [CrossRef]
- Huang, F.; Jia, H.; Zou, Y.; Yao, Y.; Deng, Z. Exosomes: An Important Messenger in the Asthma Inflammatory Microenvironment. J. Int. Med. Res. 2020, 48, 300060520903220. [Google Scholar] [CrossRef]
- Esser, J.; Gehrmann, U.; D’Alexandri, F.L.; Hidalgo-Estévez, A.M.; Wheelock, C.E.; Scheynius, A.; Gabrielsson, S.; Rådmark, O. Exosomes from Human Macrophages and Dendritic Cells Contain Enzymes for Leukotriene Biosynthesis and Promote Granulocyte Migration. J. Allergy Clin. Immunol. 2010, 126, 1032–1040, 1040.e1-4. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 860915. [Google Scholar] [CrossRef]
- Vroman, H.; Hendriks, R.W.; Kool, M. Dendritic Cell Subsets in Asthma: Impaired Tolerance or Exaggerated Inflammation? Front. Immunol. 2017, 8, 941. [Google Scholar] [CrossRef]
- Yamasaki, A.; Okazaki, R.; Harada, T. Neutrophils and Asthma. Diagnostics 2022, 12, 1175. [Google Scholar] [CrossRef]
- Seys, S.F.; Lokwani, R.; Simpson, J.L.; Bullens, D.M.A. New Insights in Neutrophilic Asthma. Curr. Opin. Pulm. Med. 2019, 25, 113–120. [Google Scholar] [CrossRef]
- De Volder, J.; Vereecke, L.; Joos, G.; Maes, T. Targeting Neutrophils in Asthma: A Therapeutic Opportunity? Biochem. Pharmacol. 2020, 182, 114292. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Roux-Dalvai, F.; Droit, A.; Lavoie, J.-P. Neutrophil-Derived Exosomes: A New Mechanism Contributing to Airway Smooth Muscle Remodeling. Am. J. Respir. Cell Mol. Biol. 2016, 55, 450–461. [Google Scholar] [CrossRef]
- Xie, Y.; Abel, P.W.; Casale, T.B.; Tu, Y. TH17 Cells and Corticosteroid Insensitivity in Severe Asthma. J. Allergy Clin. Immunol. 2022, 149, 467–479. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Di, C.; Zhao, C.; Chen, J.; Su, W.; Wu, Q.; Wu, M.; Su, X.; Xia, Z. Increased Airway Epithelial Cell-Derived Exosomes Activate Macrophage-Mediated Allergic Inflammation via CD100 Shedding. J. Cell Mol. Med. 2021, 25, 8850–8862. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Houser, M.C.; Feng, M.; Thorp, E.B.; Nusrat, A.; Parkos, C.A.; Sumagin, R. Deposition of Microparticles by Neutrophils onto Inflamed Epithelium: A New Mechanism to Disrupt Epithelial Intercellular Adhesions and Promote Transepithelial Migration. FASEB J. 2016, 30, 4007–4020. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Chen, Z.-C.; Li, N.; Wang, Z.-H.; Guo, Y.-L.; Tian, C.-J.; Cheng, D.-J.; Tang, X.-Y.; Zhang, L.-X. Exosomal Transfer of Activated Neutrophil-Derived lncRNA CRNDE Promotes Proliferation and Migration of Airway Smooth Muscle Cells in Asthma. Hum. Mol. Genet. 2022, 31, 638–650. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their Role, Activation and Polarization in Pulmonary Diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the Innate and Adaptive Immune System for Tissue Repair and Regeneration: Considering More than Macrophages. Acta Biomater. 2021, 133, 208–221. [Google Scholar] [CrossRef]
- Komlósi, Z.I.; van de Veen, W.; Kovács, N.; Szűcs, G.; Sokolowska, M.; O’Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and Molecular Mechanisms of Allergic Asthma. Mol. Asp. Med. 2022, 85, 100995. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M. Th1/Th2 Paradigm Extended: Macrophage Polarization as an Unappreciated Pathogen-Driven Escape Mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively Activated Macrophages; a Double-Edged Sword in Allergic Asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef]
- Barnes, P.J. Immunology of Asthma and Chronic Obstructive Pulmonary Disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Gharavi, A.T.; Hanjani, N.A.; Movahed, E.; Doroudian, M. The Role of Macrophage Subtypes and Exosomes in Immunomodulation. Cell. Mol. Biol. Lett. 2022, 27, 83. [Google Scholar] [CrossRef]
- Alobaidi, A.H.; Alsamarai, A.M.; Alsamarai, M.A. Inflammation in Asthma Pathogenesis: Role of T Cells, Macrophages, Epithelial Cells and Type 2 Inflammation. Antiinflamm. Antiallergy Agents Med. Chem. 2021, 20, 317–332. [Google Scholar] [CrossRef]
- Nagano, T.; Katsurada, M.; Dokuni, R.; Hazama, D.; Kiriu, T.; Umezawa, K.; Kobayashi, K.; Nishimura, Y. Crucial Role of Extracellular Vesicles in Bronchial Asthma. Int. J. Mol. Sci. 2019, 20, 2589. [Google Scholar] [CrossRef]
- Hansbro, P.M.; Kim, R.Y.; Starkey, M.R.; Donovan, C.; Dua, K.; Mayall, J.R.; Liu, G.; Hansbro, N.G.; Simpson, J.L.; Wood, L.G.; et al. Mechanisms and Treatments for Severe, Steroid-Resistant Allergic Airway Disease and Asthma. Immunol. Rev. 2017, 278, 41–62. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Zhou, T.; Hu, J.; Dai, B.; Yi, F.; Tian, N.; Jiang, L.; Dong, X.; Zhu, Q.; et al. MicroRNA-370 Carried by M2 Macrophage-Derived Exosomes Alleviates Asthma Progression through Inhibiting the FGF1/MAPK/STAT1 Axis. Int. J. Biol. Sci. 2021, 17, 1795–1807. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Abbaspour-Aghdam, S.; Kamrani, A.; Valizadeh, H.; Nadiri, M.; Sadeghi, A.; Shamsasenjan, K.; Jadidi-Niaragh, F.; Roshangar, L.; et al. Chronic Obstructive Pulmonary Disease and Asthma: Mesenchymal Stem Cells and Their Extracellular Vesicles as Potential Therapeutic Tools. Stem Cell Res. Ther. 2022, 13, 262. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, L.; Zhu, Y.; Xu, Y. Mesenchymal Stem Extracellular Vesicles in Various Respiratory Diseases: A New Opportunity. J. Inflamm. Res. 2024, 17, 9041–9058. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.L.; Bang, J.-Y.; Kim, J.; Mo, Y.; Kim, Y.; Lee, C.-G.; Elias, J.A.; Kim, H.Y.; Kang, H.-R. Mesenchymal Stem Cells Exert Their Anti-Asthmatic Effects through Macrophage Modulation in a Murine Chronic Asthma Model. Sci. Rep. 2022, 12, 9811. [Google Scholar] [CrossRef]
- Mun, S.J.; Kang, S.A.; Park, H.-K.; Yu, H.S.; Cho, K.-S.; Roh, H.-J. Intranasally Administered Extracellular Vesicles from Adipose Stem Cells Have Immunomodulatory Effects in a Mouse Model of Asthma. Stem Cells Int. 2021, 2021, 6686625. [Google Scholar] [CrossRef]
- de Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human Adipose Tissue Mesenchymal Stromal Cells and Their Extracellular Vesicles Act Differentially on Lung Mechanics and Inflammation in Experimental Allergic Asthma. Stem Cell Res. Ther. 2017, 8, 151. [Google Scholar] [CrossRef]
- Worthington, E.N.; Hagood, J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020, 21, 2318. [Google Scholar] [CrossRef]
- Li, X.; Yang, N. Exosome miR-223-3p in the Bone Marrow-Derived Mesenchymal Stem Cells Alleviates the Inflammation and Airway Remodeling through NLRP3-Induced ASC/Caspase-1/GSDMD Signaling Pathway. Int. Immunopharmacol. 2023, 123, 110746. [Google Scholar] [CrossRef]
- Zhuansun, Y.; Du, Y.; Huang, F.; Lin, L.; Chen, R.; Jiang, S.; Li, J. MSCs Exosomal miR-1470 Promotes the Differentiation of CD4+CD25+FOXP3+ Tregs in Asthmatic Patients by Inducing the Expression of P27KIP1. Int. Immunopharmacol. 2019, 77, 105981. [Google Scholar] [CrossRef]
- Shan, L.; Liu, S.; Zhang, Q.; Zhou, Q.; Shang, Y. Human Bone Marrow-Mesenchymal Stem Cell-Derived Exosomal microRNA-188 Reduces Bronchial Smooth Muscle Cell Proliferation in Asthma through Suppressing the JARID2/Wnt/β-Catenin Axis. Cell Cycle 2022, 21, 352–367. [Google Scholar] [CrossRef]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.-Y.; Wang, G.; et al. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Attenuate the Inflammation of Severe Steroid-Resistant Asthma by Reshaping Macrophage Polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef]
- Gu, W.; Zheng, T.; Li, W.; Luo, X.; Xu, X.; Wang, Y.; Mao, C.; Ma, Y.; Dong, L. Migrasomes Derived from Human Umbilical Cord Mesenchymal Stem Cells: A New Therapeutic Agent for Ovalbumin-Induced Asthma in Mice. Stem Cell Res. Ther. 2025, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Luo, X.; Gao, X.; Gu, W.; Ma, Y.; Xu, L.; Yu, M.; Liu, X.; Liu, J.; et al. A Non-Invasive Strategy for Suppressing Asthmatic Airway Inflammation and Remodeling: Inhalation of Nebulized Hypoxic hUCMSC-Derived Extracellular Vesicles. Front. Immunol. 2023, 14, 1150971. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, Y.; Mao, Y.; Xu, X.; Gu, W.; Li, W.; Mao, C.; Zheng, T.; Dong, L. Nebulization of Hypoxic hUCMSC-EVs Attenuates Airway Epithelial Barrier Defects in Chronic Asthma Mice by Transferring CAV-1. Int. J. Nanomed. 2024, 19, 10941–10959. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, J.; Zhang, T. From Mesenchymal Stem Cells to Their Extracellular Vesicles: Progress and Prospects for Asthma Therapy. Asian J. Pharm. Sci. 2024, 19, 100942. [Google Scholar] [CrossRef]
- Jangam, T.C.; Desai, S.A.; Patel, V.P.; Pagare, N.B.; Raut, N.D. Exosomes as Therapeutic and Diagnostic Tools: Advances, Challenges, and Future Directions. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef]
- Dilsiz, N. A Comprehensive Review on Recent Advances in Exosome Isolation and Characterization: Toward Clinical Applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef]
- Lehrich, B.M.; Liang, Y.; Fiandaca, M.S. Foetal Bovine Serum Influence on in vitro Extracellular Vesicle Analyses. J. Extracell. Vesicles 2021, 10, e12061. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- ElKashef, S.M.M.A.E.; Ahmad, S.E.-A.; Soliman, Y.M.A.; Mostafa, M.S. Role of microRNA-21 and microRNA-155 as Biomarkers for Bronchial Asthma. Innate Immun. 2021, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Soccio, P.; Moriondo, G.; Lacedonia, D.; Tondo, P.; Pescatore, D.; Quarato, C.M.I.; Carone, M.; Foschino Barbaro, M.P.; Scioscia, G. MiRNA and Exosomal miRNA as New Biomarkers Useful to Phenotyping Severe Asthma. Biomolecules 2023, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mera, S.; Martelo-Vidal, L.; Miguéns-Suárez, P.; Saavedra-Nieves, P.; Arias, P.; González-Fernández, C.; Mosteiro-Añón, M.; Corbacho-Abelaira, M.D.; Blanco-Aparicio, M.; Méndez-Brea, P.; et al. Serum Exosome Inflamma-miRs Are Surrogate Biomarkers for Asthma Phenotype and Severity. Allergy 2023, 78, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-kappaB-Dependent Induction of microRNA miR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Jadamba, B.; Jin, Y.; Lee, H. Harmonising Cellular Conversations: Decoding the Vital Roles of Extracellular Vesicles in Respiratory System Intercellular Communications. Eur. Respir. Rev. 2024, 33, 230272. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, N.A.; Castagna, A.; Ambrosani, F.; Argentino, G.; Friso, S.; Zurlo, M.; Beri, R.; Maule, M.; Vaia, R.; Senna, G.; et al. Extracellular Vesicles in Asthma: Intercellular Cross-Talk in TH2 Inflammation. Cells 2025, 14, 542. https://doi.org/10.3390/cells14070542
Cheema NA, Castagna A, Ambrosani F, Argentino G, Friso S, Zurlo M, Beri R, Maule M, Vaia R, Senna G, et al. Extracellular Vesicles in Asthma: Intercellular Cross-Talk in TH2 Inflammation. Cells. 2025; 14(7):542. https://doi.org/10.3390/cells14070542
Chicago/Turabian StyleCheema, Naila Arif, Annalisa Castagna, Francesca Ambrosani, Giuseppe Argentino, Simonetta Friso, Marco Zurlo, Ruggero Beri, Matteo Maule, Rachele Vaia, Gianenrico Senna, and et al. 2025. "Extracellular Vesicles in Asthma: Intercellular Cross-Talk in TH2 Inflammation" Cells 14, no. 7: 542. https://doi.org/10.3390/cells14070542
APA StyleCheema, N. A., Castagna, A., Ambrosani, F., Argentino, G., Friso, S., Zurlo, M., Beri, R., Maule, M., Vaia, R., Senna, G., & Caminati, M. (2025). Extracellular Vesicles in Asthma: Intercellular Cross-Talk in TH2 Inflammation. Cells, 14(7), 542. https://doi.org/10.3390/cells14070542








